Abstract
Deer mice (Peromyscus maniculatus) and congeneric species are used in a wide variety of research applications, particularly studies of developmental, physiologic, and behavioral characteristics associated with habitat adaptation and speciation. Because peromyscine mice readily adapt to colony conditions, animals with traits of interest in the field are moved easily into the laboratory where they can be studied under controlled conditions. The purpose of this study was to determine the serum chemistry and hematologic parameters of 4 frequently used species from the Peromyscus Genetic Stock Center species (P. californicus, P. leucopus, P. maniculatus, and P. polionotus) and to determine quantitative differences in these parameters among species and between sexes. Triglyceride values were substantially higher in female compared with male mice in all 4 species. Similar cross-species differences in MCH were present. Overall there was considerable interspecific variation for most blood parameters, with little evidence for covariation of any 2 or more parameters. Because crosses of P. maniculatus and P. polionotus produce fertile offspring, segregation analyses can be applied to determine the genetic basis of any traits that differ between them, such as their 3.8- and 2.1-fold interspecific differences in cholesterol and triglyceride levels, respectively. The current data provide a set of baseline values useful for subsequent comparative studies of species experiencing different circumstances, whether due to natural variation or anthropogenic environmental degradation. To enable such comparisons, the raw data are downloadable from a site maintained by the Stock Center (http://ww2.biol.sc.edu/~peromyscus).
Abbreviations: BW, P. maniculatus bairdii; IS, P. californicus insignis; LL, P. leucopus; PO, P. polionotus subgriseus
Collectively peromyscine rodents are the most common, abundant, and speciose native North American mammals. Ranging from Alaska to Central America and from the Atlantic to the Pacific, they occur in a wide range of habitats, including sea-level wetlands, beaches, forests, prairies, and deserts and mountains of elevations to 14,000 ft.23,24 As such, peromyscine rodents are uniquely positioned as models for studying the factors, genetic and otherwise, responsible for reproductive isolation and speciation. In addition, they are useful as models to study the factors enabling adaptive responses to changing environmental conditions, to other species, and to each other.10,18,35 Peromyscus maniculatus (deer mice) and P. leucopus (white-footed mice) are the most familiar, widespread, and biologically best-known species. In addition, these particular species have drawn considerable public health interest owing to their roles as zoonotic reservoirs of infectious disease organisms,41 notably hantavirus (P. maniculatus)29 and the Borrelia spp. causing Lyme disease (P. leucopus).27
Peromyscines are reared in animal colonies incorporating caging, feeding, and maintenance regimens used for laboratory mice.21 The stocks maintained by the Peromyscus Genetic Stock Center (http://stkctr.biol.sc.edu/) were all derived from wild-caught animals and bred by random mating to maintain genetic diversity. As such, they can be considered to be closely related genetically to their wild counterparts and possess allelic combinations maximizing the physiologic and behavioral traits undergirding the species’ habitat adaptation. This situation contrasts with the standard inbred strains of laboratory mice, which are amalgams of genes derived from 3 or 4 different Mus spp.6,16 and which lack wild natural counterparts. Random admixture of genes from different species may provide a means of unmasking gene effects that would otherwise go unnoticed, such as those being studied in collaborative cross strains.8 Yet the results say little regarding the adaptive roles of such genes in wild populations and their corresponding phenotypes.
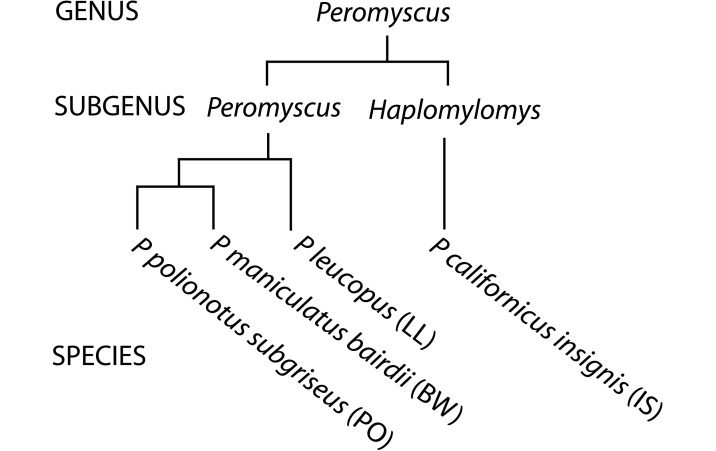
In the current report we present hematologic and biochemical comparisons of 4 species maintained by the Stock Center to determine the degree that species- and sex-associated factors affect various blood parameters. The phylogenetic relatedness of the 4 species is illustrated in Figure 1. The species examined were P. californicus insignis (IS), P. leucopus (LL), P. maniculatus bairdii (BW), and P. polionotus subgriseus (PO). Two of these (BW and PO) are interfertile sister species, whose offspring manifest disparate dysgenetic phenotypes depending on the parental sex.9,12,51 Thus, PO females mated with BW males generate offspring manifesting lethal overgrowth and which rarely develop to term. Offspring of the reciprocal cross (BW females × PO males) are viable but undersized at birth and into adulthood. We determined the hematologic and chemical parameters of the BW × PO hybrids and compared the results with those of the parental stocks.
Figure 1.
Evolutionary relationship and species designations (in parentheses) of the peromyscine species whose hematologic and serum biochemical parameters were profiled.
We undertook this study for 3 primary reasons: 1) to establish baseline values for commonly used stocks maintained by the Stock Center, thereby rendering them more useful and complementing their completed genome sequences (currently being assembled); 2) to compare species- and sex-associated differences in such values; and 3) to assess basic inheritance patterns of differences between the PO and BW stocks, which differ in numerous characteristics, including partner fidelity (PO and IS are monogamous species10,14,19), stress response and blood glucose homeostasis, repetitive behaviors, growth control, and various blood parameters documented in this study.
Materials and Methods
Animals.
All animals were obtained from the Peromyscus Genetic Stock Center and included 20 male and 20 female mice of each stock (age, 3 to 4 mo). The mice came from the following stocks: IS stock, derived from 60 ancestors collected between 1979 and 1987 in the mountains of Santa Monica, CA; LL stock, derived from 38 ancestors captured between 1982 and 1985 near Linville, NC; BW stock, descended from 40 ancestors caught in 1948 near Ann Arbor, MI; and PO Stock, derived from 21 ancestors caught in Ocala National Forest, FL. In addition, 10 male and 10 female hybrids obtained by mating BW female mice with PO male mice were analyzed.
Mice in the Peromyscus Genetic Stock Center are housed in a facility registered with the US Department of Agriculture and accredited by AAALAC. Animal procedures were in accordance with the USDA Animal Welfare Act2 and Guide for the Care and Use of Laboratory Animals19 and were reviewed and approved by the IACUC. Standard animal husbandry procedures were used throughout.21 The mice were housed in polypropylene cages with aspen bedding, fed a standard commercial rodent diet (Rodent Diet W, Harlan Teklad, Madison, WI), and provided with filtered, UV-treated water ad libitum. All cages were changed weekly. Environmental conditions in the rooms included a 16:8-h light:dark cycle, temperature maintained at 18 to 23 °C, 50% to 70% relative humidity, and 15 air changes hourly.
The mice were monitored routinely for common rodent viruses (mouse hepatitis virus, Sendai virus, pneumonia virus of mice, reovirus 3, ectromelia, mouse parvovirus, epidemic diarrhea of mice, and lymphocytic choriomeningitis virus) and Mycoplasma pulmonis. In addition, mice were monitored for intestinal parasites by direct examination of cecal or colonic contents by fecal flotation and tape test of the perianal region. The presence of mites was determined by skin scrapes and hair-tuft examinations.
Sample acquisition.
Animals were shipped from the Peromyscus Genetic Stock Center (Columbia, SC) to Comparative Clinical Pathology Services (Columbia, MO). After a 24-h acclimation period, the mice were euthanized by CO2 overdose. Approximately 1.0 mL of whole blood was collected via cardiocentesis and placed in a tube containing lithium heparin, thoroughly mixed, and analyzed immediately.
Hematologic analyses.
The CBC included red cell parameters (Hct, Hgb, RBC number, MCV, MCH, and MCHC), white cell parameters (WBC number and neutrophil, lymphocyte, monocyte, and eosinophil percentages and absolute counts), and platelet parameters (platelet number and mean platelet volume). Samples were analyzed by using an automated hematology instrument (Hemavet 950FS, Drew Scientific, Dallas, TX). Prior to CBC analysis, a blood smear was created, stained by using a Diff-Quik stain (Medical Solutions, Lakewood, NJ), and used to perform a manual WBC differential count and to assess RBC morphology and platelet clumping.
Plasma biochemistry analyses.
After CBC analyses, the samples were centrifuged at 15,000 × g for 5 min to separate the plasma from the cells. The plasma was harvested and analyzed for plasma biochemical parameters (that is, glucose, BUN, creatinine, total protein, albumin, phosphorus, sodium, chloride, potassium, total CO2, cholesterol, triglycerides, calcium, total bilirubin, ALP, ALT, and GGT) by using commercial assays (Beckman-Coulter, Brea, CA) on an automated clinical chemistry analyzer (model AU680, Beckman-Coulter). Globulins were calculated by subtracting albumin from total protein.
Statistical analyses.
For the raw data available on the Stock Center website (http://ww2.biol.sc.edu/~peromyscus), 95% reference intervals were established by using a statistical program (version 11.6.0.0, MedCalc, Ostend, Belgium). The program calculates reference intervals according by using normal distribution and by using a nonparametric percentile method. Normal distribution was determined by using the D'Agostino–Pearson test, with significance set at a P value of less than 0.05. If the data failed to achieve normal distribution, a nonparametric statistical method was applied.
All comparisons were performed by using the statistical suite in the SAS Enterprise Guide (version 4.2, SAS Institute, Cary, NC). For interspecies comparisons, male and female values were pooled and the results analyzed by ANOVA. Statistical significance was inferred at a P value of less than 0.05. Interspecies comparisons of each parameter were derived from one-way ANOVA, coupled with Tukey tests to effect pairwise comparisons. The Enterprise Guide also was used to determine the significance of sex-associated differences and those between BW, PO, and their F1 hybrids (Figure 2) according to pairwise t tests. Equality of variances was evaluated by using the folded F statistic. If the assumption of equality of variances was not reasonable, the Satterthwaite approximation for degrees of freedom was used to determine P values.
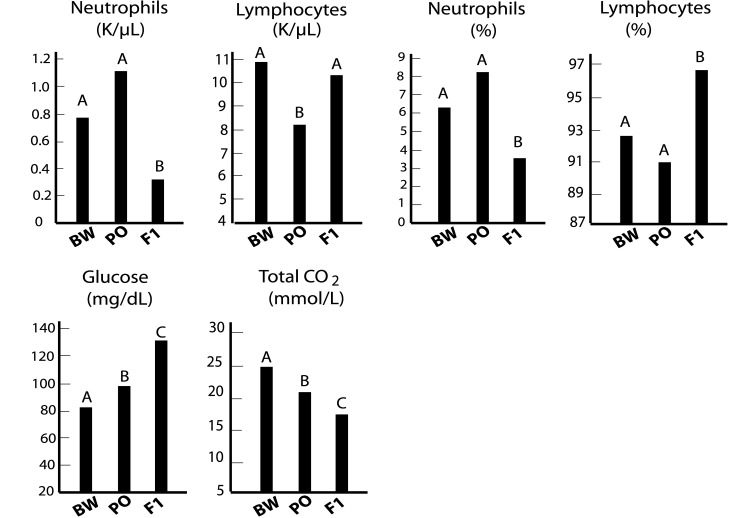
Figure 2.
Hematologic and biochemical parameters in which the hybrid values lie outside those of either parent. Data derived from pooled male and female values (BW and PO, n = 40 each; (BW × PO)F1, n = 20. Values indicated with the same letter are considered to be in the same range; values with different letters are significantly different (t test, P < 0.05).
Results
Mean values and standard deviations for each hematologic and biochemical parameter, categorized by sex, for the 4 Peromyscus species and the BW × PO (female × male) hybrids are provided (Tables 1 and 2). Roughly half of the blood parameters among the pure species differed significantly between the sexes but usually with no apparent interspecific correlations. In a couple of cases, however, a consistent sex-associated difference was noted for all 4 species. Specifically, triglyceride concentrations were significantly higher in female than male mice; the ratios of the male:female means were: IS, 1:1.56; LL, 1:1.56; BW, 1:1.52; PO, 1:1.94. For interspecies comparisons, the male and female values from each were pooled and the results analyzed by one-way ANOVA (Tables 3 through 5); values that differed significantly among the species (determined by Tukey test) are so indicated.
Table 1.
Hematologic values
| IS |
LL |
BW |
PO |
BW × PO |
|||||||
| F | M | F | M | F | M | F | M | F | M | ||
| WBC (K/μL) | Mean | 10.89 | 14.29 | 8.41 | 8.76 | 11.22 | 12.35 | 8.63 | 10.14 | 11.72 | 9.70 |
| 1 SD | 2.90 | 4.76 | 2.72 | 2.40 | 3.88 | 3.72 | 1.58 | 3.06 | 3.12 | 2.54 | |
| Neutrophils (%) | Mean | 6.00 | 9.53 | 4.26 | 7.75 | 4.00 | 8.55 | 6.75 | 9.75 | 2.10 | 4.00 |
| 1 SD | 5.02 | 5.77 | 2.83 | 8.53 | 3.00 | 8.48 | 5.04 | 9.60 | 2.88 | 3.13 | |
| n | 19 | 19 | 19 | 20 | 19 | 20 | 20 | 20 | 10 | 10 | |
| Lymphocytes (%) | Mean | 93.2 | 89.6 | 95.0 | 91.2 | 95.1 | 90.5 | 92.2 | 89.4 | 97.2 | 95.7 |
| 1 SD | 5.2 | 6.0 | 4.3 | 8.8 | 3.7 | 8.6 | 5.5 | 10.1 | 3.2 | 3.3 | |
| Monocytes (%) | Mean | 1.91 | 1.67 | 2.60 | 0.95 | 1.44 | 0.86 | 1.21 | 1.29 | 1.00 | 1.00 |
| 1 SD | 0.94 | 0.82 | 3.05 | 0.83 | 0.73 | 0.86 | 0.43 | 0.49 | 0.63 | 0.00 | |
| n | 11 | 15 | 5 | 20 | 9 | 14 | 14 | 7 | 6 | 2 | |
| Neutrophils (K/μL) | Mean | 0.62 | 1.43 | 0.35 | 0.66 | 0.47 | 1.05 | 0.58 | 1.68 | 0.22 | 0.42 |
| 1 SD | 0.51 | 1.15 | 0.27 | 0.70 | 0.50 | 1.13 | 0.42 | 2.79 | 0.27 | 0.39 | |
| n | 19 | 19 | 19 | 20 | 19 | 20 | 20 | 20 | 9 | 10 | |
| Lymphocytes (K/μL) | Mean | 10.18 | 12.75 | 8.02 | 8.00 | 10.64 | 11.21 | 7.96 | 8.91 | 11.45 | 9.26 |
| 1 SD | 2.78 | 4.20 | 2.54 | 2.39 | 3.56 | 3.50 | 1.56 | 2.31 | 3.30 | 2.33 | |
| Monocytes (K/μL) | Mean | 0.21 | 0.22 | 0.12 | 0.09 | 0.16 | 0.08 | 0.10 | 0.15 | 0.10 | 0.10 |
| 1 SD | 0.08 | 0.14 | 0.04 | 0.08 | 0.07 | 0.07 | 0.04 | 0.09 | 0.08 | 0.04 | |
| n | 11 | 15 | 5 | 20 | 9 | 14 | 14 | 7 | 6 | 2 | |
| RBC (M/μL) | Mean | 10.0 | 10.2 | 10.9 | 12.0 | 10.8 | 12.2 | 10.1 | 10.8 | 10.6 | 11.5 |
| 1 SD | 0.7 | 0.9 | 1.0 | 0.8 | 0.9 | 0.7 | 0.6 | 0.5 | 0.7 | 0.8 | |
| Hemoglobin (g/dL) | Mean | 15.0 | 14.3 | 14.3 | 13.0 | 13.2 | 13.2 | 13.9 | 14.0 | 14.1 | 14.6 |
| 1 SD | 0.8 | 0.9 | 1.4 | 1.0 | 1.2 | 0.7 | 1.0 | 0.9 | 1.2 | 0.8 | |
| Hematocrit (%) | Mean | 44.1 | 43.3 | 41.1 | 44.6 | 38.9 | 45.1 | 40.7 | 41.1 | 42.6 | 42.1 |
| 1 SD | 2.5 | 2.6 | 3.9 | 3.3 | 4.0 | 2.7 | 2.3 | 2.4 | 3.8 | 2.4 | |
| MCV (fL) | Mean | 44.3 | 42.8 | 37.5 | 37.2 | 36.1 | 37.2 | 40.1 | 38.1 | 40.4 | 36.5 |
| 1 SD | 1.2 | 3.1 | 3.0 | 2.4 | 1.3 | 1.6 | 1.9 | 1.8 | 1.3 | 1.3 | |
| MCH (pg) | Mean | 15.1 | 14.1 | 13.1 | 10.8 | 12.3 | 10.9 | 13.7 | 12.9 | 13.0 | 12.7 |
| 1SD | 0.6 | 0.8 | 0.8 | 0.7 | 0.4 | 0.5 | 1.0 | 0.7 | 0.5 | 0.7 | |
| MCHC (g/dL) | Mean | 34.1 | 33.1 | 34.9 | 29.1 | 34.0 | 29.3 | 34.1 | 34.0 | 33.0 | 34.6 |
| 1SD | 0.9 | 1.0 | 2.1 | 0.5 | 0.7 | 0.9 | 1.4 | 1.3 | 1.3 | 1.4 | |
| Platelets (M/μL) | Mean | 286 | 365 | 368 | 421 | 361 | 354 | 477 | 543 | 310 | 306 |
| 1 SD | 125 | 168 | 131 | 145 | 162 | 117 | 178 | 188 | 58 | 134 | |
| MPV (fL) | Mean | 5.53 | 5.01 | 4.83 | 4.61 | 4.60 | 4.55 | 5.48 | 5.23 | 4.95 | 6.87 |
| 1 SD | 0.85 | 0.74 | 0.86 | 0.49 | 0.66 | 0.22 | 0.64 | 0.51 | 0.68 | 0.83 | |
F, female; M, male.
Unless otherwise noted for specific parameters, n = 20 of each sex for pure species and n = 10 of each sex for the BW × PO hybrids.
Table 2.
Serum biochemical values
| IS |
LL |
BW |
PO |
BW × PO |
|||||||
| F | M | F | M | F | M | F | M | F | M | ||
| Triglycerides (mg/dL) | Mean | 493 | 315 | 463 | 296 | 226 | 149 | 526 | 271 | 261 | 205 |
| 1 SD | 233 | 236 | 214 | 114 | 56 | 61 | 253 | 113 | 127 | 100 | |
| Cholesterol (mg/dL) | Mean | 146 | 165 | 114 | 100 | 78 | 97 | 390 | 283 | 179 | 181 |
| 1 SD | 39 | 30 | 71 | 32 | 10 | 14 | 73 | 84 | 20 | 24 | |
| Glucose (mg/dL) | Mean | 121 | 102 | 107 | 63 | 72 | 93 | 95 | 101 | 122 | 137 |
| 1 SD | 25 | 41 | 26 | 27 | 24 | 25 | 26 | 31 | 39 | 34 | |
| Total CO2 (mmol/L) | Mean | 23.5 | 24.0 | 19.4 | 19.1 | 21.5 | 28.2 | 21.5 | 22.0 | 16.2 | 18.7 |
| 1 SD | 3.70 | 2.0 | 3.0 | 3.9 | 4.50 | 3.20 | 4.90 | 4.50 | 5.90 | 3.30 | |
| BUN (mg/dL) | Mean | 33.8 | 36.6 | 23.6 | 24.1 | 33.4 | 30.3 | 41.4 | 38.3 | 37.1 | 33.0 |
| 1 SD | 7.8 | 6.9 | 3.9 | 3.8 | 3.5 | 3.9 | 4.9 | 4.9 | 7.3 | 6.1 | |
| Creatinine (mg/dL) | Mean | 0.22 | 0.22 | 0.21 | 0.23 | 0.20 | 0.20 | 0.25 | 0.22 | 0.24 | 0.21 |
| 1 SD | 0.03 | 0.02 | 0.02 | 0.04 | 0.00 | 0.00 | 0.04 | 0.02 | 0.04 | 0.02 | |
| Sodium (mmol/L) | Mean | 157 | 154 | 156 | 157 | 152 | 155 | 153 | 153 | 149 | 152 |
| 1 SD | 6 | 2 | 6 | 5 | 2 | 4 | 4 | 3 | 9 | 1 | |
| Potassium (mmol/L) | Mean | 7.88 | 7.99 | 6.78 | 7.27 | 6.10 | 6.97 | 5.68 | 6.44 | 6.62 | 6.72 |
| 1 SD | 2.17 | 1.15 | 0.74 | 0.82 | 0.89 | 0.59 | 0.87 | 0.99 | 1.50 | 0.85 | |
| Chloride (mmol/L) | Mean | 112 | 111 | 112 | 108 | 108 | 110 | 108 | 108 | 109 | 110 |
| 1 SD | 4 | 2 | 4 | 2 | 2 | 5 | 3 | 3 | 3 | 2 | |
| Calcium (mg/dL) | Mean | 10.4 | 10.7 | 11.0 | 10.9 | 10.2 | 10.0 | 11.5 | 11.3 | 11.7 | 10.9 |
| 1 SD | 0.8 | 0.8 | 0.7 | 0.7 | 0.6 | 0.5 | 0.8 | 1.3 | 2.2 | 0.4 | |
| Phosphorus (mg/dL) | Mean | 10.4 | 9.9 | 12.5 | 16.7 | 10.3 | 8.1 | 11.8 | 11.7 | 12.8 | 9.2 |
| 1 SD | 3.5 | 2.3 | 2.2 | 2.4 | 2.4 | 1.4 | 1.8 | 2.1 | 2.7 | 1.0 | |
| Total protein (g/dL) | Mean | 6.08 | 6.16 | 6.52 | 6.52 | 5.92 | 5.42 | 7.33 | 6.98 | 6.29 | 5.89 |
| 1 SD | 0.43 | 0.36 | 0.42 | 0.44 | 0.35 | 0.36 | 0.47 | 0.52 | 0.24 | 0.34 | |
| Albumin (g/dL) | Mean | 3.27 | 3.16 | 3.37 | 3.45 | 3.14 | 3.03 | 3.98 | 3.82 | 3.63 | 3.41 |
| 1 SD | 0.23 | 0.15 | 0.37 | 0.21 | 0.21 | 0.19 | 0.23 | 0.27 | 0.21 | 0.15 | |
| Globulins (g/dL) | Mean | 2.82 | 3.00 | 3.15 | 3.07 | 2.78 | 2.39 | 3.35 | 3.16 | 2.66 | 2.48 |
| 1 SD | 0.24 | 0.25 | 0.26 | 0.26 | 0.24 | 0.33 | 0.32 | 0.37 | 0.20 | 0.23 | |
| Total bilirubin (mg/dL) | Mean | 0.23 | 0.26 | 0.18 | 0.16 | 0.16 | 0.14 | 0.22 | 0.18 | 0.25 | 0.17 |
| 1 SD | 0.05 | 0.08 | 0.05 | 0.05 | 0.05 | 0.05 | 0.04 | 0.04 | 0.07 | 0.05 | |
| ALT (U/L) | Mean | 141 | 202 | 214 | 328 | 189 | 366 | 336 | 333 | 182 | 213 |
| 1 SD | 62 | 190 | 209 | 146 | 96 | 225 | 206 | 151 | 71 | 131 | |
| ALP (U/L) | Mean | 369 | 325 | 252 | 204 | 231 | 282 | 386 | 404 | 354 | 367 |
| 1 SD | 48 | 59 | 53 | 41 | 38 | 101 | 66 | 56 | 68 | 32 | |
| GGT (U/L) | Mean | 4.25 | 6.60 | nd | nd | 3.30 | 3.20 | 3.90 | 3.00 | 3.30 | 3.20 |
| 1 SD | 1.64 | 4.00 | nd | nd | 0.80 | 0.70 | 2.66 | 0.00 | 0.64 | 0.40 | |
F, female; M, male; nd, not determined.
Table 3.
Interspecies comparisons of WBC parameters (mean [1 SD])
| Species | WBC (K/μL) | Lymphocytes (%) | Neutrophils (%) | Monocytes (%) | Lymphocytes (K/μL) | Neutrophils (K/μL) | Monocytes (K/μL) |
| IS | 12.6 (4.3)a | 91.4 (5.8) | 7.8 (5.6) | 1.77 (0.86) | 11.5 (3.7)a | 1.02 (0.97) | 0.21 (0.12) |
| LL | 8.6 (2.5)b | 93.1 (7.1) | 6.1 (6.6) | 1.28 (1.59) | 8.0 (2.4)b | 0.51 (0.55) | 0.09 (0.07) |
| BW | 11.8 (3.8)a | 92.8 (7.0) | 6.3 (6.7) | 1.09 (0.85) | 10.9 (3.5)a | 0.77 (0.92) | 0.11 (0.08) |
| PO | 9.3 (2.5)b | 90.8 (8.1) | 8.3 (7.1) | 1.24 (0.44) | 8.4 (2.0)b | 1.13 (2.04) | 0.12 (0.06) |
Data from male and female mice have been combined. Within a parameter, values with the same letter are considered to be within the same range; those with different letters are significantly different from each other according to Tukey tests (P < 0.05). The letter designations are size-ordered—a represents the largest values, and b, c, and d indicate successively smaller ones.
Table 4.
Interspecies comparison of RBC parameters (mean [1 SD])
| Species | RBC (M/μL) | Hgb (g/dL) | Hct (%) | MCV (fL) | MCH (pg) | MCHC (g/dL) | Platelets (M/μL) | MPV (fL) |
| IS | 10.1 (0.8)b | 14.7 (0.9)a | 43.7 (2.6)a | 43.5 (2.4)a | 14.6 (0.9)a | 33.6 (1.1)a | 325 (151)b | 5.27 (0.83)a |
| LL | 11.5 (1.1)a | 13.7 (1.4)b,c | 42.8 (4.0)a,b | 37.5 (2.7)c | 12.0 (1.4)c | 32.0 (3.3)b | 394 (138)b | 4.71 (0.70)b |
| BW | 11.5 (1.1)a | 13.2 (1.0)c | 42.0 (4.6)a,b | 36.6 (1.5)c | 11.6 (0.8)c | 31.7 (2.5)b | 357 (139)b | 4.57 (0.48)b |
| PO | 10.5 (0.6)b | 13.9 (0.9)b | 40.9 (2.3)b | 39.1 (2.1)b | 13.3 (0.9)b | 34.0 (1.3)a | 510 (183)a | 5.35 (0.88)a |
Data from male and female mice have been combined. Within a parameter, values with the same letter are considered to be within the same range; those with different letters are significantly different from each other according to Tukey tests (P < 0.05). The letter designations are size-ordered—a represents the largest values, and b, c, and d indicate successively smaller ones.
Although BW and PO are closely related sister species (Figure 1), several blood values differed significantly between these species (Tables 3 through 5). Furthermore, for most hematologic and biochemical parameters, the values for the (BW × PO) F1 hybrids fell close to or midway between those of the parental species (Tables 1 and 2). Exceptions to this pattern appeared among the values for the proportions of neutrophils and lymphocytes among WBC and the concentration of neutrophils (Figure 2). Glucose values were higher for hybrids than parental stocks, but blood CO2 concentration was higher in BW and PO mice than in their hybrid progeny (Figure 2).
Table 5.
Interspecies comparison of serum chemical values (mean [1 SD])
| Triglycerides (mg/dL) | Cholesterol (mg/dL) | Glucose (mg/dL) | Total CO2 (mmol/L) | BUN (mg/dL) | Creatinine (mg/dL) | Sodium (mmol/L) | Potassium (mm/L) | Chloride (mm/L) | |
| IS | 404 (254)a | 155.7 (36.6)b | 111.6 (35.6)a | 23.7 (3.0)a,b | 35.2 (7.6)b | 0.22 (0.03)a | 155.4 (5.3)a,b | 7.93 (1.76)a | 111.5 (3.4)a |
| LL | 380 (189)a | 107.6 (54.5)c | 84.7 (34.5)b | 19.3 (3.4)c | 23.8 (3.8)d | 0.22 (0.03)a | 156.6 (5.3)a | 7.02 (0.81)b | 109.7 (4.0)b |
| BW | 187 (69)b | 87.4 (15.6)c | 82.7 (26.7)b | 24.8 (5.1)a | 31.9 (4.0)c | 0.20 (0.00)b | 153.9 (3.7)b | 6.53 (0.87)b,c | 108.9 (3.7)b,c |
| PO | 398 (236)a | 336.6 (96.6)a | 97.8 (28.2)a,b | 21.7 (4.8)b | 40.0 (5.2)a | 0.23 (0.04)a | 152.9 (3.6)b | 6.06 (1.01)c | 107.7 (3.1)c |
| Calcium (mg/dL) | Phosphorus (mg/dL) | Total protein (g/dL) | Albumin (g/dL) | Globulins (g/dL) | Total bilirubin (mg/dL) | ALT (U/L) | ALP (U/L) | ||
| IS | 10.52 (0.82)b,c | 10.1 (3.0) | 6.12 (0.40)c | 3.21 (0.20)c | 2.91 (0.26)b | 0.240 (0.071)a | 171 (147)b | 347 (59)b | |
| LL | 10.94 (0.68)a,b | 14.5 (3.1) | 6.52 (0.42)b | 3.41 (0.30)b | 3.11 (0.26)a | 0.167 (0.052)b.c | 271 (187)a,b | 228 (53)c | |
| BW | 10.09 (0.57)c | 9.2 (2.2) | 5.67 (0.43)d | 3.09 (0.21)c | 2.58 (0.35)c | 0.147 (0.050)c | 277 (193)a | 256 (79)c | |
| PO | 11.40 (1.13)a | 11.7 (2.0) | 7.15 (0.53)a | 3.90 (0.26)a | 3.25 (0.36)a | 0.197 (0.048)b | 335 (183)a | 395 (62)a |
Data from male and female mice have been combined. For each parameter, values with the same letter are considered to be within the same range; those with different letters are significantly different from each other according to Tukey tests (P < 0.05). The letter designations are size-ordered—a represents the largest values, and b, c, and d indicate successively smaller ones.
Discussion
Despite peromyscine abundance, species richness, and diverse research applications, relatively few reports include blood chemical and cellular measurements.5,52,53 One of the primary purposes of the current study was to assess the blood cell and biochemistry values of 4 of the most frequently studied species maintained by the Peromyscus Genetic Stock Center. Of these, both BW and LL continue to be studied for their roles as reservoir species for human infectious disease organisms, particularly hantavirus in BW,1,39,40 and Borrelia burgdorferi (which is associated with Lyme disease) in LL.3,36,46 In addition, both species are of interest for host–ectoparasite studies.8,38 With a life span 3.5-fold longer than that of similar-sized Mus spp. mice, LL is an important model for longevity research.26,42 For their ubiquity throughout the country, BW and LL routinely serve as sentinel species at sites, including superfund sites, experiencing high levels of chemical or radiation contamination.14,17,34,45 Both PO and IS are monogamous species and the subjects of ongoing behavioral and physiologic partner-fidelity studies.20,37,47 In addition, IS serves as a model of metabolic syndrome.25 BW and its hybrids with PO are models for exploring the initial steps of speciation and the roles of genomic imprinting and other epigenetic processes therein.43,48-50
Each species presents with its own particular blood cellular and hematologic profile, with some interspecific overlap. There is little agreement, especially for the serum biochemistry, among the species set regarding which parameters display a female or male bias. Apart from that, many of those parameters demonstrated to be significantly different between sexes are based on small differences of questionable physiologic significance. Exceptions to this trend are the female biases for high serum triglycerides and high MCH in all 4 species (Tables 1 and 2). In addition, the 4 species are similar in that the neutrophil and monocyte counts, with no significant differences in proportions.
Interspecific total WBC counts differed significantly (IS = BW > LL = PO) and is driven by species-associated variation in lymphocyte counts (Table 3). At this level, these results fail to support a previously proposed theoretical paradigm31,32 suggesting that polygamous species have more robust immune systems than do monogamous ones. This hypothesis was based on WBC and lymphocyte counts compared among 18 primate species segregated by partner fidelity; it assumes that immunologic potency is proportional to WBC numbers. Among the species in our current panel, IS and PO are documented to be monogamous, BW and LL polygamous;4,11,15,20 their WBC counts and partner-fidelity profiles are obviously discordant. Among the RBC indices, the IS profile is the overall outlier among the 4 species. IS RBC counts are low, but the cells are 11% to 18% larger than those of the other species and contain 10% to 25% more Hgb. Phylogenetically IS is the most distantly related among the 4 species (Figure 1).
Comparative values of BW and PO are of particular interest. These 2 sister species are interfertile and produce fertile hybrid offspring; they therefore are appropriate for genetic studies of BW–PO differences.48 Furthermore, the molecular resources enabling detailed genetic and gene expression analyses have burgeoned in recent years.13 Full sequencing of the BW (6-fold redundancy) and PO (2-fold) genomes is underway (http:/www.hgsc.bcm.edu/content/peromyscus-genome-project); the completed BW genome is currently undergoing annotation. The ability of BW × PO mating to produce fertile offspring enabled the construction of a complete linkage map composed of 185 type I (gene) markers and 155 type II (microsatellite) markers.22 Furthermore, EST–transcriptome data from multiple tissues are available and steadily growing. In the current study, 2 traits draw particular attention for the degree of their BW–PO variation. PO cholesterol levels are almost 4-fold elevated over those of BW, and PO triglyceride levels are more than 2-fold higher than those of BW. Other BW–PO differences that might lend themselves to genetic analyses are total protein, albumin, globulins which all differ by 26% between the 2 species, and ALP which differs by 54% between the 2.
In Figure 2 are illustrated analytes whose (BW × PO) F1 hybrid values fell significantly outside (either greater than or less than) the values of the parental BW and PO stocks. These traits were the exceptions, in that most hybrid values fell close to or midway between the parental values. Accordingly, values for the number and proportion of neutrophils were lower in hybrids than the parental strains, and the proportion of lymphocytes—but not lymphocyte number—was greater in hybrid progeny than in the parents. This hybrid effect likely is centered on the reduced neutrophil concentration, which is responsible for the altered neutrophil and lymphocyte proportions. As noted earlier, (BW × PO) F1 hybrids manifest hybrid dysgenesis and are substantially smaller that either parent stock; whereas oversize embryos result from the reciprocal cross. Possibly the neutrophil effect is a manifestation at the cellular level of such dysgenesis. An alternate explanation is that multiple genes control a trait, with each species homozygous recessive for alternating ones. In this scenario, hybrid values would lie outside of the parental values because the hybrids would be heterozygous for both genes. Similar phenomena have been observed regarding isoniazid sensitivity in inbred mice and their hybrids44 and RBC osmotic fragility in inbred mice and their recombinant inbreds.30 Additional segregation analyses are required to resolve these 2 possibilities. Similarly, among the serum biochemical traits, CO2 concentration values were lower in hybrids than parents, whereas glucose values were higher in hybrids than parents. It is noteworthy that offspring of BW female ×PO male mice are viable but undersized at birth and into adulthood. Human infants that are born small for gestational age are likely to become diabetic with age.28 The elevated glucose values in these hybrid offspring may be a reflection of this phenomenon. However, the hybrid values are more like that of PO mice in fasted glucose challenge tests;33 therefore the interspecific differences in glucose homeostasis bear additional study as well.
The data presented provide the first comprehensive evaluation of the major blood cell and biochemical parameters of several Peromyscus species. This information serves as a useful baseline for comparisons with wild-caught specimens, to evaluate the extent to which there are similarities or differences associated with physiologic adaptation to various habitats (including contaminated sites), parasites, and microorganisms or to domestication and genetic drift. To facilitate utilization of this information, the raw data are available at http://ww2.biol.sc.edu/~peromyscus; these data can be downloaded and used for direct comparisons, as well as regression and correlation analyses.
Acknowledgments
This work was partially funded by grants from the National Science Foundation (MCB-0517754) and NIH (P40 RR014279).
References
- 1.Amman BR, Manangan AP, Flietstra TD, Calisher CH, Carroll DS, Wagoner KD, Mills JN. 2013. Association between movement and Sin Nombre virus (Bunyaviridae: Hantavirus) infection in North American deermice (Peromyscus maniculatus) in Colorado. J Wildl Dis 49:132–142 [DOI] [PubMed] [Google Scholar]
- 2.Animal Welfare Act as Amended. 2008. 7 USC §2131–2159
- 3.Baum E, Hue F, Barbour AG. 2012. Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. MBio 3:e00434–e00412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birdsall DA, Nash D. 1973. Occurrence of successful multiple insemination of females in natural populations of deer mice (Peromyscus maniculatus). Evolution 27:106–110 [DOI] [PubMed] [Google Scholar]
- 5.Blom JM, Gerber JM, Nelson RJ. 1994. Day length affects immune cell numbers in deer mice: interactions with age, sex, and prenatal photoperiod. Am J Physiol 267:R596–R601 [DOI] [PubMed] [Google Scholar]
- 6.Bonhomme F, Guenet JL, Dod B, Moriwaki K, Bulfield G. 1987. The polyphyletic origin of laboratory inbred mice and their rate of evolution. Biol J Linn Soc Lond 30:51–58 [Google Scholar]
- 7.Casci T. 2012. Mouse genetics: fruits of the collaborative cross. Nat Rev Genet 13:223 [Google Scholar]
- 8.Dallas TA, Fore SA, Kim HJ. 2012. Modeling the influence of Peromyscus leucopus body mass, sex, and habitat on immature Dermacentor variabilis burden. J Vector Ecol 37:338–341 [DOI] [PubMed] [Google Scholar]
- 9.Dawson WD, Sagedy MN, En-yu L, Kass DH, Crossland JP. 1993. Growth regulation in Peromyscus species hybrids: a test for mitochondrial–nuclear genomic interaction. Growth Dev Aging 57:121–133 [PubMed] [Google Scholar]
- 10.Dewey MJ, Dawson WD. 2001. Deer mice: “the Drosophila of North American mammalogy.” Genesis 29:105–109 [DOI] [PubMed] [Google Scholar]
- 11.Dewsbury DA. 1981. An exercise in the prediction of monogamy in the field from laboratory data on 42 species of muroid rodents. The Biologists 63:138–162 [Google Scholar]
- 12.Dice L. 1940. Speciation in Peromyscus. Am Nat 74:289–298 [Google Scholar]
- 13.Felder MR, Vrana PB, Szalai G, Shorter K, Lewandowski A. 2012. Development of resources for Peromyscus laboratory research. Integr Comp Biol 52:E242 [Google Scholar]
- 14.Ficko SA, Luttmer C, Zeeb BA, Reimer K. 2013. Terrestrial ecosystem recovery following removal of a PCB point source at a former pole-vault–line radar station in Northern Labrador. Sci Total Environ 461-462:81–87 [DOI] [PubMed] [Google Scholar]
- 15.Foltz DW. 1981. Genetic evidence for long-term monogamy in a small rodent, Peromyscus polionotus. Am Nat 117:665–675 [Google Scholar]
- 16.Frazer KA, Eskin E, Kang HM, Bogue MA, Hinds DA, Beilharz EJ, Gupta RV, Montgomery J, Morenzoni MM, Nilsen GB, Pethiyagoda CL, Stuve LL, Johnson FM, Daly MJ, Wade CM, Cox DR. 2007. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature 448:1050–1053 [DOI] [PubMed] [Google Scholar]
- 17.Hays KA, Breshears MA. 2011. Presence of hyperplastic pectoral mammary glands in a white-footed mouse (Peromyscus leucopus) from a Superfund Site in Oklahoma, USA. J Wildl Dis 47:255–258 [DOI] [PubMed] [Google Scholar]
- 18.Heideman PD, Rightler M, Sharp K. 2005. A potential microevolutionary life-history trade-off in white-footed mice (Peromyscus leucopus). Funct Ecol 19:331–336 [Google Scholar]
- 19.Institute for Laboratory Animal Research. 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press [Google Scholar]
- 20.Jasarevic E, Bailey DH, Crossland JP, Dawson WD, Szalai G, Ellersieck MR, Rosenfeld CS, Geary DC. 2013. Evolution of monogamy, paternal investment, and female life history in Peromyscus. J Comp Psychol 127:91–102 [DOI] [PubMed] [Google Scholar]
- 21.Joyner CP, Myrick LC, Crossland JP, Dawson WD. 1998. Deer mice as laboratory mice. ILAR J 39:322–330 [DOI] [PubMed] [Google Scholar]
- 22.Kenney-Hunt J, Lewandowski A, Glenn TC, Glenn JL, Tsyusko OV, O'Neill RJ, Brown J, Ramsdell CM, Nguyen Q, Phan T, Shorter KS, Dewey MJ, Szalai G, Vrana PB, Felder MR. 2014. A genetic map of Peromyscus with chromosomal assignment of linkage groups. Mamm Genome 25:160–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King JA. 1968. Biology of Peromyscus. Brookings (SD): American Society of Mammalogists [Google Scholar]
- 24.Kirkland GL, Jr, Layne JN. 1989. Advances in the study of Peromyscus (Rodentia). Lubbock (TX): Texas Tech University Press. [Google Scholar]
- 25.Krugner-Higby L, Caldwell S, Coyle K, Bush E, Atkinson R, Joers V. 2011. The effects of diet composition on body fat and hepatic steatosis in an animal (Peromyscus californicus) model of the metabolic syndrome. Comp Med 61:31–38 [PMC free article] [PubMed] [Google Scholar]
- 26.Labinskyy N, Mukhopadhyay P, Toth J, Szalai G, Veres M, Losonczy G, Pinto JT, Pacher P, Ballabh P, Podlutsky A, Austad SN, Csiszar A, Ungvari ZI. 2009. Longevity is associated with increased vascular resistance to high-glucose–induced oxidative stress and inflammatory gene expression in Peromyscus leucopus. Am J Physiol Heart Circ Physiol 296:H946–H956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levin ML, Fish D. 1998. Density-dependent factors regulating feeding success of Ixodes scapularis larvae (Acari: Ixodidae). J Parasitol 84:36–43 [PubMed] [Google Scholar]
- 28.Levy-Marchal C, Jaquet D. 2004. Long-term metabolic consequences of being born small for gestational age. Pediatr Diabetes 5:147–153 [DOI] [PubMed] [Google Scholar]
- 29.Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann H, Sanchez A, Childs J, Zaki S, Peters CJ. 1993. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science 262:914–917 [DOI] [PubMed] [Google Scholar]
- 30.Norman NK, Dewey MJ. 1985. Genetic control of red cell osmotic fragility. J Hered 76:31–35 [DOI] [PubMed] [Google Scholar]
- 31.Nunn CL. 2002. A comparative study of leukocyte counts and disease risk in primates. Evolution 56:177–190 [DOI] [PubMed] [Google Scholar]
- 32.Nunn CL, Gittleman JL, Antonovics J. 2000. Promiscuity and primate immune system. Science 290:1168–1170 [DOI] [PubMed] [Google Scholar]
- 33.Oriel RC, Wiley CD, Dewey MJ, Vrana PB. 2008. Adaptive genetic variation, stress, and glucose regulation. Dis Model Mech 1:255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phelps KL, Mcbee K. 2010. Population parameters of Peromyscus leucopus (white-footed deer mice) inhabiting a heavy-metal–contaminated superfund site. Southwest Nat 55:363–373 [Google Scholar]
- 35.Powell FL. 2003. Functional genomics and the comparative physiology of hypoxis. Annu Rev Physiol 65:203–230 [DOI] [PubMed] [Google Scholar]
- 36.Rogic A, Tessier N, Legendre P, Lapointe FJ, Millien V. 2013. Genetic structure of the white-footed mouse in the context of the emergence of Lyme disease in southern Quebec. Ecol Evol 3:2075–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenfeld CS, Johnson SA, Ellersieck MR, Roberts RM. 2013. Interactions between parents and parents and pups in the monogamous California mouse (Peromyscus californicus). PLoS ONE 8:e75725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rynkiewicz EC, Hawlena H, Durden LA, Hastriter MW, Demas GE, Clay K. 2013. Associations between innate immune function and ectoparasites in wild rodent hosts. Parasitol Res 112:1763–1770 [DOI] [PubMed] [Google Scholar]
- 39.Schountz T, Acuna-Retamar M, Feinstein S, Prescott J, Torres-Perez F, Podell B, Peters S, Ye C, Black WC, 4th, Hjelle B. 2012. Kinetics of immune responses in deer mice experimentally infected with Sin Nombre virus. J Virol 86:10015–10027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schountz T, Shaw TI, Glenn TC, Feldmann H, Prescott J. 2013. Expression profiling of lymph node cells from deer mice infected with Andes virus. BMC Immunol 14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schrag SJ, Wiener P. 1995. Emerging infectious disease: what are the relative roles of ecology and evolution. Trends Ecol Evol 10:319–324 [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Pulliam DA, Liu Y, Hamilton RT, Jernigan AL, Bhattacharya A, Sloane LB, Qi W, Chaudhuri A, Buffenstein R, Ungvari Z, Austad SN, Van Remmen H. 2013. Reduced mitochondrial ROS, enhanced antioxidant defense, and distinct age-related changes in oxidative damage in muscles of long-lived Peromyscus leucopus. Am J Physiol Regul Integr Comp Physiol 304:R343–R355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shorter KR, Crossland JP, Webb D, Szalai G, Felder MR, Vrana PB. 2012. Peromyscus as a mammalian epigenetic model. Genet Res Int 2012:179159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor BA. 1976. Genetic analysis of susceptibility to isoniazid-induced seizures in mice. Genetics 83:373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tovar-Sanchez E, Cervantes LT, Martinez C, Rojas E, Valverde M, Ortiz-Hernandez ML, Mussali-Galante P. 2012. Comparison of 2 wild rodent species as sentinels of environmental contamination by mine tailings. Environ Sci Pollut Res Int 19:1677–1686 [DOI] [PubMed] [Google Scholar]
- 46.Tsao K, Fish D, Galvani AP. 2012. Predicted outcomes of vaccinating wildlife to reduce human risk of Lyme disease. Vector Borne Zoonotic Dis 12:544–551 [DOI] [PubMed] [Google Scholar]
- 47.Turner LM, Young AR, Rompler H, Schoneberg T, Phelps SM, Hoekstra HE. 2010. Monogamy evolves through multiple mechanisms: evidence from V1aR in deer mice. Mol Biol Evol 27:1269–1278 [DOI] [PubMed] [Google Scholar]
- 48.Vrana P, Shorter K, Szalai G, Felder M, Crossland J, Veres M, Dewey M, Dawson W. 2013. Peromyscus (deer mice) as developmental models. WIREs Dev Biol [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 49.Vrana PB. 2007. Genomic imprinting as a mechanism of reproductive isolation in mammals. J Mammal 88:5–23 [Google Scholar]
- 50.Vrana PB. 2012. Disruption of developmental and epigenetic programs in Peromyscus hybrids. Integr Comp Biol 52:E184 [Google Scholar]
- 51.Vrana PB, Fossella JA, Matteson P, del Rio T, O'Neill MJ, Tilghman SM. 2000. Genetic and epigenetic incompatibilities underlie hybrid dysgenesis in Peromyscus. Nat Genet 25:120–124 [DOI] [PubMed] [Google Scholar]
- 52.Wu PJ, Greeley EH, Hansen LG, Segre M. 1999. Hematology values from clinically healthy Peromycus leucopus. J Zoo Wildl Med 30:589–590 [PubMed] [Google Scholar]
- 53.Wu PJ, Greeley EH, Hansen LG, Segre M. 1999. Immunological, hematological, and biochemical responses in immature white-footed mice following maternal Aroclor 1254 exposure: a possible bioindicator. Arch Environ Contam Toxicol 36:469–476 [DOI] [PubMed] [Google Scholar]