Abstract
Background
The purpose of this study was to evaluate in vitro cytotoxicity and antifibrotic effects of mitomycin C on normal and scarred human vocal fold fibroblasts.
Methods
Fibroblasts were subjected to mitomycin C treatment at 0.2, 0.5, or 1 mg/mL, or serum control. Cytotoxicity, immunocytochemistry, and Western blot for collagen I/III were performed at days 0, 1, 3, and 5.
Results
Significant decreases in live cells were measured for mitomycin C-treated cells on days 3 and 5 for all doses. Extracellular staining of collagen I/III was observed in mitomycin C-treated cells across all doses and times. Extracellular staining suggests apoptosis with necrosis, compromising the integrity of cell membranes and release of cytosolic proteins into the extracellular environment. Western blot indicates inhibition of collagen at all doses except 0.2 mg/mL at day 1.
Conclusion
A total of 0.2 mg/mL mitomycin C may provide initial and transient stimulation of collagen for necessary repair to damaged tissue without the long-term risk of fibrosis.
Keywords: mitomycin C, larynx, vocal folds, fibroblasts, surgical scar
Introduction
Mitomycin C is a natural compound isolated from gram-negative bacteria Streptomyces caespitosus.1 Because of the alkylating ability in its reduced state, mitomycin C can form carbonium ions that cross-link double-stranded DNA at adenosine and guanine during late G1 or S phases preventing DNA strands from separating during their replication, thus halting mitosis. Mitomycin C also binds to the promoter sites of inducible genes to suppress cellular RNA and protein synthesis.1 With these multiple properties, mitomycin C is widely used as antibacterial antibiotic and chemotherapeutic drugs to modulate cell replication and protein synthesis for disease control.2–4 Use of mitomycin C has recently been explored as an antifibrotic drug to control postoperative scarring, particularly for circular structures of organs such as the eye and airway.5–9
Scarring is an unpredictable, dynamic, and undesired byproduct of surgical, radiation-induced, thermal, or any other injury that results in detrimental changes in tissue viscoelasticity and physiology. The scarring response to injury includes proliferation of fibroblasts and their excessive secretion of collagen matrix proteins into the wound site, resulting in the formation of fibrosis, clinically recognized as a scar. In the airway, postoperative scarring can compromise an individual's breathing, swallowing, and communication. Mitomycin C has been studied in vivo and clinically for various fibrotic diseases in the airway, such as glottis and subglottic stenosis as well as tracheal and esophageal conditions.6,7,10–14 Mitomycin C has been shown to have antifibrotic functions in the airway through the inhibition of fibroblast proliferation and inhibition of their collagen synthesis.15–19 In the airway literature, mitomycin C has been used in a wide range of doses (range, 0.1–1.0 mg/mL), exposure durations (range, 2–5 minutes), and frequencies (a single dose to 4 reapplications).7 Benefits of mitomycin C topical application in reducing scar formation have been reported empirically. The effectiveness of topical mitomycin C application in the prevention of anterior glottis stenosis after microre-section of anterior commissure carcinomas has been evaluated in a noncontrolled, nonrandomized study.14 After surgery, 33% of patients developed acceptably small webs in the anterior glottis that had no influence on the voice quality. Vocal fold atrophies were not observed. In a prospective randomized double-blind placebo-controlled study, a single versus double topical application of mitomycin C was compared for effectiveness in reducing scarring or restenosis of the airway in patients with laryngotracheal stenosis after endoscopic CO2 laser and dilation procedures.6 The dose and application time of mitomycin C was 0.5 mg/mL and 5 minutes, respectively. The 2-application group showed longer relapse rate of airway stenosis than the single-application group. Authors suggested that mitomycin C may delay but does not necessarily prevent the recurrence of stenosis. Animal studies including canines,20–24 rabbits,25 and swine26,27 also showed the improvement of airway wound healing after mitomycin C application. Specific to the management of vocal fold scarring, topical application of mitomycin C has been shown to lead to (1) reduced anterior glottic web formation, (2) decreased total collagen deposition, (3) improved cricoarytenoid joint mobility, and (4) decreased granulation tissue development.6,7
The risk and benefit ratio of mitomycin C application remains a concern in clinical practice. Mitomycin C has been shown to be toxic through the generation of oxygen radicals28 and the induction of apoptosis.29,30 Apoptotic cells release inflammatory mediators, such as tumor necrosis factor and high mobility group box 1 that amplify or trigger inflammation causing prolonged tissue damage.31–33 In the airway literature, both clinical and animal studies have demonstrated possible risks with the use of mitomycin C. In a retrospective study, 5% of patients who underwent airway dilation procedures were reported to have fibrinous debris accumulated at the mitomycin C application site and causing airway obstruction.34 This complication was even more pronounced in pediatric patients who have smaller airways.17 Use of mitomycin C may also call for carcinogenic caution because of its alkylating property. In a case report, a nonsmoking patient who was treated with repeated application of topical mitomycin C to the larynx for an anterior glottis web, developed laryngeal cancer 2.5 years after mitomycin C treatment.35 Animal studies suggest that a high concentration of mitomycin C could be a factor in the occurrence of adverse events. In a rabbit study, a high dose of mitomycin C (1 mg/mL) or saline was topically applied to the injured trachea. Early complications of mitomycin C were found in 45% of the animals whose airways were obstructed by unresolved scabs at the mitomycin C-treated site, leading to early death.36 The cross-sectional area of the lumen of the trachea was similar between the mitomycin C-treated and saline-control wounds. Interestingly, re-epithelization was significantly delayed in all mitomycin C-treated wounds. In a canine study, a single dose of mitomycin C at 0.4 mg/mL was topically applied to the surgically injured vocal folds for 3 minutes.37 Four weeks after, the mucosal wave of the vocal folds was found to be impaired on the mitomycin C-treated side of the vocal folds in 3 of the 6 dogs as examined by videolaryngostroboscopy. Histologic evaluation demonstrated signs of atrophy and a reduction in the number of fibroblasts on the mitomycin C-treated side compared to controls. Conversely, low doses of mitomycin C may be ineffective in the prevention of scar formation. In a randomized controlled trial, a single dose of 0.2 mg/mL mitomycin C or isotonic sodium chloride was applied to children for 2 minutes after laryngotracheal reconstruction in the prevention of postintubation stenosis.38 An interim analysis showed that the size of granulation tissue was not different between the mitomycin C-treated and the control groups. The study was prematurely terminated on the counsel of the Data Safety and Monitoring Committee.
Collectively, an optimal dose of mitomycin C should allow the necessary repair process to replace the lost tissue after injury but also be able to control scar formation and wound contracture. The wide range of treatment doses, drug exposure duration, and application methodologies in clinical practice and research studies yield conflicting outcomes and uncertain risk benefit ratio for mitomycin C intervention. Specific to the vocal folds, there is a paucity of data available regarding the toxicity of mitomycin C. More controlled in vitro studies are needed to provide a better understanding of the mechanism of action of mitomycin C in the modulation of vocal fold wound repair. The purpose of this study was to evaluate in vitro cytotoxicity and antifibrotic effects of mitomycin C on fibroblasts isolated from human normal and scarred vocal folds. Clinically relevant doses of mitomycin C were tested. Results may provide insights into optimal mitomycin C application for the control of postoperative vocal fold scarring.
Materials and Methods
Human vocal fold fibroblast cell cultures
Cytotoxicity and the antifibrotic effect of mitomycin C on human normal and scarred vocal fold fibroblasts (nVFFs and sVFFs, respectively) were evaluated. Primary cultures of nVFF and sVFF were obtained from explants from normal and scarred human vocal fold tissue, respectively. The normal human vocal fold specimen was harvested from an autopsy of a 21-year-old donor within 4 hours of death.39,40 The scarred human vocal fold specimen was harvested during phonosurgery of removing the vocal fold scar from a 56-year-old female patient.41 All tissue was received in compliance with the University of Wisconsin Madison Institutional Review Board. To isolate and culture the cells, vocal fold lamina propria tissue was cut into small pieces and re-suspended in Dulbecco's modified Eagle's medium (DMEM) cell culture medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 0.01 mg/mL streptomycin, and 1× nonessential amino acid (all materials were from Sigma, St. Louis, MO). Cells were cultured at 37°C in a humidified 5% CO2 atmosphere. These cells were identified as fibroblasts using standard morphological and immunohistochemical criteria.42 Full immortalization details and characterization of nVFF is described in previous reports.39,40 All experiments described in this article were carried out using immortalized nVFF and primary sVFF from passages P7 to P11.
Cytotoxity analysis
To quantitatively evaluate cytotoxicity of mitomycin C, viability of mitomycin C-treated nVFF and sVFF was measured using Cytotox-Glo Cytotoxicity Assay (Promega, Madison,WI). This assay uses sequential luminescent measures to detect both the dead and live cell population in a sample through the use of a luminogenic peptide substrate (alanyl-alanylphenylalanyl-aminoluciferin) to measure a dead-cell protease activity, which are released from dead cells that have lost membrane integrity. The luminescent signal (relative light unit) reflects the dead-cell protease level and is proportional to the number of dead cells. Followed by a lysis procedure, the assay measures the total luminescence in the sample and is proportional to the total population of cells. Viability, which is expressed in percentage of live cells from the total cell population, can then be calculated from subtracting the luminescent signal results from experimental cell death from total luminescent values.
The nVFF and sVFF were seeded and incubated in 96-well plates at 10,000 cells/well with DMEM-10% FBS for 48 hours to ensure cell adhesion and growth in the well. Cells were then starved with serum-free DMEM for 24 hours. After starvation, cells were treated with mitomycin C (0, 0.2, 0.5, 1.0 mg/mL) for 2 minutes, washed with serum-free DMEM 3 times, and then followed with incubation in serum-free DMEM. On days 0, 1, 3, and 5 of culture, cell populations were monitored in quadruplicate for each condition using the aforesaid Cytotox-Glo Cytotoxicity Assay in accord with the manufacturer's instructions. In brief, 50 μL CytoTox-Glo assay reagent was added to each well. Plates were mixed briefly by orbital shaking and incubated for 15 minutes at room temperature. Luminescent output was read on a Flex Station III plate reader (Molecular Devices, Sunnyvale, CA). After subtracting the background of negative control (serum free-DMEM only without cells), the relative dead cell number was calculated. Subsequently, 50 μL of digitonin lysis agent was added to all wells. Plates were mixed briefly by orbital shaking and incubated for 15 minutes at room temperature and scanned by a plate-reader. Relative total cell population was calculated after subtracting the background control. Percentage of live cells was calculated as (relative total cell population – relative dead cell population)/relative total cell population × 100.
Immunocytochemistry
Spatial localization of collagen I and III in mitomycin C-treated vocal fold fibroblasts was assessed using immunofluorescent staining. The nVFF and sVFF were seeded into sterile Permanox 8-chamber slides (Lab-Tek, Thermo Fisher Scientific, Rochester, NY) at a density of 5 × 104 cells per cm2 in DMEM-10% FBS. Cells were incubated until 100% confluence was reached (approximately 4 days). Cells were then starved with serum-free DMEM for 24 hours. After starvation, cells were treated with mitomycin C (0, 0.2, 0.5, 1.0 mg/mL) for 2 minutes and then washed with serum-free DMEM 3 times. Cells were incubated in serum-free DMEM for 1, 3, or 5 days. After incubation, cells were washed 3 times with ice-cold phosphate-buffered saline (PBS) for 15 minutes each. Cells were then fixed with 4% paraformaldehyde for 15 minutes at room temperature, washed 3 times with PBS for 10 minutes each, and permeabilized for 60 minutes in 0.5% Triton X-100 (Sigma, St Louis, MO). After permeabilization, cells were incubated with 5% normal goat serum for 60 minutes, with the primary antibodies directly against rabbit polyclonal antihuman collagen I and III (ab34710 for collagen I and ab7778 for collagen III; Abcam, Cambridge, MA) both in 1:1000 dilution for 90 minutes and corresponding fluorescence-conjugated (Alexa 488 and 568; A11008 and A11011 from Invitrogen, Carlsbad, CA) secondary antibodies for 60 minutes. Cells were then washed 3 times with 0.1% phosphate-buffered saline Tween for 10 minutes. Chambers and gaskets were carefully removed and the slides were mounted with VECTASHIELD Mounting Medium with diamidino-phenylindole (DAPI; Vector Lab, Burlingame, CA) to preserve fluorescence and to label cell nuclei. Negative control cells were processed identically without application of primary antibodies. Mounted slides were examined on a Nikon Eclipse E600 fluorescent microscope (Nikon, Melville, NY) and images were captured on a Pixera color camera (Pixera, Los Gatos, CA).
A supplementary experiment was carried out to confirm if the localization of the collagen staining was extracellular or intracellular. The sVFF was treated with mitomycin C and incubated in serum-free DMEM for the duration, as aforementioned. Only the mitomycin C dose of 0 and 1.0 mg/mL were included in this validation experiment. The aforesaid staining procedures were performed, except the cells were subjected to either the permeabilization buffer or not.
Preparation of cell culture lysates for Western blot
The nVFF and sVFF were seeded into 100-mm polysty-rene plastic plates in DMEM with 10% FBS and were grown in a 37°C incubator with 5% CO2 until 100% confluence was reached. Cells were then starved with serum-free DMEM for 24 hours. After starvation, cells were treated with mitomycin C (0, 0.2, 0.5, 1.0 mg/mL) for 2 minutes and then washed with serum-free DMEM 3 times. Cells were incubated in serum-free DMEM for 1, 3, or 5 days. At the end of incubation, cells were washed with PBS and trypsinized. Live cells in each plate were counted using trypan blue. Total cell protein was then extracted by using the M-Per protein extraction kit (Pierce, Rockford, IL) in accord with the manufacturer's instructions. Protein concentration was measured using BCA protein assay (Thermo Scientific, Rockford, IL) in duplicates. Protein aliquots were stored at −80°C for downstream Western blot analysis.
Western blot
For each protein sample, concentration of collagen I and III were measured using Western blots. Total protein (30 μg for collagen I and 50 μg for collagen III) was subjected to standard denaturing precast NuPAGE 4% to 12% Bis-Tris gel electrophoresis (Invitrogen) using NuPAGE MOPS SDS buffer. Proteins were then transferred onto nitrocellulose membranes using an XCELL II Blot Module (Invitrogen) and blocked using Invitrogen blocking buffer overnight at 4°C. Membranes were probed for rabbit polyclonal antihuman collagen I (ab292, 1:500 dilution; Abcam) or mouse-monoclonal antihuman collagen III (ab6310, 1:167 dilution; Abcam) for 1 hour at room temperature. MagicMark XP Western protein standard (Invitrogen, Carlsbad, CA) was used to validate bands in the range of 20 to 220 kDa. Bound antibodies on the nitrocellulose membrane were detected using the WesternBreeze antirabbit or antimouse chemiluminescent kit (Invitrogen) for collagen I and collagen III, respectively. Chemiluminescent signal was captured by a charge-coupled digital camera system (LAS-4000m Fuji-film, Japan). The next day, membranes were incubated either with rabbit polyclonal antihuman GAPDH (ab9485, 1:1,000 dilution; Abcam) for the collagen I membrane, or with mouse monoclonal antihuman glyceraldehyde 3-phosphate dehydrogenase (GAPDH; G8173, 1:50,000 dilution; Invitrogen) for the collagen III membrane. Same signal detection and capture procedures for GAPDH loading controls were performed as aforesaid. Relative densities of the bands were measured using ImageJ analysis software (http://rsb.info.nih.gov/ij) and normalized to the respective band density of GAPDH loading control and the number of live cells.
Statistical analysis
Three-way analysis of variance was used to test the differences in the percentage of live cells' time points, mitomycin C dose, and cell type. If F-tests revealed significant differences, post-hoc pairwise comparisons using Fisher protected least squares difference were carried out. An α-level of 0.05 was used for all post-hoc comparisons.
Results
Mitomycin C cytotoxity
Cell viability was evaluated by determining the percentage of live cell using cytotoxity assays (Figure 1). No significant differences in cell viability were noted for the serum control group (0 mg/mL) throughout the period. No significant dose effects were noted for either nVFF or sVFF at each time point. After averaging the data from the 3 dose groups at each time point, the mean (SD) percentage of live nVFF cells was estimated to be 78% (±1.8), 63% (±5.4), and 40% (±12.8) of the total cell population at days 1, 3, and 5, respectively. For sVFF, the mean (SD) percentage of live cells was estimated to be 65% (±4.6), 47% (±7.8), and 31% (±7.9) of the total cell population at days 1, 3, and 5, respectively. Compared to day 0, cell viability in mitomycin C-treated sVFF and nVFF cultures decreased significantly from day 3 and day 5, respectively (p < .0001; Figure 1). In addition, compared to the control group, cell viability in both mitomycin C-treated nVFF and sVFF cultures decreased significantly in all doses at both days 3 and 5 (p < .0001; Figure 1).
Figure 1.
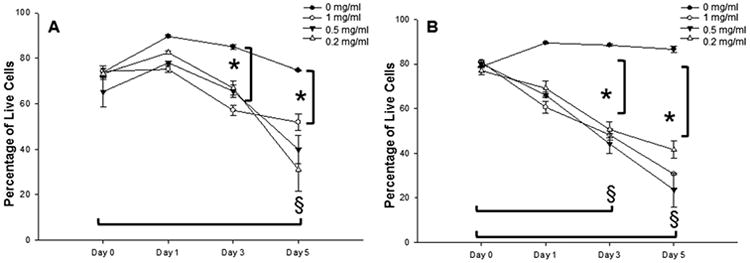
Percentage of live cells in mitomycin C-treated fibroblast cultures. (A and B) Show human normal vocal fold fibroblasts (nVFFs) and scarred vocal fold fibroblasts (sVFFs), respectively. Line and error bars represent means and the SEs of the data (n=4 for each group). *Denotes a statistically significant dose effect of that time point compared to 0 mg/mL. §Denotes a statistically significant time effect of all doses except 0 mg/mL compared to day 0.
Collagen I and III expression
Immunofluorscence staining
Immunofluorescence indicated differentiated collagen distribution between control and mitomycin C-treated groups (Figures 2 and 3). Collagen staining was observed around cell nuclei in the control group, whereas a more spreading and web-like pattern sprouting from cell nuclei was observed in all mitomycin C-treatment groups. Such patterns were consistent across doses, cell types, and time points. A validation experiment was carried out to determine if the collagen distribution was intracellular or extracellular (Figure 4). Cells were exposed to either with or without permeabilization buffer before staining. In comparing the controls under the two permeabilization conditions (Figures 4A, 4E, 4I, and 4M), a spreading collagen web was observed in the nonpermeabilization but not the permeabilization group. The cell membranes were expected to remain intact in the absence of permeabilization and thus the distinctive web-like pattern seen was likely because of the secreted collagen being precipitated into the extracellular space. For all mitomycin C-treated groups, similar web-like collagen staining patterns were observed in both permeabilization conditions. A bright fluorescent purplish or pinkish DAPI stain was also observed qualitatively in mitomycin C-treated groups (Figures 4G, 4H, 4K, 4L, 4O, and 4P) compared with the control groups.
Figure 2.
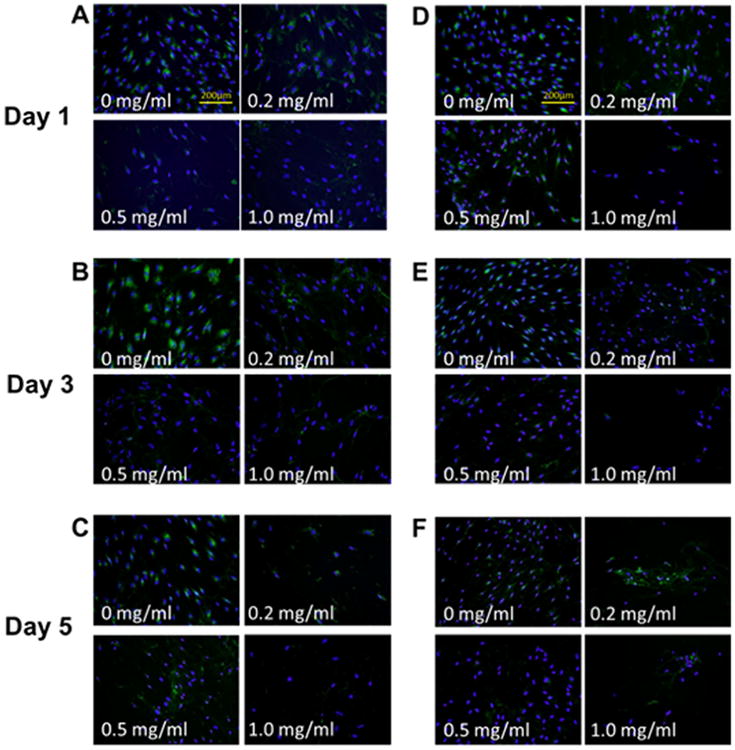
Collagen type I expression of fibroblasts after mitomycin C treatments. (A–C) Representative immunocytochemistry (ICC) staining of normal vocal fold fibroblasts (nVFFs) over 5 days after various doses of mitomycin C administration (original magnification ×200). (D–F) Representative ICC staining of scarred vocal fold fibroblasts (sVFFs). Collagen type I was stained green and cell nuclei was stained blue. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
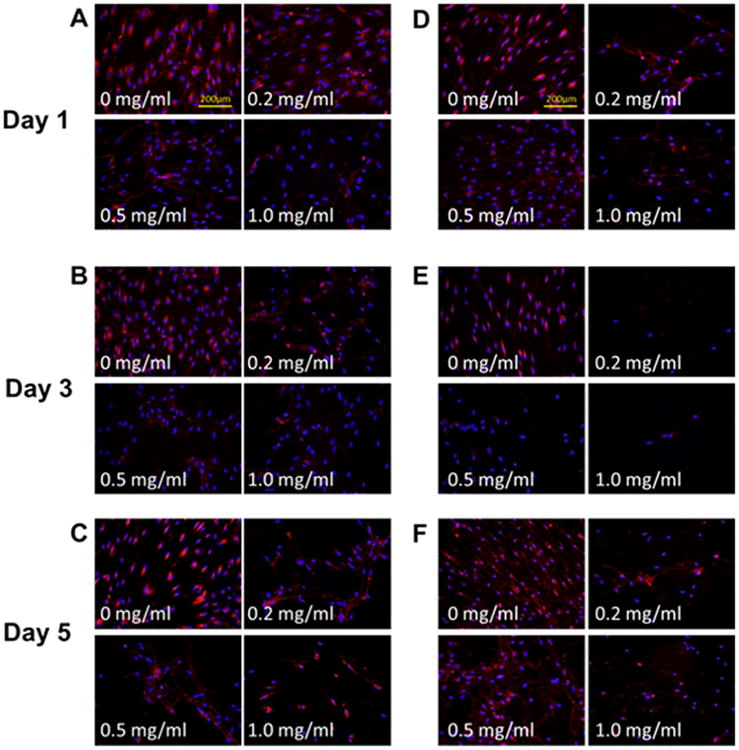
Collagen type III expression of fibroblasts after mitomycin C treatments. (A–C) Representative immunocytochemistry (ICC) staining of normal vocal fold fibroblasts (nVFFs) over 5 days after various doses of mitomycin C administration (original magnification ×200). (D–F) Representative ICC staining in scarred vocal fold fibroblasts (sVFFs). Collagen type III was stained red and cell nuclei were stained blue. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 4.
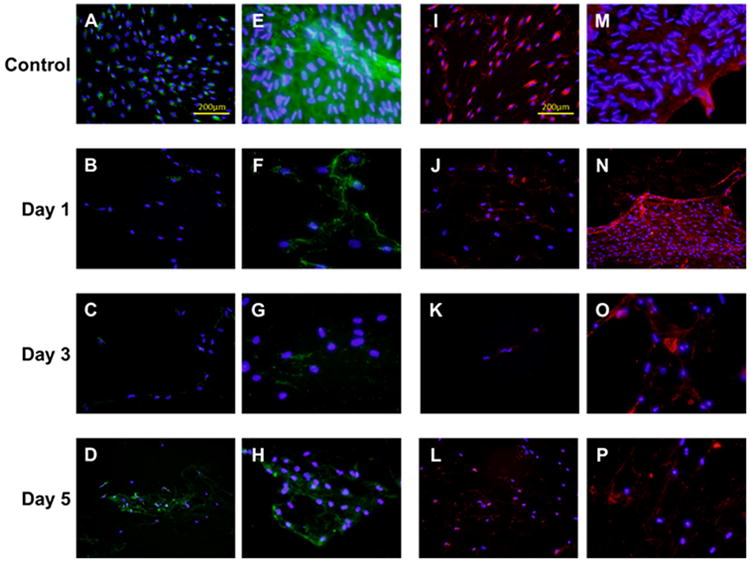
Collagen type I and III expression of scarred vocal fold fibroblasts (sVFFs) after mitomycin C treatments in the validation experiment (original magnification ×400). All cells were treated with 1.0 mg/ml mitomycin C except the serum controls. (A–H) Representative immunocytochemistry (ICC) staining of collagen I (A–D) with cell membrane permeabilization (E–H) in the absence of permeabilization. (I–P) Representative ICC staining of collagen III (I–L) with cell membrane permeabilization (M–P) in the absence of permeabilization. Collagen type I and III was stained green and red respectively. Cell nuclei were stained blue. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Western blotting
In certain dose and time conditions (0.5 mg/mL of sVFF at day 3 and all treatment doses of both cultures at day 5), the amount of total protein extracted was insufficient for running any single Western blot lanes secondary to the significant reduction in cell viability. Western blots (Figures 5 and 6) of collagen type I and III were thus performed for days 1 and 3 only. For these 2 time points, mitomycin C showed modulation of collagen types I and III expression in both nVFF and sVFF. Dose-dependent treatment with mitomycin C downregulated collagen type I and III in sVFF and nVFF in a linear manner within the same time period. The effective dose for nVFF to decrease collagen type I was not reached until 1.0 mg/mL at days 1 and 3 (Figures 5C and 5D), as this was the first mitomycin C dose in which collagen type I production decreased by more than half compared to no-treatment controls (Figure 5C vs 5D). Expression of collagen III in mitomycin C-treated nVFF at day 3 was qualitatively higher than day 1 (Figure 6C vs 6D) across doses, whereas comparable mitomycin C effects were not seen in both collagen type I and III production for sVFF. Last, for both time points, a qualitative increase at the 0.2 mg/mL dose was measured for collagen type I from nVFF and collagen type III from sVFF compared to no-treatment controls.
Figure 5.
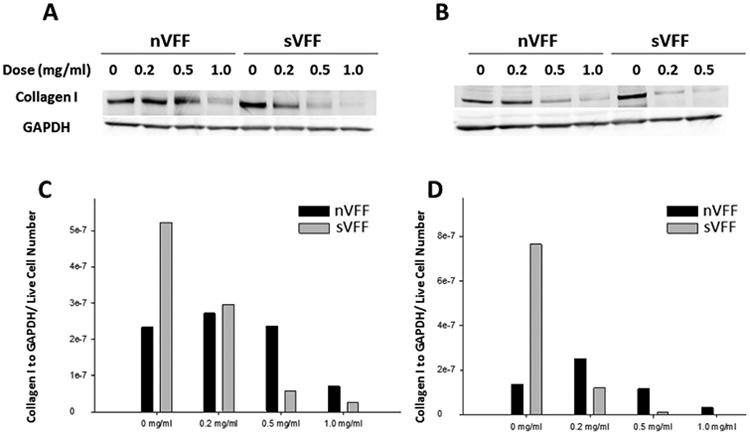
Western blot analysis of collagen I in mitomycin C-treated normal vocal fold fibroblasts (nVFFs) and scarred vocal fold fibroblasts (sVFFs). (A and B) Western immunoblots of mitomycin C-treated fibroblasts at 1 day and 3 days after treatment, respectively. Blots were analyzed by densitometry and collagen type I expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and live cell numbers by trypan blue. (C and D) Show the densitometric results of Western blots at day 1 and day 3, respectively.
Figure 6.
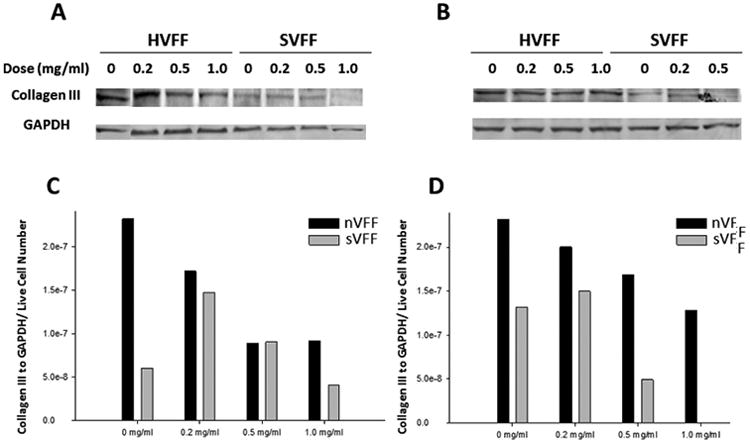
Western blot analysis of collagen III in mitomycin C-treated normal vocal fold fibroblasts (nVFFs) and scarred vocal fold fibroblasts (sVFFs). Western blot analysis of collagen III in mitomycin C-treated nVFF and sVFF. (A and B) Show the Western immunoblots of mitomycin C-treated fibroblasts at 1 day and 3 days after treatment, respectively. Blots were analyzed by densitometry and collagen type III expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and live cell numbers by trypan blue. (C and D) Show the densitometric results of Western blots at day 1 and day 3, respectively.
Discussion
Wound healing is an innate tissue response to injuries with the primary intention to restore normal architecture and function of the damaged or lost tissue. The response to injury triggers a highly complex acute inflammation and healing response. During wound healing, immune and repair cells (eg, neutrophils, macrophages, fibroblasts) and signaling molecules (eg, inflammatory cytokines, growth factors) locate damage tissue, eliminate necrotic cells and debris, and produce extracellular matrix (ECM) substances for eventual tissue repair.43–48 Antifibrotic agents are designed to target fibroblasts and collagen production with the purpose of minimizing postoperative scarring risk in order to make the surgery safer and more successful. An ideal drug for tissue healing would be to accelerate the repair process while minimizing the risk of long-term fibrosis.
Mitomycin C has been used as an antifibrotic drug clinically in surgery of laryngotracheal stenosis. Use of mitomycin C has, however, accounted for adverse effects, including healing delays, mucosal atrophy, and cell toxicity.37 Optimizing the parameters of mitomycin C delivery, including its concentration, exposure duration, delivery method, and timing of application have been researched in many other tissue types but not systematically in the vocal folds.15,16,29,49–53 Fibroblasts from different tissues can produce dissimilar ratios of collagens and respond uniquely to the signaling molecules.54 A better understanding of the mitomycin C effects on the actual tissue involved is necessary to optimize the parameters for drug delivery.
In the current study, cell culture experiments using human vocal fold fibroblasts were used to better define the effects of mitomycin C concentration on cytotoxity and collagen production. Vocal fold fibroblasts are the dominant cell types in healthy vocal fold mucosa and are attracted to the wound site after injury.55 Vocal fold fibroblasts produce essential ECM proteins including hyaluronan, fibronectin, and collagen forming the interstitial matrix to maintain vocal fold structure and function.56 Type I and type III collagen are major structural proteins in vocal fold mucosa. Collagen type III fibers were found more wavy and thicker than type I fibers in human vocal folds.57 Collagen type III fibers were suggested for maintaining lamina propria structure, whereas collagen type I were for providing tensile strength around the basement membrane zone of the vocal folds. Excessive and disorganized collagen deposition is one hallmark of postoperative vocal fold scarring,56,58 resulting in an increase of the stiffness of the vocal fold mucosa, affecting the propagation of normal mucosal wave, and, in turn, causing impairments of vocal fold vibration for proper voice quality. In normal vocal folds, there is more collagen type I than type III but the ratio is reversed in scarred vocal folds.57,59–63 Upon surgical injury, levels of both collagen types are elevated initially in the vocal folds. Starting from day 7, collagen type I expression declines but collagen type III stays elevated throughout the later tissue remodeling process.60–63 Our results showed that both nVFF and sVFF produced less collagen type I at the later time points than the earliest across all mitomycin C doses, which corroborated in the aforesaid postinjury healing response. An ideal antifibrotic agent is thus able to modify fibroblast activities for specific collagen-type secretion thereby reducing excessive scar formation while allowing normal healing.
Mitomycin C reduces collagen production of fibroblasts by interacting with both nuclear DNA and ribosomal RNA of the cells.64 mitomycin C forms cross-links to the double-stranded DNA, arrests the cell cycle, and slows fibroblast proliferation.65 Unfortunately, mitomycin C can also induce cell death via apoptosis and necrosis depending on the dose and exposure time.15,30,53,65–67 In the present study, doses of 0.2 mg/mL, 0.5 mg/mL, or 1.0 mg/mL mitomycin C were applied to nVFF and sVFF for 2 minutes. A significant reduction of cell viability was observed in mitomycin C–treated sVFF and nVFF from day 3 and day 5 compared to serum controls. Dose-response effect was not statistically significant in the range of doses tested in this study. Signs of apoptosis were also observed in the aforesaid time points as observed from the immunocytochemistry (ICC). Oxidative stress has been reported to account for 22% of the observed cell death at 5 minutes of 0.4 mg/mL mitomycin C application to mouse lens epithelial cells.66 In this study, apoptosis increased cell membrane permeability and uptake of DAPI, leaving a strong purplish or pinkish stain. Secondary necrosis was evident such that the necrotic cells lost membrane integrity and the intracellular collagen was leaked out from the cytoplasm, as indicated by the distinctive collagen web patterning.
Our results suggest that a concentration of mitomycin C as low as 0.2 mg/mL could cause cytotoxicity in both nVFF and sVFF through apoptosis and secondary necrosis. Compared to other studies, visible focal necrosis and disintegration was observed in human nasal mucosa fibro-blasts immediately after a 5-minute exposure of mitomycin C at 0.4 mg/mL.15 A 4-minute exposure of 4 mg/mL mitomycin C application caused a rapid cell death of human dermal fibroblasts.67 The dose of 0.4 mg/mL or 0.04 mg/mL resulted in a decreased cell proliferation but no rapid cell death as seen in the dose of 4 mg/mL. These results suggested that the cytotoxicity effect of mitomycin C seem to be tissue-specific, even though the target cells were all fibroblasts.
Our results of collagen expression were normalized to the number of live cells and, thus, the change of the collagen expression was by means of mitomycin C effects on RNA and not cell number. rRNA constitutes 71% of total cellular RNA in eukaryotes and has G/C-rich region of nuclear DNA for preferred mitomycin C cross-linking.64 Thus, rRNA is the other potential nucleic acid target of mitomycin C in addition to nuclear DNA. Several other studies also showed that mitomycin C interacted with rRNA as the other mechanism of inhibiting protein production.54,64,68 For instance, in vitro polymer-ase chain reaction and gel-shift experiments have shown that transcript levels of rRNA decreases by 1.5-fold after mitomycin C treatments within 30 minutes, leading to deficient ribosomal formation or function as well as genome-wide translational inhibition for cell growth and protein synthesis.64
Specific to collagen production, our study demonstrated that at a 0.2 mg/mL dose of mitomycin C, expression of collagen type I was elevated in nVFF at day 1 but then decreased at day 3 compared to the serum controls. The same pattern of collagen type III expression was seen for sVFF. This transient increase of collagen secretion may be favorable for the necessary repair of lost collagen in injured tissue. The rapid decrease of collagen type I expression may also be favorable in terms of minimizing the long-term risk of fibrosis. The exact mechanism for the transient increase of collagen production at a 0.2 mg/mL dose observed is unclear and warrants further investigation. In addition, the differentiated effects of mitomycin C on collagen type I and III expressions observed in this study were not found in other mitomycin C reports. In one study of investigating tendon's capsule fibroblasts, mitomycin C reduced both collagen types I and III to a similar degree.54 In another study, however, mitomycin C was found to reduce the production of collagen type I significantly in tendon's capsule fibroblasts throughout the 48-day culture period.68 Conversely, the production of collagen type III was significantly elevated at the start and then decreased toward control levels during the remainder of the culture period.
An interesting finding of this study was that sVFF seem to be more sensitive than nVFF to mitomycin C treatments for measures of cytotoxity and collagen production. Although these two cell types showed similar morphology and contractile properties, sVFF have been reported to have lower proliferative rates and higher a-smooth muscle actin expression than nVFF. In addition, 15 ECM genes were differentially expressed between sVFF and nVFF.41 In the present study, the same culture medium was used for both cell types. Results indicated that once the phenotype of fibroblasts changed from normal to scar during the disease process, the phenotype and the intrinsic functions of the cells were still retained even after being isolated from the native tissue environment. Developing immortalized cell lines of sVFF is warranted for research to study the drug effects in the treatment of vocal fold scarring. Another possible explanation of the observed difference between sVFF and nVFF in their sensitivity to mitomycin C is that sVFFs were primary cells whereas nVFFs were from immortalized cell lines. Primary cell lines and their immortalized counterparts have been reported to have differentially expressed gene profiles of cell cycle control and apoptosis.69–71 Immortalized cell lines showed a more expressive profile of cell cycle proliferation and a suppressive profile of cell death compared to their primaries, making immortalized cells more robust against a cell cycle inhibitor, such as mitomycin C in this study. Further research is warranted to compare primary versus immortalized VFF in their response of cell cycle control and apoptosis.
Normal wound healing involves 3 overlapping phrases: (1) inflammation, (2) matrix cell migration and proliferation, and (3) scar remodeling and maturation. In upper airway wound healing, fibroblasts proliferate and secrete collagen to the wound area within days after injury.72 One would assume mitomycin C to be effective when fibroblasts are present in the wound site. However, fibro-blasts are not present in a wound immediately during the acute phase of inflammation. Thus, clinically, one would expect a better result of mitomycin C intervention if the drug is applied during the cell proliferation phrase, such as within a week after surgery instead of at the time of surgery or an immediate application after surgery as this timing better represents when fibroblasts in the tissue are present and are in active secretion of collagen. Further, we observed that the amount of collagen production was effectively decreased in nVFF by half if high mitomycin C dose 1.0 mg/ml was used. The effect was seen as early as 1 day after application. Clinicians, however, have to be cautious that such rapid decrease of collagen production may be contraindicated for the necessary restoration of the damaged tissue in the long term. The present work represents a necessary first step in providing bench to bedside translation that would dictate a more targeted window for in vivo testing of mitomycin C. Future research is warranted to establish the effective time window for mitomycin C application to exert its effect on inhibiting fibroblast proliferation in vivo.
Conclusions
A better understanding of the consequences of mitomycin C is imperative to allow effective and precise clinical usage to maximize success and minimize complications. Our results demonstrate that a 2-minute application of mitomycin C at 0.2 mg/mL dose on vocal fold fibroblasts was the lowest concentration to achieve the desired cytotoxicity effect, an initial transient stimulation of collagen secretion and subsequent suppression of collagen secretion in vitro. This dose fits the expectation of optimizing collagen dynamics by reducing excessive scar formation while allowing the necessary healing. Ongoing research is carried out in our laboratory to evaluate the effect of the timing of mitomycin C application in vivo.
Acknowledgments
The authors would like to acknowledge Xia Chen and Craig Berchtold for the technical advice to the research methodology and Glen Leverson for the statistical consultation of this project. All are in the Department of Surgery, University of Wisconsin Madison.
Source of Funding: The study was funded by the National Institute on Deafness and Other Communication Disorders (R01DC4336, R01 DC9600).
Footnotes
Part of this work was presented at the 8th International Conference on Voice Physiology and Biomechanics, Erlangen, Germany, July 2012.
References
- 1.Talwar GP, Srivastava LM, editors. Textbook of biochemistry and human biology. New Delhi: Prentice–Hall of India Private Limited; 2004. [Google Scholar]
- 2.Galm U, Hager MH, Van Lanen SG, Ju J, Thorson JS, Shen B. Antitumor antibiotics: bleomycin, enediynes, and mitomycin. Chem Rev. 2005;105:739–758. doi: 10.1021/cr030117g. [DOI] [PubMed] [Google Scholar]
- 3.Reddy MV, Randerath K. 32P-analysis of DNA adducts in somatic and reproductive tissues of rats treated with the anticancer antibiotic, mitomycin C. Mutat Res. 1987;179:75–88. doi: 10.1016/0027-5107(87)90043-1. [DOI] [PubMed] [Google Scholar]
- 4.Verweij J, Pinedo HM. Mitomycin C: mechanism of action, usefulness and limitations. Anticancer Drugs. 1990;1:5–13. [PubMed] [Google Scholar]
- 5.Santhiago MR, Netto MV, Wilson SE. Mitomycin C: biological effects and use in refractive surgery. Cornea. 2012;31:311–321. doi: 10.1097/ICO.0b013e31821e429d. [DOI] [PubMed] [Google Scholar]
- 6.Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope. 2009;119:272–283. doi: 10.1002/lary.20056. [DOI] [PubMed] [Google Scholar]
- 7.Veen EJ, Dikkers FG. Topical use of MMC in the upper aerodigestive tract: a review on the side effects. Eur Arch Otorhinolaryngol. 2010;267:327–334. doi: 10.1007/s00405-009-1151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen SH, Feng YF, Stojanovic A, Wang QM. Meta-analysis of clinical outcomes comparing surface ablation for correction of myopia with and without 0.02% mitomycin C. J Refract Surg. 2011;27:530–541. doi: 10.3928/1081597X-20110112-02. [DOI] [PubMed] [Google Scholar]
- 9.Teus MA, de Benito–Llopis L, Alió JL. Mitomycin C in corneal refractive surgery. Surv Ophthalmol. 2009;54:487–502. doi: 10.1016/j.survophthal.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Rahbar R, Valdez TA, Shapshay SM. Preliminary results of intraoperative mitomycin-C in the treatment and prevention of glottic and subglottic stenosis. J Voice. 2000;14:282–286. doi: 10.1016/s0892-1997(00)80037-5. [DOI] [PubMed] [Google Scholar]
- 11.Ward RF, April MM. Mitomycin-C in the treatment of tracheal cicatrix after tracheal reconstruction. Int J Pediatr Otorhinolaryngol. 1998;44:221–226. doi: 10.1016/s0165-5876(98)00061-5. [DOI] [PubMed] [Google Scholar]
- 12.Cortés de Miguel S, Cabeza Barrera J, Gallardo Medina M, Cassini Gómez de Cádiz LF, Salmerón–García A, Rodríguez Lucas F. Topical endotracheal mitomycin C as a complementary treatment for endoscopic treatment of recurrent laryngotracheal stenosis. Farm Hosp. 2011;35:32–35. doi: 10.1016/j.farma.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Roediger FC, Orloff LA, Courey MS. Adult subglottic stenosis: management with laser incisions and mitomycin-C. Laryngoscope. 2008;118:1542–1546. doi: 10.1097/MLG.0b013e318179247a. [DOI] [PubMed] [Google Scholar]
- 14.Roh JL, Yoon YH. Prevention of anterior glottic stenosis after transoral microresection of glottic lesions involving the anterior commissure with mitomycin C. Laryngoscope. 2005;115:1055–1059. doi: 10.1097/01.MLG.0000163341.67553.B9. [DOI] [PubMed] [Google Scholar]
- 15.Hu D, Sires BS, Tong DC, Royack GA, Oda D. Effect of brief exposure to mitomycin C on cultured human nasal mucosa fibroblasts. Ophthal Plast Reconstr Surg. 2000;16:119–125. doi: 10.1097/00002341-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson B, Gray SD, Thibeault S. Time and dose effects of mitomycin C on extracellular matrix fibroblasts and proteins. Laryngoscope. 2005;115:110–115. doi: 10.1097/01.mlg.0000150694.08259.80. [DOI] [PubMed] [Google Scholar]
- 17.Rahbar R, Jones DT, Nuss RC, et al. The role of mitomycin in the prevention and treatment of scar formation in the pediatric aerodigestive tract: friend or foe? Arch Otolaryngol Head Neck Surg. 2002;128:401–406. doi: 10.1001/archotol.128.4.401. [DOI] [PubMed] [Google Scholar]
- 18.Djordjevic B, Kim JH. Different lethal effects of mitomycin C and actinomycin D during the division cycle of HeLa cells. J Cell Biol. 1968;38:477–482. doi: 10.1083/jcb.38.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seong GJ, Park C, Kim CY, et al. Mitomycin-C induces the apoptosis of human Tenon's capsule fibroblast by activation of c-Jun N-terminal kinase 1 and caspase-3 protease. Invest Ophthalmol Vis Sci. 2005;46:3545–3552. doi: 10.1167/iovs.04-1358. [DOI] [PubMed] [Google Scholar]
- 20.Eliashar R, Eliachar I, Esclamado R, Gramlich T, Strome M. Can topical mitomycin prevent laryngotracheal stenosis? Laryngoscope. 1999;109:1594–1600. doi: 10.1097/00005537-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Spector JE, Werkhaven JA, Spector NC, Huang S, Sanders D, Reinisch L. Prevention of anterior glottic restenosis in a canine model with topical mitomycin-C. Ann Otol Rhinol Laryngol. 2001;110:1007–1010. doi: 10.1177/000348940111001103. [DOI] [PubMed] [Google Scholar]
- 22.Spector JE, Werkhaven JA, Spector NC, et al. Preservation of function and histologic appearance in the injured glottis with topical mitomycin-C. Laryngoscope. 1999;109(7 Pt 1):1125–1129. doi: 10.1097/00005537-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Correa AJ, Reinisch L, Sanders DL, et al. Inhibition of subglottic stenosis with mitomycin-C in the canine model. Ann Otol Rhinol Laryngol. 1999;108(11 Pt 1):1053–1060. doi: 10.1177/000348949910801106. [DOI] [PubMed] [Google Scholar]
- 24.Roh JL, Yoon YH. Prevention of anterior glottic stenosis after bilateral vocal fold stripping with mitomycin C. Arch Otolaryngol Head Neck Surg. 2005;131:690–695. doi: 10.1001/archotol.131.8.690. [DOI] [PubMed] [Google Scholar]
- 25.Roh JL. Prevention of posterior glottic stenosis by mitomycin C. Ann Otol Rhinol Laryngol. 2005;114:558–562. doi: 10.1177/000348940511400712. [DOI] [PubMed] [Google Scholar]
- 26.Coppit G, Perkins J, Munaretto J, Nielsen R, McKinney L, Ulnick K. The effects of mitomycin-C and stenting on airway wound healing after laryngotracheal reconstruction in a pig model. Int J Pediatr Otorhinolaryngol. 2000;53:125–135. doi: 10.1016/s0165-5876(00)00322-0. [DOI] [PubMed] [Google Scholar]
- 27.Camargo PA, Campos AC, Matias JE, Rispoli DZ, Przysiezny PE, Fonseca VR. Topical mitomycin C effect on swine vocal folds healing. Braz J Otorhinolaryngol. 2006;72:601–604. doi: 10.1016/S1808-8694(15)31015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pritsos CA, Sartorelli AC. Generation of reactive oxygen radicals through bioactivation of mitomycin antibiotics. Cancer Res. 1986;46:3528–3532. [PubMed] [Google Scholar]
- 29.Song JS, Kim JH, Yang M, Sul D, Kim HM. Mitomycin-C concentration in cornea and aqueous humor and apoptosis in the stroma after topical mitomycin-C application: effects of mitomycin-C application time and concentration. Cornea. 2007;26:461–467. doi: 10.1097/ICO.0b013e318030d217. [DOI] [PubMed] [Google Scholar]
- 30.Wu KY, Wang HZ, Hong SJ. Mechanism of mitomycin-induced apoptosis in cultured corneal endothelial cells. Mol Vis. 2008;14:1705–1712. [PMC free article] [PubMed] [Google Scholar]
- 31.Bell CW, Jiang W, Reich CF, III, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318–C1325. doi: 10.1152/ajpcell.00616.2005. [DOI] [PubMed] [Google Scholar]
- 32.Qin S, Wang H, Yuan R, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pogrebniak HW, Matthews W, Pass HI. Chemotherapy amplifies production of tumor necrosis factor. Surgery. 1991;110:231–237. [PubMed] [Google Scholar]
- 34.Hueman EM, Simpson CB. Airway complications from topical mitomycin C. Otolaryngol Head Neck Surg. 2005;133:831–835. doi: 10.1016/j.otohns.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 35.Agrawal N, Morrison GA. Laryngeal cancer after topical mitomycin C application. J Laryngol Otol. 2006;120:1075–1076. doi: 10.1017/S0022215106003008. [DOI] [PubMed] [Google Scholar]
- 36.Roh JL, Kim DH, Rha KS, Sung MW, Kim KH, Park CI. Benefits and risks of mitomycin use in the traumatized tracheal mucosa. Otolaryngol Head Neck Surg. 2007;136:459–463. doi: 10.1016/j.otohns.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Garrett CG, Soto J, Riddick J, Billante CR, Reinisch L. Effect of mitomycin-C on vocal fold healing in a canine model. Ann Otol Rhinol Laryngol. 2001;110:25–30. doi: 10.1177/000348940111000105. [DOI] [PubMed] [Google Scholar]
- 38.Hartnick CJ, Hartley BE, Lacy PD, et al. Topical mitomycin application after laryngotracheal reconstruction: a randomized, double-blind, placebo-controlled trial. Arch Otolaryngol Head Neck Surg. 2001;127:1260–1264. doi: 10.1001/archotol.127.10.1260. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Thibeault SL. Characteristics of age-related changes in cultured human vocal fold fibroblasts. Laryngoscope. 2008;118:1700–1704. doi: 10.1097/MLG.0b013e31817aec6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X, Thibeault SL. Novel isolation and biochemical characterization of immortalized fibroblasts for tissue engineering vocal fold lamina propria. Tissue Eng Part C Methods. 2009;15:201–212. doi: 10.1089/ten.tec.2008.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jette ME, Hayer SD, Thibeault SL. Characterization of human vocal fold fibroblasts derived from chronic scar. Laryngoscope. doi: 10.1002/lary.23681. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thibeault SL, Li W, Bartley S. A method for identification of vocal fold lamina propria fibroblasts in culture. Otolaryngol Head Neck Surg. 2008;139:816–822. doi: 10.1016/j.otohns.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirsner RS, Eaglstein WH. The wound healing process. Dermatol Clin. 1993;11:629–640. [PubMed] [Google Scholar]
- 44.Cohen K, Diegelmann R, Lindblad W. Wound healing: biochemical and clinical aspects. Philadelphia: W.B. Saunders; 1992. [Google Scholar]
- 45.Hackham DJ, Ford HR. Cellular, biochemical, and clinical aspects of wound healing. Surg Infect (Larchmt) 2002;3(Suppl 1):S23–S35. doi: 10.1089/sur.2002.3.s1-23. [DOI] [PubMed] [Google Scholar]
- 46.Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77:509–528. doi: 10.1016/s0039-6109(05)70566-1. [DOI] [PubMed] [Google Scholar]
- 47.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 48.Broughton G, II, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(7 Suppl):12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 49.Song JS, Kim JH, Yang M, Sul D, Kim HM. Concentrations of mitomycin C in rabbit corneal tissue and aqueous humor after topical administration. Cornea. 2006;25(10 Suppl 1):S20–S23. doi: 10.1097/01.ico.0000247208.93638.92. [DOI] [PubMed] [Google Scholar]
- 50.Pirouzian A, O'Halloran H, Scher C, Jockin Y. Early-onset scleral and corneal ectasias following low-dose mitomycin-C-augmented trabeculectomy in a uveitic glaucoma patient. Ophthalmologica. 2006;220:406–408. doi: 10.1159/000095870. [DOI] [PubMed] [Google Scholar]
- 51.Thornton I, Puri A, Xu M, Krueger RR. Low-dose mitomycin C as a prophylaxis for corneal haze in myopic surface ablation. Am J Ophthalmol. 2007;144:673–681. doi: 10.1016/j.ajo.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 52.Thornton I, Xu M, Krueger RR. Comparison of standard (0.02%) and low dose (0.002%) mitomycin C in the prevention of corneal haze following surface ablation for myopia. J Refract Surg. 2008;24:S68–S76. doi: 10.3928/1081597X-20080101-13. [DOI] [PubMed] [Google Scholar]
- 53.Roh DS, Cook AL, Rhee SS, et al. DNA cross-linking, double-strand breaks, and apoptosis in corneal endothelial cells after a single exposure to mitomycin C. Invest Ophthalmol Vis Sci. 2008;49:4837–4843. doi: 10.1167/iovs.08-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gross RL. Collagen type I and III synthesis by Tenon's capsule fibroblasts in culture: individual patient characteristics and response to mitomycin C, 5-fluorouracil, and ascorbic acid. Trans Am Ophthalmol Soc. 1999;97:513–543. [PMC free article] [PubMed] [Google Scholar]
- 55.Ling C, Yamashita M, Waselchuk EA, Raasch JL, Bless DM, Welham NV. Alteration in cellular morphology, density and distribution in rat vocal fold mucosa following injury. Wound Repair Regen. 2010;18:89–97. doi: 10.1111/j.1524-475X.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansen JK, Thibeault SL. Current understanding and review of the literature: vocal fold scarring. J Voice. 2006;20:110–120. doi: 10.1016/j.jvoice.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Tateya T, Tateya I, Bless DM. Collagen subtypes in human vocal folds. Ann Otol Rhinol Laryngol. 2006;115:469–476. doi: 10.1177/000348940611500612. [DOI] [PubMed] [Google Scholar]
- 58.Li NY, Vodovotz Y, Hebda PA, Abbott KV. Biosimulation of inflammation and healing in surgically injured vocal folds. Ann Otol Rhinol Laryngol. 2010;119:412–423. doi: 10.1177/000348941011900609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tateya T, Tateya I, Bless DM. Immuno-scanning electron microscopy of collagen types I and III in human vocal fold lamina propria. Ann Otol Rhinol Laryngol. 2007;116:156–159. doi: 10.1177/000348940711600212. [DOI] [PubMed] [Google Scholar]
- 60.Tateya I, Tateya T, Lim X, Sohn JH, Bless DM. Cell production in injured vocal folds: a rat study. Ann Otol Rhinol Laryngol. 2006;115:135–143. doi: 10.1177/000348940611500210. [DOI] [PubMed] [Google Scholar]
- 61.Tateya T, Tateya I, Sohn JH, Bless DM. Histologic characterization of rat vocal fold scarring. Ann Otol Rhinol Laryngol. 2005;114:183–191. doi: 10.1177/000348940511400303. [DOI] [PubMed] [Google Scholar]
- 62.Tateya T, Tateya I, Sohn JH, Bless DM. Histological study of acute vocal fold injury in a rat model. Ann Otol Rhinol Laryngol. 2006;115:285–292. doi: 10.1177/000348940611500406. [DOI] [PubMed] [Google Scholar]
- 63.Welham NV, Montequin DW, Tateya I, Tateya T, Choi SH, Bless DM. A rat excised larynx model of vocal fold scar. J Speech Lang Hear Res. 2009;52:1008–1020. doi: 10.1044/1092-4388(2009/08-0049). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snodgrass RG, Collier AC, Coon AE, Pritsos CA. Mitomycin C inhibits ribosomal RNA: a novel cytotoxic mechanism for bioreductive drugs. J Biol Chem. 2010;285:19068–19075. doi: 10.1074/jbc.M109.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takahashi N, Murayama T, Oda M, Miyakoshi M. Cell growth inhibition and DNA incorporation of mitomycin C in cell culture. Ophthalmic Res. 1998;30:120–125. doi: 10.1159/000055464. [DOI] [PubMed] [Google Scholar]
- 66.Park HK, Lee KW, Choi JS, Joo CK. Mitomycin C-induced cell death in mouse lens epithelial cells. Ophthalmic Res. 2002;34:213–219. doi: 10.1159/000063880. [DOI] [PubMed] [Google Scholar]
- 67.Chen T, Kunnavatana SS, Koch RJ. Effects of mitomycin-C on normal dermal fibroblasts. Laryngoscope. 2006;116:514–517. doi: 10.1097/01.MLG.0000205590.62824.0A. [DOI] [PubMed] [Google Scholar]
- 68.Occleston NL, Daniels JT, Tarnuzzer RW, et al. Single exposures to anti-proliferatives: long-term effects on ocular fibroblast wound-healing behavior. Invest Ophthalmol Vis Sci. 1997;38:1998–2007. [PubMed] [Google Scholar]
- 69.Boerma M, Burton GR, Wang J, Fink LM, McGehee RE, Jr, Hauer–Jensen M. Comparative expression profiling in primary and immortalized endothelial cells: changes in gene expression in response to hydroxy methylglutaryl-coenzyme A reductase inhibition. Blood Coagul Fibrinolysis. 2006;17:173–180. doi: 10.1097/01.mbc.0000220237.99843.a1. [DOI] [PubMed] [Google Scholar]
- 70.Pan C, Kumar C, Bohl S, Klingmueller U, Mann M. Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol Cell Proteomics. 2009;8:443–450. doi: 10.1074/mcp.M800258-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kong BW, Lee JY, Bottje WG, Lassiter K, Lee J, Foster DN. Genome-wide differential gene expression in immortalized DF-1 chicken embryo fibroblast cell line. BMC Genomics. 2011;12:571. doi: 10.1186/1471-2164-12-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirshoren N, Eliashar R. Wound-healing modulation in upper airway stenosis – myths and facts. Head Neck. 2009;31:111–126. doi: 10.1002/hed.20925. [DOI] [PubMed] [Google Scholar]


