Abstract
We examined the efficacy of enrofloxacin administered by 2 different routes in a mouse model of sepsis. Male CD1 mice were infected with a bioluminescent strain of enteropathogenic Escherichia coli and treated with enrofloxacin either by injection or in drinking water. Peak serum levels were evaluated by using HPLC. Mice were monitored for signs of clinical disease, and infections were monitored by using bioluminescence imaging. Serum levels of enrofloxacin and the active metabolite ciprofloxacin were greater in the group treated by injection than in controls or the groups treated by administration in drinking water. Survival of the group treated with enrofloxacin injection was greater than that of controls and groups treated with enrofloxacin in the drinking water. Bioluminescence in the group treated with enrofloxacin injection was less than that in the groups treated with oral administration at 12 h and in the groups treated orally and the control group at 16 h. According to these findings, we recommend the use of injectable enrofloxacin at 5 mg/kg SC for mice with systemic infections.
Abbreviation: MIC, minimal inhibitory concentration
Sepsis is defined as the systemic inflammatory response to the presence of bacterial infection.6 It affects approximately 750,000 people annually in the United States, with a mortality rate of 30% to 50% and a financial burden of US$16.7 billion dollars.1 Septic shock is persistent hypotention with hypoperfusion abnormalities or organ dysfunction secondary to sepsis and kills 10 times more people than myocardial infarction in the United States.7,38 Bacterial isolates from patients with gram-negative infections most commonly include Escherichia coli, Klebsiella species, or Enterobacter species.6 Due to the widespread and severe nature of this disease, this is an active area of scientific research.14,17
Enrofloxacin is a frequently used antibiotic approved for use in dogs, cats, cattle, and pigs in the United States. It is a fluoroquinolone antibiotic that leads to bacterial cell death through the inhibition of topoisomerase II, which controls supercoiling of bacterial DNA.33 The bactericidal effect is dependent on the concentration of antibiotic present in tissues,35 both of the parent compound and of the active metabolite ciprofloxacin.16 Enrofloxacin has been studied in a wide range of laboratory animal species including bovines,16,26,27 dogs,5,36 frogs,20,25 horses,41 macaques,4 marmosets,44 mice,22,32,34,36,40,49 rabbits,19 rats,8 sheep,15 and swine.15,37,52 Published doses in veterinary formularies range from 2.5 to 20 mg/kg for enteral and parenteral bolus dosing and for rodents at 0.05 to 0.2 mg/mL in drinking water.11,45,46 In laboratory animal medicine, fluoroquinolone antibiotics are sometimes administered as a therapeutic agent to populations of animals. One example of this practice is for eradication of an opportunistic pathogen such as Pneumocysitis carinii in genetically modified mice.32 In addition, fluroquinolones are used on a large scale prophylactically in research protocols involving bone marrow transplantation in mice.39 When large groups of animals require treatment, the route of administration can affect the personnel time required to conduct the research.
Bioluminesence imaging is a noninvasive imaging modality that has been used to study cell trafficking, tumor development, gene expression, gene therapy, inflammation and infection, protein–protein interactions, and protein stability and function in laboratory rodents.13 Bioluminescent imaging systems use specialized charge-coupled cameras to capture low amounts of light. Organisms can be genetically engineered to express firefly luciferase or other types of luciferase enzymes, which emit light when the organisms are provided an exogenous substrate, such as luciferin, in the presence of ATP and oxygen.29 In addition, specialized bacteria have been engineered to carry genes for both the luciferase and the substrate, a long-chain fatty aldehyde, and thus do not require an exogenous source of substrate.47 These types of modified bacteria have been used to study the progression and treatment of bacterial infections of the gastrointestinal tract,10,21 wounds,23,47 and surgical implants.30,51,53 Advantages of bioluminescence imaging include the ability to assess the spatial and temporal distribution of bacteria after intervention in the same animal, thus reducing animal numbers.
The purpose of the current study was to refine a model of sepsis by evaluating the efficacy of different routes of administration of prophylactic antibiotics. This work was part of a larger study evaluating the pathogenesis of hospital-acquired infection in patients receiving blood transfusions during concurrent antibiotic therapy. With an effective model system, research results can be translated to treatment of human diseases. Serial noninvasive optical imaging of a bioluminescently engineered strain of enteropathogenic Escherichia coli was used as a biomarker of bacterial infection in the presence of mitigating drug regimens. We hypothesized that there would be no difference in the severity of infection, evaluated as photons per second emitted from a region of interest over the abdomen, between animals that received enrofloxacin via different routes of administration in this model of sepsis. Our goal was to establish an effective method of preventing or treating gram-negative sepsis through antibiotic administration that minimized animal handling and manipulation, promoted animal wellbeing, and maximized the information obtained from the animals used in our research.
Materials and Methods
Animals.
Male CD1 mice (weight, 24 to 25 g) were purchased from Charles River Laboratories (Wilmington, MA). Animals were housed at a maximum of 5 per cage on autoclaved corncob bedding (Alpha-dri–Cob Blend, Shepherd Specialty Papers, WF Fisher and Son, Somerville, NJ) in static polysulfone microisolation cages. Enrichment was provided in the form of social housing and cotton nesting pads (Nestlets, Ancare, Bellmore, NY). Mice had ad libitum access to irradiated feed (Purina Lab Diet 5053, PMI, St Louis, MO) and water treated by reverse osmosis. Mice were free of Sendai virus, pneumonia virus of mice, mouse hepatitis virus, minute virus of mice, mouse parvovirus, Theiler mouse encephalomyelitis virus, reovirus type 3, epizoodic diarrhea of infant mice virus, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, mouse adenovirus, ectromelia virus, K virus, polyomavirus, and endo- and ectoparasites. Mice were maintained in accordance with the Guide for the Care and Use of Laboratory Animals28 in an AAALAC-accredited facility. All procedures were approved by the Columbia University IACUC and followed applicable governmental policies and regulations.
Drug treatments and sampling.
We randomly assigned each of 20 mice to one of 4 experimental groups. Three groups of mice received antibiotic treatment with enrofloxacin (Baytril, 22.7 mg/mL, Bayer, Pittsburgh, PA). Two of the treatment groups (n = 5 mice each) received enrofloxacin orally in drinking water at different concentrations: 0.05 mg/mL (low dose) and 0.1 mg/mL (high dose). One group (n = 5 mice) received a subcutaneous dose of enrofloxacin at 5 mg/kg diluted in 0.2 mL saline. The control group (n = 5 mice) received 0.2 mL saline subcutaneously. All mice received bottles of drinking water treated by reverse osmosis. Enrofloxacin and saline injections were given at 24, 14, and 2 h prior to blood collection (time 0, 1000). Medicated water was supplied 24 h prior to blood collection. Mice were euthanized by cervical dislocation under isoflurane anesthesia. Blood was collected by cardiocentesis post mortem. Serum was separated and stored at −80 °C until analysis. Concentrations of enrofloxacin and its active metabolite ciprofloxacin were evaluated by HPLC as previously described,16,37 except for slight modification to accommodate the small sample volume used for this analysis (150 µL). The assays for enrofloxacin and ciprofloxacin have been validated previously and published for other species by our laboratory,16,37 and a partial validation was performed for this current study in mice. Solid-phase extraction of enrofloxacin and ciprofloxacin was performed by using Oasis HLB (1 mL) extraction cartridges (Waters, Milford, MA) for plasma samples, followed by reverse-phase chromatography with fluoresence detection at an excitation wavelength of 280 nm and an emission wavelength of 500 nm. The mobile phase consisted of 80% water, 20% acetonitrile, 0.2% trifluoroacetic acid, and 0.1% triethylamine. The injection volume for serum samples was 50 μL. The limit of quantitation was 0.01 µg/mL for both enrofloxacin and ciprofloxacin. Drug concentrations were determined from calibration curves made from fortified blank serum collected from mice prior to drug administration. Enrofloxacin reference standard was supplied by Bayer, and ciprofloxacin reference standard was obtained from the United States Pharmacopeia (Rockville, MD). Our laboratory used published guidelines for method validation.50
Bacteria.
A bioluminescently engineered strain of enteropathogenic Escherichia coli (strain Xen14, parent strain EPEC WS2572 containing a stable, chromosomally integrated luxCDABE cassette from Photorhabdus luminescense) was used (PerkinElmer, Waltham, MA). Bacteria from a frozen glycerol stock were grown in lysogeny broth3 at 37 °C with shaking at 200 rpm until midlog phase (approximately 4 to 5 h). The bacteria were harvested, washed twice with sterile PBS, and enumerated by optical density measurements to determine concentration (cfu/mL) and verify midlog phase harvest. A sample of the grown bacteria was submitted to a commercial diagnostic laboratory (Antech Diagnostics, Lake Success, NY) for confirmatory culture and susceptibility testing.
Experimental infection.
We randomly allocated each of 60 mice to one of the 4 groups described earlier: enrofloxacin in drinking water at 0.05 mg/mL and at 0.1 mg/mL (14 mice each), enrofloxacin administered by subcutaneous injection at 5 mg/kg in 0.2 mL saline (16 mice), and a 0.2-mL saline injection control group (16 mice). Medicated water was supplied 24 h prior to infection and throughout the study. Enrofloxacin injections were administered at 24, 14, and 2 h prior to infection and at 12 h after infection (time 0, 1200). All mice received a single intraperitoneal injection of 1 × 108 cfu of Escherichia coli Xen14 at time 0. Bacteria were diluted in 0.2 mL sterile saline. Mice were euthanized by cervical dislocation under isoflurane anesthesia at 24 h, or earlier when observed to be moribund (weak, dehydrated, unable to right themselves) by a blinded observer (ARS). The remaining mice were euthanized by the same method immediately after the final imaging session, at 24 h after infection.
Imaging.
Mice were anesthetized with isoflurane in oxygen (2% to 5%) and underwent imaging in dorsal recumbency every 4 to 8 h by using an in vivo imaging system (IVIS Spectrum, PerkinElmer). Images were evaluated with Living Image Software (PerkinElmer). A region of interest was established over the abdomen that extended from the pelvis to the xyphoid. Bioluminescence of the region was measured as flux (photons/s).
Statistical analysis.
All statistical analysis was conducted by using Prism 6 (GraphPad Software, La Jolla, CA). Drug levels were analyzed by one-way ANOVA with Tukey posttests. Survival was analyzed by using a log-rank Mantel–Cox test. Bioluminesence was analyzed using one-way ANOVA or a Kruskall–Wallis test with Bonferroni or Dunn posttests as deemed appropriate by a KS normality test.
Results
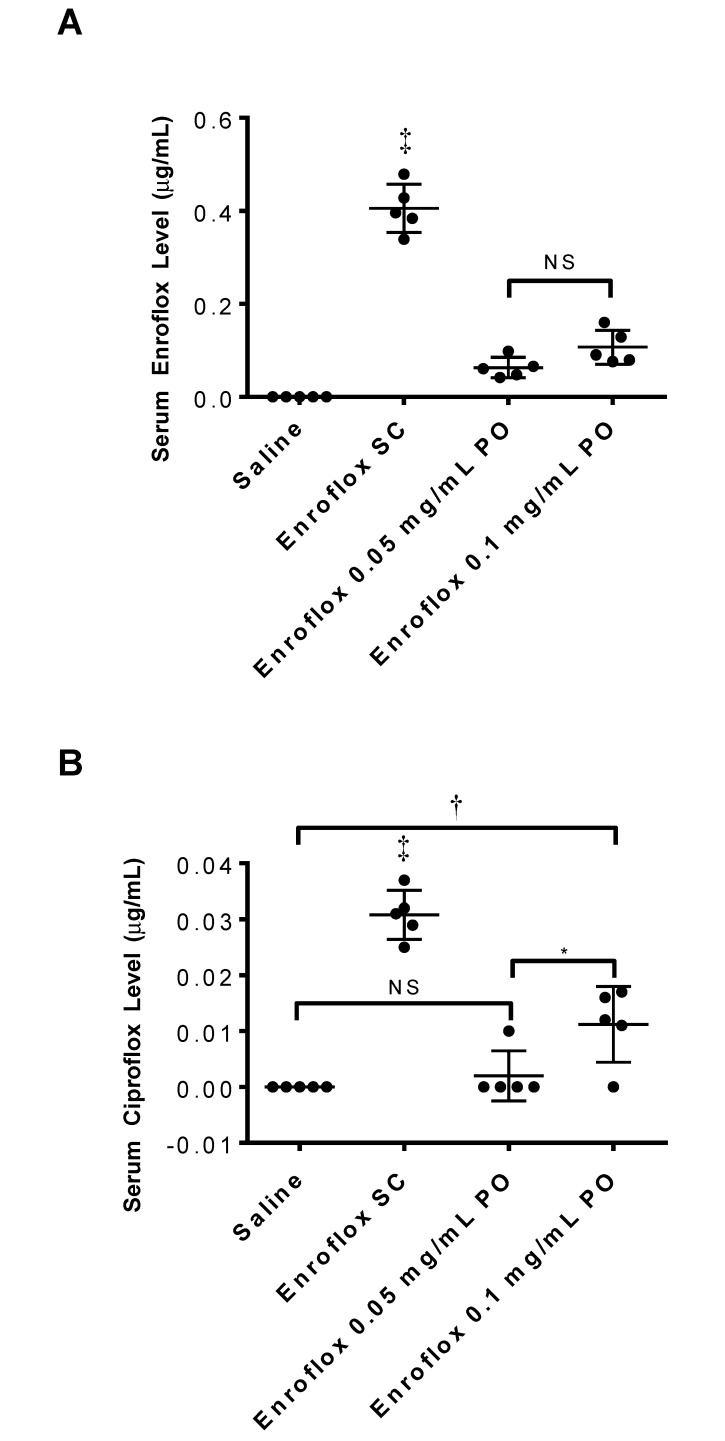
The serum enrofloxacin concentrations in the group that received subcutaneous injections of enrofloxacin (5 mg/kg) at 2 h after administration was more than 4 times greater than that of mice that received oral enrofloxacin (0.05 mg/mL or 0.1 mg/mL) at 24 h after the addition of the drug to the drinking water (P < 0.0001 for both comparisons; Figure 1 A). The enrofloxacin serum concentration (mean ± 1 SD) was 0.405 ± 0.052, 0.063 ± 0.022, and 0.107 ± 0.036 μg/mL in the groups receiving the subcutaneous, low oral, and high oral doses of enrofloxacin, respectively. There was no enrofloxacin detected in the saline control group (<0.01 μg/mL). The difference in enrofloxacin levels was significant (P < 0.01) for all groups when compared with the saline-injected control animals. There was no significant difference between the 2 groups that received oral doses.
Figure 1.
Serum (A) enrofloxacin and (B) ciprofloxacin levels in mice 2 h after the last injection or 24 h after addition of antibiotic to drinking water. (n = 5 for each group). *, P < 0.05; †, P < 0.01; ‡, P < 0.001.
The serum ciprofloxacin concentrations in the group that received subcutaneous injections of enrofloxacin (5 mg/kg) were greater than those of all other groups (P < 0.001 for all comparisons; Figure 1 B). The serum ciprofloxacin concentration (mean ± 1 SD) was 0.031 ± 0.004, less than 0.01 μg/mL, and 0.011 ± 0.007 μg/mL in the groups receiving the subcutaneous, low oral, and high oral doses of enrofloxacin, respectively, and less than 0.01 μg/mL in the saline control group (P < 0.01 for all groups compared with the saline control group). There was no difference in ciprofloxacin concentrations between the groups receiving low-dose oral enrofloxacin or saline. However, the high-dose oral enrofloxacin group did have higher ciprofloxacin levels (P < 0.01) than did saline control mice.
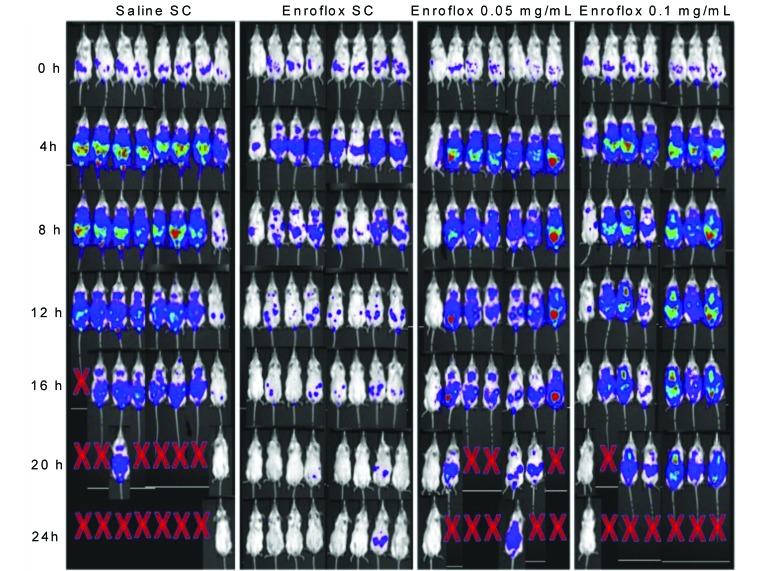
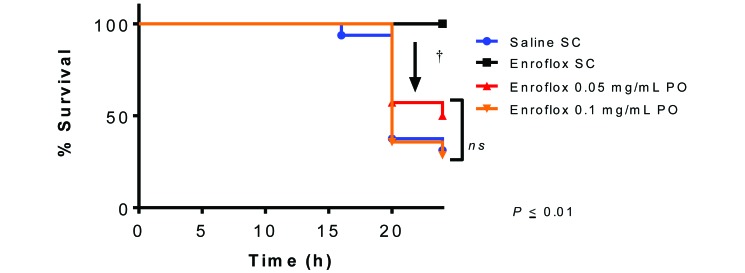
A representative 24-h time sequence of bioluminescence images of antibiotic-treated mice challenged with 1 × 108 cfu E. coli Xen 14 by intraperitoneal injection is shown (Figure 2). All mice treated subcutaneously with enrofloxacin survived for 24 h after infection, the end of the study. In contrast, the median survival of the saline, low-dose oral enrofloxacin, and high-dose oral enrofloxacin groups was 20 h, 12 h, and 20 h, respectively (Figure 3). Survival of the group that received injectable enrofloxacin was greater (P < 0.01, log-rank Mantel–Cox analysis) than that of all other groups. Survival did not differ between the 2 groups that received oral doses of enrofloxacin and the saline control group.
Figure 2.
Representative in vivo images of antibiotic treated mice obtained for 24 h after intraperitoneal bacterial challenge with 1 × 108 CFU E. coli Xen 14.
Figure 3.
Survival curve of antibiotic treated mice challenged with E. coli Xen 14. (n = 16 each for saline control group and enrofloxacin 5 mg/kg SQ, n = 14 each for enrofloxacin in drinking water at 0.05 and 0.1 mg/mL). Survival of group treated with 5 mg/kg SQ is significantly greater (†, P < 0.01) than all other groups.
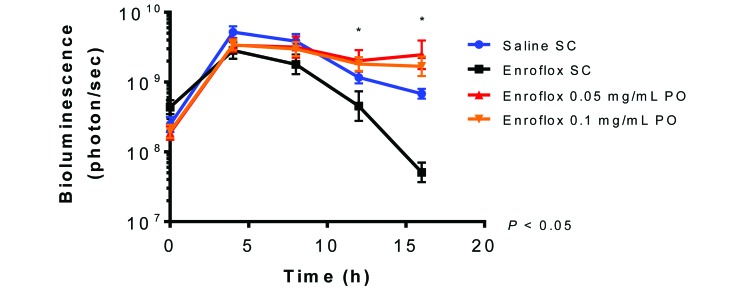
Differences in bioluminescence were noted between groups at 12 and 16 h after infection (Figure 4). Mice that received subcutaneous injections of enrofloxacin had less bioluminescent flux (mean ± 1 SD; P < 0.05) than did animals in both the low-dose and high-dose oral enrofloxacin groups (4.525 × 108 ± 1.150 × 109, 2.019 × 109 ± 3.189 × 109, and 1.815 × 109 ± 1.704 × 109 photons/s, respectively). At 16 h after infection, mice in the group that received subcutaneous enrofloxacin had less (P < 0.05) bioluminescent flux than did the low-dose and high-dose oral enrofloxacin and the saline control groups (5.078 × 107 ± 5.476 × 107, 2.468 × 109 ± 3.936 × 109, 1.682 × 109 ± 1.697 × 109, and 6.831 × 108 ± 3.145 × 108 photons/s, respectively). The bioluminescence levels of the 2 oral dosage groups and the saline control group were not significantly different from each other.
Figure 4.
Bioluminescence of region of interest (mean ± 1 SD) of antibiotic-treated mice challenged with E. coli Xen 14. (n = 16 each for saline control group and enrofloxacin 5 mg/kg SC; n = 14 each for enrofloxacin in drinking water at 0.05 and 0.1 mg/mL). Bioluminesence of group treated with 5 mg/kg SC is significantly lower than both groups dosed in drinking water at 12 h postinfection (ϕ, P < 0.05) and to both groups dosed in water as well as the saline control group at 16 h postinfection (*, P < 0.05).
The reference laboratory culture of the bacteria verified the bacteria as a strain of Escherichia coli susceptible to enrofloxacin, with a MIC of 0.5 μg/mL or less.
Discussion
Antibiotics are used by laboratory animal veterinarians for both clinical and research purposes. Cost and ease of administration are crucial when large numbers of rodents require treatment. Administration of medication in drinking water can save hours of personnel time and can minimize rodents’ pain and stress associated with handling and injections. However, appropriate drug levels are paramount for clinical efficacy and research integrity.
The results of this study suggest that subcutaneous dosing of enrofloxacin is more effective at reducing bacterial burden than are regimens based on oral dosing through the drinking water. Animals that received enrofloxacin subcutaneously survived longer and exhibited less bacteria-associated bioluminescence at 12 and 16 h after infection than did the other groups, indicating greater efficacy through the subcutaneous route of administration. Bioluminescence was not compared beyond 16 h because after that time point, the mice that had demonstrated the strongest bioluminescent flux had to be euthanized due to clinical signs of severe sepsis. A key advantage to the use of bioluminescence imaging for this study was the ability to obtain quantifiable data and statistical significance without the need for death as an experimental endpoint. Unfortunately, because the small number of animals in this study, we were unable to correlate a specific cut-off point for bioluminescence that was predictive of morbidity. Future studies of infection models using bioluminescence imaging potentially could refine endpoints and allow for euthanasia of animals before clinical illness is observed.
The bactericidal activity of fluroquinolones is correlated to the ratio of AUC24:MIC.2,31 Dosing schemes that provide for a high peak concentration relative to the MIC (Cmax:MIC or AUC:MIC) are preferable for all fluoroquinolones to maximize the bactericidal effect and minimize the selection of drug-resistant bacteria.18,42 In our current experiment, the mean serum concentrations of enrofloxacin and ciprofloxacin at the time of bacterial administration was significantly higher in mice given enrofloxacin subcutaneously than in animals that received the drug in drinking water, even though the group that received 0.1 mg/mL in drinking water theoretically received a dose that was 50% higher than that of the group dosed subcutaneously. The doses of 0.05 and 0.1 mg/mL in drinking water correlate to a doses of approximately 7.5 and 15 mg/kg daily, respectively, according to an estimated daily water consumption of 1.5 mL/10 g.24 By contrast, the enrofloxacin dose from subcutaneous administration was 10 mg/kg daily. The serum enrofloxacin concentrations that we found were higher than other published results in mice. One study found an average concentration of less than 0.1 µg/mL at 2 h after subcutaneous administration of 10 mg/kg enrofloxacin.40 This concentration is approximately 4 times lower than that we detected in the current study in the group that received 5 mg/kg SC. The cited study did not detect the metabolite ciprofloxacin.40 The differences between studies may reflect an improved sensitivity of our assay.
Fluoroquinolones are absorbed from both the gastrointestinal tract and parenteral injection sites and subsequently are distributed well in body tissues.9 However, oral absorption can be affected by the physiology of the gastrointestinal tract.42 Oral absorption is low in horses compared with other species, and oral absorption in ruminants is affected by the volume of the GI tract.42 Therefore poor absorption from the mouse GI tract may be one of the factors that affected the serum concentration in the current study. We suspect that another important factor that may explain the low concentrations of enrofloxacin observed in our mice that received the oral dose was poor consumption of the treated water. Enrofloxacin has a bitter taste12. In addition, the injectable formulation, which was added to the drinking water, is very alkaline, to maintain solubility. These factors may have made the drinking water unpalatable to the mice. This suspicion is supported by a lack of significant difference in enrofloxacin serum concentration between the low- and high-dose oral regimens. Because an increasing dose of enrofloxacin in the drinking water is associated with an increasingly bitter taste, mice given the higher dose of enrofloxacin likely drank less water than did the other groups. Furthermore, once the mice were infected, water consumption in all groups likely decreased due to the systemic inflammatory response. It is also possible that the mice drank water at a time point that would produce a peak level at a different time than the subcutaneously dosed group. Rodents consume more water during the night than during daytime hours; therefore enrofloxacin levels in the groups that received the oral dose likely fluctuated depending on the time of day.48 One study reported the terminal half-life of enrofloxacin in mice to be 0.81 h.40 Perhaps the timing of sampling (approximately 3 h after lights on) did not coincide with the peak serum concentration. We were unable to quantify water consumption in this study due to technical difficulties with socially housed animals and spillage secondary to moving the cages for dosing and imaging. In addition, we cannot rule out poor solubility of the drug in the drinking water as a possible reason for low concentrations. Enrofloxacin has poor aqueous solubility and might precipitate after being added to the drinking water. For this reason, we elected not to use crushed tablets for our oral dosing regimen.
The doses of enrofloxacin that we used reflected current recommendations in commonly used veterinary formularies. Several historic papers report the prolonged use of much higher doses, from 25 to 85 mg/kg daily in drinking water for colony-wide treatment of Pasteurella pneumotropica.22,32,34,49 However, because enrofloxacin serum concentrations did not differ between the 2 oral-treatment groups, it is unlikely that even higher doses of enrofloxacin would have been beneficial in this setting. Some sources report masking the taste of enrofloxacin with sweeteners.43 We elected to exclude this method of delivery, because alteration of glucose metabolism could have affected our sepsis model. Future studies evaluating higher doses of enrofloxacin administered in drinking water and by bolus gavage and including multiple time points would be useful to better understand the pharmacokinetics of this drug. In addition, future studies could incorporate the use of artificial sweeteners to enhance palatability.
Finally, another alternative hypothesis for the decreased effectiveness of oral dosing is that enrofloxacin and other fluoroquinolone antibiotics are degraded when exposed to light.43 We used clear water bottles in this study, but UV exposure is an unlikely cause of the reduced drug levels, because the bottles were covered by an opaque microisolation top and positioned on a solid metal rack without direct exposure to light.
Our results reflect experiments using a single strain and sex of mouse, male CD1 mice, and a single strain of Escherichia coli, Xen14. The male CD1 mouse model was selected because it has a normal immune system, eliminated the variable of estrus, and fit with the needs of the larger experimental study. The Xen14 strain was selected because it is similar to enteropathogenic Escherichia coli strains seen in human infections and because it expresses the genes necessary for bioluminescence imaging. Future studies exploring the route of administration of enrofloxacin in other mouse strains, in female animals, or to treat infections with other organisms are needed to be able to generalize the findings.
According to the dramatic results we obtained by using in vivo imaging, we recommend the use of injectable enrofloxacin at 5 mg/kg SC rather than administration in drinking water at 0.05 or 0.1 mg/mL for animals at risk for or with known sepsis. If antibiotics must be administered through drinking water, a drug that is dependent on time above the MIC may be a more prudent choice.
Acknowledgments
We thank Steven Spitalnik for his support and guidance.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 2.Aucoin D. 2007. Target: the antimicrobial reference guide to effective treatment, 3rd ed. Port Huron (MI): North American Compendiums. [Google Scholar]
- 3.Barbier M, Damron FH, Bielecki P, Suárez-Diez M, Puchałka J, Albertí S, Martins dos Santos V, Goldberg JB. 2014. From the environment to the host: re-wiring of the transcriptome of Pseudomonas aeruginosa from 22° C to 37° C. PloS One 9:e89941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black-Schultz L, Coatney RW, Warnick CL, Swif B. 1997. Lack of reactivation of shigellosis in naturally infected enrofloxacin-treated cynomolgus monkeys after exogenous immunosuppression. Lab Anim Sci 47:602–605 [PubMed] [Google Scholar]
- 5.Blondeau JM, Borsos S, Blondeau L, Blondeau B. 2012. In vitro killing of Escherichia coli, Staphylococcus pseudintermedius and Pseudomonas aeruginosa by enrofloxacin in combination with its active metabolite ciprofloxacin using clinically relevant drug concentrations in the dog and cat. Vet Microbiol 155:284–290 [DOI] [PubMed] [Google Scholar]
- 6.Bone RC. 1993. Gram-negative sepsis: a dilemma of modern medicine. Clin Microbiol Rev 6:57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein R, Sibbald WJ. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655 [DOI] [PubMed] [Google Scholar]
- 8.Bregante MA, Saez P, Aramayona J, Fraile L, Garcia M, Solans C. 1999. Comparative pharmacokinetics of enrofloxacin in mice, rats, rabbits, sheep, and cows. Am J Vet Res 60:1111–1116. [PubMed] [Google Scholar]
- 9.Brown SA. 1996. Fluoroquinolones in animal health. J Vet Pharmacol Ther 19:1–14 [DOI] [PubMed] [Google Scholar]
- 10.Burns-Guydish SM, Olomu IN, Zhao H, Wong RJ, Stevenson DK, Contag CH. 2005. Monitoring age-related susceptibility of young mice to oral Salmonella enterica serovar Typhimurium infection using an in vivo murine model. Pediatr Res 58:153–158 [DOI] [PubMed] [Google Scholar]
- 11.Carpenter JW. 2013. Exotic animal formulary, 4th ed. St Louis (MO): Elsevier Saunders. [Google Scholar]
- 12.Chun MK, Choi HK. 2004. Preparation and characterization of enrofloxacin–carbopol complex in aqueous solution. Arch Pharm Res 27:670–675 [DOI] [PubMed] [Google Scholar]
- 13.Close DM, Xu T, Sayler GS, Ripp S. 2011. In vivo bioluminescent imaging (BLI): noninvasive visualization and interrogation of biological processes in living animals. Sensors (Basel) 11:180–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J. 2002. The immunopathogenesis of sepsis. Nature 420:885–891 [DOI] [PubMed] [Google Scholar]
- 15.Cox SK, Cottrell M, Smith L, Papich M, Frazier D, Bartges J. 2004. Allometric analysis of ciprofloxacin and enrofloxacin pharmacokinetics across species. J Vet Pharmacol Ther 27:139–146 [DOI] [PubMed] [Google Scholar]
- 16.Davis JL, Foster D, Papich M. 2007. Pharmacokinetics and tissue distribution of enrofloxacin and its active metabolite ciprofloxacin in calves. J Vet Pharmacol Ther 30:564–571 [DOI] [PubMed] [Google Scholar]
- 17.Deitch EA. 1998. Animal models of sepsis and shock: a review and lessons learned. Shock 9:1–11 [DOI] [PubMed] [Google Scholar]
- 18.Dudley MN. 1991. Pharmacodynamics and pharmacokinetics of antibiotics with special reference to the fluoroquinolones. Am J Med 91:S45–S50 [DOI] [PubMed] [Google Scholar]
- 19.Elmas M, Yazar E, Uney K, Tras B. 2008. Pharmacokinetics of enrofloxacin and flunixin meglumine and interactions between both drugs after intravenous co-administration in healthy and endotoxaemic rabbits. Vet J 177:418–424 [DOI] [PubMed] [Google Scholar]
- 20.Felt S, Papich MG, Howard A, Long T, McKeon G, Torreilles S, Green S. 2013. Tissue distribution of enrofloxacin in African clawed frogs (Xenopus laevis) after intramuscular and subcutaneous administration. J Am Assoc Lab Anim Sci 52:186–188 [PMC free article] [PubMed] [Google Scholar]
- 21.Foucault ML, Thomas L, Goussard S, Branchini B, Grillot-Courvalin C. 2010. In vivo bioluminescence imaging for the study of intestinal colonization by Escherichia coli in mice. Appl Environ Microbiol 76:264–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goelz MF, Thigpen JE, Mahler J, Rogers WP, Locklear J, Weigler BJ, Forsythe DB. 1996. Efficacy of various therapeutic regimens in eliminating Pasteurella pneumotropica from the mouse. Lab Anim Sci 46:280–285. [PubMed] [Google Scholar]
- 23.Hamblin MR, O'Donnell DA, Murthy N, Contag CH, Hasan T. 2002. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem Photobiol 75:51–57 [DOI] [PubMed] [Google Scholar]
- 24.Harkness JE, Wagner JE. 1989. The biology and medicine of rabbits and rodents, 3rd ed. Philadelphia (PA): Lea and Febiger. [Google Scholar]
- 25.Howard AM, Papich MG, Felt SA, Long CT, McKeon GP, Bond ES, Torreilles SL, Luong RH, Green SL. 2010. The pharmacokinetics of enrofloxacin in adult African clawed frogs (Xenopus laevis). J Am Assoc Lab Anim Sci 49:800–804. [PMC free article] [PubMed] [Google Scholar]
- 26.Idowu O, Peggins J, Cullison R, Bredow JV. 2010. Comparative pharmacokinetics of enrofloxacin and ciprofloxacin in lactating dairy cows and beef steers following intravenous administration of enrofloxacin. Res Vet Sci 89:230–235 [DOI] [PubMed] [Google Scholar]
- 27.Idowu OR, Peggins JO. 2004. Simple, rapid determination of enrofloxacin and ciprofloxacin in bovine milk and plasma by high-performance liquid chromatography with fluorescence detection. J Pharm Biomed Anal 35:143–153 [DOI] [PubMed] [Google Scholar]
- 28.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press [Google Scholar]
- 29.Kadurugamuwa JL, Francis KP. 2008. Bioluminescent imaging of bacterial biofilm infections in vivo. Methods Mol Biol 431:225–239. [DOI] [PubMed] [Google Scholar]
- 30.Kadurugamuwa JL, Sin LV, Yu J, Francis KP, Kimura R, Purchio T, Contag PR. 2003. Rapid direct method for monitoring antibiotics in a mouse model of bacterial biofilm infection. Antimicrob Agents Chemother 47:3130–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu P, Müller M, Derendorf H. 2002. Rational dosing of antibiotics: the use of plasma concentrations versus tissue concentrations. Int J Antimicrob Agents 19:285–290 [DOI] [PubMed] [Google Scholar]
- 32.Macy JD, Weir EC, Compton SR, Shlomchik MJ, Brownstein DG. 2000. Dual infection with Pneumocystis carinii and Pasteurella pneumotropica in B cell-deficient mice: diagnosis and therapy. Comp Med 50:49–55 [PubMed] [Google Scholar]
- 33.Maddison JE, Page SW, Church DB. 2008. Small animal clinical pharmacology, 2nd ed. Philadelphia (PA): Saunders. [Google Scholar]
- 34.Matsumiya LC, Lavoie C. 2003. An outbreak of Pasteurella pneumotropica in genetically modified mice: treatment and elimination. Contemp Top Lab Anim Sc 42:26–28 [PubMed] [Google Scholar]
- 35.McKellar QA, Sanchez Bruni S, Jones D. 2004. Pharmacokinetic–pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. J Vet Pharmacol Ther 27:503–514 [DOI] [PubMed] [Google Scholar]
- 36.Meinen JB, McClure J, Rosin E. 1995. Pharmacokinetics of enrofloxacin in clinically normal dogs and mice and drug pharmacodynamics in neutropenic mice with Escherichia coli and staphylococcal infections. Am J Vet Res 56:1219–1224. [PubMed] [Google Scholar]
- 37.Messenger KM, Papich MG, Blikslager A. 2012. Distribution of enrofloxacin and its active metabolite, using an in vivo ultrafiltration sampling technique after the injection of enrofloxacin to pigs. J Vet Pharmacol Ther 35:452–459 [DOI] [PubMed] [Google Scholar]
- 38.Moore LJ, Moore FA, Todd SR, Jones SL, Turner KL, Bass BL. 2010. Sepsis in general surgery: the 2005–2007 national surgical quality improvement program perspective. Arch Surg 145:695–700. [DOI] [PubMed] [Google Scholar]
- 39.Nunamaker EA, Artwohl JE, Anderson RJ, Fortman JD. 2013. Endpoint refinement for total body irradiation of C57BL/6 mice. Comp Med 63:22–28 [PMC free article] [PubMed] [Google Scholar]
- 40.Ogino T, Arai T. 2007. Pharmacokinetic interactions of flunixin meglumine and enrofloxacin in ICR mice. Exp Anim 56:79–84 [DOI] [PubMed] [Google Scholar]
- 41.Papich MG, Van Camp S, Cole J, Whitacre M. 2002. Pharmacokinetics and endometrial tissue concentrations of enrofloxacin and the metabolite ciprofloxacin after IV administration of enrofloxacin to mares. J Vet Pharmacol Ther 25:343–350 [DOI] [PubMed] [Google Scholar]
- 42.Papich MG, Riviere JE. 2009. Fluoroquinolone antimicrobial drugs, p 983–1012. In: Riviere J, Papich MG editors. Veterinary pharmacology and therapeutics. Ames (IA): Wiley–Blackwell Publishing. [Google Scholar]
- 43.Petritz OA, Guzman DS-M, Wiebe VJ, Papich MG. 2013. Stability of 3 commonly compounded extemporaneous enrofloxacin suspensions for oral administration to exotic animals. J Am Vet Med Assoc 243:85–90 [DOI] [PubMed] [Google Scholar]
- 44.Pisharath HR, Cooper TK, Brice AK, Cianciolo RE, Pistorio AL, Wachtman LM, Mankowski JL, Newcomer CE. 2005. Septicemia and peritonitis in a colony of common marmosets (Callithrix jacchus) secondary to Klebsiella pneumoniae infection. Contemp Top Lab Anim Sc 44:35–37 [PubMed] [Google Scholar]
- 45.Plumb DC. 2011. Plumb's veterinary drug handbook, 7th ed. Ames (IA): Wiley-Blackwell [Google Scholar]
- 46.Quesenberry K, Carpenter JW. 2011. Ferrets, rabbits, and rodents: clinical medicine and surgery, 3rd ed. Philadelphia (PA): Saunders. [Google Scholar]
- 47.Rocchetta HL, Boylan C, Foley J, Iversen P, LeTourneau D, McMillian C, Contag P, Jenkins D, Parr T. 2001. Validation of a noninvasive, real-time imaging technology using bioluminescent Escherichia coli in the neutropenic mouse thigh model of infection. Antimicrob Agents Chemother 45:129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rowland NE. 2007. Food or fluid restriction in common laboratory animals: balancing welfare considerations with scientific inquiry. Comp Med 57:149–160 [PubMed] [Google Scholar]
- 49.Ueno Y, Shimizu R, Nozu R, Takahashi S, Yamamoto M, Sugiyama F, Takakura A, Itoh T, Yagami K-i. 2002. Elimination of Pasteurella pneumotropica from a contaminated mouse colony by oral administration of enrofloxacin. Exp Anim 51:401–405 [DOI] [PubMed] [Google Scholar]
- 50.United States Pharmacopeial Convention. 2014. The United States Pharmacoepia 37. Rockville (MD): US Pharmacopeia [Google Scholar]
- 51.Vuong C, Kocianova S, Yu J, Kadurugamuwa JL, Otto M. 2008. Development of real-time in vivo imaging of device-related Staphylococcus epidermidis infection in mice and influence of animal immune status on susceptibility to infection. J Infect Dis 198:258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiuff C, Lykkesfeldt J, Aarestrup FM, Svendsen O. 2002. Distribution of enrofloxacin in intestinal tissue and contents of healthy pigs after oral and intramuscular administrations. J Vet Pharmacol Ther 25:335–342 [DOI] [PubMed] [Google Scholar]
- 53.Xiong YQ, Willard J, Kadurugamuwa JL, Yu J, Francis KP, Bayer AS. 2005. Real-time in vivo bioluminescent imaging for evaluating the efficacy of antibiotics in a rat Staphylococcus aureus endocarditis model. Antimicrob Agents Chemother 49:380–387 [DOI] [PMC free article] [PubMed] [Google Scholar]