Abstract
Effective pain medication is important for animal stewardship and valid research results. We compared the pharmacokinetic assessments of standard, immediate-release buprenorphine (Bup IR) and a sustained-release buprenorphine formulation (Bup SR Lab) in male C57BL/6J mice, a mouse strain commonly used in biomedical research. We postulated that the administration of Bup SR Lab would achieve a more persistent blood drug concentration (>1 ng/mL) compared with single-dose Bup IR. The study assumed a blood buprenorphine concentration of 1 ng/mL as the minimum that may result in adequate analgesia, as previously reported. The 7 experimental groups included Bup IR (0.03, 0.05, 0.1, and 2 mg/kg), Bup SR Lab (0.3 and 1.2 mg/kg), and saline placebo (0.7 mL/100 g). Blood sampling occurred at 0.5, 1, 3, 6, 12, 24, 48, and 72 h for evaluation by using a forensic ELISA. Bup IR at 0.03 and 0.05 mg/kg and Bup SR Lab at 0.3 mg/kg failed to obtain maximal blood concentrations (Cmax) above 1 ng/mL. All other doses (0.1 and 2 mg/kg Bup IR and 1.2 mg/kg Bup SR Lab) reached a Cmax above 1 ng/mL within 3 h after injection. In addition, 1.2 mg/kg Bup SR Lab and 2 mg/kg Bup IR provided blood concentrations above 1 ng/mL for up to 12 h, and 0.1 mg/kg Bup IR achieved this criterion for as long as 3 h. In conclusion, Bup SR Lab at 1.2 mg/kg and Bup IR at 0.1 or 2.0 mg/kg achieve or surpass the published threshold for adequate analgesia in mice.
Abbreviations: Bup IR, immediate-release buprenorphine; Bup SR Lab, sustained-released buprenorphine; Cmax, maximal observed blood concentration; TThE, time to therapeutic effectiveness threshold; TLAST, time at last quantifiable blood concentration; Tmax, time to Cmax
Mice are the most widely used research animal, and providing them with adequate veterinary care during research studies is a vital component of implementing the basic tenets of ethical animal stewardship and of complying with animal welfare regulations and recommendations. Fundamental to this care is recognizing, preventing, assessing, and managing clinical pain.2,13,15 Uncontrolled pain and distress can negatively affect animals’ quality of life and adversely influence research results.15 The use of an appropriate and effective pain medication is an important tool that is often used to minimize these effects.
In the clinical setting, buprenorphine, a synthetic opiate classified as a partial μ agonist and κ antagonist, is a common systemic analgesic administered to rodents.5,7,8,11,21-23 Compared with other opioids, buprenorphine produces full analgesic effects, reduces respiratory depression, and minimally affects immune responses.5,7,18,21,25 However, previously reported buprenorphine dose ranges and administration frequencies for different mice strains vary widely, making it a challenge to determine appropriate and effective analgesic dosages.8,10,12,22 Recommended doses range from 0.05 to 0.1 mg/kg and frequencies from 1 to 8 doses daily.8,12,16 The wide variations in daily administration recommendations may lead to fluctuations in bloodstream drug concentration and inconsistent analgesic control.17
In an effort to provide consistent analgesia, sustained-release formulations of buprenorphine, including injectable forms and transdermal patches, have been developed and subsequently evaluated in mice and rats.1,9,20,26 A United States veterinary compounding pharmacy has developed an injectable sustained-release buprenorphine that provides as much as 72 h of continuous analgesia in several species after a single injection.3,9 This single injection in species other than mice results in a sustained plasma concentration over 1.0 ng/mL, the concentration associated with providing pain relief in humans, for as long as 72 h.3,4,9,11,19,24 However, a recent study in BALB/cJ and SWR/J mice specifically investigated the efficacy of a sustained-release buprenorphine formulation and of buprenorphine HCl by using thermal-contact response time methodology (hotplate test).1 The authors concluded that the sustained-release formulation at 1.0 mg/kg provided an effective analgesic period of 12 h and that the clinical buprenorphine dose of 0.1 mg/kg provided little to no analgesic effect.1 Comparing these recent findings with vendor-provided literature may lead to uncertainty regarding the appropriate dose of buprenorphine to achieve analgesia and the response of different mouse strains to similar dose–drug combinations. These findings also suggest that buprenorphine pharmacokinetics, in multidose and sustained-release formulations, are not clearly understood. Therefore, additional study is warranted to elucidate buprenorphine dosages appropriate for use in the clinical research environment.
In this study, we postulated that a single dose of sustained-release buprenorphine would achieve more persistent blood drug concentration (>1 ng/mL) in mice than would a single dose of the typically used immediate-release buprenorphine (Bup IR). To determine whether these expected differences were significant, we used a pharmacokinetic study to evaluate the performance of standard Bup IR and of a recently available sustained-released buprenorphine (Bup SR Lab). The pharmacokinetic study examined the relationship between drug dose and the transient postinjection blood concentration of buprenorphine. Using a forensic ELISA method, we determined the time course of blood drug concentration after administration.14
Materials and Methods
We obtained 120 male C57BL/6J mice (25 to 30 g) from Jackson Laboratory (Bar Harbor, ME). All mice were free of contagious ectoparasites, endoparasites, helminthes, 18 murine viruses, and 11 bacteria and mycoplasma prior to shipping. Animal housing consisted of ventilated microisolation racks (Lab Products with Enviro-Gard-B, Seaford, DE) on hardwood bedding (Harlan, Fredrick, MD), with paper nesting material provided for enrichment. Mice were group-housed initially and then transferred to single housing 2 d prior to testing. Mice were given food (NIH 31 Autoclaved Rodent Diet, Harlan) and tap water ad libitum and were maintained on a 12:12-h light:dark cycle. All mice were ear-tagged with a unique ID number. The IACUC of the National Heart, Lung, and Blood Institute, an AAALAC-accredited animal care program, reviewed and approved this protocol.
Drugs and routes of administration.
We evaluated 2 formulations of buprenorphine, Bup IR (0.3 mg/mL stock, Buprenex, Reckitt Benchiser Pharmaceuticals, Richmond, VA) and Bup SR Lab (1.0 mg/mL stock, Buprenorphine SR Lab ZooPharm, Windsor, CO). In addition, an equal volume of sterile saline for injection was used in the placebo groups. All injections were administered subcutaneously in the right flank.
Experimental design.
We designed the pharmacokinetic study as a blinded, placebo-controlled, randomized trial using 7 experimental treatment groups. Drug treatment groups were differentiated according to placebo, drug formulation, and dosage levels. Dose selection for Bup IR (0.03, 0.05, 0.1 and 2 mg/kg) was based on published data.3,22,23 The 2 Bup SR Lab dose levels were selected according to information in the supplier's insert (0.3-mg/kg dose) and published data from rats (1.2-mg/kg dose).9 Placebo dose (0.7 mL/100 g sterile saline) was selected to be equal in volume to high-dose Bup IR.10
Treatment groups representing commonly used clinical doses or those previously studied (Bup IR intermediate dose, 0.1 mg/kg; Bup SR Lab low-dose, 0.3 mg/kg; and Bup SR Lab high-dose, 1.2 mg/kg) each comprised 24 randomly selected mice. The remaining groups each included 12 randomly selected mice. Because Bup SR Lab cannot be diluted, all injections for all Bup SR Lab doses and those for low and intermediate Bup IR doses were completed without dilution by using precision syringes (model 1705, Gastight 50-µL syringe, Hamilton, Reno, NV) fitted with 23-gauge needles. High Bup IR and placebo doses were injected by using 1.0-mL tuberculin syringes and 23-gauge needles. In addition, due to the inability to dilute Bup SR Lab, the injected drug volume could not be controlled between groups.
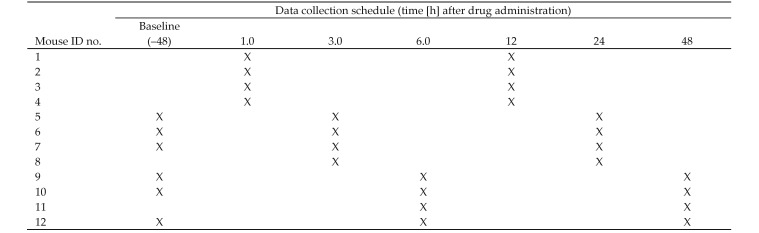
Randomly presented mice from the experimental groups were sampled at 6 or 8 postinjection time points during the test period (Figures 1 and 2). The 8-sampling time regime was used earlier in the study but a refinement to the test periods occurred when the placebo and additional Bup IR doses were introduced, that is when the 0.5- and 72-h time points were eliminated. This change allowed an additional mouse to be sampled at each of the 6 most relevant time points.
Figure 1.
Notional representation of randomly presented test group sampled at 8 time points after injection.
Figure 2.
Notional representation of randomly presented test group, sampled at 6 time points after injection.
To ensure reproducible administration of drug and reduce stress, each mouse was placed in an anesthesia chamber (Impac6, VetEquip, Pleasanton, CA) and anesthetized briefly (less than 2 min) by using isoflurane prior to injection. Mice each received a single subcutaneous injection in the right flank. The dose was calculated by using the test group's average mouse weight. For each test group, after drug injection, randomly preselected mice were subjected to submandibular blood sampling at the assigned time interval. This testing sequence was repeated until all test groups and therefore all treatment groups were injected and sampled.
On each test day, every mouse received a physical exam, with special attention to the presence of skin lesions, submandibular swelling and other abnormal clinical signs. Any abnormal findings were noted and followed longitudinally. These mice were submitted for a complete necropsy, and lesions were evaluated histologically after their last tests or sampling event.
Quantification of buprenorphine concentration.
A buprenorphine ELISA kit (no. 131919, Neogen, Lexington, KY) was used to determine the drug concentration in 20-µL blood samples.6 The drug concentration reflects the free base of buprenorphine. A 5-point standard response curve was developed by using buprenorphine standards of 0.0, 0.5, 1.0, 1.5, 3.0 ng/mL (SigmaPlot, Systat Software, San Jose, CA).14 This ELISA and standard response curve was used to study Bup IR pharmacokinetics in NMRI male mice.14 A lower limit of quantitation (0.33 ng/mL) was selected in light of instructions from the manufacturer of the ELISA kit. The samples’ absorbance readings were obtained by using a microplate reader (Rainbow ELISA reader, Tecan SLT Laboratory Instruments, Crailsheim, Germany) at a wavelength of 450 nm in accordance with the manufacturer's instructions.
Data analysis.
A noncompartmental pharmacokinetic analysis (WinNonlin, Pharmsight Products, St Louis, MO) was completed for blood buprenorphine concentration as determined by ELISA. This analysis calculated the AUC, AUC SE, peak blood buprenorphine concentration (Cmax), and time to reach Cmax (Tmax). Blood concentration was normalized by subtracting the observed placebo blood buprenorphine concentration at each time point for each treatment group. A P value of 0.05 was used to define statistical significance.
Results
Pharmacokinetics.
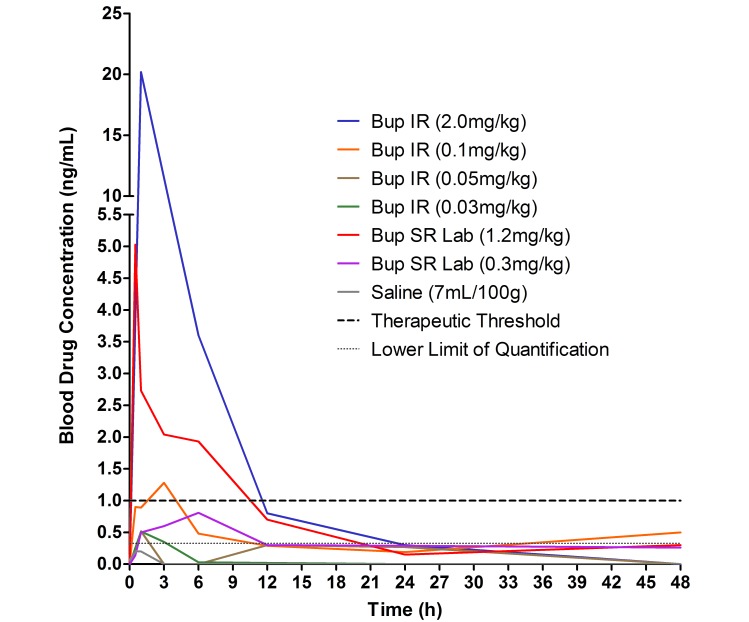
The blood buprenorphine concentration determined by using the forensic ELISA varied across all drug types and dosage levels. For the pharmacokinetics analysis, a noncompartmentalized approach was used. The last data collection time point at which the blood buprenorphine concentration exceeded the lower limit of quantification (TLAST) ranged from 1 to 24 h for all Bup IR and Bup SR Lab doses, with both the 2.0-mg/kg Bup IR and 1.2-mg/kg Bup SR doses yielding TLAST of 24 h (Figure 3). The data collection time period associated with therapeutic effectiveness threshold (TThE), assumed to be 1 ng/mL, indicated that Bup IR (0.1 and 2.0 mg/kg) doses ranged from 3 to 6 h, whereas Bup SR Lab (1.2 mg/kg) yielded therapeutic levels at 12 h (Figure 3).
Figure 3.
This graph plots the buprenorphine concentration in blood of different buprenorphine formulations over 48 h. Horizontal dashed lines represent therapeutic threshold (1 ng/mL) and lower limit of quantitation (0.33 ng/mL). Values below the lower limit of quantification are extrapolated values.
The results of the noncompartmental pharmacokinetic study are shown in Table 1. The Bup IR doses of 0.03 to 0.1 mg/kg were dose-to-AUC–proportional, but this proportionality was not observed for the 2-mg/kg dose. The low (0.3 mg/kg) and high (1.2 mg/kg) doses of Bup SR Lab failed to show the same proportionality. The time for Bup IR doses to reach Tmax ranged from 1 to 3 h; the Tmax data for Bup Lab SR ranged from 0.5 to 6 h. The 0.1-mg/kg dose of Bup IR yielded a Cmax of 1.28 ng/mL, which exceeded the TTThE of 1 ng/mL. The 2.0-mg/kg dose of Bup IR achieved a blood concentration of 20.2 ng/mL, the highest Cmax observed during the study. The 1.2-mg/kg dose of Bup Lab SR had the second highest observed Cmax value (5.03 ng/mL).
Table 1.
Pharmacokinetic data for Bup IR and Bup SR Lab
| Parameter | Unit | Bup IR | Bup SR Lab | ||||
| Dose | mg/kg | 0.03 | 0.05 | 0.1 | 2 | 0.3 | 1.2 |
| AUClast | h × ng/mL | 2.2 ± 0.6 | 5.6 ± 2.7 | 19.1 ± 5.0 | 82.0 ± 18.2 | 20.1 ± 5.7 | 62.9 ± 11.6 |
| Tmax | h | 1 | 1 | 3 | 1 | 6 | 0.5 |
| Cmax | ng/mL | 0.5 ± 0.4 | 0.5 ± 0.4 | 1.3 ± 1.4 | 20.2 ± 11.9 | 0.8 ± 0.7 | 5.0 ± 2.3 |
Adverse effects.
As previously reported, several mice developed skin lesions after subcutaneous injection of Bup Lab SR.1 The lesions were in close proximity to the injection sites and were consistent grossly and histologically with ulcerative skin lesions ranging from a mild ulcerative dermatitis to full-thickness necrosis with concurrent cellulitis, inflammation, and hemorrhage. The frequency and severity of the lesions we observed with Bup Lab SR varied with the dose level: the 1.2-mg/kg dose led to 10 lesions among 24 mice compared with 3 lesions among 24 mice with the 0.3-mg/kg dose. During the duration of this study, 2 different vials of Bup SR Lab were used. No adverse effects were observed with Bup IR.
Discussion
In this study, we assessed the pharmacokinetic response generated by escalating doses of Bup IR and Bup SR Lab in C57BL/6J male mice. The study design enabled comparisons between the various recommended clinical doses of both drugs formulations, with the intent of providing guidance regarding the selection of formulation and dosage for mice. Single-dose Bup SR Lab provided a longer time at the assumed effective blood concentration (1 ng/mL) than did single-dose Bup IR.
The last time point at which the blood concentration of buprenorphine exceeded the therapeutic threshold (1 ng/mL) was 6 h for the 2.0-mg/kg dose of Bup IR (Figure 3). Consistent with our findings, a previous study using the same dose reported an analgesic duration of 3 to 5 h.10 On the basis of our results and because there were no observed adverse effects, this increased dose of 2.0-mg/kg could be considered a viable clinical dose. In addition, the lower Bup IR doses resulted in TThE of 3 h or less, with the doses of 0.5 and 0.03 mg/kg never reaching the therapeutic threshold. In comparison, the 1.2-mg/kg dose of Bup SR Lab resulted in a TThE of 12 h which, although still an increase over that for Bup IR, was less than what we expected. However, this 12-h duration is twice the duration of pain relief compared with that achieved by using Bup IR and suggests that the use of Bup SR Lab would likely result in less mouse handling and more consistent analgesia. These findings support the study's hypothesis that the sustained-release formulation would result in extended periods of above-threshold blood concentrations and, likely, associated analgesic effects. Our pharmacokinetic results further support this conclusion in that the AUC values for the Bup IR doses were much smaller than were those observed with Bup SR Lab doses. This finding suggests that Bup SR Lab provides enhanced bioavailability, which is most evident at time periods 12 h or more after injection.
During this study, Bup SR Lab frequently produced skin lesions as an adverse effect. Therefore partway through the study, we began to allow the Bup SR Lab stock to warm to room temperature before it was injected into the mice. We suspect that the liquid viscosity of the drug carrier is highly temperature-dependent and that at low temperatures, this viscosity may be sufficiently high to prevent effective flow of the formulation into the subcutaneous space. This factor might enable the product to flow onto the mouse's skin, where an adverse reaction can occur. After we instituted the practice of warming the Bup SR Lab stock to room temperature, the frequency of adverse skin lesions seemed to decrease.
In general, this pharmacokinetic study showed that the forensic ELISA was a successful analytical tool, determining drug concentrations in near real time with reduced costs and numbers of animals. This analytical tool enables clinicians to estimate the blood concentration of buprenorphine in different species or strains or with different formulations. It is our opinion that the forensic ELISA is a viable alternative to more expensive analytical methods when evaluating performance-based objective criteria for buprenorphine analgesia. This technology required the development of a standard curve by using known concentrations of buprenorphine. Because Bup SR Lab cannot be diluted, we assumed that all drug within the bloodstream, regardless of formulation, would act like Bup IR. If the buprenorphine associated with Bup SR Lab remained bound to the sustained-release matrix while in the bloodstream, then the measurement of free buprenorphine would underestimate the actual transient drug concentration but would accurately estimate the available drug concentration. The lower limit of quantification is a characteristic of the forensic ELISA, which therefore cannot be used to accurately detect buprenorphine concentrations less than 0.33 ng/mL. In the current study, we felt that this limitation was acceptable and that the forensic ELISA kit can be used to determine blood concentrations of buprenorphine in mice.
Given the findings of this study and the assumed therapeutic level of 1 ng/mL, we make the following recommendations to investigators, animal care staff, and veterinarians when the Bup SR Lab product is used in young adult male C57BL/6J mice. First, Bup SR Lab should be administered at 1.2 mg/kg SC to maintain therapeutic blood drug concentration levels above 1 ng/mL for at least 12 h. A practical dose for clinical use is 1 mg/kg; using Hamilton syringes will provide the necessary precision for dosing. Second, Bup IR should be administered at 2.0 mg/kg SC to maintain blood drug levels above 1 ng/mL for nearly 12 h; a dose of 0.1 mg/kg SC would require readministration approximately every 3 h to maintain the same therapeutic blood drug level.
In conclusion, the 1.2-mg/kg dose of Bup SR Lab and the 0.1- and 2.0-mg/kg doses of Bup IR result in blood buprenorphine concentrations that exceed 1 ng/mL (the presumed therapeutic threshold) during the initial 3-h postinjection period, according to a forensic ELISA test method. In the clinical setting, a single injection of SR Bup Lab at 1.2 mg/kg provides an alternative to repeated dosing with Bup IR. Increased understanding of the pharmacokinetics of the new sustained-release formulations of buprenorphine will enable veterinarians, animal care staff, and researchers to improve pain-control regimens, decrease animal-welfare concerns regarding uncontrolled pain, and increase compliance with directives regarding the care of mice.
References
- 1.Carbone ET, Lindstrom KE, Diep S, Carbone L. 2012. Duration of action of sustained-release buprenorphine in 2 strains of mice. J Am Assoc Lab Anim Sci 51:815–819 [PMC free article] [PubMed] [Google Scholar]
- 2.Carbone L. 2012. Pain management standards in the eighth edition of the Guide for the Care and Use of Laboratory Animals J Am Assoc Lab Anim Sci 51:322–328. [PMC free article] [PubMed] [Google Scholar]
- 3.Catbagan DL, Quimby JM, Mama KR, Rychel JK, Mich PM. 2011. Comparison of the efficacy and adverse effects of sustained-release buprenorphine hydrochloride following subcutaneous administration and buprenorphine hydrochloride following oral transmucosal administration in cats undergoing ovariohysterectomy. Am J Vet Res 72:461–466 [DOI] [PubMed] [Google Scholar]
- 4.Chawarski MC, Schottenfeld RS, O'Connor PG, Pakes J. 1999. Plasma concentrations of buprenorphine 24 to 72 hours after dosing. Drug Alcohol Depend 55:157–163 [DOI] [PubMed] [Google Scholar]
- 5.Christoph T, Kogel B, Schiene K, Meen M, De Vry J, Friderichs E. 2005. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol 507:87–98 [DOI] [PubMed] [Google Scholar]
- 6.Cirimele V, Etienne S, Villain M, Ludes B, Kintz P. 2004. Evaluation of the One-Step ELISA kit for the detection of buprenorphine in urine, blood, and hair specimens. Forensic Sci Int 143:153–156 [DOI] [PubMed] [Google Scholar]
- 7.Davis MP. 2012. Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. J Support Oncol 10:209–219 [DOI] [PubMed] [Google Scholar]
- 8.Flecknell P. 2009. Laboratory animal anaesthesia. New York (NY): Elsevier Science. [Google Scholar]
- 9.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204 [PMC free article] [PubMed] [Google Scholar]
- 10.Gades NM, Danneman PJ, Wixson SK, Tolley EA. 2000. The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci 39:8–13 [PubMed] [Google Scholar]
- 11.Guarnieri M, Brayton C, DeTolla L, Forbes-McBean N, Sarabia-Estrada R, Zadnik P. 2012. Safety and efficacy of buprenorphine for analgesia in laboratory mice and rats. Lab Anim (NY) 41:337–343 [DOI] [PubMed] [Google Scholar]
- 12.Hawk CT, Leary S, Morris T. 2005. Formulary for laboratory animals. Hoboken (NJ): John Wiley and Sons. [Google Scholar]
- 13.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 14.Jacobsen KR, Kalliokoski O, Hau J, Abelson KSP. 2011. Voluntary ingestion of buprenorphine in mice. Anim Welf 20:591–596 [DOI] [PubMed] [Google Scholar]
- 15.Kohn DF, Martin TE, Foley PL, Morris TH, Swindle MM, Vogler GA, Wixson SK. 2007. Guidelines for the assessment and management of pain in rodents and rabbits. J Am Assoc Lab Anim Sci 46:97–108 [PubMed] [Google Scholar]
- 16.Kolstad AM, Rodriguiz RM, Kim CJ, Hale LP. 2012. Effect of pain management on immunization efficacy in mice. J Am Assoc Lab Anim Sci 51:448–457 [PMC free article] [PubMed] [Google Scholar]
- 17.Kukanich B. 2011. Clinical interpretation of pharmacokinetic and pharmacodynamic data in zoologic companion animal species. Vet Clin North Am Exot Anim Pract 14:1–20 [DOI] [PubMed] [Google Scholar]
- 18.Martucci C, Panerai AE, Sacerdote P. 2004. Chronic fentanyl or buprenorphine infusion in the mouse: similar analgesic profile but different effects on immune responses. Pain 110:385–392 [DOI] [PubMed] [Google Scholar]
- 19.Nunamaker EA, Halliday LC, Moody DE, Fang WB, Lindeblad M, Fortman JD. 2013. Pharmacokinetics of 2 formulations of buprenorphine in macaques (Macaca mulatta and Macaca fascicularis). J Am Assoc Lab Anim Sci 52:48–56 [PMC free article] [PubMed] [Google Scholar]
- 20.Park I, Kim D, Song J, In CH, Jeong S-W, Lee SH, Min B, Lee D, Kim S-O. 2008. Buprederm, a new transdermal delivery system of buprenorphine: pharmacokinetic, efficacy, and skin irritancy studies. Pharm Res 25:1052–1062 [DOI] [PubMed] [Google Scholar]
- 21.Pergolizzi J, Aloisi AM, Dahan A, Filitz J, Langford R, Likar R, Mercadante S, Morlion B, Raffa RB, Sabatowski R, Sacerdote P, Torres LM, Weinbroum AA. 2010. Current knowledge of buprenorphine and its unique pharmacological profile. Pain Pract 10:428–450 [DOI] [PubMed] [Google Scholar]
- 22.Roughan JV, Flecknell PA. 2002. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating post-operative pain in animals. Lab Anim 36:322–343 [DOI] [PubMed] [Google Scholar]
- 23.Stokes EL, Flecknell PA, Richardson CA. 2009. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim 43:149–154 [DOI] [PubMed] [Google Scholar]
- 24.Watson PJ, McQuay HJ, Bullingham RE, Allen MC, Moore RA. 1982. Single-dose comparison of buprenorphine 0.3 and 0.6 mg IV given after operation: clinical effects and plasma concentration. Br J Anaesth 54:37–43 [DOI] [PubMed] [Google Scholar]
- 25.Yassen A, Olofsen E, Kan J, Dahan A, Danhof M. 2008. Pharmacokinetic–pharmacodynamic modeling of the effectiveness and safety of buprenorphine and fentanyl in rats. Pharm Res 25:183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yun MH, Jeong SW, Pai CM, Kim SO. 2010. Pharmacokinetic–pharmacodynamic modeling of the analgesic effect of buprederm, in mice. Health 02:824–831 [Google Scholar]