Abstract
Objective
As bilateral salpingo-oophorectomy is frequently performed with hysterectomy for nonmalignant conditions, defining health outcomes associated with benign bilateral salpingo-oophorectomy performed at different ages is critical.
Methods
We assessed mortality risk associated with benign total abdominal hysterectomy or bilateral salpingo-oophorectomy among 52,846 Breast Cancer Detection and Demonstration Project Follow-up Study participants. Surgery and risk factor data were ascertained via baseline interview (1979-1986) and three questionnaires (1987-1998). During follow-up through December 2005 (mean=22.1 years), 13,734 deaths were identified. We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for overall and disease-specific mortality following total abdominal hysterectomy or bilateral salpingo-oophorectomy performed by ages 35, 40, 45, 50, or 55 years compared to not having surgery using landmark analyses and multivariable Cox regression.
Results
Undergoing bilateral salpingo-oophorectomy by age 35 was associated with increased mortality risk (HR35 years=1.20, 95% CI: 1.08, 1.34), which decreased with age (HR40 years=1.12, 95% CI: 1.04, 1.21; HR45 years=1.10, 95% CI: 1.03, 1.17). Total abdominal hysterectomy alone performed by age 40 was associated with increased mortality risk to a lesser extent (HR40 years=1.08, 95% CI: 1.01, 1.15). Analyses based on matched propensity scores related to having gynecological surgery yielded similar results. Elevated mortality risks were largely attributable to non-cancer causes.
Conclusions
Benign gynecologic surgeries among young women are associated with increased mortality risk, which attenuates with age.
Keywords: Bilateral oophorectomy, cohort studies, hysterectomy, mortality
Introduction
Defining the health outcomes associated with bilateral salpingo-oophorectomy (BSO) is important. BSO lowers breast and ovarian/fallopian tube cancer risk,1 but may increase chronic disease risk.2-5 Results of prior studies assessing associations of BSO and mortality are inconsistent.5-9 Of approximately 600,000 hysterectomy procedures performed annually in the United States, half include incidental BSO;10 thus, any adverse effects of BSO affect public health.
Parker et al. reported that oophorectomy and hysterectomy were associated with a 13% increase in mortality, primarily from coronary heart disease and lung cancer, when compared with hysterectomy alone,5 supporting conclusions of a simulated analysis.6 Another analysis found that “prophylactic” oophorectomy was unrelated to mortality overall, but was related to increased mortality if performed before age 45 years.7 Similarly, another study linked oophorectomy to increased mortality, primarily due to cardiovascular disease, among obese women who underwent surgery before age 40 and did not use menopausal hormone therapy (HT).8 Finally, one investigation found that BSO was unrelated to mortality.9 Thus, data suggest that incidental BSO may be associated with increased mortality, but clarification of risks, particularly in relation to age at surgery are needed.
Defining whether BSO is related to increased mortality and identifying factors that modify this risk would inform discussions related to considering oophorectomy as an incidental procedure concurrent with hysterectomy as well as elective cancer-risk reducing surgery. To assess mortality risks associated with benign hysterectomy and BSO, we analyzed data from the Breast Cancer Demonstration Detection Project (BCDDP) follow-up study, which included an initial assessment of 61,431 women in 1979-1986 and follow-up for outcomes through 2005. Our analysis included a landmark analysis specifically designed to assess risks associated with BSO by predetermined ages, and an analysis in which women who underwent BSO were compared to women with a similar propensity to undergo this surgery, but did not (matched propensity scores). The large size and lengthy follow-up in the BCDDP cohort offers an important opportunity to assess long-term disease-specific mortality risks, which could reflect loss of ovarian function.
Methods
Study population
Briefly, BCDDP was a breast cancer screening program co-sponsored by the American Cancer Society and the National Cancer Institute that enrolled over 280,000 women at 29 U.S. centers between 1973 and 1980.11 In 1979, 64,182 participants meeting the following criteria were enrolled in the BCDDP follow-up study: 1) breast cancer diagnosed during the screening program (n=4,275); 2) benign breast surgery (n=25,114); 3) recommended for follow-up but not biopsied (n=9,628) and 4) a subset of women who during screening had neither breast surgery performed nor recommended (n=25,165). The National Cancer Institute Institutional Review Board approved the study; all participants provided informed consent.
During BCDDP Follow-up Study Phase 1 (baseline: 1979-1986), 61,431 women (96%) completed a baseline telephone interview and up to six (usually four) annual telephone interviews. In Phases 2 (1987-1989), 3 (1993-1995) and 4 (1995-1998) participants completed self-administered, mailed questionnaires. When possible, living non-respondents were interviewed by telephone.
Exposure assessment
Each phase collected data about gynecologic surgeries, including total abdominal hysterectomy (TAH, or simple hysterectomy), unilateral salpingo-oophorectomy (USO), bilateral-salpingo oophorectomy (BSO), and surgery dates. To assess outcomes following benign gynecologic surgeries, we defined women who underwent gynecologic surgeries prior to or within 12 months of ovarian cancer diagnosis or death (n=265, including 162 deaths) or endometrial cancer diagnosis or death (n=511, including 150 deaths) as not having had benign gynecologic surgery.
BCDDP screening phase interviews collected demographic data, including race/ethnicity and education level. Measured height and weight recorded at the last screening visit were used to calculate body mass index (BMI, kg/m2). Each phase included questions about use and duration of HT; Phase 1 did not distinguish between estrogen-only and estrogen-progestin therapy as the former was the primary formulation in use at that time. Other risk factors ascertained included reproductive history (Phase 1), alcohol use and smoking status (Phase 2, with smoking status updated in Phase 3), diagnoses of cardiovascular disease, diabetes, stroke, or osteoporosis (Phases 2-4), and family histories of breast cancer (baseline with updates in Phases 1-4) and ovarian cancer (Phase 4).
Ascertainment of cancer diagnoses and vital status
Incident cancers were identified through self-report, linkage to 19 state cancer registries, and the National Death Index. Self-reported cancers were verified through medical record review with standardized data abstraction. Vital status was ascertained by regular searches of the National Death Index through December 31, 2005. The primary cause of death was coded as listed on the death certificate using the International Classification of Diseases (ICD-9 and ICD-10). Women who were not identified as deceased in the National Death Index were presumed to be alive.
Analytic population
Of 61,431 women who completed the Phase 1 interview, we excluded women for the following: unknown menopausal status (n=50); never having menstruated (n=10); unknown date of gynecologic surgery (n=1,570) or death (n=18); recorded death date earlier than that of baseline interview (n=6); and diagnosis of cancer prior to follow-up (n=6,931). The present analysis included 52,846 women, with follow-up questionnaire data at Phase 2 for 44,622 (84.4%), Phase 3 for 39,007 (73.8%), and Phase 4 for 37,171 (70.3%).
Statistical analysis
The exposure of interest in this analysis was benign gynecological surgery; therefore, women who underwent gynecological cancer surgery were considered unexposed. We categorized benign gynecological surgery as: none (i.e., intact uterus with retention of at least one ovary); TAH (with or without USO); and BSO (with or without TAH).
We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) associated with benign gynecologic surgery for all-cause and cause-specific mortality, using age as the time scale. Study entry began at the baseline interview and person-years accrued until death or December 31, 2005. Exit age was censored at 90 years (n=3,368 women) to ensure proportionality of the hazards. In all-cause mortality analyses, endometrial and ovarian cancer deaths were combined with deaths among women who did not undergo surgery because neither group had the exposure of interest (i.e., benign gynecological surgery). We evaluated cause-specific mortality for diseases that resulted in over 100 deaths. Examination of hazard plots and tests for interaction between gynecologic surgery status and the natural log of age were consistent with proportional hazards.
We examined the relation between benign gynecologic surgery status and mortality using two approaches: 1) a time-dependent exposure, in which women whose surgery status changed during follow-up contributed person-time to multiple exposure categories; and 2) landmark analyses,12 which assessed mortality risk based on whether surgery was performed at or before pre-specified landmark ages. We highlight the latter as more clinically relevant. Specifically, fixed landmark ages (35, 40, 45, 50, or 55 years) were selected to conduct survival analysis of women with and without benign gynecological surgery by that age. Women who died prior to the landmark age were excluded, and women who underwent benign surgery after that age were considered unexposed. Thus, we compared the risk of death among women who had benign gynecologic surgery by specific landmark ages to those who did not.
We adjusted for the following potential confounders in our final multivariable models: BMI (<25, 25-<30, 30+ kg/m2, unknown), alcohol use (never, ever, unknown), smoking (never, ever, unknown), HT use (never used, estrogen therapy <10 years, estrogen therapy use 10+ years, used other/unknown hormone formulations), and birth cohort (1891-<1911, 1911-<1931, 1931+). Smoking status and HT were treated as time-dependent exposures, allowing women to contribute person-time to multiple categories. Further adjustment for race/ethnicity, education, income, marital status, history of cardiovascular disease, diabetes, stroke, osteoporosis, age at menarche, parity, oral contraceptive use and duration, and family history of breast or ovarian cancers had minimal effects on risk estimates; we therefore do not show these results.
To reduce the possibility of bias due to differences in covariate distributions between women who underwent benign gynecologic surgery and those who did not, we matched women in these groups using propensity scores,13 defined as the conditional probability of undergoing TAH or BSO, given all covariates listed above. We computed deciles of the propensity score and created two matched datasets, one for TAH and another for BSO, selecting one woman who did not undergo surgery from each decile of the propensity score for every woman who underwent benign gynecologic surgery who fell into the same propensity score decile, and we repeated the landmark analyses.
In landmark sensitivity analyses, we censored exit age at 70 years to ensure that observed associations were not being driven by unique characteristics of long-term survivors. To explore whether the cumulative risk estimates observed at older landmark ages were driven by benign gynecologic surgeries conducted earlier in life, we also analyzed benign gynecologic surgery status as a time-dependent exposure in five-year age intervals in standard Cox models.
We used a likelihood ratio test, comparing models with and without interaction terms, to separately examine effect modification by HT and BMI. Probability values of <0.05 were considered statistically significant. All tests of significance were two-tailed. Analyses were performed using SAS software (SAS Institute Inc., Cary, NC).
Results
Distribution of participant characteristics by benign gynecologic surgery status
Women were predominantly non-Hispanic white (86.4%), with a mean (SD) age at baseline of 55.3 (8.7) years. At the end of follow-up, 11,247 (21%) women reported having TAH (with or without a USO), and 12,652 (24%) women reported having BSO (with or without a TAH) (Table 1). Among women categorized as having undergone benign TAH, 3,248 (29%) also reported having a USO. Among women reporting having BSO, the vast majority (98%) also reported having TAH.
Table 1. Distribution of Select Participant Characteristics by Benign Gynecologic Surgery Status Among 52,846 Women in the US BCDDP Follow-up Study With Follow-up From 1979-1986 to 2005.
| Characteristic | Benign gynecologic surgery status, N (%)a | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| None | Simple hysterectomy | Bilateral salpingo-oophorectomy | ||||
| N=28,947 | N=11,247 | N=12,652 | ||||
| Age, y | ||||||
| <50 | 9,103 | 31.4% | 3,647 | 32.4% | 3,663 | 29.0% |
| 50-54 | 6,455 | 22.3% | 2,499 | 22.2% | 2,875 | 22.7% |
| 55-59 | 5,463 | 18.9% | 2,173 | 19.3% | 2,533 | 20.0% |
| 60-64 | 3,512 | 12.1% | 1,355 | 12.0% | 1,673 | 13.2% |
| 65-69 | 2,179 | 7.5% | 854 | 7.6% | 1,052 | 8.3% |
| 70-74 | 1,496 | 5.2% | 501 | 4.5% | 605 | 4.8% |
| 75-79 | 634 | 2.2% | 194 | 1.7% | 212 | 1.7% |
| 80+ | 105 | 0.4% | 24 | 0.2% | 39 | 0.3% |
| Birth cohort | ||||||
| 1891-<1911 | 2,108 | 7.3% | 673 | 6.0% | 808 | 6.4% |
| 1911-<1931 | 17,353 | 59.9% | 6,805 | 60.5% | 8,056 | 63.7% |
| 1931+ | 9,486 | 32.8% | 3,769 | 33.5% | 3,788 | 29.9% |
| Race/ethnicity | ||||||
| Non-Hispanic white | 24,974 | 86.3% | 9,689 | 86.1% | 10,992 | 86.9% |
| Other/unknown | 3,973 | 13.7% | 1,558 | 13.9% | 1,660 | 13.1% |
| Education | ||||||
| <High school or high school graduate | 3,639 | 12.7% | 1,665 | 15.0% | 1,963 | 15.6% |
| Post-high school | 25,071 | 87.3% | 9,468 | 85.0% | 10,591 | 84.4% |
| BMI, kg/m2 | ||||||
| <25 | 18,827 | 66.8% | 6,968 | 63.1% | 7,876 | 63.8% |
| 25-<30 | 6,780 | 24.0% | 2,933 | 26.6% | 3,121 | 25.3% |
| 30+ | 2,596 | 9.2% | 1,139 | 10.3% | 1,343 | 10.9% |
| Smoking | ||||||
| Never | 13,909 | 56.4% | 5,823 | 59.6% | 6,670 | 59.8% |
| Ever | 10,772 | 43.6% | 3,951 | 40.4% | 4,491 | 40.2% |
| Alcohol | ||||||
| Never | 10,406 | 44.0% | 4,490 | 47.7% | 5,213 | 48.5% |
| Ever | 13,254 | 56.0% | 4,922 | 52.3% | 5,528 | 51.5% |
| Cardiovascular disease | ||||||
| No | 18,283 | 83.6% | 6,739 | 78.7% | 7,768 | 78.9% |
| Yes | 3,579 | 16.4% | 1,821 | 21.3% | 2,082 | 21.1% |
| Diabetes | ||||||
| No | 19,909 | 91.0% | 7,537 | 88.5% | 8,669 | 88.5% |
| Yes | 1,963 | 9.0% | 982 | 11.5% | 1,125 | 11.5% |
| Stroke | ||||||
| No | 22,303 | 96.5% | 8,630 | 94.7% | 9,853 | 95.0% |
| Yes | 820 | 3.5% | 481 | 5.3% | 522 | 5.0% |
| Osteoporosis | ||||||
| No | 16,914 | 78.9% | 6,475 | 76.9% | 7,433 | 77.4% |
| Yes | 4,532 | 21.1% | 1,947 | 23.1% | 2,174 | 22.6% |
| Parity | ||||||
| Nulliparous/missing | 4,524 | 15.6% | 1,110 | 9.9% | 1,888 | 14.9% |
| One | 3,528 | 12.2% | 1,212 | 10.8% | 1,670 | 13.2% |
| Two | 8,454 | 29.2% | 3,223 | 28.7% | 3,710 | 29.3% |
| Three or more | 12,441 | 43.0% | 5,702 | 50.7% | 5,384 | 42.6% |
| Menopausal hormone therapy | ||||||
| Never used | 13,631 | 47.1% | 2,679 | 23.8% | 1,904 | 15.0% |
| ET <10 years | 3,497 | 12.1% | 3,798 | 33.8% | 3,536 | 27.9% |
| ET 10+years | 584 | 2.0% | 2,771 | 24.6% | 4,501 | 35.6% |
| Used hormones, type other/unknown | 11,235 | 38.8% | 1,999 | 17.8% | 2,711 | 21.4% |
| Family history of ovarian | ||||||
| No | 17,881 | 94.7% | 7,179 | 94.5% | 8,012 | 92.8% |
| Yes | 1,009 | 5.3% | 419 | 5.5% | 623 | 7.2% |
BCDDP, Breast Cancer Detection and Demonstration Project; BMI, body mass index; ET, estrogen therapy
Benign gynecologic surgery status at end of follow-up. Missing values were excluded from column percentage calculations.
Compared with women who did not undergo benign gynecologic surgery, women who underwent BSO were more likely to have birth dates between 1911-1930, higher BMIs, histories of chronic disease, used HT, and family histories of ovarian cancer. Women who underwent TAH or BSO were less likely than women who did not undergo benign gynecologic surgery to have ever smoked or consumed alcohol and to have post-high school education. Gynecologic surgery status did not vary substantially by other factors. Mortality risk increased with increasing BMI; increased risks were also observed for smokers and those who reported having a history of cardiovascular disease, diabetes or stroke (Table 2). Mortality risk was inversely associated with education, alcohol use, increasing parity and HT duration.
Table 2. Survival Model Results for the Relation Between Select Participant Characteristics and All-cause Mortality Among 52,846 Women in the US BCDDP Follow-up Study With Follow-up From 1979-1986 to 2005.
| Characteristic | No. of deaths | HR | 95% CI |
|---|---|---|---|
| Birth cohort | |||
| 1891-<1911 | 1,960 | 1.00 | referent |
| 1911-<1931 | 10,042 | 0.93 | 0.88 , 0.98 |
| 1931+ | 1,732 | 0.92 | 0.85 , 1.00 |
| Race/ethnicity | |||
| Non-hispanic white | 11,961 | 1.00 | referent |
| Other/unknown | 1,773 | 1.03 | 0.98 , 1.08 |
| Education | |||
| <High school or high school | 2,696 | 1.00 | referent |
| Post-high school | 10,917 | 0.84 | 0.80 , 0.87 |
| BMI, kg/m2 | |||
| <25 | 7,742 | 1.00 | referent |
| 25-<30 | 3,791 | 1.17 | 1.12 , 1.21 |
| 30+ | 1,885 | 1.71 | 1.62 , 1.79 |
| Smokinga | |||
| Never | 5,191 | 1.00 | referent |
| Ever | 4,413 | 1.49 | 1.43 , 1.55 |
| Alcohol | |||
| Never | 4,790 | 1.00 | referent |
| Ever | 4,574 | 0.95 | 0.91 , 0.99 |
| Cardiovascular disease | |||
| No | 6,212 | 1.00 | referent |
| Yes | 2,473 | 1.30 | 1.24 , 1.36 |
| Diabetes | |||
| No | 7,138 | 1.00 | referent |
| Yes | 1,502 | 1.82 | 1.72 , 1.93 |
| Stroke | |||
| No | 8,518 | 1.00 | referent |
| Yes | 657 | 1.21 | 1.12 , 1.31 |
| Osteoporosis | |||
| No | 6,517 | 1.00 | referent |
| Yes | 1,973 | 0.81 | 0.77 , 0.85 |
| Parity | |||
| Nulliparous/missing | 2,508 | 1.00 | referent |
| One | 2,012 | 0.94 | 0.89 , 1.00 |
| Two | 3,883 | 0.84 | 0.80 , 0.89 |
| Three or more | 5,331 | 0.84 | 0.80 , 0.88 |
| Menopausal hormone therapya | |||
| Never used | 5,712 | 1.00 | referent |
| ET <10 years | 2,919 | 0.85 | 0.81 , 0.89 |
| ET 10+years | 2,021 | 0.83 | 0.79 , 0.87 |
| Used hormones, type other/unknown | 3,082 | 0.80 | 0.77 , 0.84 |
| Family history of ovarian cancer | |||
| No | 4,050 | 1.00 | referent |
| Yes | 259 | 1.00 | 0.88 , 1.13 |
BCDDP, Breast Cancer Detection and Demonstration Project; BMI, body mass index; CI, confidence interval; ET, estrogen therapy; HR, hazard ratio
Smoking status and menopausal hormone therapy were treated as time-dependent exposures.
Relationships between gynecologic surgery and all-cause mortality
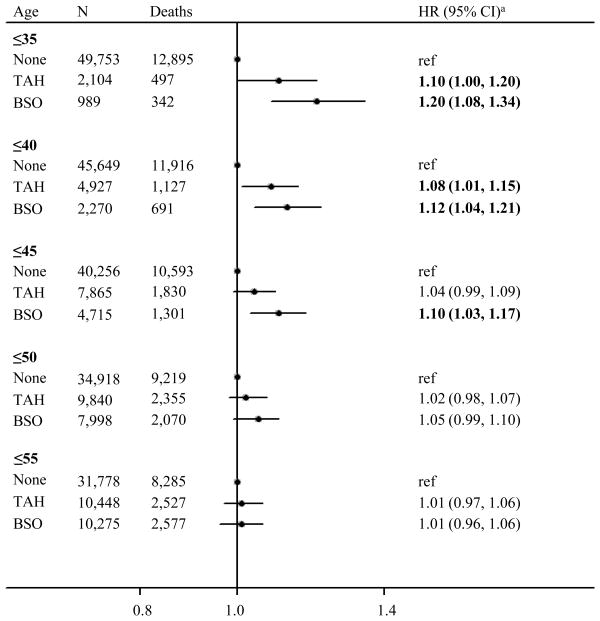
Over an average of 22 years of follow-up, 52,846 women contributed 1,167,514 person-years. Of 13,734 deaths, 4,326 (31%) were cancer-related, 9,014 (66%) non-cancer deaths, and 394 (3%) of unknown causes. Among all women, TAH and BSO were not associated with all-cause mortality (HR=1.01, 95% CI: 0.96, 1.05, and HR=0.99, 95% CI: 0.95, 1.04, respectively) in time-dependent analyses (see Table, Supplemental Digital Content 1, which shows the relation between time-dependent gynecologic surgery status and all-cause mortality). However, risk estimates varied according to age at surgery. Results from landmark analyses (Figure 1) demonstrated that women who underwent BSO by age 35 years had an increased risk of death from any cause (HR35 years=1.20, 95% CI: 1.08, 1.34), which progressively decreased if surgery was performed by later ages, until age 50 years, after which risk was not increased. TAH alone by ages 35 or 40 years was associated with increased all-cause mortality (HR35 years=1.10, 95% CI: 1.00, 1.20; HR40 years =1.08, 95% CI: 1.01, 1.15), but statistically significant associations were not observed by later landmark ages. Landmark analyses based on matched propensity scores related to having benign gynecological surgery were consistent with results for the entire cohort (see Table, Supplemental Digital Content 2, which describes results from landmark analyses with propensity score matching). Numbers were too sparse to separately examine risk associations for women who underwent TAH with USO (categorized in our benign TAH group) or for women who underwent BSO without TAH (categorized in our benign BSO group). Results from landmark analyses excluding these subgroups were also consistent with results for the entire cohort, though findings for risk associations with TAH were somewhat attenuated (data not shown).
Figure 1.
Survival Model Results for Landmark Analyses Relating Between Benign Gynecologic Surgery Status to All-cause Mortality, According to the Age by Which Surgeries Were Performed, BCDDP Follow-up Study, Follow-up From 1979-1986 to 2005 (n=52,846)
a Landmark analyses, using Cox proportional hazards models, adjusted for body mass index, alcohol use, smoking, menopausal hormone therapy use, and birth cohort.
BCDDP, Breast Cancer Detection and Demonstration Project; BSO, bilateral salpingo-oophorectomy; CI, confidence interval; TAH, transabdominal hysterectomy; HR, hazard ratio.
Sensitivity analyses in which follow-up was censored at age 70 years indicated stronger shorter-term risk estimates related to gynecologic surgery as compared with those observed in the main analysis for which follow-up was censored at age 90 years (see Table, Supplemental Digital Content 3, which shows results from sensitivity analyses with follow-up censored at age 70 years). Time-dependent analyses stratified by age at surgery implicated BSO prior to age 35 years as the main cause of excess mortality (see Table, Supplemental Digital Content 1).
Relationships between benign gynecologic surgery and disease-specific mortality
Findings for common cancer and non-cancer causes of death with potential hormonal etiology are shown in Table 3 and Table 4 (findings for less common causes of death may be found in Table, Supplemental Digital Content 4). BSO was associated with a reduction in risk of cancer-related death if performed by age 50 (HR50 years=0.89, 95% CI: 0.81, 0.98) or by age 55 years (HR55 years=0.88, 95% CI: 0.80, 0.97) (Table 3). BSO was associated with increased risk of death from colorectal and pancreatic cancers; however, findings were only statistically significant at selected ages at surgery, based on limited data. Brain cancer deaths were increased among women who underwent BSO by age 55 years (HR55 years=1.67, 95% CI: 1.05, 2.67), based on 32 fatal cases (see Table, Supplemental Digital Content 4). TAH by any of the landmark ages was not associated with cancer mortality.
Table 3. Survival Model Results for the Relation Between Benign Gynecologic Surgery Status and Cancer Mortality, According to the Age by Which Surgeries Were Performed: US BCDDP Follow-up Study With Follow-up From 1979-1986 to 2005 (n=52,846).
| Benign gynecological surgery status | All cancers (No. deaths=4,326) | Lung (No. deaths=914) | Breast (No. deaths=734) | CRC (No. deaths=384) | Pancreas (No. deaths=322) | NHL (No. deaths=236) |
|---|---|---|---|---|---|---|
| Age at surgery | ||||||
| ≤35 years | ||||||
| None | ||||||
| No. of deaths | 4,076 | 859 | 697 | 362 | 299 | 222 |
| HRa | ref | ref | ref | ref | ref | ref |
| TAH | ||||||
| No. of deaths | 153 | 33 | 25 | 7 | 9 | 10 |
| HRa | 1.00 | 1.00 | 0.93 | 0.51 | 0.76 | 1.20 |
| 95% CI | 0.85, 1.18 | 0.70, 1.42 | 0.62, 1.38 | 0.24, 1.08 | 0.39, 1.49 | 0.63, 2.28 |
| BSO | ||||||
| No. of deaths | 97 | 22 | 12 | 15 | 14 | 4 |
| HRa | 1.14 | 1.17 | 0.88 | 1.92 | 2.09 | 0.84 |
| 95% CI | 0.93, 1.39 | 0.77, 1.80 | 0.49, 1.56 | 1.13, 3.23 | 1.21, 3.60 | 0.31, 2.28 |
| ≤40 years | ||||||
| None | ||||||
| No. of deaths | 3,779 | 784 | 646 | 338 | 283 | 200 |
| HRa | ref | ref | ref | ref | ref | ref |
| TAH | ||||||
| No. of deaths | 374 | 85 | 66 | 27 | 22 | 28 |
| HRa | 1.04 | 1.12 | 1.04 | 0.83 | 0.76 | 1.50 |
| 95% CI | 0.94, 1.17 | 0.89, 1.41 | 0.80, 1.35 | 0.55, 1.23 | 0.49, 1.19 | 1.00, 2.25 |
| BSO | ||||||
| No. of deaths | 173 | 45 | 22 | 19 | 17 | 8 |
| HRa | 0.92 | 1.14 | 0.73 | 1.09 | 1.09 | 0.79 |
| 95% CI | 0.79, 1.08 | 0.84, 1.55 | 0.47, 1.12 | 0.68, 1.74 | 0.66, 1.80 | 0.39, 1.62 |
| ≤45 years | ||||||
| None | ||||||
| No. of deaths | 3,374 | 694 | 579 | 303 | 247 | 173 |
| HRa | ref | ref | ref | ref | ref | ref |
| TAH | ||||||
| # of deaths | 595 | 130 | 103 | 49 | 39 | 37 |
| HR° | 1.00 | 1.04 | 0.99 | 0.89 | 0.83 | 1.23 |
| 95% CI | 0.91, 1.09 | 0.85, 1.26 | 0.80, 1.23 | 0.65, 1.22 | 0.58, 1.18 | 0.85, 1.79 |
| BSO | ||||||
| No. of deaths | 355 | 90 | 52 | 32 | 36 | 26 |
| HRa | 0.95 | 1.16 | 0.85 | 0.90 | 1.17 | 1.36 |
| 95% CI | 0.84, 1.06 | 0.92, 1.47 | 0.63, 1.14 | 0.61, 1.32 | 0.81, 1.70 | 0.88, 2.11 |
| <50 years | ||||||
| None | ||||||
| No. of deaths | 2,957 | 612 | 500 | 268 | 199 | 150 |
| HRa | ref | ref | ref | ref | ref | ref |
| TAH | ||||||
| No. of deaths | 742 | 149 | 123 | 62 | 57 | 46 |
| HRa | 0.96 | 0.92 | 0.96 | 0.84 | 1.06 | 1.18 |
| 95% CI | 0.88, 1.04 | 0.76, 1.11 | 0.78, 1.19 | 0.62, 1.13 | 0.77, 1.45 | 0.83, 1.68 |
| BSO | ||||||
| No. of deaths | 575 | 144 | 98 | 51 | 64 | 38 |
| HRa | 0.89 | 1.08 | 0.97 | 0.81 | 1.40 | 1.17 |
| 95% CI | 0.81, 0.98 | 0.88, 1.32 | 0.76, 1.22 | 0.58, 1.12 | 1.02, 1.93 | 0.79, 1.73 |
| ≤55 years | ||||||
| None | ||||||
| No. of deaths | 2,630 | 557 | 430 | 238 | 175 | 136 |
| HRa | ref | ref | ref | ref | ref | ref |
| TAH | ||||||
| No. of deaths | 783 | 155 | 126 | 65 | 61 | 48 |
| HRa | 0.96 | 0.88 | 1.01 | 0.81 | 1.08 | 1.13 |
| 95% CI | 0.88, 1.04 | 0.73, 1.07 | 0.81, 1.25 | 0.60, 1.09 | 0.79, 1.49 | 0.79, 1.62 |
| BSO | ||||||
| No. of deaths | 723 | 169 | 127 | 64 | 72 | 47 |
| HRa | 0.88 | 0.97 | 1.05 | 0.78 | 1.26 | 1.10 |
| 95% CI | 0.80, 0.97 | 0.80, 1.18 | 0.84, 1.31 | 0.57, 1.06 | 0.92, 1.72 | 0.76, 1.60 |
BSO, bilateral salpingo-oophorectomy; CI, confidence interval; CRC, colorectal cancer; HR, hazard ratio; NHL, non-Hodgkins lymphoma; TAH, total abdominal hysterectomy.
Landmark analyses, using Cox proportional hazards models, adjusted for body mass index, alcohol use, smoking, menopausal hormone therapy use, and birth cohort.
Table 4. Survival Model Results for the Relation Between Benign Gynecologic Surgery Status and Noncancer Mortality, According to the Age by Which Surgeries Were Performed: US BCDDP Follow-up Study With Follow-up From 1979-1986 to 2005 (n=52,846).
| Benign gynecological surgery status | All non-cancers (No. deaths=9,014) | CHD (No. deaths=3,453) | Stroke (No. deaths=1,177) | COPD (No. deaths=537) | Alzheimer's disease (No. deaths=349) | Diabetes (No. deaths=295) |
|---|---|---|---|---|---|---|
| Age at surgery | ||||||
| ≤35 years | ||||||
| None | ||||||
| No. of deaths | 8,442 | 3,208 | 1,123 | 494 | 335 | 267 |
| HRa | ref | ref | ref | ref | ref | ref |
| TAH | ||||||
| No. of deaths | 334 | 131 | 32 | 28 | 10 | 18 |
| HRa | 1.17 | 1.21 | 0.85 | 1.72 | 1.01 | 1.75 |
| 95% CI | 1.04, 1.30 | 1.02, 1.44 | 0.59, 1.20 | 1.17, 2.53 | 0.54, 1.90 | 1.08, 2.83 |
| BSO | ||||||
| No. of deaths | 238 | 114 | 22 | 15 | 4 | 10 |
| HRa | 1.25 | 1.56 | 0.84 | 1.35 | 0.59 | 1.50 |
| 95% CI | 1.10, 1.42 | 1.29, 1.89 | 0.55, 1.29 | 0.81, 2.27 | 0.22, 1.57 | 0.79, 2.82 |
| ≤40 years | ||||||
| None | ||||||
| No. of deaths | 7,788 | 2,963 | 1,020 | 459 | 312 | 238 |
| HRa | ref | ref | ref | ref | ref | ref |
| TAH | ||||||
| No. of deaths | 727 | 276 | 93 | 48 | 25 | 36 |
| HRa | 1.11 | 1.13 | 1.09 | 1.25 | 1.07 | 1.67 |
| 95% CI | 1.03, 1.20 | 0.99, 1.28 | 0.88, 1.36 | 0.92, 1.70 | 0.71, 1.62 | 1.16, 2.39 |
| BSO | ||||||
| No. of deaths | 499 | 214 | 64 | 30 | 12 | 21 |
| HRa | 1.22 | 1.37 | 1.15 | 1.28 | 0.80 | 1.60 |
| 95% CI | 1.11, 1.34 | 1.19, 1.58 | 0.89, 1.48 | 0.88, 1.86 | 0.45, 1.43 | 1.02, 2.52 |
| ≤45 years | ||||||
| None | ||||||
| HRa | 6,912 | 2,626 | 901 | 429 | 282 | 212 |
| HR§ | ref | ref | ref | ref | ref | ref |
| TAH | ||||||
| No. of deaths | 1,182 | 455 | 148 | 64 | 47 | 47 |
| HRa | 1.06 | 1.10 | 1.01 | 0.89 | 1.13 | 1.33 |
| 95% CI | 1.00, 1.13 | 0.99, 1.22 | 0.85, 1.21 | 0.68, 1.17 | 0.82, 1.55 | 0.96, 1.84 |
| BSO | ||||||
| No. of deaths | 913 | 371 | 126 | 44 | 20 | 35 |
| HRa | 1.18 | 1.28 | 1.19 | 0.90 | 0.69 | 1.45 |
| 95% CI | 1.10, 1.27 | 1.14, 1.43 | 0.98, 1.45 | 0.65, 1.24 | 0.43, 1.10 | 1.00, 2.10 |
| <50 years | ||||||
| None | ||||||
| No. of deaths | 5,992 | 2,280 | 781 | 369 | 248 | 184 |
| HRa | ref | ref | ref | ref | ref | ref |
| TAH | ||||||
| No. of deaths | 1,546 | 600 | 193 | 90 | 53 | 57 |
| HRa | 1.06 | 1.11 | 0.99 | 0.96 | 0.92 | 1.26 |
| 95% CI | 1.00, 1.13 | 1.01, 1.22 | 0.84, 1.17 | 0.75, 1.23 | 0.68, 1.26 | 0.92, 1.72 |
| BSO | ||||||
| No. of deaths | 1,441 | 567 | 197 | 78 | 47 | 51 |
| HRa | 1.13 | 1.20 | 1.12 | 0.97 | 0.96 | 1.28 |
| 95% CI | 1.06, 1.20 | 1.08, 1.32 | 0.95, 1.33 | 0.74, 1.27 | 0.69, 1.35 | 0.91, 1.78 |
| <55 years | ||||||
| None | ||||||
| No. of deaths | 5,410 | 2,086 | 687 | 336 | 213 | 170 |
| HRa | ref | ref | ref | ref | ref | ref |
| TAH | ||||||
| No. of deaths | 1,677 | 642 | 214 | 98 | 67 | 56 |
| HRa | 1.04 | 1.07 | 1.02 | 0.94 | 1.12 | 1.09 |
| 95% CI | 0.99, 1.11 | 0.97, 1.17 | 0.86, 1.20 | 0.74, 1.20 | 0.83, 1.50 | 0.79, 1.51 |
| BSO | ||||||
| No. of deaths | 1,792 | 684 | 253 | 100 | 68 | 65 |
| HRa | 1.08 | 1.10 | 1.14 | 0.93 | 1.12 | 1.24 |
| 95% CI | 1.01, 1.14 | 1.00, 1.21 | 0.97, 1.34 | 0.72, 1.19 | 0.83, 1.52 | 0.90, 1.70 |
BSO, bilateral salpingo-oophorectomy; CHD, coronary heart disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio, TAH, total abdominal hysterectomy.
Landmark analyses, using Cox proportional hazards models, adjusted for body mass index, alcohol use, smoking, menopausal hormone therapy use, and birth cohort.
BSO was related to increased risk of non-cancer deaths, with the strongest associations among women undergoing surgery by age 35 years (HR35 years=1.25, 95% CI: 1.10, 1.42) (Table 4). The associations were progressively attenuated at older ages, though risk remained increased up to age 55 years (HR55 years=1.08, 95% CI: 1.01, 1.14). BSO at all landmark ages was associated with a statistically significant increased risk of death from coronary heart disease (CHD). Increased risk of death from diabetes was observed for BSO at all landmark ages, but was statistically significant only for women who underwent BSO by ages 40 and 45 years (HR40 years=1.60, 95% CI: 1.02, 2.52; HR45 years=1.45, 95% CI: 1.00, 2.10). TAH at all landmark ages was associated with increased CHD mortality, but risk estimates showed attenuation with increasing age and were lower than those observed for BSO.
Risk associations by menopausal hormone therapy use and body mass index
Ever use of HT did not statistically significantly modify the relation between gynecologic surgery and all-cause mortality (see Table, Supplemental Digital Content 5, which shows survival model results for the association between benign gynecologic surgery status and all-cause mortality for never vs. ever users of menopausal HT). However, among never users of HT, increased mortality associated with BSO persisted at older ages, whereas risks among ever users dissipated after age 50 years. All-cause mortality was particularly elevated among lean women (BMI <25 kg/m2) who underwent BSO by age 50 years (P for interaction = 0.003), whereas risks associated with TAH were strongest for overweight and obese women (BMI 25+ kg/m2) (see Table, Supplemental Digital Content 6, which shows survival model results for the relation between benign gynecologic surgery status and all-cause mortality for women with BMI <25 kg/m2 vs. BMI 25+ kg/m2). For CHD mortality, we observed similar patterns of increased risk associated with BSO among never users of HT and lean women (data not shown).
Discussion
This analysis within a large prospective cohort with lengthy follow-up shows that BSO performed at young ages is associated with an increased risk of death, which declines progressively with aging, and disappears by age 50 years. In addition, TAH alone performed by age 40 years was associated with a smaller increase in mortality risk. For both procedures, excess risk is attributable largely to non-cancer causes, especially CHD. Our results in combination with the literature support caution in recommending BSO for younger women who are not at high-risk of breast and ovarian cancers.
Prior analyses have linked BSO performed by the fifth decade to increased mortality among all women5 or among subsets of women who are obese8 or never users of exogenous hormones.5, 7-8 Our results extend these findings to younger women, demonstrating a 20% increased risk of death among women undergoing BSO by age 35 years. The California Teachers Study reported that BSO was unrelated to mortality,9 but this result was based on only 11 average years of follow-up (vs. 22 years in our study), and 97% of women who underwent BSO and 66% with intact ovaries reported taking HT. In our data and others,5, 7-8 use of HT attenuates mortality risk related to BSO; thus, patterns of exogenous hormone use may have contributed to the divergent null finding.
Prior studies have linked BSO to increased CHD mortality,4-5 with some showing a greater prevalence of CHD risk factors3 or higher CHD risk2 associated with BSO at age 50 years or younger. Surgical menopause is associated with an abrupt decrease in circulating endogenous estrogens, increases in lipids14 and subclinical atherosclerosis.15-16 Alternatively, the relationship between BSO and CHD mortality may not be causal because common risk factors may predispose to both gynecological surgery and CHD mortality. Although we adjusted for multiple factors, we cannot exclude residual confounding in our analyses of BSO and CHD mortality, and BMI was only available at baseline.
We also observed weaker CHD mortality risks associated with TAH alone, which could reflect acceleration of ovarian failure, as previously postulated,17 or misclassification of women who underwent BSO. In the Women's Health Initiative, TAH alone was not independently related to risk of CHD.18
Similar to findings from three5, 7-8 of four5, 7-9 prior cohorts, we observed higher mortality risks among women who underwent BSO and were non-users of menopausal HT. This finding has important public health implications, given the decline in HT use.19 Consistent with the mitigation of mortality risk associated with HT, we found that BSO was riskier for lean women, possibly reflecting protection associated with greater estrogen synthesis in adipose tissues20 among heavier women. However, in the National Health and Nutrition Examination Survey, a population with higher average BMI than BCDDP, excess mortality following BSO was concentrated among young obese women.8
Our data suggest that BSO by age 50 or 55 years may reduce cancer mortality, although associations were not identified for the most common tumor types. For unclear reasons, mortality risks were elevated for colorectal, pancreatic and brain cancers at selected ages.
Although use of self-reported surgical history is a potential limitation of our analysis, prior studies have reported excellent reliability and validity for these data.21-23 While we were unable to account for possible effects of surgical indications, our analysis of women matched on propensity scores related to the probability of undergoing benign gynecologic surgery yielded results similar to those for the entire cohort. In addition, we think that it is unlikely that risk information related to surgical indication that was not captured by our questionnaire data would be influencing the risk of death decades later and producing the observed monotonic relationship between age at BSO and mortality risk. Furthermore, our results are generally compatible with the literature, while extending prior findings.
We present results from a landmark analysis and a Cox model, in which benign gynecologic surgery was treated as a time-dependent exposure. Both analytic approaches avoid biases involved in comparing time-to-event data for different exposure groups when exposure group membership for an individual arbitrarily varies during the time under study. Results that used surgery as a time-dependent variable were consistent with findings from the landmark analysis showing that benign gynecologic surgeries among young women are associated with increased mortality risk. We focused on results from the landmark analysis as it is simple in its execution and its interpretation and provides the clinically relevant quantities. While the results of the landmark analysis depend on the somewhat arbitrary choice of the particular landmark time, we lessened the impact of this selection by choosing several landmark ages.
Strengths of our study include repeated risk factor and gynecological surgery data collection, the large size of the cohort, and lengthy follow-up. Sensitivity analyses demonstrated that results were not driven by deaths among the very old, where biases could come into play. We attempted to account for changes in surgical practices and treatment for chronic diseases by adjusting for birth cohort. We also observed that the prevalence of gynecologic surgeries was stable within different birth cohorts, lending relevance of our findings to current clinical practice.
Conclusions
In summary, we demonstrate that BSO among younger women is associated with a substantial increase in mortality risk, primarily due to CHD, which declines with aging and disappears by age 50 years. TAH alone at younger ages demonstrated a weaker increase in mortality, which requires confirmation given the complexities of this analysis. Although women at extremely high genetic risk for breast and ovarian cancer clearly benefit from risk-reducing BSO,1 other women must weigh the risks of developing gynecological cancer against those of CHD mortality. Further research on effects of BSO may enable women to make improved choices based on their individual risks of gynecologic cancer and CHD.
Supplementary Material
Acknowledgments
We thank James V. Lacey Jr., Aimee Kreimer, and Danny Carreon for their contributions to the development of the study design. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management and David Campbell at Information Management Services for data support.
Source of Funding: This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Abbreviations
- BSO
bilateral salpingo-oophorectomy
- BMI
body mass index
- BCDDP
Breast Cancer Demonstration Detection Project
- CI
confidence interval
- CHD
coronary heart disease
- HR
hazard ratio
- HT
hormone therapy
- TAH
total abdominal hysterectomy
- USO
unilateral salpingo-oophorectomy
Footnotes
Conflicts of Interest: None.
References
- 1.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of Risk Reduction Estimates Associated With Risk-Reducing Salpingo-oophorectomy in BRCA1 or BRCA2 Mutation Carriers. J Natl Cancer Inst. 2009;101(2):80–7. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316(18):1105–10. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 3.Dørum A, Tonstad S, Liavaag AH, Michelsen TM, Hildrum B, Dahl AA. Bilateral oophorectomy before 50 years of age is significantly associated with the metabolic syndrome and Framingham risk score: A controlled, population-based study (HUNT-2) Gynecol Oncol. 2008;109(3):377–83. doi: 10.1016/j.ygyno.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16(1):15–23. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker WH, Feskanich D, Broder MS, et al. Long-Term Mortality Associated With Oophorectomy Compared With Ovarian Conservation in the Nurses' Health Study. Obstetrics & Gynecology. 2013;121(4):709–16. doi: 10.1097/AOG.0b013e3182864350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106(2):219–26. doi: 10.1097/01.AOG.0000167394.38215.56. [DOI] [PubMed] [Google Scholar]
- 7.Rocca WA, Grossardt BR, de AM, Malkasian GD, Melton LJ., III Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7(10):821–8. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy AM, Menke A, Ouyang P, Visvanathan K. Bilateral Oophorectomy, Body Mass Index, and Mortality in U.S. Women Aged 40 Years and Older. Cancer Prevention Research. 2012;5(6):847–54. doi: 10.1158/1940-6207.CAPR-11-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan L, Xu X, Koebnick C, et al. Bilateral oophorectomy is not associated with increased mortality: the California Teachers Study. Fertil Steril. 2012;97(1):111–7. doi: 10.1016/j.fertnstert.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198(1):34.e1–7. doi: 10.1016/j.ajog.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 11.Baker LH. Breast Cancer Detection Demonstration Project: five-year summary report. CA Cancer J Clin. 1982;32(4):194–225. doi: 10.3322/canjclin.32.4.194. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–9. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum PR, Rubin DB. Reducing Bias in Observational Studies Using Subclassification on the Propensity Score. Journal of the American Statistical Association. 1984;79(387):516–24. [Google Scholar]
- 14.Lobo RA. Surgical menopause and cardiovascular risks. Menopause. 2007;14(3 Pt 2):562–6. doi: 10.1097/gme.0b013e318038d333. [DOI] [PubMed] [Google Scholar]
- 15.Mack WJ, Slater CC, Xiang M, Shoupe D, Lobo RA, Hodis HN. Elevated subclinical atherosclerosis associated with oophorectomy is related to time since menopause rather than type of menopause. Fertil Steril. 2004;82(2):391–7. doi: 10.1016/j.fertnstert.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Allison MA, Manson JE, Langer RD, et al. Oophorectomy, hormone therapy, and subclinical coronary artery disease in women with hysterectomy: the Women's Health Initiative coronary artery calcium study. Menopause. 2008;15(4 Pt 1):639–47. doi: 10.1097/gme.0b013e31816d5b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddle N, Sarrel P, Whitehead M. The effect of hysterectomy on the age at ovarian failure: identification of a subgroup of women with premature loss of ovarian function and literature review. Fertil Steril. 1987;47(1):94–100. doi: 10.1016/s0015-0282(16)49942-5. [DOI] [PubMed] [Google Scholar]
- 18.Hsia J, Barad D, Margolis K, et al. Usefulness of prior hysterectomy as an independent predictor of Framingham risk score (The Women's Health Initiative) Am J Cardiol. 2003;92(3):264–9. doi: 10.1016/s0002-9149(03)00621-0. [DOI] [PubMed] [Google Scholar]
- 19.Kim N, Gross C, Curtis J, et al. The impact of clinical trials on the use of hormone replacement therapy. A population-based study. J Gen Intern Med. 2005;20(11):1026–31. doi: 10.1111/j.1525-1497.2005.0221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr. 1987;45(1):277–82. doi: 10.1093/ajcn/45.1.277. [DOI] [PubMed] [Google Scholar]
- 21.Brett KM, Madans JH. Hysterectomy use: the correspondence between self-reports and hospital records. Am J Public Health. 1994;84(10):1653–5. doi: 10.2105/ajph.84.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colditz GA, Stampfer MJ, Willett WC, et al. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol. 1987;126(2):319–25. doi: 10.1093/aje/126.2.319. [DOI] [PubMed] [Google Scholar]
- 23.Irwin KL, Wingo PA, Lee NC. Agreement of self-reported ovarian number following gynecologic surgery with medical record reports. J Clin Epidemiol. 1990;43(2):181–7. doi: 10.1016/0895-4356(90)90182-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.