Abstract
Prolonged antibiotic and antiinflammatory therapy for complicated infections exposes the body to xenobiotics that can produce several adverse effects for which oxidative damage is the proposed underlying mechanism. In this context, we evaluated the effect of pazufloxacin, a fluoroquinolone antimicrobial, and meloxicam, a nonsteroidal antiinflammatory drug, on antioxidant parameters and lipid peroxidation in rabbits after oral administration for 21 d. Reduced glutathione levels were significantly decreased in rabbits (n = 4 per group) given pazufloxacin, meloxicam, or their combination. In addition, glutathione peroxidase activity was induced in the rabbits treated with pazufloxacin only. Administration of pazufloxacin and meloxicam, as single agents as well as in combination, produced significant lipid peroxidation compared with levels in untreated controls. In conclusion, both pazufloxacin and meloxicam potentially can induce oxidative damage in rabbits.
Abbreviations: COX, cyclooxygenase; GPx, glutathione peroxidase; LPO, lipid peroxidation; ROS, reactive oxygen species; SOD, superoxide dismutase
Fluoroquinolones are bactericidal drugs that inhibit the bacterial enzymes DNA gyrase and topoisomerase IV. The fourth-generation fluoroquinolone pazufloxacin is a fused tricyclic quinolone and has a 1-aminocyclopropyl substituent at C10 position, a unique feature of the molecule contributing to the potent broad spectrum activity to this drug.49 It may be used as the drug of choice in community acquired infections, acute exacerbation of chronic bronchitis and postabdominal infection sepsis.45 This drug has strong in vitro and in vivo activity against a broad range of bacteria, especially Gram-negative bacteria.19 Pazufloxacin mesylate, a salt of pazufloxacin, was found to be therapeutically effective in mice against systemic infections caused by mutidrug-resistant strains of bacteria.30
NSAID are widely used in both veterinary and human medicine for the management of pain due to infectious or noninfectious sources. These drugs act by inhibiting the proprostaglandin enzymes, cyclooxygenases (COX) 1 and 2. Meloxicam is an NSAID that has greater selectivity toward inhibition of the inducible COX2 isoform compared with the constitutive isoform COX1.15 Inhibition of COX2 mediates the therapeutic actions of NSAID, whereas COX1 inhibition usually results in unwanted side effects, especially of the gastrointestinal tract.15
A study published in 199423 reported adverse drug reactions as the sixth leading cause of death in the United States. Although fluoroquinolones generally are well tolerated,34 adverse effects including gastrointestinal discomfort, hepatotoxic reactions, CNS effects, juvenile joint toxicity, and phototoxic and retinopathic effects have been reported in association with their use.34,50 The physiochemical properties, pharmacokinetic characteristics, and antimicrobial activities of various fluoroquinolones vary markedly despite the similarity in their core molecular structure.28 Although NSAID, including meloxicam, typically rectify oxidative imbalance,12,15,33 several NSAID have led to alterations in antioxidant levels,5 thereby revealing oxidative stress as the mechanism of toxicity.24,44
The treatment of complicated infections often requires weeks of antibiotic therapy and a protracted course of NSAID to manage associated inflammatory conditions such as pyrexia and pain.26,38,46 The production of reactive oxygen species (ROS) supplements the antibacterial activity of fluoroquinolones3,21 and is proposed to lead to various side effects of both fluoroquinolones and NSAID.36,41,48 However, scant literature is available that addresses pazufloxacin and the simultaneous use of fluoroquinolones and NSAID. Therefore, we evaluated pazufloxacin and meloxicam for their effect on the antioxidant status and oxidative stress. We hypothesized that their simultaneous administration produces greater oxidative damage than that generated after exposure of either agent individually.
Materials and Methods
Animals.
Healthy male adult Soviet Chinchilla rabbits (age, 6 mo; n = 16) were procured from the rabbit farm of the Guru Angad Dev Veterinary and Animal Sciences University (Ludhiana, India). The specific pathogen status of the rabbits is unknown, but the source farm follows all recommended husbandry measures regarding hygiene and sanitation to prevent any infection of the animals. The rabbits were housed individually in single-tier wire mesh cages kept under ambient conditions of temperature and humidity, with an average day length of 11.5 h, in a well-lighted experimental house. The rabbits were acclimated to these housing conditions for 2 wk prior to the start of experiment. The animals were maintained on standard pelleted rabbit feed (Godrej Rabbit Feed, Khanna, India) and ad libitum water. The use of animals was approved by the University Animal Ethics Committee with order No.VMC/13/17/86 to 1806 dated 04 April 2013. The body weight (mean ± SE) of the rabbits at the start of the experiments was 3.16 ± 0.07.
Rabbits were randomly allocated to 4 groups. The control group received 5% dextrose (2 mL/kg body weight) by gavage. Additional groups of rabbits were gavaged with pazufloxacin mesylate (10 mg/kg every 12 h; Pazumac IV Infusion, Macleoids Pharma, Mumbai, India) or meloxicam (0.2 mg/kg; Melonex Injection, Intas Pharma, Ahmedabad, India). The dose for pazufloxacin was extrapoliated from the therapeutic dose in humans,7,45 whereas meloxicam was used at its therapeutic dose in rabbits.29 The rabbits in the remaining group received both pazufloxacin and meloxicam at the same dose rates used in the other groups. Meloxicam was administered every morning, whereas one dose of pazufloxacin was administered at the same time as the meloxicam, and the other dose was given at 12 h after the first dose. Each rabbit was dosed according to its individual body weight, and rabbits were weighed weekly so that necessary corrections in the total dosage of drugs or dextrose administered could be made.
In addition, rabbits were restrained manually on the morning of days 0, 7, 14, and 21 of treatment, and blood samples (1.5 mL) were collected from the ear vein by using separate heparinized scalp-vein needle (24-gauge) sets. Time 0 d refers to the day prior to the start of the first dose of either drug, whereas the blood samples collected on days 7, 14, and 21 were obtained approximately 12 h after infusion of the last dose of pazufloxacin or 24 h after the last dose of meloxicam. Once blood samples had been obtained on days 7 and 14, the next corresponding dose was administered.
Biochemical analysis.
The blood glutathione concentration was determined according to a previously described method.4 Briefly, 0.2 mL of whole blood was added to 1.8 mL of distilled water; 3 mL of the precipitating solution (glacial metaphosphoric acid) then was added; the mixture was incubated at room temperature for approximately 5 min and then centrifuged at 4500 × g for 15 min. We then combined 2 mL of the supernatant with 8 mL 0.3 M phosphate solution (Na2HPO4 2H2O) and 1 mL of 5–5′-dithiobis-(2-nitrobenzoic acid) reagent were added. The absorbance at 412 nm was recorded, and the concentration of glutathione in blood was determined according to the standard curve of glutathione in distilled water.
The activity of superoxide dismutase (SOD) was determined according to a previously described method.27 To this end, we combined 1.5 mL 100 mM Tris-HCl buffer, 0.5 mL 6 mM EDTA, and 1 mL 0.6 mM pyrogallol solution in a cuvette. The rate of auto-oxidation of pyrogallol was measured as the increase in absorbance at 420 nm was recorded every 30s for 4 min for reading of the blank. For the test, an appropriate amount of enzyme (in 20 µL erythrocyte lysate) was added to inhibit the auto-oxidation of pyrogallol to about 50%. A unit of enzyme activity was defined as the amount of enzyme producing 50% inhibition of the auto-oxidation of the pyrogallol measured in the blank.
The activity of glutathione peroxidase (GPx) was assayed as described previously.17 We combined 0.1 mL erythrocyte lysate, 1 mL 20 mM glutathione, 1 mL 0.4 M sodium phosphate (pH 7), and 0.5 mL 10 mM sodium azide and then brought the total volume to 4 mL by using distilled water. After a 5-min preincubation, 1 mL H2O2 (prewarmed to 37 °C) was added; after a 1-min interval, 1-mL aliquots of the incubation mixture were removed and added to 4 mL precipitation solution (m-phosphoric acid). This mixture was centrifuged at 3000 rpm for 15 min. The glutathione content in the protein-free supernatant was determined by mixing 2 mL supernatant with 2 mL 0.4 M Na2HPO4 and 1 mL dithionitrobenzoic acid reagent (40 mg dithionitrobenzoic acid [Sigma–Aldrich, St Louis, MO] in 100 mL 1% trisodium citrate) and the absorbance at 412 nm was recorded within 2 min after mixing. The GSH concentration at time 0 was determined by using an aliquot from a similar sample except that H2O2 was replaced with water. The activity of GPx was determined as:
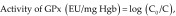 |
where C0 is the concentration of glutathione at time 0, and C is the glutathione concentration after a 1-min incubation.
The activity of catalase was determined according to a previously described method.1 We added 20 µL of erythrocyte lysate to 2 mL 50 mM sodium phosphate buffer (pH 7.0) in a cuvette and mixed well. The reaction was started by the addition of 1 mL 30 mM H2O2, and the absorbance at 240 nm was recorded at every 10 s for 1 min. The results were expressed as µmol H2O2 decomposed/min/g Hgb by using 36 as the molar extinction coefficient of H2O2.
Lipid peroxidation (LPO), that is, the amount of malondialdehyde, was estimated in 33% packed-cell erythrocytes.37 We added 1 mL 10% w/v trichloroacetic acid to 1 mL of sample and vortexed. The mixture was centrifuged at 4500 × g for 15 min, then 1 mL 0.67% w/v 2-thiobarbituric acid was added to 1 mL supernatant and the mixture boiled for 10 min. The final reaction mixture was cooled and diluted with 1 mL distilled water. The absorbance at 535 nm was recorded, and the results were expressed as nmol malondialdehyde formed/mL erythrocytes. The molar extinction coefficient of the malondialdehyde–thiobarbituric acid complex at 535 nm is 1.56 × 108/M/cm.
Statistical analysis.
All assays were done in triplicate. The average value for each triplicate result for an animal represented the individual observation from that animal. The statistical significance of the difference between group means based on individual observations was determined by ANOVA of a completely randomized design by using the Duncan multiple-range test.11 Statistical significance was defined as a P value of less than 0.05.
Results
The results for various oxidative status parameters in rabbits that received pazufloxacin mesylate, meloxicam, or both drugs are presented in Table 1. The levels of glutathione on day 7 of administration increased significantly (P < 0.05) in rabbits given pazufloxacin compared with other groups. However, on day 21 of treatment, the glutathione levels of all treated groups were significantly lower than that of the control group. The activity of SOD did not differ significantly between groups throughout the study period. The activity of GPx on day 21 of treatment was increased significantly (P < 0.05) in pazufloxacin-treated rabbits compared with all other groups and in rabbits treated with both pazufloxacin and meloxicam compared with animals given meloxicam only. On day 14, catalase activity was increased (P < 0.05) in meloxicam-treated rabbits compared with other groups, whereas on day 21, the catalase activity of meloxicam-treated rabbits was greater (P < 0.05) than that of rabbits given pazufloxacin. When compared with the control group, a significant increase in LPO was noted in the pazufloxacin-treated rabbits beginning on day 14 of treatment, in meloxicam-treated rabbits beginning on day 7, and in the animals that received both drugs beginning on day 21.
Table 1.
Effects of 21-day oral administration of pazufloxacin, meloxicam, and their combination on various markers of oxidative status (mean ± SE; n = 4) in rabbit blood
| Untreated controls | Pazufloxacin | Meloxicam | Pazufloxacin + meloxicam | |
| Reduced glutathione (nmol/mL) | ||||
| Day 0 | 431.43 ± 20.58a | 431.43 ± 23.88a | 490.43 ± 24.50a | 421.29 ± 5.71a |
| Day 7 | 464.62 ± 41.74a | 555.88 ± 51.65b | 395.48 ± 18.82a | 478.44 ± 13.91a |
| Day 14 | 490.43 ± 51.60a | 551.27 ± 29.37a | 493.19 ± 20.33a | 487.66 ± 49.75a |
| Day 21 | 487.66 ± 29.67a | 392.71 ± 20.34b | 379.80 ± 12.86b | 408.38 ± 4.36b |
| Superoxide dismutase (U/g Hgb) | ||||
| Day 0 | 597.00 ± 127.95a | 578.55 ± 76.78a | 534.31 ± 92.39a | 601.39 ± 25.41a |
| Day 7 | 579.03 ± 35.45a | 444.34 ± 66.22a | 429.10 ± 10.14a | 455.80 ± 38.71a |
| Day 14 | 647.22 ± 50.76a | 773.41 ± 82.01a | 824.29 ± 155.70a | 742.52 ± 62.50a |
| Day 21 | 590.96 ± 21.97a | 633.10 ± 98.83a | 778.74 ± 69.83a | 696.01 ± 33.76a |
| Glutathione peroxidase (U/mg Hgb) | ||||
| Day 0 | 2.10 ± 0.06a | 1.89 ± 0.10a | 2.03 ± 0.04a | 1.85 ± 0.037a |
| Day 7 | 2.07 ± 0.33a | 1.99 ± 0.35a | 1.71 ± 0.09a | 1.57 ± 0.06a |
| Day 14 | 2.12 ± 0.19a | 2.29 ± 0.10a | 1.71 ± 0.26a | 2.08 ± 0.05a |
| Day 21 | 2.32 ± 0.19ac | 3.43 ± 0.14b | 2.08 ± 0.11a | 2.82 ± 0.08c |
| Catalase (μmole H2O2 decomposed/min/mg Hgb) | ||||
| Day 0 | 1671.04 ± 86.37a | 1509.74 ± 87.40a | 1504.00 ± 174.80a | 1550.88 ± 27.59a |
| Day 7 | 1664.30 ± 96.45a | 1631.50 ± 39.55a | 1549.12 ± 175.18a | 1815.24 ± 80.42a |
| Day 14 | 1576.25 ± 68.60a | 1348.38 ± 143.93a | 2493.87 ± 289.60b | 1494.874 ± 72.93a |
| Day 21 | 1596.01 ± 51.54ab | 1379.00 ± 185.42b | 2039.53 ± 222.02a | 1786.106 ± 129.91ab |
| Lipid peroxidation (nmole malondialdehyde formed/mL erythrocytes) | ||||
| Day 0 | 5.14 ± 0.68a | 4.06 ± 0.49a | 5.46 ± 1.36a | 4.66 ± 0.21a |
| Day 7 | 5.87 ± 0.42a | 6.50 ± 0.19ab | 7.84 ± 1.10b | 6.15 ± 0.42ab |
| Day 14 | 5.96 ± 0.58a | 8.44 ± 0.98b | 7.99 ± 0.39bc | 6.19 ± 0.31ac |
| Day 21 | 6.38 ± 0.60a | 10.34 ± 1.03b | 9.87 ± 0.96b | 10.22 ± 1.00b |
Within a row, values with different superscripted letters differ significantly (P < 0.05).
Discussion
Several fluoroquinolones16,34 and meloxicam5 have been reported to deplete glutathione levels. Glutathione is the primary intracellular water soluble antioxidant that participates in the detoxification of toxic peroxides, maintenance of protein SH groups, and conjugation of xenobiotics to enable their elimination.18 Because glutathione depletion increases the susceptibility of cells and tissues to oxidative injury, it is considered an early hallmark in the progression of cell death in response to various apoptotic stimuli.2,6,13
SOD is the first line of defense against the action of O2– and other ROS.8-10 Similar to the effect associated with pazufloxacin in the current study, ofloxacin is known to induce GPx activity,34 whereas a nonsignificant change of GPx activity is reported with meloxicam.5 GPx catalyzes the detoxification of a wide range of peroxides by using glutathione as reducing equivalent.20 SOD is dismutated rapidly to H2O2 by Mn-SOD (SOD2) in the mitochondrial matrix, a process catalyzed in the intermembrane space or cytosol by CuZn-SOD (SOD1).39 Final detoxification of mitochondrial superoxides can occur by conversion of H2O2 to H2O by catalase at high H2O2 concentrations or by GPx at low H2O2 concentrations.2
Increased lipid peroxidation after fluoroquinolone treatment has been reported by several studies.25,34,40 Rofecoxib, a COX2-selective NSAID, predisposes human LDL and cell-membrane lipids to oxidative modification.47 An in vitro study revealed that NSAID may increase cardiovascular risk by inducing oxidative stress in the vasculature, with nonselective NSAID having a greater effect than do coxibs.24 The extent of lipid peroxidation indirectly reflects the degree to which biomembrane lipids have been attacked by free radicals. Antioxidant enzymes are inactivated by malondialdehyde crosslinking, which results in an increased accumulation of ROS and aggravation of macromolecular damage.32 Oxidative stress can be regarded as the imbalance between pro- and antioxidant mechanisms. This notion has led to the evaluation of indicators of each side of this balance by using ROS and reactive nitrogen species as markers of prooxidant activity, antioxidants as markers of antioxidant balance and lipid peroxidation, and proteins and DNA oxidation as markers of oxidative damage.2 Simultaneous evaluation of the markers of oxidative stress, such as the endogenous antioxidant glutathione and various antioxidant enzymes, are considered to determine oxidative stress,35,45 due to the nonspecificity of the malondialdehyde assay.14
During energy transduction from the mitochondrial electron transport chain, a small number of electrons ‘leak’ prematurely to oxygen, forming O2–.22,42 This process is exacerbated with increased xenobiotics metabolism, an energy-expending process. The biologic outcome of mitochondrial ROS production and their potential involvement in physiologic (signal transduction) compared with pathologic (oxidative imbalance or stress) processes depends on the critical equilibrium between their production and detoxification.43 The response of the body to ROS is the expression of transcription factor Nrf2, which increases the expression of proantioxidant genes.35 This response might account for the increased glutathione levels and GPx activity we noted in pazufloxacin-treated rabbits on days 7 and 21 of treatment, respectively, as well as the increased activity of catalase in meloxicam-treated animals on day 14. However, the increased activity of these antioxidants was insufficient to completely prevent oxidative damage. Another probable reason for the significant lipid peroxidation in the pazufloxacin- and meloxicam-treated groups is the depletion of an important antioxidant, glutathione. Both increases and decreases in the expression or activity of antioxidant enzymes are indicative of oxidative stress.2 However, inhibiting the activity or sufficient synthesis of antioxidants by consistently increasing exposure to prooxidative xenobiotics overwhelms the antioxidant status, which predisposes cells to oxidative damage.31
In conclusion, the results from the current study indicate that repeated oral administration of pazufloxacin and meloxicam produces oxidative stress in rabbits, as indicated by their elevated malondialdehyde levels and alteration of various antioxidant parameters. Several studies have reported the ameliorating effect of NSAID on oxidative stress. However, in our study, meloxicam yielded a prooxidative effect, perhaps because of the prolonged duration of drug administration. Because patients with bacterial prostatitis or various respiratory infections require prolonged courses of therapy with these agents, their predisposition to various diseases mediated through oxidative stress will be increased. Furthermore, studies to evaluate the production of free radicals and oxidative damage by these drugs in diverse tissues are warranted to assess their associated predisposition to various diseases and adverse drug reactions.
Acknowledgments
The current research was done in the Department of Veterinary Pharmacology and Toxicology (Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, India). The study is a part of the research project “Evaluation of retinopathic potential of fluoroquinolones” assigned by University Grants Commission, India, to the Department of Veterinary Pharmacology and Toxicology (GASVASU) under letter no. 40-279/2011 (to SR). Financial assistance to the project has been made by the University Grants Commission (India).
References
- 1.Aebi HE. 1983. Catalase, p 276–286. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York (NY): Academic Press [Google Scholar]
- 2.Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. 2009. A positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev 89:27–71 [DOI] [PubMed] [Google Scholar]
- 3.Becerra MC, Eraso AJ, Albesa I. 2003. Comparison of oxidative stress induced by ciprofloxacin and pyoverdin in bacteria and in leukocytes to evaluate toxicity. Luminescence 18:334–340 [DOI] [PubMed] [Google Scholar]
- 4.Beutler E. 1975. Red cell metabolism: a manual of biochemical methods, 2nd ed, p 67–69. New York (NY): Grune and Stratton. [Google Scholar]
- 5.Burak Cimen MY, Cimen OB, Eskandari G, Sahin G, Erdogan C, Atik U. 2003. In vivo effects of meloxicam, celecoxib, and ibuprofen on free-radical metabolism in human erythrocytes. Drug Chem Toxicol 26:169–176 [DOI] [PubMed] [Google Scholar]
- 6.Circu ML, Aw TY. 2008. Glutathione and apoptosis. Free Radic Res 42:689–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croisier D, Chavanet P, Lequeu C, Ahanou A, Nierlich A, Neuwirth C, Piroth L, Duong M, Buisson M, Portier H. 2002. Efficacy and pharmacodynamics of simulated human-like treatment with levofloxacin on experimental pneumonia induced with penicillin-resistant pneumococci with various susceptibilities to fluoroquinolones. J Antimicrob Chemother 50:349–360 [DOI] [PubMed] [Google Scholar]
- 8.Dar MA, Khan AM, Raina R, Verma PK, Sultana M. 2013. Effect of repeated oral administration of bifenthrin on lipid peroxidation and antioxidant parameters in Wistar rats. Bull Environ Contam Toxicol 91:125–128 [DOI] [PubMed] [Google Scholar]
- 9.Dubey N, Khan AM, Raina R. 2013. Subacute deltamethrin and fluoride toxicity-induced hepatic oxidative stress and biochemical alterations in rats. Bull Environ Contam Toxicol 91:334–338 [DOI] [PubMed] [Google Scholar]
- 10.Dubey N, Raina R, Khan AM. 2012. Toxic effects of deltamethrin and fluoride on antioxidant parameters in rats. Fluoride 45:242–246 [Google Scholar]
- 11.Duncan DB. 1995. Multiple range and multiple F-tests. Biometrics 11:1–14 [Google Scholar]
- 12.Edfawy M, Hassan MH, Mansour A, Hamed AA, Amin HA. 2012. Meloxicam modulates oxidative stress status, inhibits prostaglandin E2, and abrogates apoptosis in carbon tetrachloride-induced rat hepatic injury. Int J Toxicol 31:276–286 [DOI] [PubMed] [Google Scholar]
- 13.Franco R, DeHaven WI, Sifre M, Bortner CD, Cidlowski JA. 2007. Glutathione depletion and disruption of intracellular ionic homeostasis regulate lymphoid cell apoptosis. J Biol Chem 283:36071–36087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grotto D, Maria LS, Valentine J, Paniz C, Schmitt G, Garcia SC, Pomblum VJ, Rocha JB, Farina M. 2009. Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quim Nova 32:169–174 [Google Scholar]
- 15.Gunes V, Cinar M, Onmaz AC, Atalan G, Yavuz U. 2011. Effects of meloxicam on oxidative deterioration due to exercise in horses. Rev Med Vet (Toulouse) 162:258–264 [Google Scholar]
- 16.Gurbay A, Hincal F. 2004. Ciprofloxacin-induced glutathione redox status alterations in rat tissues. Drug Chem Toxicol 27:233–242 [DOI] [PubMed] [Google Scholar]
- 17.Hafeman DG, Sunde RA, Hoekstra WG. 1974. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rats. J Nutr 104:580–587 [DOI] [PubMed] [Google Scholar]
- 18.Hayes JD, McLellan LI. 1999. Glutathione and glutathione-dependent enzymes represent a coordinately regulated defense against oxidative stress. Free Radic Res 31:273–300 [DOI] [PubMed] [Google Scholar]
- 19.Higa F, Akamine M, Haranaga S, Tohyama M, Shinzato T, Tateyama M, Koide M, Saito A, Fujita J. 2005. In vitro activity of pazufloxacin, tosufloxacin, and other quinolones against Legionella species. J Antimicrob Chemother 56:1053–1057 [DOI] [PubMed] [Google Scholar]
- 20.Khan AM, Sultana M, Raina R, Dubey N, Dar SA. 2013. Effect of subacute toxicity of bifenthrin on antioxidant status and hematology after its oral exposure in goats. Proc Natl Acad Sci India Sect B Biol Sci 83:545–549 [Google Scholar]
- 21.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810 [DOI] [PubMed] [Google Scholar]
- 22.Kovacic P, Pozos RS, Somanathan R, Shangari N, O'Brien PJ. 2005. Mechanism of mitochondrial uncouplers, inhibitors, and toxins: focus on electron transfer, free radicals, and structure–activity relationships. Curr Med Chem 12:2601–2623 [DOI] [PubMed] [Google Scholar]
- 23.Lazarou J, Pomeranz BH, Corey PN. 1998. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279:1200–1205 [DOI] [PubMed] [Google Scholar]
- 24.Li H, Hortmann M, Daiber A, Oelze M, Ostad MA, Schwarz PM, Xu H, Xia N, Kleschyov AL, Mang C, Warnholtz A, Munzel T, Forstermann U. 2008. Cyclooxygenase 2-selective and nonselective nonsteroidal antiinflammatory drugs induce oxidative stress by upregulating vascular NADPH oxidases. J Pharmacol Exp Ther 326:745–753 [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Peng S, Sheng Z, Wang Y. 2010. Ofloxacin induces oxidative damage to joint chondrocytes of juvenile rabbits: excessive production of reactive oxygen species, lipid peroxidation and DNA damage. European J Pharmacol 626: 146–153 [DOI] [PubMed] [Google Scholar]
- 26.Lipsky BA, Byren I, Hoey CT. 2010. Treatment of bacterial prostatitis. Clin Infect Dis 50:1641–1652 [DOI] [PubMed] [Google Scholar]
- 27.Marklund S, Marklund M. 1974. Involvement of superoxide anion radical in autoxidation of pyrogallol and a convenient assay of superoxide dismutase. Eur J Biochem 47:469–474 [DOI] [PubMed] [Google Scholar]
- 28.Martinez M, McDermott P, Walker R. 2006. Pharmacology of the fluoroquinolones: a perspective for the use in domestic animals. Vet J 172:10–28 [DOI] [PubMed] [Google Scholar]
- 29.Plumb DC, editor. 2008. Plumb's veterinary drug handbook. Ames: Blackwell Publishing [Google Scholar]
- 30.Mitsuyama J, Takahata M, Yamashiro Y, Kitayama R, Muratani T, Matsumura N, Nakata M, Fukuta T, Yamada H, Maehana J, Hamada S, Sugiyama H, Shimakura M, Minami S, Watanabe Y, Narita H. 1999. Antibacterial activity of a new injectable quinolone, pazufloxacin mesilate (PZFX mesilate), in vitro and in vivo. Japanese J Chemother 47:37–64 [Google Scholar]
- 31.Mytilineou C, Kramer BC, Yabut JA. 2002. Glutathione depletion and oxidative stress. Parkinsonism Relat Disord 8:385–387 [DOI] [PubMed] [Google Scholar]
- 32.Noeman SA, Hamooda HE, Baalash AA. 2011. Biochemical study of oxidative stress markers in the liver, kidney, and heart of high-fat diet-induced obesity in rats. Diabetol Metab Syndr 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozgocmen S, Ardicoglu O, Erdogan H, Fadillioglu E, Gudul H. 2005. In vivo effect of celecoxib and tenoxicam on oxidant/anti-oxidant status of patients with knee osteoarthritis. Ann Clin Lab Sci 35:137–143 [PubMed] [Google Scholar]
- 34.Rampal S, Kaur R, Sethi R, Singh O, Sood N. 2008. Ofloxacin-associated retinopathy in rabbits: role of oxidative stress. Hum Exp Toxicol 27:409–415 [DOI] [PubMed] [Google Scholar]
- 35.Ristow M, Schmeisser S. 2011. Extending life span by increasing oxidative stress. Free Radic Biol Med 51:327–336 [DOI] [PubMed] [Google Scholar]
- 36.Schmidt U, Schlüter G. 1996. Studies on the mechanism of phototoxicity of Bay y3118 and other quinolones. Adv Exp Med Biol 387:117–120 [DOI] [PubMed] [Google Scholar]
- 37.Shafiq-Ur-Rehman 1984. Lead-induced regional lipid peroxidation in brain. Toxicol Lett 21:333–337 [DOI] [PubMed] [Google Scholar]
- 38.Stevermer JJ, Easley SK. 2000. Treatment of prostatitis. Am Fam Physician 61:3015–3022 [PubMed] [Google Scholar]
- 39.Storz P. 2006. Reactive oxygen species-mediated mitochondria-to-nucleus signaling: a key to aging and radical-caused diseases. Sci STKE 2006:re3. [DOI] [PubMed] [Google Scholar]
- 40.Talla V, Veerareddy PR. 2011. Oxidative stress induced by fluoroquinolones on treatment for complicated urinary tract infections in Indian patients. J Young Pharm 3:304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thuong-Guyot M, Domale O, Pocidalo JJ, Hayem G. 1994. Effects of fluoroquinolones on cultured articular condrocytes flow cytometric analysis of free radical production. J Pharmacol Exp Ther 271:1544–1549 [PubMed] [Google Scholar]
- 42.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. 2004. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266:37–56 [DOI] [PubMed] [Google Scholar]
- 43.Valko M, Leibfritz D, Moncola J, Cronin MTD, Mazura M, Telser J. 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84 [DOI] [PubMed] [Google Scholar]
- 44.Villegas I, Martín MJ, La Casa C, Motilva V, De La Lastra CA. 2002. Effects of oxicam inhibitors of cyclooxygenase on oxidative stress generation in rat gastric mucosa. A comparative study. Free Radic Res 36:769–777 [DOI] [PubMed] [Google Scholar]
- 45.Vora A. 2009. Pazufloxacin. J Assoc Physicians India 57: 722–723 [PubMed] [Google Scholar]
- 46.Wagenlehner FM, Krieger JN. 2011. Treatment of chronic bacterial prostatitis. Pelviperineol 30:17–26 [Google Scholar]
- 47.Walter MF, Jacob RF, Day CA, Dahlborg R, Weng Y, Mason RP. 2004. Sulfone COX2 inhibitors increase susceptibility of human LDL and plasma to oxidative modification: comparison to sulfonamide COX2 inhibitors and NSAIDs. Atherosclerosis 177:235–243 [DOI] [PubMed] [Google Scholar]
- 48.Yazar E, Tras B. 2001. Effects of fluoroquinolone antibiotics on hepatic superoxide dismutase and glutathione peroxidase activities in healthy and experimentally induced peritonitis mice. Rev Med Vet (Toulouse) 152:235–238 [Google Scholar]
- 49.Zhanel GG, Fontaine S, Adam H, Schurek K, Mayer M, Noreddin AM, Gin AS, Rubinstein E, Hoban DJ. 2006. A review of new fluoroquinolones: focus on their use in respiratory tract infections. Treat Respir Med 5:437–465 [DOI] [PubMed] [Google Scholar]
- 50.Zhao B, Chingnell CF, Rammal M, Smith F, Hamilton MG, Andley UP, Roberts JE. 2010. Detection and prevention of ocular phototoxicity of ciprofloxacin and other fluoroquinolone antibiotics. Photochem Photobiol 86:798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]


