Abstract
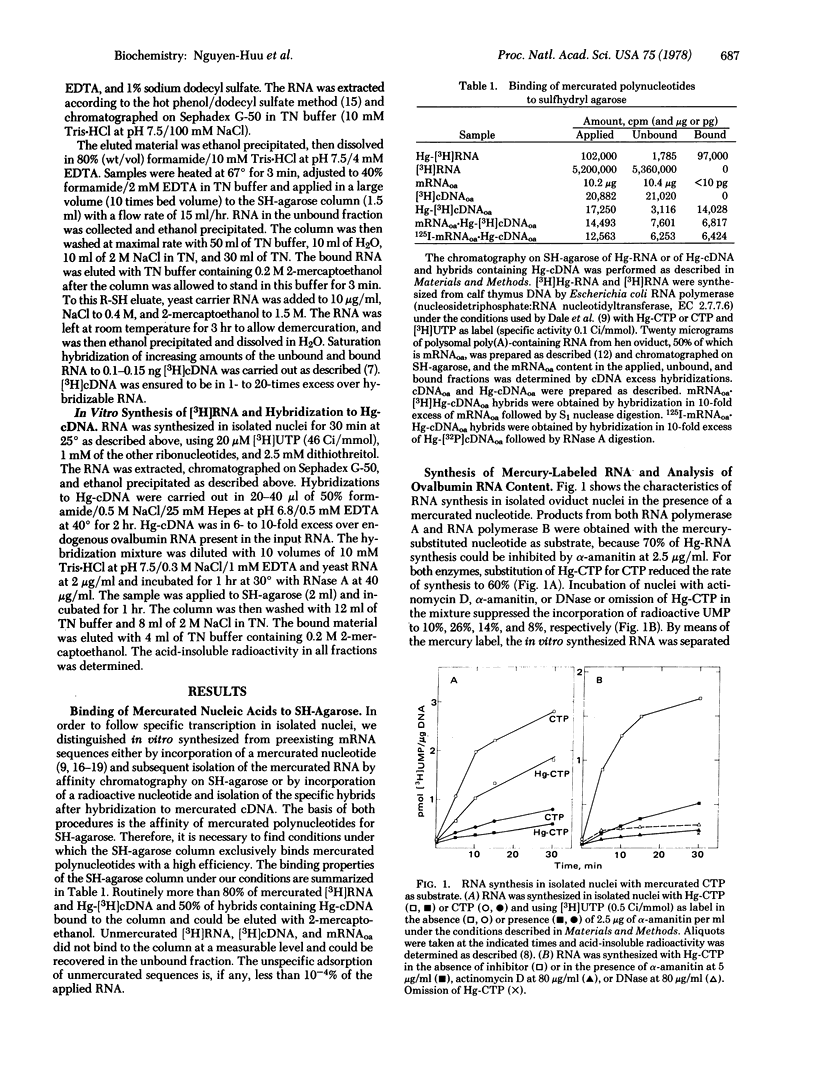
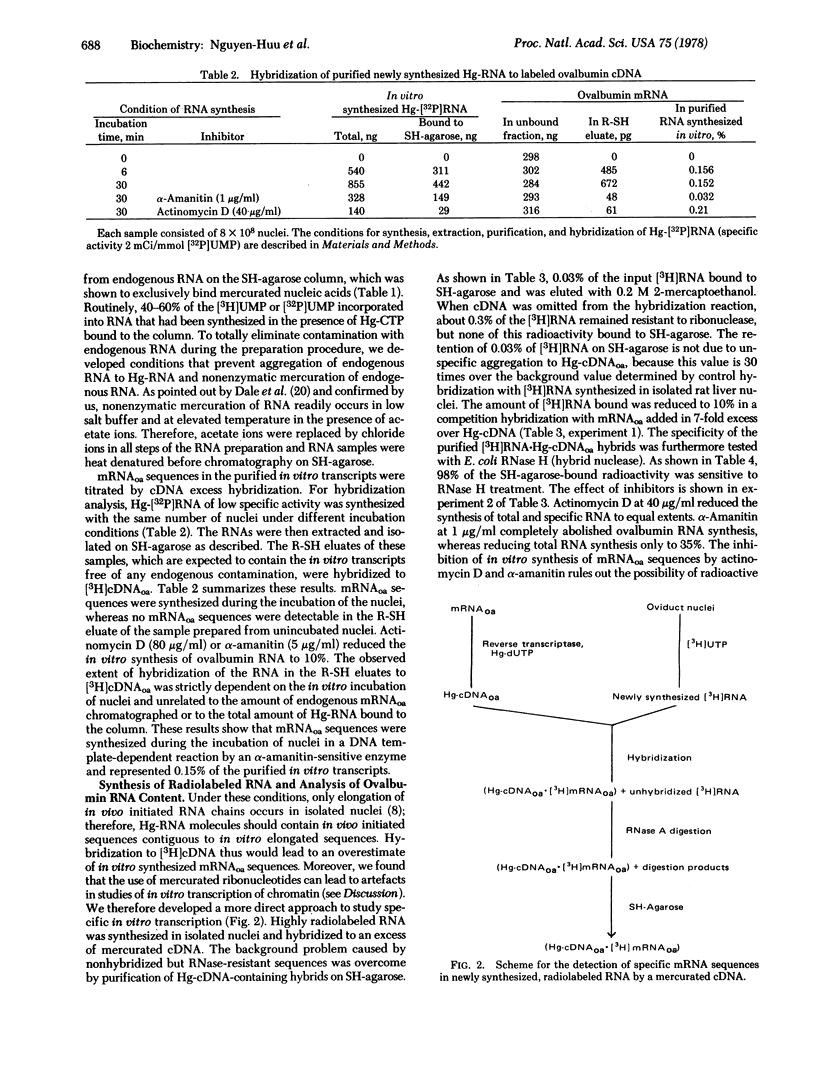
The synthesis of ovalbumin mRNA sequences was studied in isolated nuclei from hen oviduct. Two different methods of analysis were used to distinguish in vitro synthesized from preexisting mRNA sequences: (i) Mercurated ribonucleotides were used for in vitro RNA synthesis, and the newly synthesized RNA was purified by chromatography on sulfhydryl-agarose and hybridized to radioactive ovalbumin cDNA. (ii) [3H]UTP was used to label the in vitro synthesized RNA. Hybridization to unlabeled mercurated cDNA, RNase A digestion, and subsequent purification of the hybrids on SH-agarose allowed the quantitation of newly synthesized ovalbumin mRNA sequences. Approximately 0.1% of the newly synthesized RNA was identified as ovalbumin RNA by both methods. The synthesis of ovalbumin RNA progressed during the incubation of nuclei and was sensitive to actinomycin D and low concentrations of alpha-amanitin. The preferential in vitro transcription of the ovalbumin gene (1000-fold over random transcription of the chicken genome) by RNA polymerase B (nucleosidetriphosphate:RNA nucleotidyltransferase, EC 2.7.7.6) suggests that the specificity of in vitro RNA synthesis is retained in isolated nuclei.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Cedar H., Felsenfeld G. Synthesis of globin ribonucleic acid from duck-reticulocyte chromatin in vitro. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2029–2032. doi: 10.1073/pnas.70.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebee T. J., Butterworth P. H. The use of mercurated nucleoside triphosphate as a probe in transcription studies in vitro. Eur J Biochem. 1976 Jul 15;66(3):543–550. doi: 10.1111/j.1432-1033.1976.tb10580.x. [DOI] [PubMed] [Google Scholar]
- Biessmann H., Gjerset R. A., Levy B., McCarthy B. J. Fidelity of chromatin transcription in vitro. Biochemistry. 1976 Oct 5;15(20):4356–4363. doi: 10.1021/bi00665a002. [DOI] [PubMed] [Google Scholar]
- Chan L., Means A. R., O'Malley B. W. Rates of induction of specific translatable messenger RNAs for ovalbumin and avidin by steroid hormones. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1870–1874. doi: 10.1073/pnas.70.6.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. F., Haines M. E., Emtage J. S. Quantitation of ovalbumin mRNA in hen and chick oviduct by hybridization to complementary DNA. Accumulation of specific mRNA in response to estradiol. Eur J Biochem. 1974 Nov 1;49(1):225–236. doi: 10.1111/j.1432-1033.1974.tb03827.x. [DOI] [PubMed] [Google Scholar]
- Cox R. F. Quantitation of elongating form A and B RNA polymerases in chick oviduct nuclei and effects of estradiol. Cell. 1976 Mar;7(3):455–465. doi: 10.1016/0092-8674(76)90176-8. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., Fodor J. B., Doty P. In vitro transcription of chromatin in the presence of a mercurated nucleotide. Proc Natl Acad Sci U S A. 1976 May;73(5):1564–1567. doi: 10.1073/pnas.73.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Curtis P. J., Weissmann C. Purification of globin messenger RNA from dimethylsulfoxide-induced Friend cells and detection of a putative globin messenger RNA precursor. J Mol Biol. 1976 Oct 5;106(4):1067–1075. doi: 10.1016/0022-2836(76)90353-3. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Livingston D. C., Ward D. C. The synthesis and enzymatic polymerization of nucleotides containing mercury: potential tools for nucleic acid sequencing and structural analysis. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2238–2242. doi: 10.1073/pnas.70.8.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., Martin E., Livingston D. C., Ward D. C. Direct covalent mercuration of nucleotides and polynucleotides. Biochemistry. 1975 Jun 3;14(11):2447–2457. doi: 10.1021/bi00682a027. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C. Mercurated polynucleotides: new probes for hybridization and selective polymer fractionation. Biochemistry. 1975 Jun 3;14(11):2458–2469. doi: 10.1021/bi00682a028. [DOI] [PubMed] [Google Scholar]
- Ernest M. J., Schutz G., Feigelson P. RNA synthesis in isolated hen oviduct nuclei. Biochemistry. 1976 Feb 24;15(4):824–829. doi: 10.1021/bi00649a015. [DOI] [PubMed] [Google Scholar]
- Giesecke K., Sippel A. E., Nguyen-Huu M. C., Groner B., Hynes N. E., Wurtz T., Schütz G. A RNA-dependent RNA polymerase activity: implications for chromatin transcription experiments. Nucleic Acids Res. 1977 Nov;4(11):3943–3958. doi: 10.1093/nar/4.11.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. Tissue-specific transcription of the globin gene in isolated chromatin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3440–3442. doi: 10.1073/pnas.70.12.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N. E., Sippel A. E., Jeep S., Chi-Nguyen-Huu M., Schütz G. Immunoadsorption of specific chicken oviduct polysomes. Isolation of ovalbumin, ovomucoid, and lysozyme messenger RNA. J Biol Chem. 1977 Oct 10;252(19):6666–6674. [PubMed] [Google Scholar]
- Harris S. E., Rosen J. M., Means A. R., O'Malley B. W. Use of a specific probe for ovalbumin messenger RNA to quantitate estrogen-induced gene transcripts. Biochemistry. 1975 May 20;14(10):2072–2081. doi: 10.1021/bi00681a006. [DOI] [PubMed] [Google Scholar]
- Harris S. E., Schwartz R. J., Tsai M. J., O'Malley B. W., Roy A. K. Effect of estrogen on gene expression in the chick oviduct. In vitro transcription of the ovalbumin gene in chromatin. J Biol Chem. 1976 Jan 25;251(2):524–529. [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Sippel A. E., Nguyen-Huu M. C., Schütz G. mRNA complexity and egg white protein mRNA content in mature and hormone-withdrawn oviduct. Cell. 1977 Aug;11(4):923–932. doi: 10.1016/0092-8674(77)90303-8. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Groner Y., Monroy G., Hurwitz J. The in vitro synthesis of avian myeloblastosis viral RNA sequences. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3045–3049. doi: 10.1073/pnas.71.8.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A. J., Burgoyne L. A. Interpretation of the properties of chromatin extracts from mammalian nuclei. Nucleic Acids Res. 1976 Apr;3(4):1101–1110. doi: 10.1093/nar/3.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Pennequin P., Schimke R. T. Induction of ovalbumin mRNA sequences by estrogen and progesterone in chick oviduct as measured by hybridization to complementary DNA. J Biol Chem. 1975 Oct 25;250(20):8105–8110. [PubMed] [Google Scholar]
- Miller O. L., Jr, Beatty B. R. Visualization of nucleolar genes. Science. 1969 May 23;164(3882):955–957. doi: 10.1126/science.164.3882.955. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Moore P. B., Mulvihill E. R. A significant lag in the induction of ovalbumin messenger RNA by steroid hormones: a receptor translocation hypothesis. Cell. 1976 Aug;8(4):557–572. doi: 10.1016/0092-8674(76)90224-5. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. Transcription of the adenovirus genome by an -amanitine-sensitive ribonucleic acid polymerase in HeLa cells. J Virol. 1972 Apr;9(4):621–626. doi: 10.1128/jvi.9.4.621-626.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Quantitative measurement of ovalbumin messenger ribonucleic acid activity. Localization in polysomes, induction by estrogen, and effect of actinomycin D. J Biol Chem. 1973 Mar 25;248(6):2031–2039. [PubMed] [Google Scholar]
- Rymo L., Parsons J. T., Coffin J. M., Weissmann C. In vitro synthesis of Rous sarcoma virus-specific RNA is catalyzed by a DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2782–2786. doi: 10.1073/pnas.71.7.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. M., Huang R. C. Transcription in vitro of immunoglobulin kappa light chain genes in isolated mouse myeloma nuclei and chromatin. Proc Natl Acad Sci U S A. 1976 Mar;73(3):775–779. doi: 10.1073/pnas.73.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Giza P. E. Accentuated expression of silk fibroin genes in vivo and in vitro. J Mol Biol. 1976 Nov 5;107(3):183–206. doi: 10.1016/s0022-2836(76)80001-0. [DOI] [PubMed] [Google Scholar]
- Towle H. C., Tsai M. J., Tsai S. Y., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. J Biol Chem. 1977 Apr 10;252(7):2396–2404. [PubMed] [Google Scholar]
- Tsai M. J., Towle T. C., Harris S. E., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. J Biol Chem. 1976 Apr 10;251(7):1960–1968. [PubMed] [Google Scholar]
- Weinmann R., Raskas H. J., Roeder R. G. Role of DNA-dependent RNA polymerases II and III in transcription of the adenovirus genome late in productive infection. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3426–3439. doi: 10.1073/pnas.71.9.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M., Felsenfeld G. Use of mercury-substituted ribonucleoside triphosphates can lead to artefacts in the analysis of in vitro chromatin transcrits. Biochem Biophys Res Commun. 1977 Apr 11;75(3):598–603. doi: 10.1016/0006-291x(77)91514-5. [DOI] [PubMed] [Google Scholar]


