Abstract
Current housing guidelines for laboratory rodents include recommendations for enrichment. Working with guinea pigs, we have developed an open-field enrichment paradigm that provides several aspects of this species’ natural environment. These naturalistic aspects include access to increased space for exploration, access to western timothy (Phleum pratense L.) hay, and grouping as a herd to facilitate social interaction. To determine the immediate effect on behavior from access to the enriched environment, female guinea pigs from 2 strains, IAF Hairless and NIH Hartley, were observed in both standard home cages and an open-field enriched environment. Subjects were housed with cagemates in pairs for the home-cage observation and were grouped as a herd when in the open-field arena. Behaviors were videorecorded for 1 h and then scored. Salivary cortisol levels were measured both prior to and immediately after behavioral observations. Analyses revealed higher levels of activity and social interaction in the open-field arena compared with the home cage, with no significant change in salivary cortisol levels. These results suggest that exposure to the open-field environment provide increased opportunities for exercise and social enrichment. Although additional studies are needed to determine long-term effects on experimental outcomes, the open-field configuration holds promise as a laboratory enrichment paradigm for guinea pigs.
Abbreviation: IAF, Institute Armand Frappier
Guinea pigs have been widely used in research to study developmental patterns of behavior.1,19,23,31,40 Similar to humans, guinea pigs have prolonged gestation, pregnancy complications, the need for vitamin C supplementation, precocial sensory development, and social dependence.33,39 Due to their common use in research, interest has been generated in developing programs of enrichment for laboratory guinea pigs.21,38 Although there is currently no specific protocol for guinea pigs, environmental enrichment for laboratory rodents in general has demonstrated effects such as reduction of barbering,6 delayed progression of neurologic disease,25 and recovery of function after stroke12,29 and traumatic brain injury.13
Past research also has demonstrated effects on corticosterone and cortisol levels in association with environmental enrichment.7 For example, when rats are housed individually, levels of corticosterone are increased markedly. Elevated corticosterone or cortisol, in turn, can lead to a host of deleterious outcomes in rodents, including longer recovery time for wound healing,27 increased inflammatory response,3 and decreased immune functioning.26 However, providing enrichment in the form of toys, in addition to nesting materials, significantly reduced concentrations of corticosterone in rats.7 This reduction illustrates physiologic effects of enrichment in laboratory rodents.
However, not all effects from environmental enrichment have been positive. Reviews of the literature have generally reported mixed and conflicting results.5,38 Some studies have even reported increased aggression, stress, and altered endocrine function.15,16
The purpose of the current study was 3-fold. First, we investigated a potential new form of environmental enrichment for laboratory guinea pigs. Although typical enrichment programs focus on aspects of the home cage, in this study, guinea pigs were observed in a familiar open-field environment, with access to species-relevant enrichment. The main objective in using a familiar open field was to replicate the natural herd environment typically seen in wild species of guinea pigs.32,33 By using an enclosure markedly larger than the standard laboratory cage, an environment capable of accommodating a herd of guinea pigs was created.
Second, although several strains of guinea pigs currently are available, behavioral research traditionally uses the Hartley strain.39 Due to this heavy reliance on a single strain, little is known about the behavior of other strains. For example, the Institute Armand Frappier (IAF) Hairless guinea pig may be particularly useful for ultrasound studies of fetal behavior and observations of neurobehavioral functioning after dermal exposure to neurotoxins. Despite this utility, no behavioral studies of IAF guinea pigs have been reported to date. This Hairless strain of guinea pigs is derived from a spontaneous mutation in the Hartley line. They are euthymic and immunocompetent,10 thus providing a Hairless alternative to the standard laboratory guinea pig. Even though this strain has been used in nonbehavioral research for more than 3 decades,10 little is known about the behavior of the IAF Hairless strain. To correct this deficit in knowledge, the current study compared activity level, consumption of food and water, and social behaviors between IAF Hairless and Hartley guinea pig strains.
Third, we measured salivary cortisol as a measure of hypothalamic–pituitary–adrenal activity in the guinea pigs. This measurement could indicate whether exposure to an open-field arena such as the apparatus used in this study might elicit activation of this axis as one effect of enrichment. Rodents tend to avoid large open areas due to fear of predation.30 Consequently, placing rodents in an open-field arena could elicit a stress response associated with the release of corticotrophin-releasing hormone, which triggers adrenal gland release of glucocorticoid hormones.9 Behavioral indicators of a stress response in laboratory animals include vocalization, crouched stance, closure of the eyes, and piloerection.20 These behaviors also occur after intracerebroventricular injections of corticotrophin-releasing hormones.7,11,36 In terms of the present study, stressed guinea pigs might be expected to express high levels of cortisol, spend less time in the central area of the open field, or show behavioral signs of fear. Such signs would include increased frequency of freezing behaviors, startle responses, and altered activity.
Taken together, these aims led naturally to 3 hypotheses. First, we hypothesized that the open-field environment would provide laboratory guinea pigs with enrichment in the form of exercise and social interface, as evidenced by increased levels of activity and social interaction. Our second hypothesis was that because the Hairless strain was derived from the Hartley line, both strains would behave similarly in the enriched environment. For example, both Hairless and Hartley strains should show similar levels of activity, consumption of food or water, and social behaviors in the open field, as compared with the home cage. Third, we hypothesized that although frequencies of behavior in the open field would be altered due to enrichment, glucocorticoid levels would not differ significantly from baseline levels. To test these hypotheses, we observed and quantified activity, consumption, and social interaction of 2 strains of laboratory guinea pigs in an enriched open-field apparatus and a home-cage environment. In addition, salivary cortisol was measured both before and after exposure to the enriched open-field arena.
Materials and Methods
Female NIH multicolored Hartley (n = 10) and IAF Hairless (n = 11) guinea pigs (age, 6 to 8 mo) were obtained from Charles River (Kingston, NY) and Elm Hill Labs (Chelmsford, MA), respectively. Hartley guinea pigs weighed 781 to 883 g and Hairless animals weighed 638 to 833 g. The 2 strains were maintained in separate vivariums. Both Hartley and Hairless guinea pigs were housed in standard polycarbonate laboratory housing (76 cm × 56 cm) with a cagemate from the same strain. Food (Teklad-7006 guinea pig pellets, Harlan Teklad, Madison, WI) and water were provided ad libitum. However, due to constraints in the animal rooms, Hartley guinea pigs were maintained on an automatic watering system, and the Hairless strain was housed with access to water bottles. All other aspects of the housing environment were identical. Cages were maintained in temperature (23 °C) and humidity-controlled (46% to 56%) rooms. Housing areas were on a 12:12-h light:dark cycle (lights on at 0700), and each cage contained paper bedding (TEK-Fresh, Harlan, Indianapolis, IN).
As part of an enrichment program, guinea pigs were placed in a 140 cm × 70 cm open-field arena (Figure 1) for approximately 1 h daily in a temperature (23 °C) and humidity-controlled (46% to 56%) room. The open-field arena was constructed of metal caging material 35 cm high, and lined to a height of 20 cm with Coroplast material (Fastsigns, Dayton, OH). Guinea pigs from both strains were acclimated to the open field through daily exposure on weekdays of 1 h for at least 1 mo prior to testing. The open-field arena was lined with Polar-Tek fleece (Monsanto, Creve Couer, MO), and four 3-cm3 wood blocks were distributed evenly for additional environmental enrichment. Wire spheres containing western timothy hay (Pheleum pratense L.) were located at the 2 long ends of the arena, and a water bottle was placed centrally along one wall of the open-field arena. Guinea pigs were provided ad libitum accesses to both the timothy hay and water for the duration of the hour spent in the open-field arena.
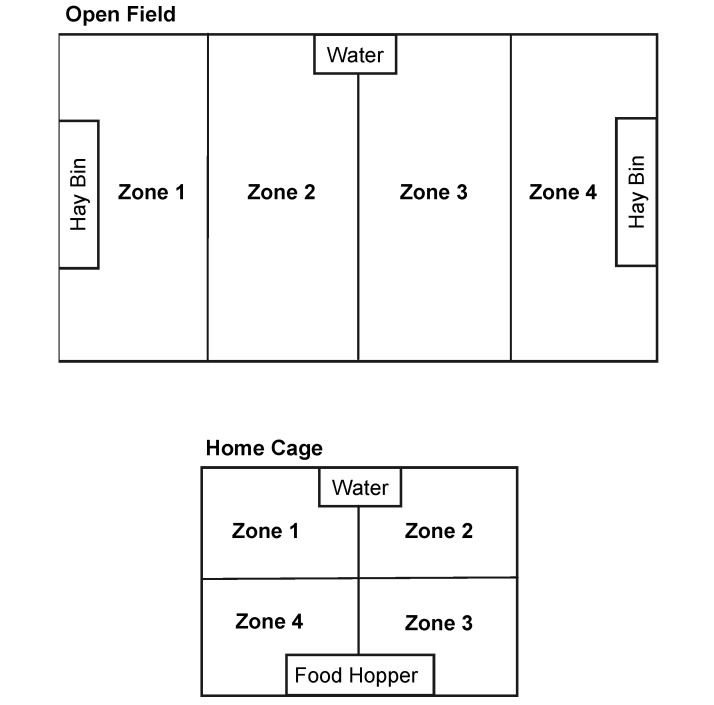
Figure 1.
Depiction of the open field and home cage. Locations for food pellets (Food Hopper), timothy hay (Hay Bin), and water are depicted on scale drawings of the open field (top) and home cage (bottom) environments. Zones 1 through 4 depict the boundaries used during scoring of activity.
At all times, guinea pigs were cared for in accordance with the Guide for the Care and Use of Laboratory Animals.22 All procedures, husbandry, and care for the guinea pigs in this study were reviewed and approved by the Wright State University Animal Care and Use Committee.
Behavioral observations.
Two aims of the behavioral observations in this study were to determine 1) whether an enriched open-field arena facilitates exercise and social behavior in laboratory guinea pigs and 2) the behavioral differences and similarities between Hartley and Hairless guinea pigs. Environmental enrichment is defined by the US Department of Agriculture as, “the invention and installation of apparatus which can be used for work or play.”24 In the current study, we expand this definition to include social interaction and diet as enrichment. Social enrichment was provided through exposure to a large arena with multiple free-roaming animals that were permitted ad libitum access to water and timothy hay, a form of dietary enrichment. The independent variables of this experiment formed a 2 × 2 design of Environment (Home Cage and Open Field) and Strain (Hartley and Hairless). The dependent variables included measures of activity, consumption, and social interaction.
Behavioral observations of both the Hartley and Hairless guinea pigs were recorded for 1 h in their standard home-cage environment. The guinea pigs were observed and recorded in groups of 2, according to their typical housing arrangement on a day, when neither cagemate was in estrus. Estrus was determined through visual inspection of the vaginal membrane.39 The 2 herds (Hartley, n = 10; Hairless, n = 11) also were observed and recorded for 1 h in the open-field arena. On the day of testing, the entire herd was moved on a cart to the adjoining observation room and allowed to acclimate in their cages on the cart for 15 min. To reduce the potential stress of a novel environment, guinea pigs were familiarized to the open-field arena. All subjects of a single strain were placed together in the open field for 1 h daily at least 1 mo prior to the start of the experiment. The day of testing was chosen to coincide with as few of the animals in estrus as possible. Estrus was determined by visually inspecting the vaginal opening membrane daily.39 The day chosen, one for each strain, resulted in only one Hairless and no Hartley guinea pigs in estrus. All observations for both the home cage and open field were done between 1300 and 1500.
Conditions and behavior in both the open field and home cage were videorecorded (1080p, 3MOS HD camcorder, Panasonic, Osaka, Japan) for 1 h. Videos were transferred to a computer, split into 4 segments (15 min each), and rendered into an appropriate (MPEG-2 HD) video format by using Premier Pro CS4 (Adobe, San Jose, CA).
Scoring and analysis.
Activity, consumption, and social behaviors were scored from rendered video files by using JWatcher video software.8 Definitions of behaviors were derived from those commonly used in prior behavioral studies involving wild and domestic guinea pigs.18,33 Each guinea pig was scored individually on a separate pass of the video. Individual guinea pigs were identified by unique markings (multicolored fur patterns in Hartley animals and tattoos on the hindquarters of Hairless animals). Behaviors were coded from continuous viewing.
Activity.
Numerous measures were used in this study to describe activity in the guinea pig. General activity level was defined as the frequency of movement from area to area within the home cage or the open-field arena. To score activity, both the home cage and the open-field arena were split up into 4 zones of equal area (Figure 1). In the open field, zone 1 was located on one side and zone 4 located on the opposite end of the arena. Zones for the home cage were designated 1 to 4 in a clockwise manner, because of the nearly square shape of standard guinea pig housing. For both environmental conditions, the frequency of transition from one zone to the next zone was scored. These frequencies depict relative activity level, due to the size disparity between the home cage and open-field apparatus.
To determine whether the guinea pigs used the entire area, or engaged in some form of thigmotaxis, a measure of guinea pig density was calculated. For this measure, the 4 zones were subdivided into 3 equal sections, creating a total of 12 areas. The 1-h behavioral observation videos for both Hartley and Hairless strains were viewed at 1-min intervals, with a clear overlay delineating the 12 areas. At each of the 1-min time points, the total number of guinea pigs in each of the 12 sections was tallied. A guinea pig was considered to be in an area when the centerpoint of its back was within the corresponding section of the overlay. A mean density for each section was calculated from the 1-min tallies.
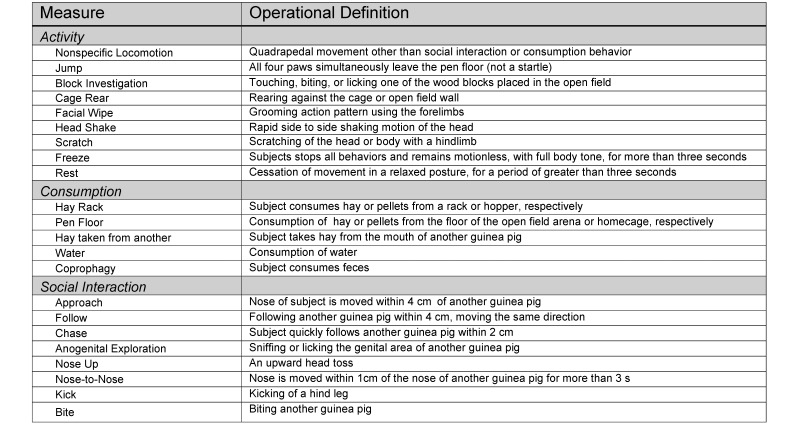
Additional indicators of general activity were scored during a separate viewing of the video files. These measures were nonspecific locomotion, jump, block investigation, cage rear, facial wipe, head shake, scratch, freeze, and rest. The operational definitions for these behaviors are described in Figure 2.
Figure 2.
Description of behavioral codes. Operational definitions used to score the observed behaviors of both Hartley and Hairless guinea pigs. Behavioral coding was applied to observations of subjects in both the home cage and open-field arena. Definitions were based on those from prior studies.18,33
Consumption.
Consumption behaviors included drinking water, engaging in coprophagy, and feeding on either timothy hay (open field) or commercial pellets (home cage; Figure 2). This final category was subdivided further according to the source of the hay (the hay rack or pen floor or taken from another guinea pig). Pellet consumption was defined as the event when an observed guinea pig consumed chow pellets from the bin in the home cage. Both behaviors were scored as durations and expressed as proportions of total time observed.
Social interaction.
Social behaviors observed included measures of approach to another guinea pig, following another, chasing, anogenital exploration, giving nose up (a social signal of hierarchy), nose-to-nose interaction, kicking directed toward another, and biting (Figure 2). Proximity to another guinea pig was defined when one guinea pig's nose came within 4 cm of another guinea pig. A proportional grid was used to score behaviors of proximity (for example, approach, follow, and chase). The grid was scaled separately to the dimensions of the home cage and the open-field arena and placed over the computer screen, with each grid block representing 4 cm. The behavior was scored when the head of the observed guinea pig came within one grid block of another guinea pig. Social behaviors were quantified as frequencies per 1-h observation period or 15-min segment.
Behavioral sequence.
To further assess guinea pig behavior in the home cage and open field, sequential analyses of Markovian transition probabilities were processed by using JWatcher Video software.8 Sequential analysis is a useful tool for assessing the temporal patterning of behaviors.2,17 These analyses quantify the probabilities of a successive behavior after an initial behavior. All coded observations (Figure 2) were included in the analysis, and significant behavioral transitions were determined from Bonferroni corrected P values.
Unless otherwise stated, all statistical analyses were performed by using Statistica software version 10.35 General activity data were analyzed by using 4-way repeated-measures ANOVA of Type (Hairless and Hartley) × Environment (Home Cage and Open Field) × Segment (1 to 4, 15-min segments) × Zone (1 to 4), with an α of P less than 0.05. Environment, Segment, and Zone were treated as within factors, and Type as a between factor. Effect sizes were calculated by using partial eta-squared (ηp2). Only significant ANOVA involving all factors were assessed subsequently in pairwise comparisons by using Fisher protected least square difference (PLSD). Frequency measures for the open field were analyzed in a series of 2-tailed t tests. Post hoc pairwise comparisons were made by using Bonferroni correction. For behavioral measures not meeting statistical assumptions for parametric tests, Yates corrected χ2 analyses were performed.
Salivary cortisol.
To evaluate whether stress due to the novelty of the open-field arena was a factor, salivary cortisol samples were collected prior to placement in the open-field arena and again at the conclusion of the observation. Salivary samples were collected by using 2 or 3 sponges per subject (BD Visispear, Beaver Visitec, Waltham, MA). Collection was accomplished for all subjects in less than 5 min, with one investigator holding each subject and a second investigator swabbing the mouth with sponges. Saliva was extracted from the sponges by centrifugation and stored at −80 °C until processing. Cortisol levels were quantified by ELISA by using a salivary cortisol kit (Salimetrics, State College, PA). Salimetrics reports antibody reactivity for cortisol at 100% and crossreactivity for endogenous hormones at 100 ng/mL to be less than 0.013%.34 Results were analyzed in a 2-way repeated-measures ANOVA with Pre–Post as a within-groups factor and Type as the between-groups factor.
Results
Behavioral observations.
Activity.
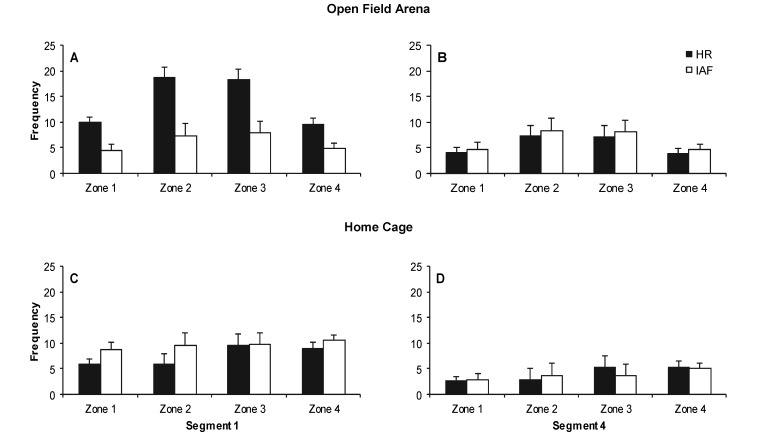
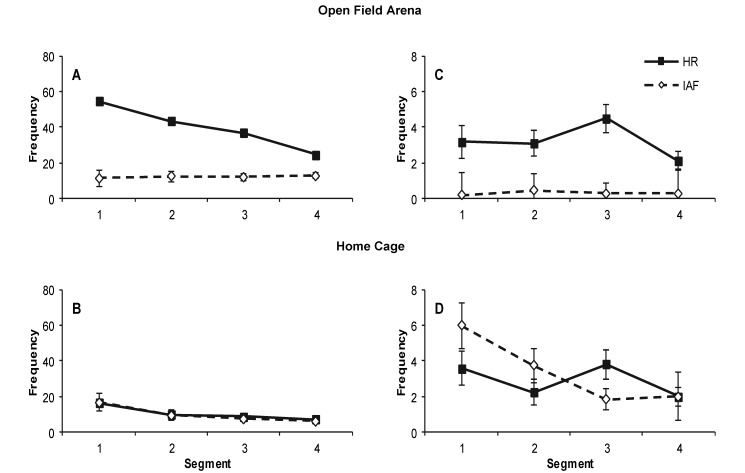
Guinea pigs from both strains were observed to exhibit varying levels and forms of activity. Analyses of overall activity as defined by zone transitions (Figure 3) revealed significant (P < 0.05) main effects of Type (Hartley and Hairless), Environment (Open Field and Home Cage), Segment (Segments 1 to 4), and Zone (Zones 1 to 4); 2-way interactions of Type × Environment, Type × Zone, and Environment × Zone; and 3-way interactions of Type × Environment × Segment and Type × Environment × Zone. No other 2-way or 3-way interactions involving Segment or Environment were significant (Table 1). Post hoc comparisons revealed no significant differences in activity for any time segment or zone transition between Hartley and Hairless guinea pigs when observed in the home cage. However, when observed in the open-field arena, Hartley guinea pigs made significantly (P < 0.05) more frequent transitions across zones 2 and 3 than did the Hairless group, except during the final 15-min segment (Figure 3). Measures of guinea pig density revealed means in the 12-zone subdivisions ranging from 0.55 ± 0.09 to 2.23 ± 0.10. Measures of guinea pig density across the 3 subdivisions of zones 2 and 3 in general revealed no significant differences between the bottom wall area of these zones and the central-mosft areas (P = 0.366 and P = 0.484 for Hartley and Hairless, respectively). The density measures between the top wall area of zones 2 and 3, where the drinking water was located, and the central area were not significantly different, for the Hairless strain (P = 0.111). Density measures for the Hartley guinea pigs in zones 2 and 3 were significantly (P < 0.001) higher than in the central areas. For both strains, areas proximal to the hay bins had significantly (P < 0.001) higher densities than did central areas.
Figure 3.
Frequency of zone transitions. Bars depict the mean frequency/15 min of movement activity into each of 4 zones in the (A and B) open field and (C and D) home cage for Hartley (HR) and Hairless (IAF) guinea pigs. Plots A and C depict the first 15-min segment of the 1 h observation (segment 1), and plots B and D represent the final 15-min segment (segment 4). Bonferroni-corrrected significant differences (P < 0.001) in panel A were detected between Hartley crossings of zones 2 and 3 in both the open field and home cage and the other 2 zones for the 1st 15-min segment for the open field only. In addition, frequencies for Hartley subjects were elevated during this period in the open field relative to Hairless values for all zones. Errors bars depict the SEM.
Table 1.
Results of repeated-measures ANOVA of observed behaviors
| df | F | P | ηp2 | |
| Activity level: frequency of zone crossing | ||||
| Type | 1, 19 | 4.886 | 0.029 | 0.035 |
| Environment | 1, 19 | 9.735 | 0.002 | 0.067 |
| Type × Environment | 1, 19 | 9.338 | <0.001 | 0.171 |
| Segment | 3, 57 | 9.338 | <0.001 | 0.171 |
| Zone | 3, 57 | 51.295 | <0.001 | 0.274 |
| Type × Segment | 3, 57 | 1.0966 | 0.353 | 0.024 |
| Type × Zone | 3, 57 | 5.396 | 0.001 | 0.038 |
| Environment × Segment | 3, 57 | 0.244 | 0.865 | 0.005 |
| Environment × Zone | 3, 57 | 51.431 | <0.001 | 0.274 |
| Type × Environment × Segment | 3, 57 | 3.060 | 0.030 | 0.063 |
| Type × Environment × Zone | 3, 57 | 4.578 | 0.004 | 0.033 |
| Environment × Segment × Zone | 9, 171 | 1.022 | 0.422 | 0.022 |
| Type × Environment × Segment × Zone | 9, 171 | 1.211 | 0.286 | 0.026 |
| Proportion of time spent consuming hay from rack | ||||
| Type | 1, 19 | 7.578 | 0.007 | 0.053 |
| Environment | 1, 19 | 109.897 | <0.001 | 0.445 |
| Type × Environment | 1, 19 | 10.797 | 0.001 | 0.074 |
| Segment | 3, 57 | 5.764 | 0.001 | 0.113 |
| Type × Segment | 3, 57 | 0.128 | 0.943 | 0.003 |
| Environment × Segment | 3, 57 | 5.211 | 0.002 | 0.103 |
| Type × Environment × Segment | 3, 57 | 0.200 | 0.896 | 0.004 |
| Proportion of time spent drinking water | ||||
| Type | 1, 19 | 24.674 | 0.001 | 0.154 |
| Environment | 1, 19 | 17.769 | <0.001 | 0.1116 |
| Segment | 3, 57 | 0.876 | 0.455 | 0.019 |
| Type × Environment | 1, 19 | 11.084 | 0.001 | 0.075 |
| Type × Segment | 3, 57 | 0.226 | 0.878 | 0.005 |
| Environment × Segment | 3, 57 | 1.290 | 0.281 | 0.028 |
| Type × Environment × Segment | 3, 57 | 0.404 | 0.750 | 0.001 |
| Approach frequency | ||||
| Type | 1, 19 | 67.468 | <0.001 | 0.332 |
| Environment | 1, 19 | 83.891 | <0.001 | 0.382 |
| Type × Environment | 1, 19 | 62.950 | <0.001 | 0.316 |
| Segment | 3, 57 | 9.019 | <0.001 | 0.166 |
| Type × Segment | 3, 57 | 3.340 | 0.022 | 0.069 |
| Environment × Segment | 3, 57 | 0.533 | 0.661 | 0.012 |
| Type × Environment × Segment | 3, 57 | 4.041 | 0.009 | 0.082 |
| Frequency of nose-to-nose behavior | ||||
| Type | 1, 19 | 7.678 | 0.006 | 0.053 |
| Environment | 1, 19 | 9.978 | 0.068 | 0.002 |
| Type × Environment | 1, 19 | 15.047 | 0.001 | 0.100 |
| Segment | 3, 57 | 2.388 | 0.072 | 0.050 |
| Type × Segment | 3, 57 | 2.170 | 0.094 | 0.046 |
| Environment × Segment | 3, 57 | 1.890 | 0.134 | 0.040 |
| Type × Environment × Segment | 3, 57 | 0.917 | 0.435 | 0.020 |
Between factors: Type. Within factors: Environment, Segment, and Zone.
In addition to zone transitions as a measure of general activity level, several other observations were quantified to more fully describe and compare behaviors between Hartley and Hairless guinea pigs in the open-field arena (Table 2). Bouts of nonspecific locomotion (not socially directed) were significantly (P < 0.05) more frequent in the Hartley group, as were investigations of the small blocks distributed throughout the arena and periods of rest. All locomotion, for both strains, occurred at the rate of a moderate trot, with no stampeding behavior observed. Analyses of Hairless data revealed significantly (P < 0.05) greater frequency of the freezing response when compared with that in the Hartley strain. Freezing responses were very brief, lasting less than 8 s in duration. No differences were observed between groups for frequencies of scratching, jumps, cage rearing, facial wiping, or head shakes (Table 2). All jumps were of the ‘popcorn’ variety, and no startles were observed.
Table 2.
Frequencies of guinea pig behaviors in the open-field arena
| Frequently seen behaviors | Hartley |
Hairless |
t(19) | P | d | 1-β | ||
| Mean | 1 SD | Mean | 1 SD | |||||
| Activity | ||||||||
| Nonspecific locomotion | 66.000 | 12.166 | 29.182 | 15.191 | 6.088 | <0.001a | 2.793 | 1.000 |
| Block investigation | 5.500 | 3.028 | 0.818 | 0.982 | 4.866 | <0.001a | 2.233 | 1.000 |
| Scratch | 2.300 | 2.710 | 5.091 | 3.727 | 1.945 | 0.067 | 0.892 | 0.629 |
| Rest | 45.200 | 13.620 | 19.818 | 8.244 | 5.224 | <0.001a | 2.397 | 1.000 |
| Consumption | ||||||||
| Hay rack | 25.800 | 13.307 | 31.455 | 15.384 | 0.896 | 0.381 | 0.411 | 0.229 |
| Pen floor | 12.200 | 6.596 | 31.455 | 11.431 | 4.661 | <0.001a | 2.139 | 1.000 |
| Hay taken from another | 2.200 | 1.751 | 1.091 | 1.136 | 1.738 | 0.098 | 0.797 | 0.523 |
| Social interaction | ||||||||
| Approach | 135.200 | 37.821 | 35.545 | 19.821 | 7.670 | <0.001a | 3.519 | 1.000 |
| Anogenital exploration | 2.800 | 1.687 | 4.364 | 3.722 | 1.218 | 0.238 | 0.559 | 0.350 |
| Nose up | 6.200 | 4.022 | 1.000 | 2.408 | 3.636 | 0.002a | 1.668 | 0.972 |
| Nose to nose | 10.800 | 9.750 | 0.909 | 1.136 | 3.348 | 0.003a | 1.536 | 0.939 |
| Rarely seen behaviors | n | % | n | % | Χ2(1) | P | φ | 1-β |
| Activity | ||||||||
| Jump | 1 | 4.762 | 0 | 0.000 | 0.000 | 0.961 | 0.235 | 0.137 |
| Cage rear | 0 | 0.000 | 4 | 19.048 | 2.440 | 0.118 | 0.463 | 0.374 |
| Facial wipe | 7 | 33.333 | 7 | 33.333 | 0.020 | 0.877 | 0.070 | 0.050 |
| Head shake | 2 | 9.524 | 7 | 33.333 | 2.490 | 0.115 | 0.440 | 0.363 |
| Freeze | 0 | 0.000 | 10 | 47.619 | 13.900 | <0.001a | 0.909 | 0.840 |
| Consumption | ||||||||
| Water | 10 | 47.619 | 2 | 9.524 | 11.170 | 0.001a | 0.826 | 0.629 |
| Coprophagy | 0 | 0.000 | 10 | 47.619 | 13.900 | <0.001a | 0.909 | 0.840 |
| Social interaction | ||||||||
| Follow | 10 | 47.619 | 2 | 9.524 | 11.170 | 0.001a | 0.826 | 0.629 |
| Chase | 0 | 0.000 | 2 | 9.524 | 0.450 | 0.501 | 0.310 | 0.203 |
| Kick | 1 | 4.762 | 2 | 9.524 | 0.010 | 0.929 | 0.118 | 0.106 |
| Bite | 0 | 0.000 | 0 | 0.000 | — | — | — | — |
n, number of subjects exhibiting the behavior among 10 Hartley and 11 Hairless guinea pigs; %, percentage of total (n = 21); Χ2, Yates corrected χ2.
Bonferroni-corrected significant P value.
Consumption.
Guinea pigs were observed performing several consumption behaviors. Analyses of the proportion of time spent consuming timothy hay from the hay rack in the open-field arena or of guinea pig pellets from the food hopper in the home cage (Figure 4) revealed significant (P < 0.05) main effects of Environment, Segment, and Type and 2-way interactions of Environment × Segment and Environment × Type. No other significant interactions with Segment or Type were revealed (Table 1). Likewise, post hoc comparisons found no differences in the proportion of time spent consuming food pellets for all time segments between Hartley and Hairless guinea pigs when observed in home cages (Figure 4). When compared across environments, however, post hoc comparisons revealed significant (P < 0.05) increases in proportion of time consuming hay in the open-field arena for both Hartley and Hairless guinea pigs across all but the last time segment. In addition, Hairless guinea pigs spent an even greater (P < 0.05) percentage of their time consuming hay than did the Hartley group (Figure 4).
Figure 4.
Consumption of timothy hay and food pellets during the observation period. Points depict the mean percentage of total time that Hartley (HR) and Hairless (IAF) animals spent consuming either (A) timothy hay from the rack in the open field or (B) food pellets in the home cage during each of the four 15-min segments (segments 1 through 4). Bonferroni-corrected significant differences were revealed between open field and home cage consumption (P < 0.001) during segments 1 through 3 by both strains. In addition, Hairless subjects showed increased time spent consuming hay during the 4th segment for the open field relative to home cage (P = 0.006). In the open field, values for Hairless subjects were increased significantly (P < 0.006) relative to Hartley during all but the third segment. Error bars represent the SEM.
Significant (P < 0.05) decreases over time in hay consumption from the rack for the Hairless group were partially offset by increases in hay consumption from the pen floor, with mean proportions and 95% CI of 0.111 (95% CI, 0.054 to 0.167), 0.192 (0.106 to 0.277), 0.213 (0.134 to 0.293), and 0.217 (0.126 to 0.308) for the four 15-min segments respectively. In comparison, proportions of hay consumed from the pen floor for Hartley guinea pigs remained relatively constant, however, with mean proportions and 95% CI of 0.063 (0.018 to 0.108), 0.071 (0.037 to 0.106), 0.036 (0.009 to 0.016), and 0.056 (0.016 to 0.019) for the four 15-min segments respectively. These strain dissimilarities also were reflected in analyses of mean frequencies for hay consumption, with no differences between Hartley and Hairless animals for rack consumption or taking hay from another guinea pig (Table 2). However, the Hairless group had more bouts of hay consumption from the pen floor of the open-field arena. In addition, bouts of coprophagy were observed only in Hairless guinea pigs in the open-field arena (Table 2).
Analysis of the proportion of time subjects spent drinking from the water source provided revealed significant (P < 0.05) main effects of Type, Environment, and Type × Environment interaction. No main effect of or any interaction with Segment was detected (Table 1). Post hoc analysis revealed no differences across any segment or between Hartley and Hairless subjects for the open-field environment. Means and 95% CI for the proportion of time spent during each of the 4 segments in the open-field arena for Hartley were 0.021 (0.000 to 0.043), 0.016 (0.003 to 0.029), 0.024 (0.008 to 0.040), and 0.016 (−0.002 to 0.035), respectively, and for Hairless animals were 0.010 (−0.011 to 0.030), 0.001 (−0.002 to 0.006), 0.006 (−0.007 to 0.019), and 0.005 (−0.001 to 0.011), respectively. Although no differences were detected in the proportions of time spent drinking within the open-field arena, a significantly (P < 0.05) greater proportion of the Hartley guinea pigs (48%) consumed water as compared with Hairless subjects (10%, Table 2).
Home cage proportions of Hairless guinea pigs that consumed water did not differ across segments or between environments. In contrast, greater proportions of the Hartley group consumed water (P < 0.05) during all 15-min segments, but only in the home cage. Means and CIs for the proportion of time spent during each of the 4 segments in the home cage for Hartley guinea pigs were 0.070 (0.016 to 0.123), 0.128 (0.023 to 0.234), 0.055 (0.030 to 0.080), and 0.120 (0.048 to 0.192), respectively, and for Hairless animals were 0.004 (−0.004 to 0.012), 0.003 (−0.004 to 0.010), 0.018 (−0.007 to 0.043), and 0.015 (−0.016 to 0.047) respectively.
Social interaction.
Guinea pigs expressed a number of social behaviors, which varied by environment and strain. The most frequent social behavior was approach. Analysis of approach frequency revealed significant (P < 0.05) main effects in all factors and interactions except Environment × Segment. Significant factors were Type, Environment, and Segment; 2-way interactions were Type × Segment and Type × Environment, and the 3-way interaction was Type × Environment × Segment (Table 1, D). Post hoc comparisons revealed differences in approach frequency during all time segments between Hartley and Hairless guinea pigs when observed in the open-field arena (Figure 5). No significant differences were detected for any time segment between Hartley and Hairless guinea pigs when observed in the home cage.
Figure 5.
Frequency of social behavior. Points depict the mean frequency (per 15 min) of (A and B) approach and (C and D) nose-to-nose behaviors for Hartley (HR) and Hairless (IAF) guinea pigs during each of the four 15-min segments (segments 1 through 4) of (A and C) open-field and (B and D) home-cage observations. Bonferroni-corrected differences were detected in (A and B) the approach behavior between Hartley subjects in the open field and home cage and between Hartley and Hairless subjects in both environments (P < 0.001). Significant differences also were detected in the nose-to-nose behavior between Hartley and Hairless subjects in all but the final segment of the open-field observation (P < 0.003). Hairless levels in the open field for the first 2 segments were depressed relative to frequencies in their home cage (P < 0.004). Error bars depict the SEM.
A similar pattern of results was obtained from data of nose-to-nose frequency (Figure 5). Analysis of frequency revealed significant main (P < 0.05) effects of Type and Environment and a 2-way interaction of Type × Environment (Table 1). No significant effects of Segment, either as main or interaction factors, were detected. Post hoc comparisons revealed no significant differences in nose-to-nose frequency for any time segments between Hartley and Hairless guinea pigs when observed in the home cage. However, in the open-field arena, post hoc comparisons revealed significantly (P < 0.05) more nose-to-nose frequency during time segments 1 through 3 for Hartley guinea pigs as compared with the Hairless group. Post hoc pairwise analyses also revealed that the Hairless group rates in the open-field arena were significantly (P < 0.05) less than those in the home cage for the first 2 segments.
The Hartley strain also exhibited higher (P < 0.05) rates of nose up during the open-field observation when compared with the Hairless strain (Table 2). No significant differences were found between Hartley and Hairless groups in the open field for anogenital exploration and several rarely observed behaviors, such as chasing or kicking another guinea pig. Aggressive biting behavior was not observed in the open field for either guinea pig strain (Table 2).
Behavioral sequence.
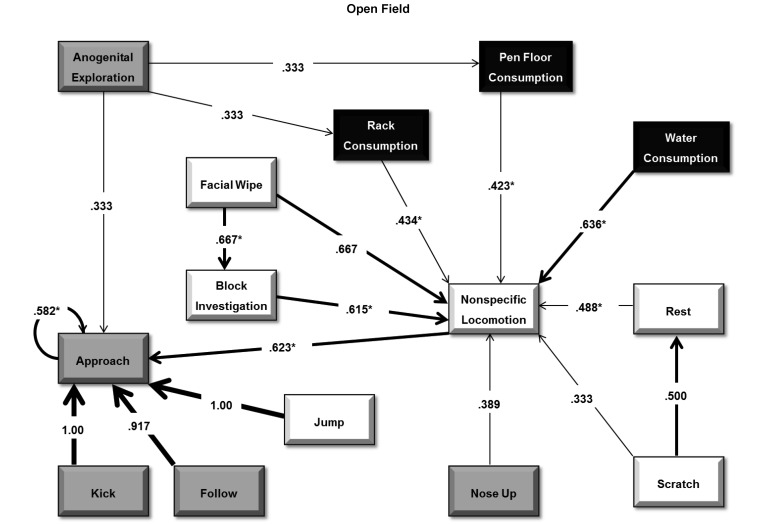
Sequential analysis of Hartley behavior for the open-field observation (Figure 6) revealed significant (P < 0.05) transition probabilities between 3 consumption behaviors (hay from both the rack and pen floor, and water consumption) and locomotion. Locomotion, in turn, transitioned on approach to another guinea pig, which was likely to transition on additional approaches. Analysis also revealed that facial wiping was likely to transition to block investigation, which in turn led to locomotion. In the home-cage observation, however, transition probability analysis revealed resting and locomotion for the Hartley strain to be mutually reciprocal (P = 0.550 and 0.602, respectively) with water (P = 0.674) and pellet consumption (P = 0.946), and scratching activity (P = 0.900) transitioned on rest behavior.
Figure 6.
Transition probabilities for observed behavior of Hartley guinea pigs in an open-field arena. The flow diagram depicts transition probabilities from one behavior to subsequent behaviors for Hartley guinea pigs during the open-field observation. Behaviors are grouped and highlighted according to the general categories: white for activity, black representing consumption, and gray for social interaction. Arrows depict the direction and strength of the probability of successive behaviors. Significant probabilities from Bonferroni-corrected P values are highlighted with an asterisk. Transition probabilities below 0.30 are not shown.
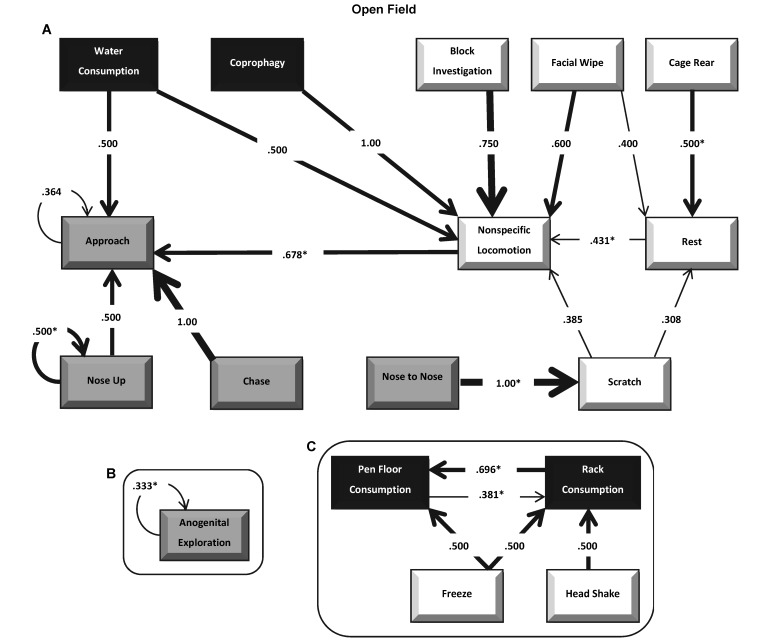
Markovian analyses of transition probabilities for Hairless behaviors in the open-field arena are depicted in Figure 7. Significant (P < 0.05) transition probabilities were revealed from cage rearing (for example, rearing against the arena wall) to resting, locomotion, and then to approach of another guinea pig. Pen floor and rack consumption of timothy hay were significantly (P < 0.05) reciprocal in nature. For social behaviors, nose up and anogenital exploration were likely to repeat, and nose-to-nose was likely to lead to scratching activity. In the home-cage environment, significant (P < 0.05) transition probabilities were revealed only for a mutually reciprocal interchange between resting and nonspecific locomotion (P = 0.510 and 0.541, respectively).
Figure 7.
Transition probabilities for observed behavior of the Hairless guinea pigs in an open-field arena. The flow diagram depicts transition probabilities from one behavior to subsequent behaviors for Hairless guinea pigs during the open-field observation. Behaviors are grouped and highlighted according to the general categories: white for activity, black representing consumption, and gray for social interaction. Isolated behaviors that were not part of the main set of transition probabilities (A) are depicted in B and C. Arrows depict the direction and strength of the probability of successive behaviors. Significant probabilities from Bonferroni-corrected P values are highlighted with an asterisk. Transition probabilities below 0.30 are not shown.
Salivary cortisol.
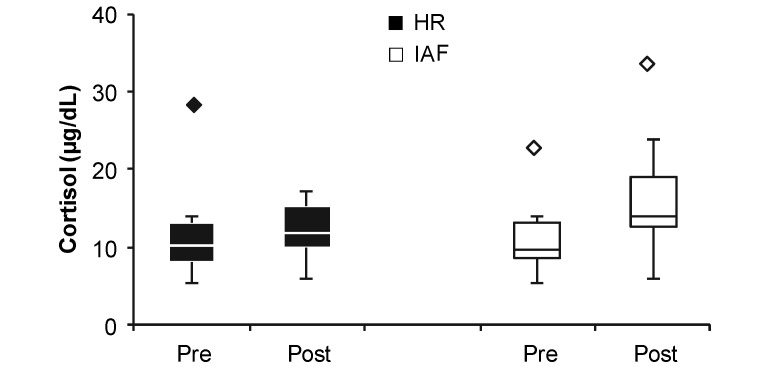
The interassay coefficient of variability across the 2 assay plates analyzed was 12.57%, and the intraassay for within-plate coefficient of variability was 4.87%. There were no significant differences in cortisol levels in saliva collected either before and after 1 h in the open-field arena (Figure 8). Similarly, levels did not differ between Hartley and Hairless guinea pigs. Analyses of salivary cortisol levels revealed no significant effects of Type (F1,17 = 1.766, P = 0.201, ηp2 = 0.094) or time of sample (F1,17 = 2.428, P = 0.138, ηp2 = 0.125) or type × time interaction (F1, 17 = 1.548, P < 0.230, ηp2 = 0.083).
Figure 8.
Salivary cortisol levels. Box plots of mean cortisol (µg/dL) levels for Hartley (HR) and Hairless (IAF) guinea pigs prior to observations in the open field (Pre) and after completion of observations (Post). Plots depict the full range of data for each group, excluding outliers, which are represented by points above the plots. Lines within the plots depict the median, and boxes represent the interquartile ranges. Bonferroni-corrected post hoc analyses failed to detect significant differences between Pre and Post salivary cortisol measures for either Hartley or Hairless subjects (P = 0.466 and P = 0.301, respectively). Error bars depict the SEM.
Discussion
Guinea pigs displayed a wide range of behaviors that varied by strain in the enriched open-field arena compared with home-cage activity but did not have elevated cortisol levels (Figure 8). Hartley guinea pigs displayed a greater number of zone transitions across all time segments in the open field compared with the home cage (Figure 3). In fact, both strains transitioned across all zones in the open field at frequencies at least equal to those of the smaller home cage. Furthermore, subjects had to travel longer distances to transition all 4 zones, due to the larger size of the enriched open field. These results suggest that guinea pigs actively entered all quadrants of the larger open field, even though it was not a novel environment. Density measures across zones 2 and 3 also suggest active entry into all areas of the arena, because subdivision of these centrally located zones into wall and interior areas did not typically reveal differences in guinea pig density. The exception to this finding was for the Hartley strain in the wall area of zones 2 and 3 proximate to the drinking water.
Higher frequencies in transitions across zones 2 and 3 for the Hartley strain may not represent more locomotion as compared with the Hairless group. The frequent zone transitions of the Hartley guinea pigs are more likely due to the proximate location of the water bottle (Figure 1), as suggested by large numbers of water consumption bouts (Table 2) when compared with those of Hairless subjects. The Hartley group also had more water consumption during the home-cage observation. A difference in husbandry between the 2 guinea pig strains that may explain the greater water consumption by the Harley group is that the Hartley guinea pigs were housed with an automated water system, whereas the Hairless group had water bottles identical to those used in this study. Consequently, the water bottles were an atypical object for the Hartley subjects, and the greater frequencies of zone transitions and consumption associated may represent exploration of this unusual object. Although the greater water consumption may have been a displacement activity in reaction to stress, this interpretation is unlikely in light of the stable cortisol levels reported in this study. Rather, the increased interest the Hartley group displayed for the water location suggests that ordinary water bottles may represent a form of enrichment for laboratory guinea pigs typically housed on an automated watering system.
As with water consumption in Hartley animals, Hairless guinea pigs spent a large portion of the observation period consuming timothy hay (Figure 4), a food item that was not provided in the home cage. However, the Hartley group did not similarly prefer timothy hay. Hairless guinea pigs are likely to have higher metabolic requirements, because they lack the hair coat of the Hartley strain. At the ambient temperature used in this study (23 °C), the Hairless strain, which lacks the insulating properties of hair, may have had a greater caloric need than did the Hartley group, due to higher metabolic rates necessary to maintain body temperature. To date, the metabolic rate of Hairless guinea pig has not been quantified, and this question remains unanswered. Furthermore, nearly all Hairless subjects were coprophagic, whereas coprophagy was not observed in Hartley guinea pigs. This suggests that the Hairless animals may have dietary needs beyond those of Hartley guinea pigs and might require more than simple increases in caloric intake. However, Hairless guinea pigs did not consume more food pellets in the home cage as compared with the Hartley group, suggesting that the Hairless strain simply may prefer timothy hay. Studies with additional food choices may shed more light on this hypothesis.
One final explanation for the greater consumption of hay by Hairless animals involves freezing responses, which were observed for only the Hairless guinea pigs in the open-field arena. Markovian analyses revealed a 0.500 probability that Hairless guinea pigs would consume hay from either the pen floor or the hay rack after a freezing response. Although freezing was observed infrequently, a small proportion of the hay consumption observed in the Hairless subjects may be displacement activity. Such activities typically are thought to relieve stress.37 However, the Hairless guinea pigs did not have elevated salivary cortisol at the conclusion of the open-field observation. Consequently, stress seems unlikely to have lead to the higher hay consumption revealed in this study. Additional investigation into the basis for greater hay consumption by the Hairless guinea pigs in the open-field arena is necessary to answer these questions.
Freezing responses were observed in nearly all Hairless animals despite cortisol levels that did not diverge significantly from baseline. These seemingly disparate results have 2 possible explanations. First, subjects exhibited only 1 or 2 freezing responses throughout the entire 1-h observation in the open-field arena. This small frequency may be insufficient to elevate cortisol levels. Second, muscle tonus can be observed more easily in the Hairless animals compared with the haired Hartley guinea pigs. Consequently, slight freezes may have been more visually apparent in the Hairless strain than in Hartley animals, favoring the identification of freezing responses in the Hairless strain.
Both guinea pig strains exhibited numerous social interactions while in the enriched open field. The Hartley guinea pigs showed higher frequencies of approach (Figure 5), nose up to other guinea pigs (Table 2), and nose-to-nose behaviors (Figure 6) than did the Hairless animals. In the open field, the Hartley group performed 3.8 times as many approaches, 6.2-fold more nose ups, and more than 12 times the number of nose-to-noses than did the Hairless group. These results confirm, as least for the Hartley strain, that the open-field arena acts as social enrichment for laboratory guinea pigs. The fact that both strains experienced a higher animal density in the open field (980 cm2 per guinea pig compared with 2128 cm2 per guinea pig in the home cage) yet only the Hartley animals showed increased social behaviors suggests that factors other than increased higher animal density led to the greater frequency of social behaviors by the Hartley group. In addition, levels of social behaviors between the 2 strains did not differ in the home-cage environment. This finding suggests that for the Hairless strain, the open-field environment does not stimulate additional social interaction above levels typically expressed. In fact, the analysis of nose-to-nose behavior revealed that this activity may actually be lower in the open-field arena relative to the home cage for Hairless animals (Figure 5). Taken together, these results demonstrate that environmental enrichment can have strain-specific effects.4,14,28
Most behaviors were evident during the first 15 min of observation, yet several were observed only rarely (Table 2). Due to the low rate of occurrence, differences between the 2 strains perhaps were not detected because of low statistical power in the analyses. A larger sample size that extends the number of observations may reveal additional differences between the 2 strains not found in this study.
Although the guinea pigs were placed in the open field in herds of 10 for Hartley animals and 11 for the Hairless guinea pigs, almost no aggressive behaviors, such as chasing, kicking, and biting, were observed (Table 2). Vocalizations were not scored because of the difficulty in uniquely identifying animals in the herd. We did not observe aggressive teeth chattering during the study. This lack of aggression, despite the large number of guinea pigs placed together in the open-field arena, may be related to the tendency for herd formation that has been identified in both domesticated and wild guinea pig populations. Guinea pigs have been observed to naturally form herds of 10 or fewer animals.32,33 Typically such a herd would include a male guinea pig, his harem, and young offspring. Consequently, 10 or fewer animals may be a natural herd size for guinea pigs. The reduction in some social behaviors in Hairless guinea pigs, this strain suggests that this strain may require a different size herd to facilitate social interaction. Additional research investigating other herd sizes for this strain may prove useful.
Additional studies examining negative social interactions also may be useful. Although we did not note overt forms of aggression in this study, more subtle forms of these behaviors perhaps were present but not identified. For example, a guinea pig initiating locomotion may be moving away from an approaching aggressor or may be forced from a location due to lack of space. Future studies examining these types of subtle interactions in the enriched open-field arena may reveal other forms of aggression and negative social interactions.
Markov transition probabilities of behavioral sequence revealed additional aspects of guinea pig behavior in the enriched open field compared with the home cage and between the 2 strains (Figures 6 and 7). In general, behavioral sequences were more varied in the open-field arena than in the home cage, particularly for the Hairless strain (Figure 7). In the home cage, locomotion and consumption behaviors were significantly likely to be followed by a period of rest. In the open-field arena, these same behaviors typically resulted in locomotion followed by approach social interaction (Figures 6 and 7). Analyses also revealed several other behavioral sequences for Hairless guinea pigs. Probabilities of hay consumption from the rack and pen floor were reciprocal. Likewise, performance of some social behaviors, such as anogenital exploration and giving a nose up, were significantly likely to result in repetitions of the same behavior. Although not strong evidence for stereotypy, follow-up investigations into repetitive behaviors in the Hairless may clarify these interesting behavioral sequences.
Overall, the open-field arena increased activity, consumption, social interaction, and more varied sequences of behavior in both Hairless and Hartley guinea pig strains without significantly elevating salivary cortisol. These results suggest that exposure to the open-field arena may be useful as enrichment for laboratory guinea pigs. Most of the behaviors reported in this study occurred most frequently during the first 15 min after placement in the open field, after which frequencies fell quickly to levels similar to those in the home cage. This pattern of results suggests that as little as 15 min of exposure to the open field may provide sufficient time for the guinea pigs to fully benefit from this form of enrichment. Due to the busy nature of most laboratories, 15 min per group of 10 guinea pigs may be more reasonable than the 1-h observation period chosen for this study. For example, by using the 15-min allotment per herd of 10 animals, a colony of 50 guinea pigs could be exposed to the enriched environment in less than 1.5 h daily, including clean up. If multiple pens are used, even greater numbers of guinea pigs could be offered enrichment simultaneously, thus further reducing the time required for this task. In addition to the time-consuming nature of this form of enrichment, having sufficient space available may be an issue for some facilities. The apparatus itself occupies more than twice the floor space of many standard guinea-pig cages. In situations of limited space, our laboratory has used a modular form of the open field that can be disassembled quickly after the completion of the daily enrichment session. Another consideration is that because the guinea pigs have been removed from standard housing when placed in the open field, they must be monitored throughout the enrichment to ensure the safety of the animals. This need for monitors may add markedly to the cost of the enrichment program, depending on availability of laboratory personnel. Finally, whether the enrichment will affect experimental data obtained from these guinea pigs is unknown. Additional studies are needed to determine exactly what, if any, permanent benefits are derived from this form of enrichment and how these alter the outcome of experimental data.
Acknowledgments
We thank Ashley Downs, Lindsey Keene, Levi Rosenthal, and Marco Voogd, for help with animal handling and behavioral scoring and the Wright State University Laboratory of Animal Resource technicians for the care of our guinea pigs, with special thanks to Emily Dudley for her veterinary expertise. This research was supported by grants NR010798 (to GAK) and GM086257 (to JSB and SAB).
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research, the National Heart Lung and Blood Institute, or the National Institutes of Health.
Portions of this work were previously presented at the 2010 annual meeting of the International Society for Developmental Psychobiology (San Diego, CA) and in 2011 at the annual meetings of the Midwestern Psychological Association (Chicago, IL), the Animal Behavior Society (Bloomington, IN), and the International Society for Developmental Psychobiology (Washington, DC).
References
- 1.Albers PCH, Timmermans PJA, Vossen JMH. 1999. Maternal behaviour in the guinea pig (Cavia aperea f. porcellus): a comparison of multiparous, primiparous, and hand-reared primiparous mothers. Neth J Zool 49:275–287 [Google Scholar]
- 2.Bakeman R, Gottman JM. 1986. Observing interaction: an introduction to sequential analysis. New York (NY): Cambridge University Press [Google Scholar]
- 3.Barnum CJ, Pace TW, Hu F, Neigh GN, Tansey M. 2012. Psychological stress in adolescent and adult mice increases neuroinflammation and attenuates the response to LPS challenge. J Neuroinflammation 9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumans V. 2005. Environmental enrichment for laboratory rodents and rabbits: requirements of rodents, rabbits, and research. ILAR J 46:162–170 [DOI] [PubMed] [Google Scholar]
- 5.Bayne K. 2005. Potential for unintended consequences of environmental enrichment for laboratory animals and research results. ILAR J 46:129–139 [DOI] [PubMed] [Google Scholar]
- 6.Bechard A, Meagher R, Mason G. 2011. Environmental enrichment reduces the likelihood of alopecia in adult C57BL/6J mice. J Am Assoc Lab Anim Sci 50:171–174 [PMC free article] [PubMed] [Google Scholar]
- 7.Belz EE, Kennell JS, Czambel RK, Rubin RT, Rhodes ME. 2003. Environmental enrichment lowers stress responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav 76:481–486 [DOI] [PubMed] [Google Scholar]
- 8.Blumstein DT, Daniel JC, Evans CS. [Internet]. 2006. JWatcher 1.0. [Cited 15 May 2012]. Available at: http://www.jwatcher.ucla.edu
- 9.Britton DR, Koob GF, Rivier J, Vale W. 1982. Intraventricular corticotropin-releasing factor enhances behavioral effects on novelty. Life Sci 31:363–367 [DOI] [PubMed] [Google Scholar]
- 10.Charles River. [Internet]. 2012. Research animal models. Hairless guinea pig. [Cited 15 May 2012]. Available at: www.criver.com/en-us/prodserv/bytype/resmodover/resmod/pages/HairlessHairlessguineapig.aspx [Google Scholar]
- 11.Cole BJ, Koob GF. 1988. Propranalol antagonizes the enhanced conditioned fear produced by corticotrophin-releasing factor. J Pharmacol Exp Ther 247:902–910 [PubMed] [Google Scholar]
- 12.Dahlqvist P, Rönnbäck A, Bergström SA, Söderström I, Olsson T. 2004. Environmental enrichment reverses learning impairment in the Morris water maze after focal cerebral ischemia in rats. Eur J Neurosci 19:2288–2298 [DOI] [PubMed] [Google Scholar]
- 13.de Witt BW, Ehrenberg KM, McAloon RL, Panos AH, Shaw KE, Raghavan PV, Skidmore ER, Kline A. 2011. Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous exposure after traumatic brain injury. Neurorehabil Neural Repair 25:343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galef BG., Jr 1999. Environmental enrichment for laboratory rodents: animal welfare and the methods of science. J Appl Anim Welf Sci 2: 267–280 [DOI] [PubMed] [Google Scholar]
- 15.Haemisch A, Gartner K. 1997. Effects of cage enrichment on territorial aggression and stress physiology in male laboratory mice. Acta Physiol Scand Suppl 640:73–76 [PubMed] [Google Scholar]
- 16.Haemisch A, Voss T, Gartner K. 1994. Effects of environmental enrichment on aggressive behavior, dominance hierarchies, and endocrine states in male DBA/2J mice. Physiol Behav 56:1041–1048 [DOI] [PubMed] [Google Scholar]
- 17.Hailman ED, Hailman JP. 1993. UNCERT user's guide. Madison (WI): University of Wisconsin Zoology Department. [Google Scholar]
- 18.Hennessy MB, Bullinger KL, Neisen G, Kaiser S, Sachser N. 2006. Social organization predicts nature of infant–adult interactions in 2 species of wild guinea pigs (Cavia aperea and Galea monasteriensis). J Comp Psychol 120:12–18 [DOI] [PubMed] [Google Scholar]
- 19.Hennessy MB, Hornschuh G, Kaiser S, Sachser N. 2006. Cortisol responses and social buffering: a study throughout the life span. Horm Behav 49:383–390 [DOI] [PubMed] [Google Scholar]
- 20.Hennessy MB, Schiml-Webb PA, Deak T. 2009. Separation, sickness, and depression: a new perspective on an old animal model. Curr Dir Psychol Sci 18:227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchinson E, Avery A, Vandewoude S. 2005. Environmental enrichment for laboratory rodents. ILAR J 46:148–161 [DOI] [PubMed] [Google Scholar]
- 22.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press [Google Scholar]
- 23.Kapoor A, Mathews SG. 2008. Prenatal stress modifies behavior and hypothalamic–pituitary–adrenal function in female guinea pig offspring: effects of timing of prenatal stress and stage of reproductive cycle. Endocrinology 149:6406–6415 [DOI] [PubMed] [Google Scholar]
- 24.Kulpa-Eddy JA, Taylor S, Adams KM. 2005. USDA perspective on environmental enrichment for animals. ILAR J 46:83–94 [DOI] [PubMed] [Google Scholar]
- 25.Nithianantharajah J, Hannan A. 2006. Enriched environments, experience-dependent plasticity, and disorders of the nervous system. Nat Rev Neurosci 7:697–709 [DOI] [PubMed] [Google Scholar]
- 26.Oishi K, Nishio N, Konishi K, Shimokawa M, Okuda T, Kuriyama T, Machida K. 2003. Differential effects of physical and psychological stressors on immune functions of rats. Stress 6:33–40 [DOI] [PubMed] [Google Scholar]
- 27.Padgett DA, Marucha PT, Sheridan J. 1998. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun 12:64–73 [DOI] [PubMed] [Google Scholar]
- 28.Pasalic I, Bosnjak B, Tkalcevic VI, Jaran DS, Javorscak Z, Markovic D, Hrvacic B. 2011. Cage enrichment with paper tissue, but not plastic tunnels, increases variability in mouse model of asthma. Lab Anim 45:121–123 [DOI] [PubMed] [Google Scholar]
- 29.Plane JM, Whitney JT, Schallert T, Parent J. 2008. Retinoic acid and environmental enrichment alter subventricular zone and striatal neurogenesis after stroke. Exp Neurol 214:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prut L, Belzung C. 2003. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3–33 [DOI] [PubMed] [Google Scholar]
- 31.Rehling A, Trillmich F. 2008. Changing supply and demand by cross-fostering: effects on the behaviour of pups and mothers in guinea pigs, Cavia aperea f. porcellus, and cavies, Cavia aperea. Anim Behav 75:1455–1463 [Google Scholar]
- 32.Rood JP. 1972. Ecological and behavioral comparisons of 3 genera of Argentine cavies. Animal Behaviour Monographs 5:1–83 [Google Scholar]
- 33.Sachser N. 1998. Of domestic and wild guinea pigs: studies in sociophysiology, domestication, and social evolution. Naturwissenschaften 85:307–317 [DOI] [PubMed] [Google Scholar]
- 34. Salimetrics. [Internet]. 2011. High-sensitivity salivary cortisol immunoassay kit, p 13–15. [Cited 15 May 2012]. Available at: http://www.salimetrics.com/documents/Cortisol_Kit_Insert.pdf.
- 35. StatSoft. [Internet]. 2011. STATISTICA (data analysis software system), version 10. [Cited 15 May 2012]. Available at: www.statsoft.com.
- 36.Swerdlow NR, Geyer MA, Vale WW, Koob GF. 1986. Corticotrophin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychopharmacology (Berl) 88:147–152 [DOI] [PubMed] [Google Scholar]
- 37.Tinbergen N. 1952. Derived activities; their causation, biological significance, origin, and emancipation during evolution. Q Rev Bioi 27:1–32 [DOI] [PubMed] [Google Scholar]
- 38.Toth LA, Kregel K, Leon L, Musch T. 2011. Environmental enrichment of laboratory rodents: the answer depends on the question. Comp Med 61:314–321 [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner JE, Manning PJ. 1976. The biology of the guinea pig. New York (NY): Academic Press. [Google Scholar]
- 40.Willis FN, Levinson DM, Buchanan DR. 1977. Development of social behavior in the guinea pig. Psychol Rec 27:527–536 [Google Scholar]