Abstract
CO2 is one of the most commonly used euthanasia agents for laboratory animals. Considerable research has gone into the effect of the agent on animals, but little has been done to examine potential human exposure during these procedures. In this study, we examine the CO2 concentrations to which personnel are exposed while euthanizing rodents with CO2. To examine the environmental levels of CO2 generated during euthanasia, we examined several variables including flow rate, inclusion of a cage in the euthanasia chamber, inversion of the euthanasia chamber, chamber size, distance from the euthanasia chamber, and room size. Under all conditions, CO2 concentrations in the room temporarily increased significantly to 600 to 4000 ppm. The results of this study show that, under several testing scenarios, occupational levels of CO2 did not exceed governmentally mandated allowable exposure limits during routine rodent euthanasia procedures.
Abbreviations: STEL, short-term exposure limit; TWA, time-weighted average
CO2 is one of the most commonly used euthanasia agents in laboratory animal facilities. Euthanasia typically is performed by using a compressed gas cylinder to supply CO2 to a small chamber. Considerable research has gone into the CO2 effect on animals, but little has been done to examine the potential effects of human exposure. To protect workers exposed to CO2, several organizations have set CO2 exposure levels with which employers must comply. The Occupational Safety and Health Administration has set a permissible exposure limit,19 American Conference of Governmental Industrial Hygienists set a threshold limit value,2 and the National Institute of Safety and Health7 has a recommended exposure limit. Normal atmospheric CO2 is approximately 300 to 500 ppm. These 3 entities have all set the time-weighted average (TWA) for CO2 as 5000 ppm and short-term exposure limit (STEL) is 30,000 ppm. TWA is an average exposure over 8 h, and STEL is the maximal exposure, typically over a 15-min interval. The National Institute of Safety and Health considers 40,000 ppm to be immediately dangerous to life.16
Rooms dedicated for euthanasia typically are small, and in some institutions, dedicated personnel are responsible for overseeing all euthanasia activities. This is especially evident during rodent colony health surveillance, in which rodent CO2 euthanasia may involve several days. This situation potentially can place employees at risk for both exposures to STEL and TWA limits. To our knowledge, there are no reports of CO2 over-exposure incidents in animal care facilities. However, there are multiple incidents of CO2 mortality and morbidity in other settings, including a research laboratory.3,8 Events are commonly associated with CO2 gas-line leaks in restaurants and manufacturing facilities1 and pooling of CO2 during fermentation processes in basements or low areas. One case report involved 25 people at an ice factory who required hospitalization when the discharge valve of a truck containing liquid CO2 was knocked open in an enclosed environment.10 These events warn us that exposure to harmful and potentially lethal CO2 levels can occur.
The current study examined personnel CO2 exposure in an animal research facility during mock mice euthanasia procedures. We hypothesized that the CO2 room concentrations depend on gas flow rate, room size, room ventilation, chamber position, and chamber size. We also hypothesized that CO2 room concentrations remain below 5000 ppm.
Materials and Methods
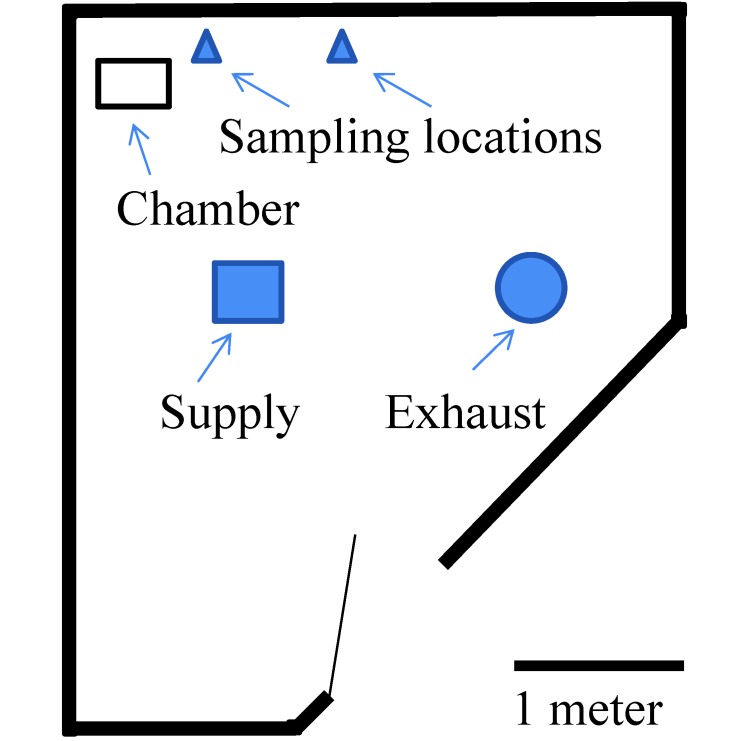
A dedicated room (30 m3; Figure 1) at Wright State University (Dayton, OH) was used for most experiments. The room had negative pressure to the corridor and 8.8 air changes hourly. The door to the room remained open during all procedures, as is standard practice at the facility. Informed consent was obtained from 2 people (AAA and SMDM), who were present in the room during all measurements, and both persons remained relatively inactive (standing or sitting in one location) while recording data, to minimize air disturbance. The CO2 concentrations in the room were measured every 15 s by using a Veloci Calc meter (model 9555-P, TSI, Shoreview, MN). The monitor was placed on a shelf 0.48 m above and 0.62 m away from a euthanasia chamber (1.42 m above the floor). Additional measurements were obtained at a shelf 1.52 m away from the chamber at the same height. The chamber was on a counter 0.94 m above the floor. A plastic tube connected to the CO2 tanks was inserted under the chamber lid.
Figure 1.
Schematic of the test room at Wright State University.
Preliminary testing.
Previous studies6,11,12,18 and a preliminary test of our system were used to determine the length of time for the CO2 gas to be turned on and reach a peak level. Several studies have shown that a CO2 displacement rate of 20%/min produces unconsciousness in rodents in about 106 s.6,11,12,18 The time to onset of unconsciousness increased to 156 s when the slower displacement rate of 10% CO2/min was used.5 The length of time to unconsciousness and death was examined to model real-time mouse euthanasia. The preliminary data were obtained from IACUC-approved protocols. Gas exposure times for euthanasia were determined according to these preliminary results (data not shown) and were consistent with previous reports.5,6,11,12,18
Testing procedure.
All trials were conducted without animals in the chamber. Tests were performed in a 22-L euthanasia chamber volume (44 cm × 23.5 cm × 21 cm) with different variables. The chamber had a removable lid that was removed (except during the lid-on experiment) after the CO2 tank was turned off. CO2 escaped the chamber through a 1-cm hole in the lid and emptied directly into the room. Prior to the initiation of a new trial, the chamber CO2 concentration was 500 ppm or lower, and room CO2 levels were 600 ppm or lower. Five replicates of each experimental condition were performed.
Two flow rates were analyzed, to model the most common euthanasia techniques. In the first paradigm, the CO2 gas cylinder was turned to a flow rate of 3 L/min (about a 15% fill rate of 100% CO2) for 7 min, thus replicating a continuous slow-fill rate. The second method (3 to 10 L/min) consisted of a flow rate of 3 L/min of 100% CO2 for 2 min; the rate then increased to 10 L/min (about a 50% fill rate) for 3 min to replicate a slow fill rate until unconsciousness of the mouse, followed by a fast fill rate. In both cases, the lid was removed 15 s after turning the gas off. The procedure was performed with the chamber empty or containing a 5.8-L cage (Allentown Caging, Allentown, NJ), to model direct placement of a mouse into the chamber compared with placement of a shoebox-style cage containing a mouse. When present, the cage was removed directly after lid removal.
Because CO2 is heavier than air, we considered that inverting the chamber might affect room CO2 levels. This additional condition was tested by repeating the previous experimental conditions. For the trials in which the chamber was turned over, the inversion was performed right after the lid was removed. The chamber then was placed so that 17.8 cm (40% of its length) extended over the counter edge in all trials involving the upside-down orientation.
To assess the effects of leaving the lid on after euthanasia, an experiment was performed in which the 3-L/min CO2 flow rate was used for 7 min and the lid was kept on for 20 min after the gas was turned off.
To evaluate the effect of chamber size on the room CO2, the 4 experimental conditions described were repeated with a 7.6-L chamber (32 cm × 19 cm × 12.5 cm; Anesthesia Chamber, Braintree Scientific, Braintree, MA). With this smaller chamber, both 1.3-L/min and 1.3-to-10–L/min flow rates were analyzed at distances of 0.62 m and 1.52 m. The lower flow rate was used to maintain consistency in the percentage fill rate between the 7.6- and 22-L chambers.
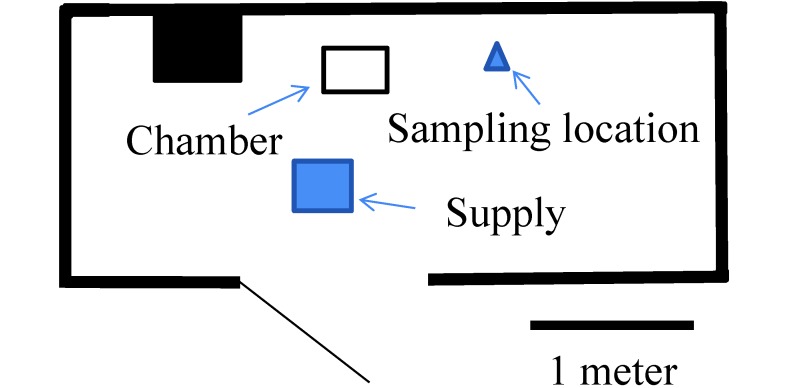
To evaluate the effect of room size, measurements were repeated by using the 22-L chamber at the Cincinnati Veterans Affairs Medical Center (Cincinnati, OH), where the room size was only 13.5 m3, with 13.1 air changes hourly and positive pressure to the hallway (Figure 2). The door to the procedure room remained open during testing, consistent with practice at this facility. We measured CO2 concentrations at a distance of 0.53 m from and 0.31 m above the chamber (0.94 m above the floor) with a flow rate of 3 L/min, without a cage in the chamber, and the chamber right side up.
Figure 2.
Schematic of the test room at Cincinnati Veterans Affairs Medical Center.
Statistical analysis.
In total, 22 combinations of the described variables were tested. Each combination was replicated 5 times. The averages of the 5 replicates are reported for the 22 groups. All statistical analyses were completed by using SAS software (version 9.3, SAS Institute, Cary, NC). Prior to interpretation of results, data were checked for normality and homogeneity of variance. Data that were in violation were transformed before additional testing. The only transformation required was one that squared the outcome values in the 3-way ANOVA for the 0.62 m distance. In one case for which transformations were not helpful (comparing the 2 distances for the 3-to-10–L/min rate with cage included and chamber right side up, for which variances were not equal), a Kruskal–Wallis test was used instead. The outcome variable for all analyses was peak CO2, and the means were compared by using 3-way ANOVA under different conditions involving cage presence, chamber position, and flow rate. t tests were used to compare the peak CO2 at 2 different distances from the source of CO2, at 2 locations, and with 2 chamber sizes. Significance was set at an α value of 0.05, and, where appropriate, the location of significant effects was determined by using a Tukey post hoc test or a Holm–Bonferroni correction. Data are presented as group means ± 1 SD.
Results
Peak CO2 levels were measured for 22 trials involving 6 variables (Table 1). The CO2 levels increased steadily in the room at both distances measured when the CO2 tank was turned on (Figure 3). The room CO2 levels reached peak values shortly after the gas was turned off and the lid was removed from the chamber. The decline in room CO2 back to baseline levels took less than 10 min. In the 5 trials examining the chamber with the lid on, CO2 levels peaked at 728 ppm on average and were slightly above ambient CO2 levels for 13 of the 27 monitored minutes.
Table 1.
Peak CO2 room concentrations (mean ± 1 SD, n = 5) during mock euthanasia procedures
| Room size (m3) | Distance (m) | Flow rate (L/min) | Cage present? | Chamber size (L) | Chamber position | CO2 concentration (ppm) |
| 30 | 0.62 | 3 | No | 22 | Up | 1114 ± 68 |
| 30 | 0.62 | 3–10 | No | 22 | Up | 1435 ± 61 |
| 30 | 0.62 | 3 | Yes | 22 | Up | 1059 ± 101 |
| 30 | 0.62 | 3–10 | Yes | 22 | Up | 1331 ± 32 |
| 30 | 0.62 | 3 | No | 22 | Up | 728 ± 38a |
| 30 | 0.62 | 3 | No | 22 | Down | 986 ± 42 |
| 30 | 0.62 | 3–10 | No | 22 | Down | 1461 ± 44 |
| 30 | 0.62 | 3 | Yes | 22 | Down | 1085 ± 59 |
| 30 | 0.62 | 3-10 | Yes | 22 | Down | 1486 ± 94 |
| 30 | 1.52 | 3 | No | 22 | Up | 2047 ± 306 |
| 30 | 1.52 | 3–10 | No | 22 | Up | 1956 ± 224 |
| 30 | 1.52 | 3 | Yes | 22 | Up | 1663 ± 229 |
| 30 | 1.52 | 3–10 | Yes | 22 | Up | 2429 ± 522 |
| 30 | 1.52 | 3 | No | 22 | Down | 1605 ± 228 |
| 30 | 1.52 | 3–10 | No | 22 | Down | 2277 ± 233 |
| 30 | 1.52 | 3 | Yes | 22 | Down | 1910 ± 157 |
| 30 | 1.52 | 3–10 | Yes | 22 | Down | 2440 ± 223 |
| 30 | 0.62 | 1.3 | No | 7.6 | Up | 657 ± 22 |
| 30 | 0.62 | 1.3–10 | No | 7.6 | Up | 1144 ± 133 |
| 30 | 1.52 | 1.3 | No | 7.6 | Up | 716 ± 56 |
| 30 | 1.52 | 1.3–10 | No | 7.6 | Up | 2638 ± 251 |
| 13.5 | 0.53 | 3 | No | 22 | Up | 3613 ± 226 |
Lid left on the chamber.
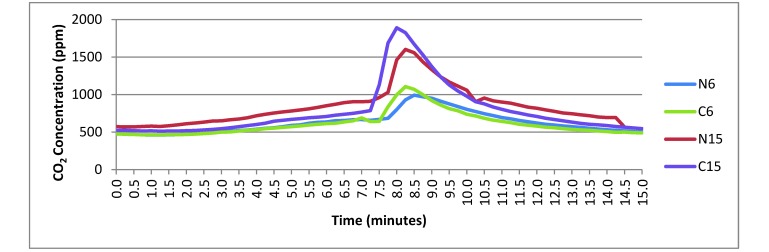
Figure 3.
Average room CO2 concentrations with a 3-L/min flow rate in a 22-L chamber turned upside down. The CO2 was turned off at 7 min and the lid removed 15 s later. N, no cage; C, cage in chamber; 6, 0.62 m; 15, 1.52 m.
Peak CO2 levels in the smaller euthanasia room (13.5 m3) with 3-L/min flow rate, chamber right side up, and measurements taken 0.53 m from the chamber averaged 3613 ± 226 ppm (Table 1). The peak value for similar conditions in the larger room (measurements taken at 0.62 m) was 1114 ± 68 ppm (P < 0.0001).
The highest mean peak CO2 concentration (2440 ppm) for the large chamber in the 30-m3 room occurred with the chamber turned upside down, a 3-to-10–L/min flow rate, a cage in the chamber, and measurement of the CO2 concentration at 1.52 m (Table 1). There was a significant (P = 0.0022) increase due to distance with all conditions at 1.52 m compared with 0.62 m. Increasing the flow rate and turning over the chamber (Figure 3) both resulted in significant (P < 0.0001 and P = 0.0436, respectively) increases in peak CO2 at 0.62 m. Having a cage in the chamber and removing it when the lid was taken off had no significant impact on CO2 levels compared with trials with no cage in the chamber (P = 0.4790). With the lid on, the chamber was tested at the 0.62-m distance only. There was a significant decrease in peak CO2 when the lid was left on (P < 0.0001). The highest mean peak with the small chamber in the 30-m3 room was 2638 ppm at 1.52 m with a 1.3-to-10–L/min flow rate. There were only mild increases in the room CO2 levels when the 1.3-L/min flow rate was used with the small chamber.
At 1.52 m, the 3-way interaction among flow rate, chamber, and cage was significant (P = 0.0092), but the significant differences were due only to flow rate. Increasing flow rate with a cage in the chamber positioned right side up increased peak CO2 compared with those at the lower flow rate (P = 0.0038). A similar increase was seen without a cage and the chamber turned upside down (P = 0.0152). The main effect for flow rate was significant (P < 0.0001).
The effect of chamber size on room CO2 levels was evaluated in the 30-m2 room by using both flow rates, chamber right side up, and no cage (Table 1). There was a significant increase in peak CO2 in the room when comparing the large chamber with the small chamber at low (3 L/min and 1.3 L/min, respectively) flow (P < 0.0001) and low-to-high (3 to 10 L/min and 1.3 to 10 L/min, respectively) flow (P < 0.0022) at 0.62 m and at low flow at 1.52 m (P = 0.0005). However, at 1.52 m with low-to-high flow, there was an increase in peak CO2 with the small chamber compared with the large chamber (P = 0.0019).
Discussion
In this study, we found that the peak CO2 concentrations, under all conditions, remained well below the OSHA threshold limit values: 5000 ppm for TWA and 30,000 ppm for STEL. TWA is an 8-h exposure and to reach this exposure level, the CO2 concentration would have to average more than 5000 ppm throughout the entire work day. In the current study, peak exposure levels averaged near 2000 ppm and reached 3690 ppm in one situation. The CO2 peaks were transitory, and even if personnel were exposed to the increased CO2 levels several times during a single day, they would still not approach TWA limits.
Exposures to increased CO2 levels can lead to a variety of symptoms (Figure 4). Although there are no limitations below the 5000 ppm for indoor CO2, the American Society of Heating, Refrigerating, and Air Conditioning Engineers suggests that indoor CO2 can be used as an indicator of odor levels, that 1000 to 1200 ppm CO2 is an indication of inadequate ventilation, and that odors often are associated with this CO2 level.17 Despite CO2 levels that remain below accepted exposure limits, there is concern that long-term exposure to CO2 in excess of 300 to 500 ppm may have a negative effect on people. This concern was raised in regard to classrooms with high student density, in which CO2 levels frequently were elevated above 1000 ppm and occasionally reached 3000 ppm.9 Classroom air quality had a significant effect on error rates in students taking a d2 test4 when CO2 increased from 1045 ppm to 2115 ppm, although no change in concentration performance was shown.21 A study examining cognitive ability in young adults showed that increasing room CO2 levels from 600 to 2500 ppm led to a significant decline in 7 of 9 decision-making performance parameters.20 At 600 ppm, subjects performed at average levels. However, at 2500 ppm CO2, subjects performed at dysfunctional levels for initiative and basic strategy and at marginal to dysfunctional levels for information usage and breadth approach. In another study, proofreading was significantly worse when subjects were exposed to 5000 ppm, with a marginally significant reduction at 3000 ppm compared with 600 ppm CO2.13 These experiments demonstrate that the CO2 levels in rooms with a euthanasia chamber could lead to cognitive impairment, even though CO2 levels are below the TWA of 5000 ppm and STEL of 30,000 ppm.
Figure 4.
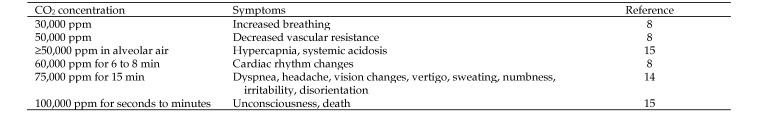
Symptoms associated with increasing CO2 concentrations.
We noted significant differences in room peak CO2 concentrations with alterations of flow rate, lid removal, chamber position, chamber size, room size, and distance from the chamber. Not surprisingly, there was no significant difference associated with inclusion of a cage in the chamber. Many of the alterations in room CO2 levels were expected. For example, increasing the flow rate of CO2 increased room CO2 levels, as anticipated. Similarly, gas release led to increased CO2 in the small room compared with the large room. The number of room-air changes were higher in the small room but likely were insufficient to reduce the acute elevations in room CO2 levels. A slight increase in room CO2 was observed when the chamber was inverted. In this orientation, the chamber was 40% off of the table and released more CO2 into the room, given that CO2 is heavier than air and therefore more easily escapes an inverted chamber, to contribute to overall room CO2 levels.
Surprisingly, CO2 peak levels were higher at 1.52 m than at 0.62 m from the euthanasia chamber. Room ventilation and air circulation patterns likely played a role in this finding, although this hypothesis was not investigated further. Another surprising result was the increased level of CO2 at 1.52 m for the 1.3-to-10–L/min flow rate in the small chamber compared with the 3-to-10–L/min flow rate in the large chamber. The CO2 peak in the room occurred earlier when the small chamber was used instead of the large chamber. These results may be due to differences in how the lid was removed (slid off for the small chamber compared with lifted off for the large chamber).
There were a few limitations in our study. First, the room door was left open. Closing the door may reduce air changes, and higher levels of CO2 may occur. In addition, there were 2 people in the room who contributed to the total CO2 of the room. However, we believe this situation had little effect on overall results, given that room CO2 concentrations returned to baseline levels prior to subsequent tests in all trials. A third deficiency was the absence of animals in the chamber. We do not believe that the CO2 produced by animals in the euthanasia chamber would significantly contribute to room levels. Chamber leakage was a factor, because placement of the interior chamber meter for a companion study prevented the lid from closing completely. This feature likely had little effect because there are already holes in the chamber to allow gas escape, and peak CO2 levels occurred after the lid was removed. In addition, we did not measure the CO2 concentrations at floor level. Therefore, whether subsequent trials were affected by an overall accumulation of CO2 is unknown. However, because CO2 did not exceed 5000 ppm, this accumulation likely had minimal effect on the study. Finally, differences in ventilation patterns and air replacement rates between the 2 rooms could have influenced the observed CO2 levels, so that direct comparisons are limited. We did not evaluate optimal placement of the CO2 meter in the room to detect the highest CO2 levels possible but rather selected distances at which personnel commonly stand relative to the chamber. We considered these distances to most accurately reflect the human exposure potential.
Despite these minor limitations, we believe our data are a fair representation of the CO2 exposure of personnel involved with euthanasia procedures using compressed gas cylinders. Note that the results of this study are specific to the room sizes, frequency of euthanasia procedures, and variables tested; results in other facilities may be different. It is important that personnel who deal with potentially lethal agents, such as CO2, are monitored closely to ensure their safety. According to our findings, the current practice of CO2 euthanasia of rodents by using a chamber is safe in regard to regulatory standards. However, we caution that published reports suggest that even the moderately increased CO2 levels present in our experiments could alter human cognitive ability. Additional research in this area may clarify risks involved with chronic exposure to moderately increased CO2 levels.
Acknowledgments
This research was supported in part by the Wright State University STREAMS program (NIH grant no. 5R25HL103168-02).
References
- 1. ABC7 KGO-TV San Francisco CA. [Internet]. 2013. Over 70 workers fall ill at Vallejo food company. [Cited 6 September 2013]. Available at: http://abclocal.go.com/kgo/story?section=news/local/north_bay&id=8602169.
- 2.American Conference of Governmental Industrial Hygienists. 2012. Threshold limit values for chemical substances and physical agents and biological exposure indices. Cincinnati (OH): American Conference of Governmental Industrial Hygienists [Google Scholar]
- 3.Analox Sensor Technology. [Internet]. 2013. Carbon dioxide incidence reports. [Cited 6 September 2013]. Available at: http://www.analox.net/incidents.php
- 4.Bates ME, Lemay EP., Jr 2004. The d2 test of attention: construct validity and extensions in scoring techniques. J Int Neuropsychol Soc 10:392–400 [DOI] [PubMed] [Google Scholar]
- 5.Burkholder TH, Niel L, Weed JL, Brinster LR, Bacher JD, Foltz CJ. 2010. Comparison of carbon dioxide and argon euthanasia: effects on behavior, heart rate, and respiratory lesions in rats. J Am Assoc Lab Anim Sci 49:448–453 [PMC free article] [PubMed] [Google Scholar]
- 6.Danneman PJ, Stein S, Walshaw SO. 1997. Humane and practical implications of using carbon dioxide mixed with oxygen for anesthesia or euthanasia of rats. Lab Anim Sci 47:376–385 [PubMed] [Google Scholar]
- 7.Department of Health and Human Services. 2006. NIOSH pocket guide to chemical hazards (Pub. No. 2005-149). Cincinnati (OH): NIOSH publications dissemination [Google Scholar]
- 8.Gill JR, Ely SF, Hau Z. 2002. Environmental gas displacement: 3 accidental deaths in the workplace. Am J Forensic Med Pathol 23:26–30 [DOI] [PubMed] [Google Scholar]
- 9.Godwin C, Batterman S. 2007. Indoor air quality in Michigan schools. Indoor Air 17:109–121 [DOI] [PubMed] [Google Scholar]
- 10.Halpern P, Raskin Y, Sorkine P, Oganezov A. 2004. Exposure to extremely high concentrations of carbon dioxide: a clinical description of a mass casualty incident. Ann Emerg Med 43: 196–199 [DOI] [PubMed] [Google Scholar]
- 11.Hewett TA, Kovacs MS, Artwohl JE, Bennett BT. 1993. A comparison of euthanasia methods in rats, using carbon dioxide in prefilled and fixed flow-rate filled chambers. Lab Anim Sci 43:579–582 [PubMed] [Google Scholar]
- 12.Hornett TD, Haynes AP. 1984. Comparison of carbon dioxide–air mixture and nitrogen–air mixture for the euthanasia of rodents: design of a system for inhalation euthanasia. Anim Technol 35:93–99 [Google Scholar]
- 13.Kajtar L, Herczeg I, Lang E. 2003. Examination of influence of CO2 concentration by scientific methods in the laboratory, p 176–181. In: Proceedings of healthy buildings 2003, 7–11 December. Singapore: Stallion Press. [Google Scholar]
- 14.Kaye J, Buchanan F, Kendrick A. 2004. Acute carbon dioxide exposure in healthy adults: evaluation of a novel means of investigating the stress response. J Neuroendocrinol 16:256–264 [DOI] [PubMed] [Google Scholar]
- 15.Laferty EA, McKay RT. 2006. Physiologic effects and measurements of carbon dioxide and oxygen levels during qualitative respirator-fit testing. J Chem Health Saf 13:22–28 [Google Scholar]
- 16.National Institute for Occupational Safety and Health. 1992. Criteria for a recommended standard occupational exposure to carbon dioxide (pub. no. 92-100). Cincinnati, OH: NIOSH (DHHS) [Google Scholar]
- 17.National Institute for Occupational Safety and Health. 2010. ASHRAE standard: ventilation for acceptable indoor air quality. ANSI/ASHRAE standard 62.1-2010. ISSN 1041-2336. Indoor environmental quality: building ventilation. Appendix C p 37–38
- 18.Niel L, Weary DM. 2006. Behavioural responses of rats to gradual-fill carbon dioxide euthanasia and reduced oxygen concentrations. Appl Anim Behav Sci 100:295–308 [Google Scholar]
- 19.Occupational Health and Safety Standards—Labor. [Internet]. 2002. Air contaminants. CFR. Title 29. Part 1910.1000. [Cited 6 September 2013]. Available at: http://www.gpo.gov/fdsys/granule/CFR-2011-title29-vol6/CFR-2011-title29-vol6-sec1910-1000/content-detail.html
- 20.Satish U, Mendell MJ, Shekhar K, Hootchi T, Sullivan D, Streufert S, Fisk WJ. 2012. Is CO2 an indoor pollutant? Direct effects of low-to-moderate CO2 concentrations on human decision-making performance. Environ Health Perspect 120:1671–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Twardella D, Matzen W, Lahrz T, Burghardt R, Spegel H, Hendrowarsito L, Frenzel AC, Fromme H. 2012. Effect of classroom air quality on students’ concentration: results of a cluster-randomized cross-over experimental study. Indoor Air 22:378–387 [DOI] [PubMed] [Google Scholar]