Abstract
CO2 euthanasia is used widely for small laboratory animals, such as rodents. A common necessity in many animal research facilities is to euthanize mice in sequential batches. We assessed the effects of several variables on the time it took for CO2 to dissipate within a chamber. Using standard euthanasia time, changes in flow rate were compared between a slow 15% fill rate for 7 min, and a slow 15% followed by a rapid 50% filling for a total of 5 min. Additional variables assessed included the effects of opening the lid after the completion of chamber filling, turning the chamber over after completion of filling, and the use and removal of a cage from within the chamber. For all trials, CO2 levels in the chambers peaked between 50% and 80%. After the gas was turned off, the concentration of CO2 dropped to below 10% CO2 within 2 min, except when the lid was left on the chamber, where concentration levels remained above 10% after 20 min. CO2 dissipation was significantly faster when the chamber was turned upside down after filling. Significant interaction effects occurred among the factors of cage presence within the chamber, flow rate, and chamber position. Only leaving the lid on the chamber had any practical implication for delaying CO2 dissipation. We recommend that users allow 2 min for CO2 to clear from the chamber before subsequent euthanasia procedures, unless the chamber is manipulated to increase the dissipation rate.
The American Veterinary Medical Association guidelines for the euthanasia of animals provide new recommendations for the use of CO2 for rodent euthanasia.21 Rodent euthanasia with CO2 by using a gradual 10% to 30% vol/min displacement rate is considered acceptable with conditions.21 The AVMA regulations additionally clarify that immersion of conscious animals into a container prefilled with 100% CO2 is unacceptable.21
Justification for the recommendation is based on several reports in the literature showing aversion and pain associated with high CO2 levels in mice5,8,14,22 and rats.3,5,6,8,14,17,18 In addition, other studies have revealed the complexity of the signs associated with the use of CO2 as both an anesthetic and euthanizing agent.4,7,16,20,23,24 Mice exposed to levels of CO2 above 50% had pulmonary lesions including edema and hemorrhage.6 Rats found CO2 concentrations of 13.0% to18.4% aversive and were more likely to leave an area with these CO2 levels.17,19 Similar results are found in people. At CO2 concentrations of approximately 8%, dyspnea begins in humans and becomes severe at 15%.15 Humans had mild pain when exposed to 50% CO2 and experienced more pain at higher CO2 concentration levels.6
The current study examines how to practically implement the euthanasia recommendations21 by establishing the time required for CO2 dissipation, thus ensuring that animals are not placed into a chamber filled with 100% CO2. This concern arose because a common necessity in many research facilities is to euthanize mice in sequential batches. CO2 is heavier than air (specific gravity, 1.52), thus diffusion of the gas from a euthanasia chamber can be prolonged. The aim of this study was to identify how long it would take, after one group of mice had been euthanized, for the CO2 in a euthanizing chamber to dissipate before a second animal or group of animals could be put into the chamber. Several variables (flow rate, chamber position, whether the lid was left on, and whether a cage was in the chamber) were examined to measure the time for CO2 dissipation. We hypothesized that the dissipation of CO2 to an acceptable level would take less than 1 min and that sequential events would not be delayed unduly because of the new AVMA recommendation.
Materials and Methods
Equipment.
The euthanasia chamber tested was a 22-L transparent polycarbonate shoebox cage (44 cm × 23.5 cm × 21 cm) with an acrylic glass lid that had two 1-cm holes for CO2 input and gas escape. In some experiments, a mouse shoebox cage (5.8 L, Allentown Caging, Allentown, NJ) was placed inside the chamber to model euthanasia of a mouse in its home cage. An indoor-air, 0% to 100% CO2 meter (CM-0003, CO2meter.com, Ormond Beach, FL) with accuracy of ±30 ppm and ±5% of the measured value was used to measure CO2 levels. The meter consisted of 2 tubes, one for the intake of gas and one for the return of gas to the chamber. These were placed under the lid, and measurements were taken at the bottom of the chamber. Measurements were delayed approximately 7 s, the time it took for the gas to travel through the tube to the sensor. Compressed CO2 gas was provided from a cylinder (Weiler Welding, Moraine, OH) and controlled by using a regulator (Western Medica, Westlake, OH). There were 8.8 room air changes hourly, and the room pressure was negative to the corridor.
Preliminary testing.
Previous studies6,9,11,18 and a preliminary test of our system were used to determine the length of time needed for the CO2 gas to reach a peak level. Several studies have shown that CO2 displacement at 20%/min produces unconsciousness in about 106 s;6,9,11,16 the time to onset of unconsciousness increased to 156 s when 10%/min was used.2 The lengths of time to unconsciousness and death were examined to model real-time mouse euthanasia. The preliminary data were obtained by using IACUC-approved protocols. Gas exposure times for euthanasia were determined based on these preliminary results (data not shown) and were consistent with previous reports.2,6,9,11,16
Testing procedure.
All trials were conducted without animals in the chamber. Two flow rates were analyzed to model the most common euthanasia techniques. In the first paradigm, the CO2 gas cylinder was turned on for 7 min to a flow rate of 3 L/min (about a 15% fill rate of 100% CO2), replicating a continuous, slow, fill rate. The second method (3 to 10 L/min) consisted of a flow rate of 3 L/min of 100% CO2 for 2 min followed by 10 L/min (about a 50% fill rate) for 3 min, replicating a slow fill rate until a mouse was rendered unconscious and followed by a fast fill rate. At the end of each trial, the CO2 was turned off and, except for the lid-on experiment, the lid was removed 15 s later. Four variables were assessed during the study: chamber position (turned over or not); cage in the chamber until lid removal (yes or no); lid (on or off); and flow rate (3 L/min or 3 to 10 L/min). For the trials in which the chamber was turned over, the inversion was performed right after the lid was removed. The chamber then was placed so that 17.8 cm (40% of its length) extended over the counter edge in all upside-down trials. Five trials were done for each combination of variables. With the lid on, only a 3 L/min flow rate was assessed.
The chamber CO2 concentration was at or below 0.05% before a trial started. Measurements were collected every 15 s until the lid was removed. After lid removal, the CO2 concentration within the chamber was recorded every 5 s until the CO2 concentration fell below 1%. The time for dissipation was measured as the time elapsed from peak CO2 to 50%, 30%, 20%, 10%, and 1% CO2 concentrations. The trials in which the lid remained on were stopped at 20 min, because the chamber CO2 concentration did not reach 10%.
Statistical analysis.
Statistical analyses were done by using SAS software (version 9.3, SAS Institute, Cary, NC). Prior to the interpretation of results, data were checked for normality and homogeneity of variance. Data that were in violation were transformed before additional testing. The outcome variables were peak CO2 and time from peak CO2 to specified dissipation level. t-tests were used to compare mean time to dissipation levels of 50%, 30%, and 20% for lid on compared with lid off at the 3 L/min flow rate. For the remaining tests, means were compared by using 3-way ANOVA involving factors of cage presence, chamber position, and flow rate. Significant interaction effects were analyzed, followed by significant main effects that were not involved in any significant interactions. Significance was set at α = 0.05, and, where appropriate, the location of significant effects was determined by using a Tukey post hoc test. Data are presented as group mean ± 1 SD.
Results
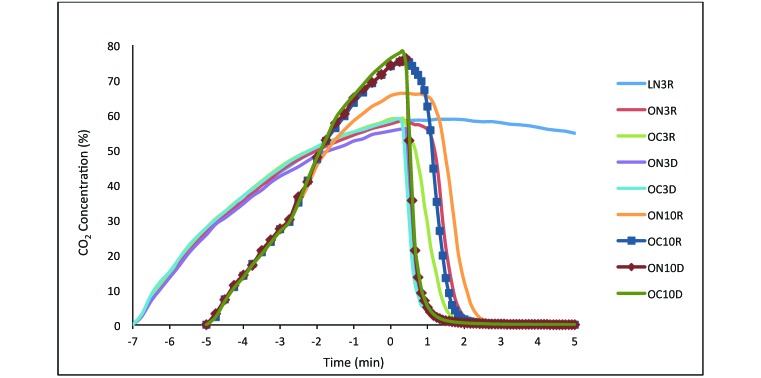
Peak CO2 values in the chamber occurred at the completion of filling the chamber (5 or 7 min) and ranged between 50% and 80% (Table 1, Figure 1). The average peak for the experiments using 3 L/min flow rates was 59% and for the 3–10 L/min flow rates was 70%. These values represent a significant (P < 0.0001) increase due to flow rate. In addition, there were significant 2-way interactions between flow rate and cage (P = 0.0092) and between flow rate and chamber (P = 0.0453; Table 1).
Table 1.
CO2 levels in a 22-L chamber
| Chamber turned over after CO2 turned off? | Cage in chamber? | Lid | Flow rate (L/min) | Peak CO2 (%; mean ± 1 SD) | Time (s; mean ± 1 SD) needed after the gas was turned off for CO2 concentration to fall below the indicated concentration |
||||
| 50% | 30% | 20% | 10% | 1% | |||||
| No | No | On | 3 | 59 ± 5a,b | 363 ± 151a | 960 ± 169a | 1125a,e | >1200a,f | >1200a,f |
| No | No | Off | 3 | 59 ± 1a,b | 45 ± 16b | 59 ± 19b | 65 ± 19b,c | 72 ± 19b,c | 109 ± 19b,c |
| No | Yes | Off | 3 | 59 ± 2a,b | 19 ± 9c | 38 ± 6c | 44 ± 6c | 54 ± 6c | 91 ± 7c,d |
| No | No | Off | 3–10 | 66 ± 5b,c | 68 ± 20b | 79 ± 18b | 85 ± 19b | 92 ± 19b | 128 ± 19b |
| No | Yes | Off | 3–10 | 76 ± 2d | 46 ± 2b | 57 ± 5b | 63 ± 8b,c | 69 ± 7c | 108 ± 6b,c |
| Yes | No | Off | 3 | 56 ± 5a | 8 ± 5c,d | 15 ± 0d | 19 ± 2d | 26 ± 7d | 73 ± 3d |
| Yes | Yes | Off | 3 | 59 ± 2a,b | 9 ± 2d | 13 ± 3d | 17 ± 5d | 26 ± 7d | 82 ± 16d |
| Yes | No | Off | 3–10 | 71 ± 7c,d | 9 ± 2d | 14 ± 2d | 20 ± 0d | 27 ± 3d | 77 ± 3d |
| Yes | Yes | Off | 3–10 | 79 ± 2d | 11 ± 2c,d | 16 ± 2d | 21 ± 2d | 30 ± 0d | 81 ± 6d |
Values in the same column with different superscript letters (a through d) differed significantly (P < 0.05 with adjustment for multiple comparisons) from each other.
e2-trial average; the other 3 trials were > 20 min (actual time not observed).
fData collection stopped 20 min after CO2 was turned off.
Figure 1.
CO2 concentrations in a 22-L euthanasia chamber. Data collection started when the gas was turned on. The time at which the gas was turned off is time 0. CO2 levels in the chamber dropped rapidly after the CO2 was turned off in all experiments in which the lid was removed. L, lid on; O, lid off; N, no cage in chamber; C, cage in chamber; 3, CO2 flow rate of 3 L/min; 10, CO2 flow rate of 3–10 L/min; R, chamber right side up; D, chamber upside down.
We also examined the time needed for CO2 levels within the chamber to drop below 50%, 30%, 20%, 10%, and 1% after the gas was turned off (Table 1). For all CO2 concentrations, there was a significant delay in CO2 dissipation when the lid was left on compared with when the lid was removed (P < 0.009). The time for dissipation when the lid was left on was 6 min to reach 50% and more than 20 min to reach 10% (Figure 1). When the lid was removed, dissipation to 50% CO2 took less than 1 min for most experiments, dissipation to 10% took less than 1.5 min, and dissipation to below 1% CO2 occurred within 2 min for most scenarios (Table 1).
There were only slight variations in the patterns of significance as CO2 dissipated. There was a main effect of turning the cage over in the effect on dissipation. The mean time to CO2 dissipation was faster when the chamber was turned upside down (P < 0.0001), except for nonsignificant comparisons at the 50% and 1% level. In the trials with the cage turned over after the gas was turned off, the CO2 dissipated to below 10% in 30 s or less. In comparison, the dissipation rate to 10% CO2 was 2 to 3 times longer when the chamber was not inverted. When the chamber was left in a normal position, inserting and then removing a cage from the chamber and using the low flow rate led to the fastest dissipation of CO2. In contrast, using a high flow rate and an empty upright chamber had the slowest CO2 dissipation rate. Significant differences in CO2 dissipation in the upright chamber are presented in Table 1.
Discussion
CO2 is a favorable agent because it induces rapid loss of consciousness6 and rapid onset of death.17 However, the AVMA recommendations for its use stipulate that placement in 100% CO2 is unsatisfactory. We were concerned that after using a dedicated euthanasia chamber for one group of animals, the level of CO2 left in the chamber would delay subsequent euthanasia efforts. Using our described methods, we found that the levels of CO2 never achieved 100% in the chamber. This result is likely due to the short exposure times (5 or 7 min of CO2 input) and to escape of CO2 from the chamber through the small openings in the lid and the gap between the lid and chamber. Because CO2 peaked between only 50% and 80%, subsequent euthanasia events would meet the AVMA guidelines because no rodents would be immersed into 100% CO2. However, we do not believe this result meets the intention of the regulation, and we speculate that the intention was to minimize pain associated with high levels of CO2. This notion is corroborated by a consensus report on CO2 euthanasia, which stated that it is more important to avoid or minimize pain and distress than to ensure a rapid loss of consciousness.8 This statement is repeated in the 2013 AVMA Euthanasia Guidelines.21
To avoid pain, it is clear that CO2 concentrations must be at least below 50%.6 Pain thresholds for CO2 were found to average 47% in a study of 12 human subjects exposed in both ascending and descending series of exposures.1 Interestingly, the duration of CO2 pulse exposure did not affect pain ratings.1 Distress related to dyspnea typically occurs between 7% and 15% CO2 in people.10,15 Adult male Wistar rats showed increasing behavioral alterations indicative of aversion likely due to dyspnea and not pain at 15% CO2 in a study that used an approach–avoidance test to assess responses to CO2 exposure.19 Rats were given food rewards in a chamber with CO2 or they could leave the area. The rats had minimal alteration in food consumption at 5% or 10% CO2 compared with normal air. However, when CO2 was 15%, there was a reduction in food consumption, and the rats avoided the CO2-filled area.19 At 20% CO2, rats immediately departed the chamber without eating.19 In another study, rats spent less time in cages when the CO2 level was as low as 3% compared with adjacent cages with normal CO2 levels.13 It is unknown whether the reduction in time spent in the areas is due to pain or distress. It should be noted that mice can detect CO2 levels at near-atmospheric conditions.12
Therefore to avoid aversive and/or painful CO2 exposure levels, it appears important to reduce the concentration of CO2 to 10% or lower prior to introduction of an animal to the euthanasia chamber. In our current study, we examined the time required for a 22-L chamber to reach 50%, 30%, 20%, 10% and 1% CO2. When the lid was removed, the decline in chamber CO2 was rapid in all cases, leading to concentrations of 10% CO2 or lower typically within 30 to 90 s. The slowest reduction in chamber CO2 occurred when the chamber was left undisturbed and did not contain a cage and the gas was turned up from a 15% /min fill rate to 50%/min fill rate. Under these conditions, there was a moderate delay in dissipation until the CO2 started mixing with the air. We found that CO2 dissipation to less than 10% was faster when the gas fill rate remained at 15%/min, when a cage was removed from the chamber, and when the chamber was turned over. In fact, turning the chamber over and placing it over the edge of the counter led to the fastest dissipation of CO2. These results are logical, given that (1) a slower CO2 flow rate leads to a lower peak concentration and faster dissipation; (2) the removal of a cage causes mixing of the air due to the motion of the removal, thus enabling faster dissipation; and (3) CO2 is heavier than air, such that turning over the chamber leads to better mixing of air and CO2 and thus improves dissipation.
There were several minor limitations of the study. First was the delay in measurement due to the tube length to the monitor; this feature delayed all readings by 7 s. In addition, no animals were present in the chamber during testing, a factor that affects the volume of the chamber and total CO2 present in the chamber. However, we believe that the contribution of having mice in the chamber would have a negligible effect on these experiments. In addition, the act of removing animals from the chamber after euthanasia was not modeled. In light of the increased dissipation rate of the CO2 in response to cage removal from inside the chamber, we believe that the actions of reaching in and removing euthanized animals would speed dissipation. Another variable that affected measurements was the small gap between the chamber and lid that was created by the insertion of the tube for measurement. The opening probably allowed increased gas escape and reduced the peak CO2 levels in the chamber. We feel that none of these limitations significantly altered the results.
In conclusion, we found that CO2 cleared from the euthanasia chamber within 90 s, and this time was decreased to as little as 30s by any movement of air around or into the chamber. We recommend that users allow a short period of time (maximum, 2 min) for CO2 dissipation between the euthanasia of 2 groups of animals. The duration of this delay depends on the technique used to clear the chamber, the chamber size, and the surface area of the chamber opening.
Acknowledgments
This research was supported in part by the Wright State University STREAMS program (NIH grant no. 5R25HL103168-02).
References
- 1.Anton F, Euchner I, Handwerker HO. 1992. Psychophysical examination of pain induced by defined CO2 pulses applied to the nasal mucosa. Pain 49:53–60 [DOI] [PubMed] [Google Scholar]
- 2.Burkholder TH, Niel L, Weed JL, Brinster LR, Bacher JD, Foltz CJ. 2010. Comparison of carbon dioxide and argon euthanasia: effects on behavior, heart rate, and respiratory lesions in rats. J Am Assoc Lab Anim Sci 49:448–453 [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang IC, Dong HP, Yang RC, Wang TH, Tsai JH, Yang PH, Huang MS. 2010. Effect of carbon dioxide on pulmonary vascular tone at various pulmonary arterial pressure levels induced by endothelin 1. Lung 188:199–207 [DOI] [PubMed] [Google Scholar]
- 4.Coenen AML, Drinkenburg WH, Hoenderken R, van Luijtelaar EL. 1995. Carbon dioxide euthanasia in rats: oxygen supplementation minimized signs of agitation and distress. Lab Anim 29:262–268 [DOI] [PubMed] [Google Scholar]
- 5.Conlee KM, Stephens ML, Rowan AN, King LA. 2005. Carbon dioxide for euthanasia: concerns regarding pain and distress, with special reference to mice and rats. Lab Anim 39:137–161 [DOI] [PubMed] [Google Scholar]
- 6.Danneman PJ, Stein S, Walshaw SO. 1997. Humane and practical implications of using carbon dioxide mixed with oxygen for anesthesia or euthanasia of rats. Lab Anim Sci 47:376–385 [PubMed] [Google Scholar]
- 7.Hackbarth H, Kuppers N, Bohnet W. 2000. Euthanasia of rats with carbon dioxide: animal welfare aspects. Lab Anim 34:91–96 [DOI] [PubMed] [Google Scholar]
- 8.Hawkins P, Playle L, Golledgee H, Leach M, Banzett R, Coenen A, Cooper J, Danneman P, Flecknell P, Kirden R, Niel L, Raj M. [Internet]. 2006. Newcastle Consensus Meeting on Carbon Dioxide Euthanasia of Laboratory Animals. London (UK): National Centre for the Replacement, Refinement, and Reduction of Animals in Science. [Cited 10 June 2014]. Available at: www.nc3rs.org.uk/downloaddoc.asp?id=416&page=292&skin=0 [Google Scholar]
- 9.Hewett TA, Kovacs MS, Artwohl JE, Bennett BT. 1993. A comparison of euthanasia methods in rats, using carbon dioxide in prefilled and fixed flow-rate filled chambers. Lab Anim Sci 43:579–582 [PubMed] [Google Scholar]
- 10.Hill L, Flack M. 1908. The effects of excess carbon dioxide and of want of oxygen upon the respiration and the circulation. J Physiol 37:77–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hornett TD, Haynes AP. 1984. Comparison of carbon dioxide–air mixture and nitrogen–air mixture for the euthanasia of rodents: design of a system for inhalation euthanasia. Anim Technol 4:93–99 [Google Scholar]
- 12.Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. 2007. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science 317:953–957 [DOI] [PubMed] [Google Scholar]
- 13.Krohn TC, Hansen AK, Dragsted N. 2003. The impact of low levels of carbon dioxide on rats. Lab Anim 37:94–99 [DOI] [PubMed] [Google Scholar]
- 14.Leach MC, Bowell VA, Allan TF, Morton DB. 2002. Aversion to gaseous euthanasia agents in rats and mice. Comp Med 52:249–257 [PubMed] [Google Scholar]
- 15.Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, Robillard R, Lancaster J, Zamarripa FE, Fox PT, Denton D. 2001. Brain responses associated with consciousness of breathlessness (air hunger). Proc Natl Acad Sci USA 98:2035–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makowska IJ, Vickers L, Mancell J, Weary MD. 2009. Evaluating methods of gas euthanasia for laboratory mice. Appl Anim Behav Sci 121:230–235 [Google Scholar]
- 17.Niel L, Stewart SA, Weary DM. 2008. Effect of flow rate on aversion to gradual-fill carbon dioxide exposure in rats. Appl Anim Behav Sci 109:77–84 [Google Scholar]
- 18.Niel L, Weary DM. 2006. Behavioural responses of rats to gradual fill carbon dioxide euthanasia and reduced oxygen concentrations. Appl Anim Behav Sci 100:295–308 [Google Scholar]
- 19.Niel L, Weary DM. 2007. Rats avoid exposure to carbon dioxide and argon. Appl Anim Behav Sci 107:100–109 [Google Scholar]
- 20.Ormandy EH, Schuppli CA, Weary DM. 2009. Worldwide trends in the use of animals in research: the contribution of genetically modified animal models. ATLA 37:63–68 [DOI] [PubMed] [Google Scholar]
- 21.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre CB, Gwaltney-Bran S, McCrackin MA, Meyer R, Miller D, Shearer J, Yanong R. [Internet]. 2013. AVMA guidelines for the euthanasia of animals: 2013 edition. [Cited 10 June 2014]. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf
- 22.Thomas AA, Flecknell PA, Golledge HDR. 2012. Combining nitrous oxide with carbon dioxide decreases the time to loss of consciousness during euthanasia in mice—refinement of animal welfare? PLoS ONE 7:e32290 10.1371/journal.pone.0032290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Widdicombe J. 1988. Nasal and pharyngeal reflexes, p 233–258. In: Mathew OP, Sant'Ambrogio G, editors. Respiratory function of the airway. New York (NY): Marcel Dekker. [Google Scholar]
- 24.Yavari P, McCulloch PF, Panneton WM. 1996. Trigeminally mediated alteration of cardiorespiratory rhythms during nasal application of carbon dioxide in the rat. J Auton Nerv Syst 61:195–200 [DOI] [PubMed] [Google Scholar]