Abstract
Animal allergens constitute a serious health risk in laboratory animal facilities. To assess possibilities for allergen reduction by technical and organizational measures, we studied personnel exposure to mouse urinary aeroallergens in an animal facility with a holding capacity of 30,000 cages. Short-term (2 h) and intermediate-term (12 h) stationary samples (n = 107) and short-term (2 h) personnel samples (n = 119) were collected on polytetrafluorethylene filters by using air pumps. Long-term (14 d) stationary dust samples containing airborne allergens (n = 165) were collected with electrostatic dust fall collectors (EDC). Mouse allergens were quantified by ELISA. Personnel samples were collected during bedding disposal and refilling of clean cages as well as during cage changing with and without use of cage-changing station. Animal rooms were equipped with either open cages, cages with a soft filter top, cages with a rigid filter top (static microisolation caging), or with individually ventilated cages (IVC) with either a sealed or nonsealed lid, each in positive- or negative-pressure mode. Highest personnel allergen exposure was detected during cage change and emptying of soiled cages. Allergen concentrations were lowest in rooms with sealed IVC under positive or negative pressure, with unsealed IVC under negative pressure, and with static microisolation caging. The use of cage-changing stations and a vacuum bedding-disposal system reduced median personnel exposures 14- to 25-fold, respectively. Using sealed IVC and changing stations minimized allergen exposure, indicating that state-of-the-art equipment reduces exposure to mouse allergens and decreases health risks among animal facility personnel.
Abbreviations: CS, cage-changing station; EDC, Electrostatic dust fall collector; IVC, Individually ventilated cages; LAA, Laboratory animal allergy; MUA, Mouse urinary aeroallergen; NSL, nonsealed lid; SL, sealed lid
The use of laboratory mice in contemporary biomedical research is constantly increasing. In particular, research with genetically modified mice, which serve as model organisms for functional studies of human diseases, has grown markedly recently.2,14 The number of rodent facilities involved in systemic phenotyping, archiving, and distribution of mouse models has increased during the last 2 decades, and the mode of interactions between researchers and mice has changed simultaneously. Whereas previously mice typically were bred and held in barrier facilities with restricted access by scientific staff, an increasing number of researchers are demanding direct access to their animals. This need is particularly relevant in phenotyping facilities, in which mouse rooms are connected directly to procedure rooms and in which mice are handled extensively during phenotyping.17 Under such experimental conditions, both researchers and animal care staff have intense contact with mice and, therefore, to mouse allergens. Consequently, the number of personnel directly exposed to laboratory animal allergens is growing constantly, and an even greater number of persons may be exposed indirectly to mouse allergens.
To accommodate the increasing access of scientific and animal care staff to mouse facilities as well as for the growing exchange of transgenic mice between institutions and the associated increased health risks, individually ventilated cages (IVC) have become the state-of-the-art caging system. In addition, IVC are used particularly to reduce the exposure of staff to airborne allergens in the animal rooms, to better control the potential for spread of infections within and between facilities.
Cross-sectional surveys indicate that between 5% and 40% of persons in contact with laboratory animals report allergic symptoms within the first year of exposure, and as many as 10% develop occupational asthma.10 In a study of newly employed animal facility workers, 55% developed laboratory animal allergy within the first 2 y5 (for a review, see reference 7). Because allergic reactions constitute a serious health problem for persons in contact with laboratory animals, and about one third of exposed staff develops symptoms of laboratory animal allergy,3,4,12,15,29,30 the prevention of these allergies should be a major objective in occupational health and safety programs.10
The goal of the current study was to assess the exposure of scientific and animal care staff to mouse allergens in mouse breeding and holding rooms using 5 different types of caging and to evaluate the effect of using cage-changing stations (CS) on mouse allergen reduction. In addition, levels of mouse allergens were evaluated in different areas of the facility, including mouse breeding and holding rooms, corridors, wash rooms, a procedure room, and offices. Particular emphasis was placed on allergen levels in the breathing zone of individual employees and the assessment of background values by long-term passive sampling by using electrostatic dust fall collectors (EDC) compared with stationary pump sampling.
Materials and Methods
Facility structure and mouse rooms.
During the study, an average of 53,000 mice (male:female, 30:70) of various inbred and transgenic strains were bred and kept at a mean density of 1.8 mice per cage in the animal facility of the Helmholtz Center Munich. The average lifespan of the mice was 125 d. Approximately 400 persons including 67 animal caretakers had access to the mouse rooms. Animal usage in the facility is very similar to that at universities or other government research facilities. Except for one office, all rooms included in this study were within a single large building and including breeding and holding rooms, corridors, wash rooms, an autopsy room, and an office near the mouse breeding and holding rooms; the other office evaluated was in a different building and not associated with laboratory animals.
The animal rooms were ventilated at positive pressure relative to the corridors, the wash rooms, and the ambient pressure outside the facility, with approximately a 20-Pa difference at each step. Incoming air was filtered by using HEPA class 13 filters. Access to the mouse quarters required either wet or air showers. On one side, the wash rooms were directly adjacent to the barrier units and connected by autoclaves. On the other side, they were connected to the outside by large sliding doors.
Mice and husbandry.
Mice were bred and held in various caging behind full barriers with wet shower access and in conventional units with air shower access at a temperature of 20 to 24 °C, humidity of 50% to 60%, approximately 15 air exchanges per hour, and a 12:12-h light:dark cycle. Wood shavings (Lignocel 3-4, Rettenmaier, Rosenberg, Germany) were provided as bedding. Mice were fed a standardized mouse diet (type 1314, Altromin, Lage, Germany) and provided sterile filtered drinking water ad libitum. When entering a mouse room, staff wore clean cotton suits and shoes, disposable gloves, bonnets, and mouth masks. Cages and water bottles were changed weekly either on mobile stainless-steel tables or in class II cage-changing stations (CS). Mice were transferred to new cages by use of forceps padded with silicone tubing. Forceps were disinfected after each cage change with 70% ethanol. All materials were autoclaved before use. Microbiologic examination was performed every 3 mo by using male Crl:CD1(Icr) sentinels as described.1 All animal studies were approved by the Helmholtz Center Munich IACUC and by the Government of Upper Bavaria, Germany.
Cage types.
The following 5 cage types (Figure 1 A through E) were used: open cages, type II polycarbonate cages (floor area, 344 cm2; height, 13.5 cm) with wire lid; soft filter-top cages, as for open cages but with an additional soft filter top composed of a flexible polyethylene frame holding a woven polypropylene filter (filtration class F7 EN779.2002, PTS KG, Boetzingen, Germany) and for which the water bottle was placed halfway across the cage through a cross cut in the the filter top; static microisolation caging, as for open cages but with a rigid filter top composed of a rigid polycarbonate frame holding a 100% polyester filter (atmospheric dust efficiency, 92% retention of 8- to 10-μm particles); and nonsealed individually ventilated cages, NSL IVC), as for open cages but covered with a nonsealed, solid stainless-steel lid and used in an IVC rack as described.16 These first four types of caging had no seal between cage and lid. The remaining type of cage used was sealed-lid (SL) IVC, which were polyphenylsulfone cages (floor area, 501 cm2; height, 13.5 cm) with a polyphenylsulfone lid carrying a rescue filter (hydrophobic polytetrafluorethylene membrane with a 0.2-µm pore size) and used in an IVC rack; silicone gasket seals were placed between cages and lids.
Figure 1.
Cage types and sampling methods. (A) Type II open polycarbonate cage (floor area, 344 cm2; height, 13.5 cm) with wire lid holding chow and water bottle. (B) Same cage setup as in panel A but with an additional soft filter top composed of a flexible polyethylene frame holding a polypropylene filter. The water bottle is positioned halfway across the cage, through a cross cut in the paper filter of the filter top. (C) Same cage setup as in panel A but with an additional rigid filter top composed of a rigid polycarbonate frame holding a polyester filter (static microisolation caging). (D) Same cage set up as in panel A but covered with a nonsealed, solid stainless-steel lid and used in an IVC rack as described. The cages shown in panels A through D had no seal between cages and lids. (E) Type II long, sealed, polyphenylsulfone cage (floor area, 501cm2; height, 13.5 cm) with polyphenylsulfone lid holding a polytetrafluorethylene membrane filter and used in an IVC rack. Silicone gaskets were placed between cages and lids. (F) Stationary air pump and sampling head carrying a filter for the collection of ambient samples. (G) Portable air pump with sampling head attached to a staff member for the collection of samples fom the breathing zone at the upper chest level during cage changing on a stainless steel table. (H) Electrostatic dust fall collectors (EDC) for the collection of ambient samples. (I) EDC (arrow) positioned on top of an IVC rack. (J) 2 EDC positioned on a sampling table in the wash room.
Two different pressurized IVC rack systems were used: Ventiracks (Biozone, Margate, UK), which held the NSL IVC (Figure 1 D), and Sealsafe Plus (Green Line) racks (Tecniplast, Hohenpeissenberg, Germany), which held the SL IVC (Figure 1 E). In addition, both systems were used in positive- and negative-pressure modes; differential pressure with the Ventirack system was 0.5 to 1 Pa in both positive- and negative-pressure modes at 120 air changes per hour. For the Sealsafe system, the pressure differential was 10 Pa in both positive- and negative-pressure modes at 60 air changes per hour. Air from both IVC systems was exhausted directly from the air handler to the exhaust tubes of the room ventilation (HVAC) system; air from the class II cage-changing stations was filtered and exhausted into the room. In total, 7 conditions were investigated: open cages, soft filter-top cages, and static microisolation caging (these cages were placed in racks hanging on the walls) and NSL and SL IVC in both positive- and negative-pressure modes.
Mouse holding rooms equipped with open cages, filter-top cages, or static microisolation caging contained 280 to 350 cages. IVC-equipped mouse holding rooms contained 164 to 252 NSL IVC or 280 SL IVC.
Cage changing.
Open, filter-top, and microisolation cages were changed on mobile stainless-steel tables. A stack of 10 to 13 clean cages with new bedding was placed on the table. One soiled cage at a time was removed from the rack, placed on the table, and opened. The wire grid and mice were transferred to a new cage; mice were handled by using forceps. When applicable, the cage was closed with a soft or rigid filter top and brought back to the rack. As many as 13 cages with soiled bedding were stacked, placed on a trolley, and passed through the autoclave to the washroom. Dirty wire grids were exchanged for clean ones every 4 wk. Static microisolation cages were changed either on stainless-steel tables or in a CS, to test its effect on allergen levels.
All IVC were changed in CS (Nuaire, Minneapolis, MN) equipped with 4-stage prefilters and supply- and exhaust HEPA filters. Two stacks of 5 to 7 clean cages containing new autoclaved bedding were placed on the work surface inside the CS. One soiled cage at a time was removed from the rack, placed on the work surface, and opened. Depending on the cage type, the rigid filter top and wire grid of static microisolation cages, solid stainless-steel lid and wire grid of NSL IVC, or polysulfone top and wire grid of SL IVC and mice were transferred to a new cage. The cage was closed and returned to the rack. Wire grids and lids were changed every 4 wk. Stacks of 10 to 13 soiled cages were placed outside the CS on a mobile stainless-steel table, transferred to a trolley, and passed through the autoclave to the washroom.
Sampling sites and dust collection.
Airborne allergen samples were collected from 5 types of rooms: mouse holding rooms, each equipped with one of the described caging systems and either a mobile stainless-steel table or CS for cage changes; corridors of various dimensions and connecting 6 to 16 mouse holding rooms; washrooms in which soiled cages were emptied into a waste container or into the funnel of a vacuum bedding-disposal system, washed in tunnel washers, filled with new bedding, and autoclaved; a procedure room used for experiments and necropsies on mice; an office near the animal rooms and used by 2 staff members with regular mouse contact; and an office of the same size and occupancy as that near the animals rooms but in a different building and without any association with mice. The office near the animal rooms was occupied by 2 scientists, who worked for 6 days each week in a barrier requiring air-shower and a complete change of clothing for access to mouse rooms containing open and filter-top cages.
Static samples were collected by air samplers fitted with Gesamtstaubprobenahme sampling heads for inhalable dust (Figure 1 F) and used at a flow rate of 3.5 L/min with preweighed and coded 3.7-cm polytetrafluorethylene filters with a 1-µm pore size (FALP03700, Millipore). The 107 stationary samples obtained at floor level or at the table surface were collected either for 2 h (28 short-term samples) or for 12 h (79 long-term samples). The 119 personnel samples, representing 28 volunteers, were taken in the breathing zone at the upper chest level during 2 h of regular cage-changing (Figure 1 G) performed in one of the mouse holding rooms or of emptying soiled cages in the washroom.
In addition, 165 samples were collected by using EDC (Figure 1 H),19,20,31 consisting of frames holding 4 tissues with 0.0209 m2 sampling area each, positioned on sampling tables (Figure 1 J) and on top of cage racks (Figure 1 I) during 14 d of sampling in the 5 room types. These samples represented the ambient exposure, defined as the exposure generated by the mice and all activities of the caretakers during the measured period. All sampling was done during routine activities in the respective rooms and during routine animal care procedures.
Filters and EDC were sent to the Institute for Prevention and Occupational Medicine (Bochum, Germany), where filters were extracted individually in 5 mL PBS 0.05% Tween-20 and one tissue from each EDC was extracted in 20 mL of this solution by shaking for 1 h at room temperature. After extraction, the filter or tissue was removed, the extract was centrifuged at 3000 × g for 15 min, and the supernatant was stored in aliquots at −80 °C until analysis.
Allergen quantification.
Polyclonal rabbit antibodies and the mouse urinary allergen (MUA) standard were obtained from the Institute for Risk Assessment Sciences (Utrecht, The Netherlands)13 and used with an amplification protocol as described.26 Briefly, 96-well plates (Maxisorp, Nunc, Denmark) were coated with ammonium-sulfate–precipitated antiMUA antibodies (2 µg/mL in carbonate–bicarbonate buffer, pH 9.6) and blocked with casein buffer (PHRPDILX, Fitzgerald Industries International, Concord, MA). Serial dilutions of MUA standard (concentrations ranging from 1 to 0.008 ng/mL) and samples in casein buffer were incubated for 1 h at room temperature. MUA binding was detected by using biotinylated antiMUA antibodies followed by polymeric peroxidase-conjugated streptavidin (SA-poly-HRP80, Fitzgerald; diluted 1:20,000 in casein buffer) and Enhanced K-blue Substrate Solution (Neogen, Lexington, KY). The enzyme reaction was stopped after 10 min by using 1 M H2SO4, and the absorbance was read at 450 nm and 650 nm. Sample concentrations were obtained by interpolation of OD values (OD450 – OD650) on a 4-parameter dose–response curve of the standard preparation by using Softmax Pro 5.4 (Molecular Devices, Sunnyvale, CA). The limit of detection was the concentration corresponding to 0.1 OD unit above the estimated minimal value (that is, parameter A) of the 4-parameter curve fit function.
Statistical analysis.
Allergen levels below the detection limit of assays were assigned a value of 2/3 of the detection limit of the plate. Calculations and correlation (Spearman, Pearson) analyses were performed by GraphPad Prism (GraphPad Software, San Diego, CA).
Results
Ambient allergen exposure in different rooms.
A total of 107 stationary airborne dust samples and 165 EDC samples were taken in the 5 types of rooms. In the procedure room and corridors, allergen levels reached maxima of 1.2 and 3.1 ng/m3 (59 and 181 ng per tissue), respectively, with median values of 0.9 ng/m3 (21 ng per tissue) and 0.5 ng/m3 (14 ng per tissue), respectively. Washrooms and particularly mouse rooms yielded high levels of allergens. In washrooms, allergen concentrations ranged from below the limit of detection to11.5 ng/m3 (6 to 4800 ng per tissue), with median values of 0.4 ng/m3 (42 ng per tissue). In mouse rooms, allergen levels ranged from below the limit of detection to 48 ng/m3 (2 to 16,400 ng per tissue), with median values of 1.7 ng/m3 (106 ng per tissue). In contrast, all measurements from offices, regardless of proximity to animal rooms, were below the detection limit. Allergen levels measured by using stationary pumps differed significantly (P < 0.01; Dunn multiple-comparison test) only between mouse rooms and offices. Table 1 contains the P values of the Dunn multiple-comparison analyses of the 14-d data obtained by using EDC.
Table 1.
Comparison (Dunn multiple-comparison analysis) of ambient mouse allergen levels obtained by using EDC for 14 d among the 5 sampling locations
| Offices | Procedure room | Washroom | Mouse rooms | Corridor | |
| Offices | not applicable | P < 0.05 | P < 0.001 | P < 0.001 | P < 0.05 |
| Procedure room | P < 0.05 | not applicable | not significant | not significant | not significant |
| Washroom | P < 0.001 | not significant | not applicable | not significant | P < 0.05 |
| Mouse rooms | P < 0.001 | not significant | not significant | not applicable | P < 0.01 |
| Corridor | P < 0.05 | not significant | P < 0.05 | P < 0.01 | not applicable |
Ambient allergen exposure in mouse rooms with different cage types.
Ambient allergen exposure in rooms with different cage types was measured by EDC over 14 d. The highest allergen levels occurred in rooms with open cages (median, 3624 ng per tissue), followed by rooms with NSL IVC under positive pressure (733 ng per tissue) and rooms with filter-topped open cages (174 ng per tissue). Measuring ambient exposure by using 12-h stationary pumps yielded the same pattern: exposures were highest for rooms open cages (median, 26.9 ng/m3), followed by NSL IVC under positive pressure and filter-topped open cages (10.6 ng/m3). Markedly lower allergen levels were found in rooms with microisolation caging (median, 14 ng per tissue), NSL IVC under negative pressure (9 ng per tissue) and SL IVC in positive (12 ng per tissue) and negative (9 ng per tissue) pressure modes. Low ambient concentrations were confirmed by using stationary pumps for sampling (median: microisolation caging, 0.2 ng/m3; SL IVC in positive-pressure mode, 0.5 ng/m3; and SL IVC as well as NSL IVC, both in negative-pressure mode, below the limit of detection). Table 2 summarizes the results of the comparison of allergen levels obtained by using EDC for 14 d among cage types and conditions.
Table 2.
Comparison (Dunn multiple-comparison analysis) of allergen levels obtained by using EDC for 14 d among mouse rooms containing various cage types and conditions
| Open | Filter top | Microisolation | NSL IVC Neg | NSL IVC Pos | SL IVC Neg | SL IVC Pos | |
| Open | na | ns | P < 0.001 | P < 0.01 | ns | P < 0.001 | P < 0.001 |
| Filter top | ns | na | ns | ns | ns | P < 0.05 | ns |
| Microisolation | P < 0.001 | ns | na | ns | P < 0.01 | ns | ns |
| NSL IVC Neg | P < 0.01 | ns | ns | na | P < 0.05 | ns | ns |
| NSL IVC Pos | ns | ns | P < 0.01 | P < 0.05 | na | P < 0.001 | P < 0.01 |
| SL IVC Neg | P < 0.001 | P < 0.05 | ns | ns | P < 0.001 | na | ns |
| SL IVC Pos | P < 0.001 | ns | ns | ns | P < 0.01 | ns | na |
na, not applicable; ns, not significant.
Mouse activity and ambient allergen levels.
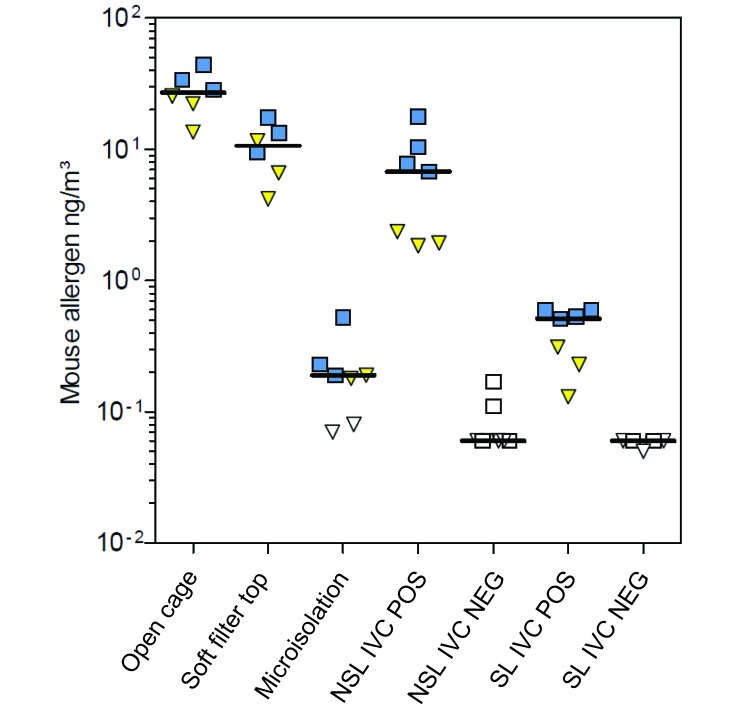
Mice are predominantly active during the dark phase of the light cycle. To determine whether ambient allergen concentrations reflected these differences in activity, 3 or 4 stationary 12-h ambient samples were obtained from rooms containing each type of caging during both the dark and light phases. In most rooms, median allergen concentrations were 1.5 to 4.7 times higher during the dark phase as compared with the light phase (Figure 2). All measurements were below the detection limit in rooms with cage systems in negative-pressure mode.
Figure 2.
Mouse allergen levels in holding rooms equipped with different cage types and conditions according to phase of the light cycle as measured during long-term stationary sampling. Squares, dark phase; triangles, light phase; open symbols, concentration below detection level. Horizontal bars show median values. NEG, cages under negative pressure; POS, cages under positive pressure.
Correlation of allergen concentrations measured by 14-d EDC and by 12-h stationary sampling.
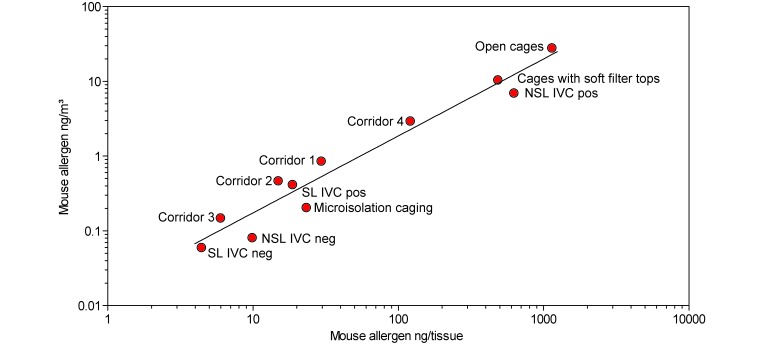
In addition to EDC sampling, ambient allergen exposure in rooms with different cage types was measured over 12 h by using stationary pumps. Means of samples from same locations were correlated and a regression line of log-transformed values was calculated (Pearson of log values = 0.95, Spearman r = 0.95, Pearson = 0.96; Figure 3). From these data, we established that allergen concentrations of 1 ng/m3 as measured by air-pump samples are equivalent to about 55 ng allergen per EDC tissue obtained during 14 d of sampling in mouse rooms and connected corridors.
Figure 3.
Correlation of mean ambient mouse allergen concentrations obtained either by stationary pumps over 12 h (y axis) or by 14-d passive sampling with EDC in same rooms (x axis). The function logy = –1.79 + 1.03logx describes the regression line shown in the graph. Corridor 1 was connected to rooms containing NSL IVC, corridor 2 to those with SL-IVC, corridor 3 to those with microisolation caging, and corridor 4 to those with open cages. neg, negative-pressure mode; pos, positive pressure mode.
Use of CS and allergen exposure.
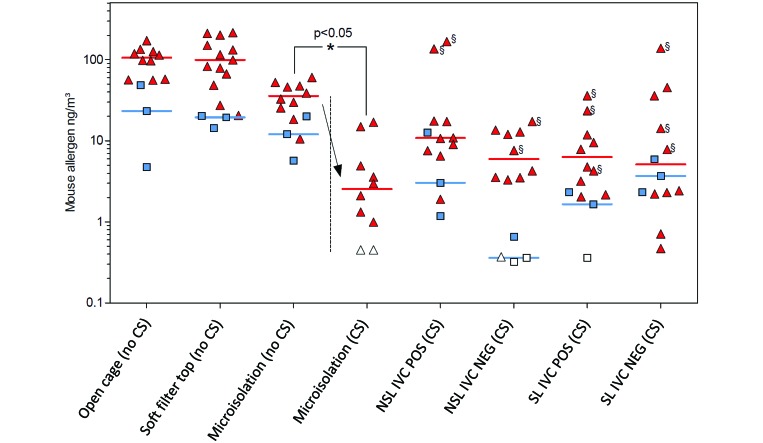
Personnel and ambient exposure levels were obtained simultaneously during cage-changing procedures (Figure 4). In general, allergen concentrations of personnel samples, which were collected over 2 h by using air pumps attached to the breathing zone of animal caretakers, were approximately 5-fold higher than were corresponding stationary samples taken by using air pumps near the back wall of the same room. Among personnel samples, the highest allergen concentrations were detected when open cages, cages with soft filter-tops, and microisolation caging were changed in the holding rooms on stainless-steel tables (that is, without CS). The personnel values associated with open cages ranged from 56 to 171 ng/m3 (median, 106 ng/m3), with filter-top cages ranged from 20 to 215 ng/m3 (median, 99 ng/m3), and with microisolation caging ranged from 11 to 60 ng/m3 (median, 36 ng/m3) in the absence of CS. Significant differences in personnel exposure levels between cage types and conditions are summarized in Table 3.
Figure 4.
Simultaneous personnel (sampled from the breathing zone of workers) and ambient (sampled from the back of a holding room) mouse allergen concentrations measured by using portable air pumps during 2 h of cage changing with or without the use of CS in mouse holding rooms equipped with different types of caging. Triangles, personnel samples; squares, ambient samples; open symbols, below the detection limit (0.5 ng/m3). Horizontal bars show median values. Arrow indicates the effect of changing static microisolation cages in a CS on mouse allergen concentrations. §, measurements corresponding to one individual caretaker among a group of 8 caretakers who changed cages in CS.
Table 3.
Summary of significant (P < 0.05) differences in personnel exposure levels during cage changing according to cage type and condition
| Microisolation (no CS) | Microisolation (CS) | NSL IVC Neg | NSL IVC Pos | SL IVC Neg | SL IVC Pos | |
| Open cages | ns | P < 0.001 | P < 0.01 | ns | P < 0.01 | P < 0.01 |
| Soft filter top | ns | P < 0.001 | P < 0.001 | ns | P < 0.01 | P < 0.01 |
| Microisolation (no CS) | na | P < 0.05 | ns | ns | ns | ns |
| Microisolation (CS) | P < 0.05 | na | ns | ns | ns | ns |
All comparisons not shown were nonsignificant.
Open cages and cages with soft filter tops were changed on the table only (that is, no CS). All IVC were changed in CS only.
Allergen levels typically were much lower when IVC were changed in CS than when CS were not used. Using a CS in a room containing NSL IVC in positive-pressure mode resulted in ambient mouse allergen exposures from 2 to 167 ng/m3 (median, 11 ng/m3); personnel exposure levels associated with using a CS for changing NSL IVC in negative-pressure mode ranged from 0.4 to 17 ng/m3 (median, 6 ng/m3), for changing SL IVC in positive-pressure mode ranged from 2 to 36 ng/m3 (median, 6 ng/m3), and for changing SL IVC in negative-pressure mode ranged from 0.5 to 138 ng/m3 (median, 5 ng/m3). Particularly high levels were measured when one individual caretaker changed IVC by using a CS (Figure 4, data points marked with §), although the difference between this caretaker and the other seven caretakers doing the same procedure was nonsignificant (Dunn multiple-comparison test).
Mouse allergen levels in personnel samples obtained when microisolation cages were changed within a CS in the holding room were significantly (P < 0.05) lower than those when they were changed on a stainless-steel table (that is, without CS) in that same room. Specifically, median values dropped 14-fold, from 36 ng/m3 (without CS) to 2.5 ng/m3 (with CS).
Allergen dissemination from mouse holding rooms to connecting corridors.
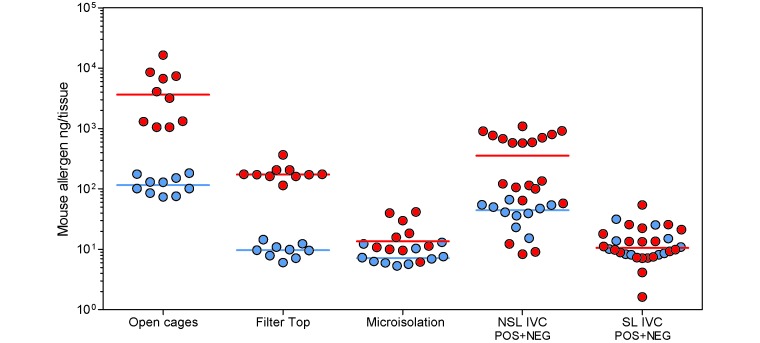
The HVAC system in the facility generated an air pressure gradient from the holding rooms in the barriers to the corridors and further to the outside of the barrier. To test whether allergens are disseminated along this gradient, we obtained 14-d ambient EDC samples. Two patterns emerged. In barriers with open cages, cages with soft filter tops, or NSL IVC, the allergen concentrations in the corridors were about 1 log lower than those in the animal rooms connected to these corridors (Figure 5 and Table 4). However, in barriers with microisolation caging or SL IVC, the allergen concentrations in the corridors and animal rooms were very similar. In detail, whereas holding rooms with open cages, cages with soft filter tops, and NSL IVC showed median allergen levels of 3624, 173, and 354 ng per EDC tissue, respectively, the median levels in adjacent corridors were 115, 10, and 44 ng per tissue, respectively. Holding rooms with microisolation cages and SL IVC showed median allergen levels of 14 and 10 ng per EDC tissue; the median levels in adjacent corridors were 7 and 10 ng per tissue, respectively.
Figure 5.
Mouse allergen room ambient concentrations measured after 14 d of EDC sampling in mouse rooms (red circles) and in their connecting corridors (blue circles). Horizontal bars show median values. Data for IVC in negative- (NEG) and positive- (POS) pressure modes were pooled because they were connected by the same corridor.
Table 4.
Summary of significant (P < 0.05; Mann–Whitney test) differences in allergen levels between mouse rooms and their connecting corridors
| P | |
| Open cages | < 0.0001 |
| Soft filter top | < 0.0001 |
| Microisolation | < 0.05 |
| NSL IVC | < 0.01 |
| SL IVC | not significant |
Effect of vacuum bedding disposal on allergen levels in washrooms.
Two-hour samples from the breathing zone of washroom staff showed median allergen levels of 197 ng/m3 when soiled bedding was disposed into a waste container and of 8 ng/m3 when it was dropped into the funnel of a vacuum disposal system. These levels differed 25-fold (P < 0.0001). The concurrent ambient levels in the washroom, measured by 14-d EDC, were 42 ng per tissue.
Discussion
Myriad animal facility staff and researchers worldwide are exposed to laboratory animal allergens. Occupational sensitization and laboratory animal allergy are paramount health issues in the laboratory animal field. Therefore we carried out a study measuring mouse allergen concentrations in our facility to evaluate the exposure of scientific and animal care staff to mouse allergens and to indicate structural and organizational measures to improve occupational safety. We included mouse breeding and holding rooms using 5 different types of caging and evaluated the effect of using CS on mouse allergen levels. Ambient concentrations of mouse allergens in different areas of the facility were compared, and particular emphasis was placed on the allergen levels in the breathing zones of employees.
In the present study, we showed that ambient allergen levels in mouse holding rooms during routine animal care procedures reached a maximum of 48 ng/m3 whereas the maximum personnel exposure level was 215 ng/m3. Because cage changing is a highly repetitive task (1 to 2 operations per minute), the personnel exposure values, which integrate 2 h of measurements, are likely to incorporate peak exposure values. Although important for risk analysis, peak exposure levels are difficult to measure with the methods we used.
Similar or higher levels were detected in other studies of manual emptying of cages, cage changing on unventilated tables,28 or different cage types.23,25 In addition, EDC have been described to generate meaningful data for assessing exposure levels.20,31 In the present study, 14-d sampling of mouse rooms and connected corridors by using EDC yielded 55 ng per EDC tissue, which correlated to 1 ng/m3 as sampled over 12 h by using a stationary air pump. Although the correlation was not clearly linear in all instances, the EDC method accommodates the measurement of low allergen concentrations and the comparison of concentrations by using back-calculation. Recently, ion-charging devices have been used to quantify ambient allergen and endotoxin exposures in rooms holding mice in different cage types.23 Not only were the previous findings similar to ours, but the ion-charging device has also been shown to be a suitable tool for the measurement of low exposure rates, although the method is unsuitable for obtaining samples from the breathing zone of personnel. Whereas the reduction of allergen loads in procedure rooms and washrooms typically requires controlled discharge of exhaust air or structural alterations, specialized equipment in mouse-holding rooms appears to represent an effective means for decreasing allergen exposure. As shown in numerous studies6,8,9,11,18,21,22,24,25,27,32 IVC and CS play key roles in reducing allergen levels, despite the fact that some IVC show considerable leakage.11,24,25,27
Here we showed that ambient allergen concentrations in rooms with IVC with sealed lids under positive or negative pressure or IVC with nonsealed lids under negative pressure were about 400 times lower than those of rooms with open cages. Rooms with nonsealed IVC under positive pressure yielded exposure levels that were nearly as high as those of rooms with open cages, indicating that this IVC system does not readily reduce allergen levels. This finding was not unexpected because, according to the manufacturer, this system leaks about one third of the total air running through the system in positive-pressure mode. It is interesting to note that mouse holding rooms equipped with static microisolation caging showed allergen levels comparable to those of rooms with SL IVC. This result reflects the lack of a pressure differential in static microisolation cages. However, note that the microenvironment of static microisolation caging requires more frequent cage changing and therefore more occupational exposure than do SL IVC.
Several studies have shown that the use of negative-pressure IVC systems from various manufacturers is an effective method for reducing allergen exposure.11,24,27 However, for hygienic reasons (that is, to protect mice from airborne infections), running the IVC systems in positive-pressure mode is advisable. Therefore, nonsealed IVC systems may not be particularly suitable for allergen reduction when hygienic considerations require positive-pressure ventilation. This dilemma may be overcome by using IVC systems with sealed lids, which then could be run in either positive- or negative-pressure mode.
In our current study, the use of CS reduced personnel and ambient room allergen concentrations by a factor of about 10. This effect is suboptimal, taking into account the ability of modified class II stations to retain particles with very high efficiency. This result may be explained by the specific cage-changing procedures of this study, which included stacking soiled cages in the mouse room outside the CS, a procedure that liberates and disseminates particles into the room air from the bedding, the mice, or both. It is interesting to note the high variation of values from all cage types and conditions shown in Figure 4. We found strong interindividual differences. This feature is the so-called ‘technician effect.’ The same operation executed by different persons may yield large differences in the amount of allergen liberated and measured in the breathing zone. This finding supports the idea that the level of training of the technician can significantly affect exposure levels. Ideally, IVC are changed and stacked only within a CS, to avoid dissemination of particles into the room. Accordingly, completely assembled clean cages with bedding are autoclaved into a barrier and transported to the mouse rooms, and dirty cages are closed before they exit the CS to be transported to the washroom. However, although preferable for a number of reasons, this procedure requires about 3 to 4 times more autoclave capacity than does the one we used in this study.
Alternatively, stacking dirty cages within a CS and transporting only stacks of dirty cages represents a practicable option in facilities with limited autoclaving capacity. However, this procedure increases the risk of contaminating surfaces within the CS. Another option is using laminar air-flow cabinets, which provide a larger protected work area than do CS.
Comparison of allergen levels showed a maximum of only 10-fold lower levels in connecting corridors than in adjacent mouse holding rooms, indicating that allergen exposure follows the air pressure gradient from mouse rooms to corridors when doors are opened to permit movement of persons or materials. In addition, the doors in our facility are not air-tight and allow minimal airflow even when closed. Despite these relatively high allergen levels in corridors, allergen levels were below the detection limit in the office that was occupied by facility staff and in the remote room that had no association with animal use. These data point to the beneficial effects on allergen transmission of clothing changes and the use of air showers or wet shower sluices during entry into and exit from mouse holding areas.
In many facilities, used cages are emptied in the washroom by using containers, stationary bedding-disposal cabinets, or vacuum waste-disposal systems. Consequently, washrooms are likely to show higher allergen levels than those in the holding rooms of the facility.28 In the present study, emptying cages into a vacuum bedding-disposal system instead of using a waste container reduced personal exposures of washroom staff by a factor of about 25, to median values of 8 ng/m3. Therefore, the allergen exposure associated with these 2 tasks was similar to that measured in the holding rooms during cage changing on stainless-steel tables or by using a CS.
Although the threshold allergen concentration at which staff become sensitized is unknown currently, there seems to be a strong correlation between allergen concentration and the provocation of allergic symptoms. One group8 reported preliminary data showing that the risk of sensitization to MUA and the development of symptoms are increased at concentrations exceeding 5 ng/m3. A recent study22 showed that the relationship between mouse allergen exposure and mouse-specific immune responses may not be linear. A cohort of 179 newly employed mouse facility staff underwent repeated examinations of allergen exposure, measurement of mouse-specific IgG and development of positive skin-prick tests. The incidence of a positive skin-prick test increased with low (0.01 to 0.1 ng/m3) to moderate (1 ng/m3) levels of exposure but fell with exposure levels beyond 10 to 100 ng/m3. In addition, the more variable the exposure was between repeated tests, the lower was the incidence of positive skin-prick tests.22 The authors summarized their study by saying that stable moderate exposure was most strongly associated with allergic sensitization, whereas a pattern of variable high-level exposure was most strongly associated with an IgG4 response, which they regarded as protective.
In comparison to other mouse allergen exposure measurements based on sheep antibodies to Mus m 1,22,27 airborne MUA concentrations in our study were rather high. In most of the mouse rooms and connecting corridors, the median ambient exposure was in a range between 0.1 and 27ng/m3. These data indicate the need for measures to further reduce exposure, including reviewing of procedures, equipment, and facility design.3,5,10 Among the most productive measures would be to avoid stacking used cages outside the CS and to use nonsealed cages in positive-pressure mode.
Allergen levels associated with IVC in negative-pressure mode were below 1 ng/m3, confirming the protective effect of this caging system. Interestingly, the combined use of static microisolation caging and CS showed protective effects similar to those of IVC and indicate their use as an economical alternative to IVC systems. However, this economy should be balanced against potential suboptimal conditions for the animals.
In summary, housing laboratory mice in different types of cages resulted in different ambient allergen concentrations. The combined use of sealed IVC and cage-changing stations yielded the lowest allergen levels, indicating that the use of state-of-the-art caging and bedding-disposal equipment considerably reduces exposure to mouse allergens and contributes to reducing health risks of staff in laboratory animal facilities. A vacuum waste-disposal reduced allergen levels in washrooms. The transfer of allergens to nonmouse areas and offices can be minimized further by the strict use of air showers or wet shower sluices.
Acknowledgments
We appreciate the animal caretakers ‘cooperation in determining personal exposures. We thank Gert Doekes, Inge Wouters, and Dick Heederik (Institute of Risk Assessment Sciences, Utrecht, The Netherlands) for antibodies and standards for ELISA to assess mouse allergen concentrations. We gratefully acknowledge the financial support of Verwaltungs–Berufsgenossenschaft and the DGUV (German Social Accident Insurance) for the project IPA-92-Tierallergene and the support of Dr. Jens Petersen and Stefan Stegmaier from theVerwaltungs–Berufsgenossenschaft. We thank Jim Wallace (Science Associates) for critically reading and commenting on the manuscript.
References
- 1.Brielmeier M, Mahabir E, Needham JR, Lengger C, Wilhelm P, Schmidt J. 2006. Microbiological monitoring of laboratory mice and biocontainment in individually ventilated cages: a field study. Lab Anim 40:247–260 [DOI] [PubMed] [Google Scholar]
- 2.Bundesministerium fur Ernahrung, Landwirtschaft und Verbraucherschutz. [Internet]. 2011. Tierschutzbericht der Bundesregierung 2011/ Animal Welfare Report 2011, p 52–62. [Cited 20 October 2013]. Available at: http://www.bmelv.de/SharedDocs/Downloads/Broschueren/Tierschutzbericht_2011.pdf;jsessionid=4B31C0151C9D51F12C1F29247EEB8C16.2_cid367?__blob=publicationFile
- 3.Bush RK. 2001. Assessment and treatment of laboratory animal allergy. ILAR J 42:55–64 [DOI] [PubMed] [Google Scholar]
- 4.Bush RK. 2001. Mechanism and epidemiology of laboratory animal allergy. ILAR J 42:4–11 [DOI] [PubMed] [Google Scholar]
- 5.Bush RK, Stave GM. 2003. Laboratory animal allergy: an update. ILAR J 44:28–51 [DOI] [PubMed] [Google Scholar]
- 6.Clough G, Wallace J, Gamble MR, Merryweather ER, Bailey E. 1995. A positive, individually ventilated caging system: a local barrier system to protect both animals and personnel. Lab Anim 29:139–151 [DOI] [PubMed] [Google Scholar]
- 7.Corradi M, Ferdenzi E, Mutti A. 2012. The characteristics, treatment, and prevention of laboratory animal allergy. Lab Anim (NY) 42:26–33 [DOI] [PubMed] [Google Scholar]
- 8.Curtin-Brosnan J, Paigen B, Hagberg KA, Langley S, O'Neil EA, Krevans M, Eggleston PA, Matsui EC. 2010. Occupational mouse allergen exposure among non-mouse handlers. J Occup Environ Hyg 7:726–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon S, Fisher SW, Raymond RH. 2001. Elimination of mouse allergens in the working environment: assessment of individually ventilated cage systems and ventilated cabinets in the containment of mouse allergens. J Allergy Clin Immunol 108:288–294 [DOI] [PubMed] [Google Scholar]
- 10.Gordon S, Preece R. 2003. Prevention of laboratory animal allergy. Occup Med (Lond) 53:371–377 [DOI] [PubMed] [Google Scholar]
- 11.Gordon S, Wallace J, Cook A, Tee RD, Newman Taylor AJ. 1997. Reduction of exposure to laboratory animal allergens in the workplace. Clin Exp Allergy 27:744–751 [PubMed] [Google Scholar]
- 12.Harrison DJ. 2001. Controlling exposure to laboratory animal allergens. ILAR J 42:17–36 [DOI] [PubMed] [Google Scholar]
- 13.Hollander A, Van Run P, Spithoven J, Heederik D, Doekes G. 1997. Exposure of laboratory animal workers to airborne rat and mouse urinary allergens. Clin Exp Allergy 27:617–626 [PubMed] [Google Scholar]
- 14.Home Office Statistics. [Internet]. 2011. Statistics of Scientific Procedures on Living Animals Great Britain, p 8–13. [Cited 20 October 2013]. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/115853/spanimals11.pdf
- 15.Hunskaar S, Fosse RT. 1990. Allergy to laboratory mice and rats: a review of the pathophysiology, epidemiology, and clinical aspects. Lab Anim 24:358–374 [DOI] [PubMed] [Google Scholar]
- 16.Kallnik M, Elvert R, Ehrhardt N, Kissling D, Mahabir E, Welzl G, Faus-Kessler T, de Angelis MH, Wurst W, Schmidt J, Holter SM. 2007. Impact of IVC housing on emotionality and fear learning in male C3HeB/FeJ and C57BL/6J mice. Mamm Genome 18: 173–186 [DOI] [PubMed] [Google Scholar]
- 17.Kollmus H, Post R, Brielmeier M, Fernández J, Fuchs H, McKerlie C, Montoliu L, Otaegui P, Rebelo M, Riedesel H, Ruberte J, Sedlacek R, Habré d'Angelis M, Schughart K. 2012. Structural and functional concepts in current mouse phenotyping and archiving facilities. J Am Assoc Lab Anim Sci 51:418–435 [PMC free article] [PubMed] [Google Scholar]
- 18.Korpi A, Mantyjarvi R, Rautiainen J, Kaliste E, Kalliokoski P, Renstrom A, Pasanen AL. 2004. Detection of mouse and rat urinary aeroallergens with an improved ELISA. J Allergy Clin Immunol 113:677–682 [DOI] [PubMed] [Google Scholar]
- 19.Liebers V, van Kampen V, Bunger J, Duser M, Stubel H, Bruning T, Raulf-Heimsoth M. 2012. Assessment of airborne exposure to endotoxin and pyrogenic active dust using electrostatic dustfall collectors (EDCs). J Toxicol Environ Health A 75:501–507 [DOI] [PubMed] [Google Scholar]
- 20.Noss I, Wouters IM, Visser M, Heederik DJ, Thorne PS, Brunekreef B, Doekes G. 2008. Evaluation of a low-cost electrostatic dust-fall collector for indoor-air endotoxin exposure assessment. Appl Environ Microbiol 74:5621–5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pacheco KA, McCammon C, Thorne PS, O'Neill ME, Liu AH, Martyny JW, Vandyke M, Newman LS, Rose CS. 2006. Characterization of endotoxin and mouse allergen exposures in mouse facilities and research laboratories. Ann Occup Hyg 50:563–572 [DOI] [PubMed] [Google Scholar]
- 22.Peng RD, Paigen B, Eggleston PA, Hagberg KA, Krevans M, Curtin-Brosnan J, Benson C, Shreffler WG, Matsui EC. 2011. Both the variability and level of mouse allergen exposure influence the phenotype of the immune response in workers at a mouse facility. J Allergy Clin Immunol 128: 390.e7–396.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platts-Mills J, Custis N, Kenney A, Tsay A, Chapman M, Feldman S, Platts-Mills T. 2005. The effects of cage design on airborne allergens and endotoxin in animal rooms: high-volume measurements with an ion-charging device. Contemp Top Lab Anim Sci 44:12–16 [PubMed] [Google Scholar]
- 24.Reeb-Whitaker CK, Harrison DJ, Jones RB, Kacergis JB, Myers DD, Paigen B. 1999. Control strategies for aeroallergens in an animal facility. J Allergy Clin Immunol 103:139–146 [DOI] [PubMed] [Google Scholar]
- 25.Renstrom A, Bjoring G, Hoglund AU. 2001. Evaluation of individually ventilated cage systems for laboratory rodents: occupational health aspects. Lab Anim 35:42–50 [DOI] [PubMed] [Google Scholar]
- 26.Renstrom A, Gordon S. 2008. Assay of air sample eluates. Methods Mol Med 138:217–225 [DOI] [PubMed] [Google Scholar]
- 27.Schweitzer IB, Smith E, Harrison DJ, Myers DD, Eggleston PA, Stockwell JD, Paigen B, Smith AL. 2003. Reducing exposure to laboratory animal allergens. Comp Med 53:487–492 [PubMed] [Google Scholar]
- 28.Thulin H, Bjorkdahl M, Karlsson AS, Renstrom A. 2002. Reduction of exposure to laboratory animal allergens in a research laboratory. Ann Occup Hyg 46:61–68 [DOI] [PubMed] [Google Scholar]
- 29.Wolfle TL, Bush RK. 2001. The science and pervasiveness of laboratory animal allergy. ILAR J 42:1–3 [DOI] [PubMed] [Google Scholar]
- 30.Wood RA. 2001. Laboratory animal allergens. ILAR J 42:12–16 [DOI] [PubMed] [Google Scholar]
- 31.Zahradnik E, Sander I, Kendzia B, Fleischer C, Bruning T, Raulf-Heimsoth M. 2011. Passive airborne dust sampling to assess mite antigen exposure in farming environments. J Environ Monit 13:2638–2644 [DOI] [PubMed] [Google Scholar]
- 32.Ziemann B, Corn M, Ansari AA, Eggleston P. 1992. The effectiveness of the Duo-Flo BioClean unit for controlling airborne antigen levels. Am Ind Hyg Assoc J 53:138–145 [DOI] [PubMed] [Google Scholar]