Abstract
Background
Enhanced recovery after surgery (ERAS) programmes aim to improve postoperative outcomes. They are being utilized increasingly in hepatic surgery. This review aims to evaluate the impact of ERAS programmes on outcomes following liver surgery.
Methods
EMBASE, MEDLINE, PubMed and the Cochrane Database were searched for trials comparing outcomes in patients undergoing liver surgery utilizing ERAS principles with those in patients receiving conventional care. The primary outcome was occurrence of postoperative complications within 30 days. Secondary outcomes included length of stay (LoS), functional recovery and adherence to ERAS protocols.
Results
Nine articles were included in the review, of which two were randomized controlled trials (RCTs). Overall complication rates were 25.0% (range: 11.5–46.4%) in ERAS patients, and 31.0% (range: 11.8–46.2%) in conventional care patients. Significantly reduced overall complication rates following ERAS care were demonstrated by a meta-analysis of the data reported in the two RCTs (odds ratio: 0.49, 95% confidence interval 0.28–0.84; P = 0.01) The median LoS reported by the studies was 5.0 days (range: 2.5–7.0 days) in ERAS patients, and 7.5 days (range: 3.0–11.0 days) in non-ERAS patients. Recovery milestones, when reported, were improved following ERAS care.
Conclusions
The adoption of ERAS protocols improves morbidity and LoS following liver surgery. Future ERAS programmes should accommodate the unique requirements of liver surgery in order to optimize postoperative outcomes.
Introduction
Enhanced recovery after surgery (ERAS) programmes were introduced initially in colorectal surgery, in which they have been associated with improvements in postoperative length of stay (LoS) and morbidity.1 They have since been adopted by multiple specialties, including orthopaedic surgery,2 gynaecology3 and breast surgery.4
The underlying principle of ERAS is a multimodal perioperative protocol to attenuate the inflammatory response and potentiate patient rehabilitation following major surgery.5 The intention is to prevent the problems associated with an exaggerated inflammatory reaction to surgery, such as poor healing, infective complications and organ dysfunction.6 This approach, incorporating intensive optimization of mobility, gut function and analgesia,7 contributes to expediting recovery and minimizing morbidity.
Enhanced recovery after surgery programmes reduce postoperative morbidity rates following a variety of surgical procedures.1 Liver resections have traditionally been associated with high mortality and morbidity rates. With current surgical and perioperative management, mortality rates of <5% can be achieved.8 However, morbidity rates remain high at 15–50%.9 Adopting ERAS protocols may facilitate further improvement in surgical outcomes in hepatic resection.
Recently, a number of publications have examined the application of ERAS programmes to hepatic surgery. This review evaluates the impacts of these programmes on morbidity and recovery rates following liver surgery.
Materials and methods
This study was conducted according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines for meta-analysis.10 A literature search of EMBASE, MEDLINE, PubMed and the Cochrane Database was performed independently by two researchers in May 2013.
The databases were searched for the period 1966–2013 using the key terms ‘enhanced recovery’, ‘fast track’, ‘ERAS’ and ‘liver’, ‘hepatobiliary’, ‘hpb’. All abstracts were reviewed for relevance. The full texts of relevant articles were subsequently reviewed.
All trials assessing enhanced recovery following liver surgery were included. Inclusion criteria required that the study should clearly state the ERAS protocol, which should contain at least four items of care considered to be contributory to an enhanced recovery programme.11 Exclusion criteria discounted any studies involving children aged 16 years and younger, and any studies that reported the use of a non-standard care pathway or compared ERAS protocols in both arms of the study.
All studies included in the final analysis were assessed by two independent reviewers. Study quality and bias were assessed independently using the Downs and Black score.12 Data were extracted directly from the papers according to data extraction forms.
The primary outcome was the occurrence of any complication within 30 days postoperatively. The following markers were assessed as secondary outcomes: LoS; time to the achievement of functional recovery; time to independent mobility; time to resumption of diet, and time until first bowel motion/flatus.
The meta-analysis was performed using RevMan Version 5.2 (Nordic Cochrane Centre, Copenhagen, Denmark). Dichotomous data were analysed using the fixed-effects odds ratio. Heterogeneity was assessed using I2 and chi-squared tests and judged to be significant if the I2-value was >50% and according to a P-value of <0.05. The cut-off for statistical significance was set at P < 0.05. When continuous quantitative data were not distributed normally, meta-analysis was not performed and a qualitative assessment was utilized.
Results
Study characteristics
A total of 257 papers were identified. The PRISMA diagram is shown in Fig. 1. Nine studies were included for review.13–21
Figure 1.
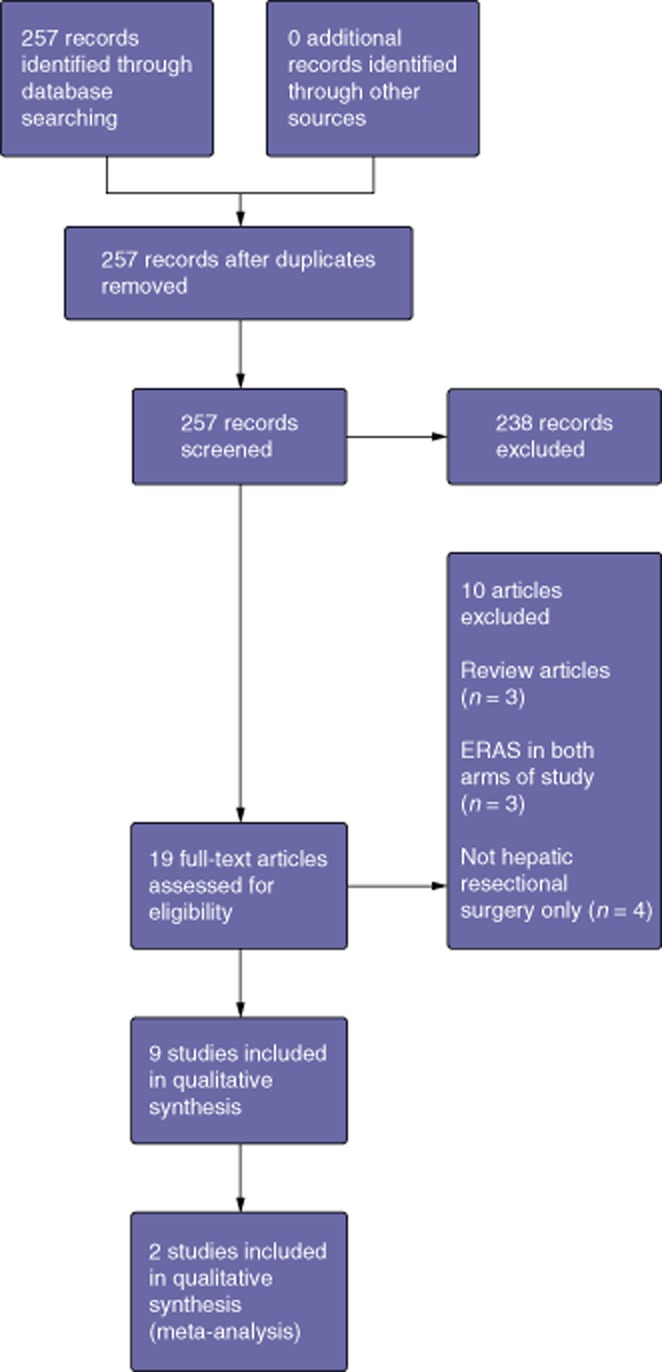
PRISMA diagram illustrating the identification and selection of studies for review
Studies investigating outcomes in open hepatic surgery included two randomized controlled trials (RCTs),16,17 two prospective cohort studies 18,19 and one retrospective cohort study21 and two case–control studies.13,20 Two case–control trials compared outcomes of ERAS protocols with those of conventional care after laparoscopic surgery.14,15
The trials included spanned the period from 2008 to 2013. A total of 522 patients underwent liver resection according to an ERAS protocol and 316 were managed on a conventional care pathway following liver resection. The median patient age was 60.0 years (range: 48.4–64.0 years) in the ERAS group and 52.5 years (range: 45.0–67.0 years) in the conventional care group. The majority of the operations were for colorectal liver metastases or hepatocellular carcinoma. Details of participant characteristics are shown in Table 1. All studies explicitly described an ERAS protocol. A median of 11 (range: 8–19) ERAS items were utilized. The individual components utilized and rates of adherence to the protocol are displayed in Table 2.
Table 1.
Demographic and operative details reported in the studies of outcomes of enhanced recovery after surgery (ERAS) programmes covered in this review
| van Dam et al13 | Sànchez-Pérez et al14 | Stoot et al15 | Jones et al16 | |
|---|---|---|---|---|
| ERAS/C | ERAS/C | ERAS/C | ERAS/C | |
| Patients, n | 61/100 | 26/17 | 13/13 | 46/45 |
| Age, years, median (range) | 62 (24–82)/60 (20–81) ns | 58.3 (29–77)/52.5 (29–84) ns | 55 (34–82)/45 (26–70) ns | 64 (IQR 27–83)/67 (IQR 27–84) ns |
| ASA class, n (%) | ||||
| I | 11 (18)/14 (14) | 0/0 | 3 (23)/6 (46) | 0/2 (4.5) |
| II | 42 (69)/64 (64) | 13 (50)/8 (47) | 9 (69)/6 (46) | 43 (93)/38 (84.5) |
| III | 8 (13)/22 (22) | 13 (50)/9 (53) | 1 (8)/1 (8) | 3 (7)/5 (11) |
| IV | –/– | –/– | –/– | –/– |
| ns | ns | ns | ns | |
| Resections, n (%) | ||||
| ≥3 segments | 51 (84)/79 (79) (≥2 segments) | 0/0 | 0/0 | 21 (46)/12 (27) |
| <3 segments | 10 (16)/21 (21) (<2 segments) ns | 26 (100)/17 (100) ns | 12 (92)/12 (92) Segmentectomy (≥2) 1 (8)/1 (8) Ns | 25 (54)/33 (73) POSSUM score higher in ERAS group (P = 0.012) |
| Pathology, n (%) | ||||
| CLM | 51 (84)/72 (72) | – | Malignant | 35 (76)/26 (58) |
| HCC | 4 (7)/9 (9) | 3 (12)/3 (18) | 8 (62)/3 (23) | – |
| Cholangio | – | 1 (4)/0 | Benign | – |
| Benign | 4 (7)/14 (14) | 14 (54)/14 (82) | 5 (38)/10 (77) | 1 (2)/9 (20) |
| Other metastases | 2 (3)/4 (4) ns | 8 (31)/0 (P < 0.05) | ns | 10 (22)/10 (22) (P = 0.021) |
| Neoadjuvent therapy, n (%) | 38 (62)/33 (33) (P ≤ 0.001) | NA | NA | 36 (78)/25 (56) (P = 0.021) |
| Cirrhosis | NA | NA | NA | NA |
| Sex, n (%) | ||||
| Male | 35 (57)/51 (51) ns | 15 (58)/10 (59) ns | 3 (23)/2 (15) ns | 31 (67)/23 (51) ns |
| EBL, ml, median (range) | 750 (0–5000)/800 (0–6000) ns | Transfusion required ERAS 19.2%; C 5.8% (P < 0.05) | 50 (50–200)/250 (50–800) (P = 0.002) | 350 (IQR 174–900)/340 (IQR 150–645) ns |
| Operating time, mins, median (range) | 220 (60–420)/270 (106–510) (P < 0.001) | 180 (60–345)/177 (80–300) ns | 118 (85–192)/180 (51–340) ns | NA |
| Pringle time, mins, mean (SD) | NA | 7 (26.9)/5 (29.4) ns | NA | NA |
| Quality (Downs and Black) | 20/32 | 18/32 | 19/32 | 31/32 |
| Ni et al17 | Mackay & O'Dwyer18 | Schultz et al19 | Lin et al20 | Connor et al21 |
|---|---|---|---|---|
| ERAS/C | ERAS/C | ERAS/C | ERAS/C | ERAS/C |
| 80/80 | 12/– | 100/– | 56/61 | 128/– |
| Mean 48.4 (±15.6)/mean 50.1 (±21.8) (P = 0.57) | 60 (43–74)/– | 64 (16–91)/– | 57 (23–73)/55 (22–81) ns | 63 (35–82)/– |
| 7 (95)/78 (98) | 4 (33.3)/– | 29 (29)/– | (I + II) 43 (76.5)/50 (82) | (I + II) 104 (81)/– |
| 4 (5)/2 (2) | 7 (58.3)/– | 46 (46)/– | 11 (19.5)/10 (16) | –/– |
| –/– | 1 (8.3)/– | 25 (25)/– | 2 (4)/1 (2) | 24 (19)/– |
| –/– | –/– | –/– | –/– | –/– |
| ns | ns | |||
| 73 (91)/69 (86) | 3 (25)/– | 32 (32)/– | 19 (34)/21 (34) | 64 (50)/– |
| 7 (9)/11 (14) ns | 9 (75)/– | 68 (68)/– | 37 (66)/40 (66) ns | 64 (50)/– |
| – | 12 (100) | 77 (77) | NA | 84 (66)/– |
| 71 (89)/76 (95) | – | 12 (12) | 9 (7)/– | |
| 9 (11)/4 (5) | – | – | 11 (9)/– | |
| – | – | – | 10 (8)/– | |
| – ns | – | Other 11 (11) | 14 (11)/– | |
| NA | NA | NA | NA | NA |
| 62 (78)/58 (73) ns | NA | NA | NA | NA |
| 66 (83)/59 (74) ns | 8 (66.7)/– | 63 (63)/– | 31 (58)/34 (56) | NA |
| Mean 313 (±223.9)/mean 358.2 (±311.7) ns | NA | NA | 760 (IQR 0–2100)/850 (IQR 0–2300) ns | >0.5 l 67%, 0.5–1.0 l 26%, >1 l 7% |
| Mean 141.2 (±57.4)/mean 132.1 (±36.9) ns | 130 IQR (110–160) | NA | 110 (IQR 60–160)/125 (IQR 81–187) ns | NA |
| NA | NA | NA | NA | NA |
| 27/32 | 18/32 | 22/32 | 21/32 | 21/32 |
ASA, American Society of Anesthesiologists, C, conventional care; CLM, colorectal liver metastases; EBL, estimated blood loss; IQR, interquartile range; HCC, hepatocellular carcinoma; NA, not assessed; ns, no statistically significant difference; SD, standard deviation. Statistically significant results are highlighted in bold.
Table 2.
Care components of enhanced recovery after surgery (ERAS) programmes and adherence data (adherence rates are shown in parentheses)
| van Dam et al13 | Sànchez-Pérez et al14 | Stoot et al15 | Jones et al16 | Ni et al17 | Mackay & O'Dwyer18 | Schultz et al19 | Lin et al20 | Connor et al21 | |
|---|---|---|---|---|---|---|---|---|---|
| Preoperative counselling | X | X (100%) | X | X (100%) | X | X | X | ||
| Avoid bowel prep | X (100%) | X | X | X | |||||
| Carb drinks up to 2 h preoperatively | X | X | X (100%) | X | X (100%) Clear fluids | X | X | ||
| Avoid anaesthetic pre-med | X | X | X (100%) | X | X | X | |||
| VTE prophylaxis | X (100%) | X | |||||||
| Antibiotic prophylaxis | X (100%) | X (100%) | X | ||||||
| Standard anaesthetic protocol | X (95%) | X | X (100%) | X | |||||
| Ileus avoidance | X | X | X (100%) | X | X Laxatives and chewing gum | ||||
| NGT avoidance | X (3/61 had NGT) | Removal at end of surgery | X | X (100%) | X | X Removed immediately postoperatively | X | ||
| Intraoperative warming | X | X (100%) | X | ||||||
| Minimization of preoperative fluids | X | X | X | X (100%) | X | X 25% continued IVI beyond PoD 1 | X | X | X |
| Avoid routine drains | X (1 drain inserted) | Drains removed 24–48 h postop when used | X | X (100%) | X | X except major resection and this was removed PoD 1 | X | X | |
| Early removal of IDC | X | No IDC in procedures <180 mins | X | X (65%) | X | X | X PoD 1 | X | X |
| Multimodal analgesic | X Epidural (95%) | i.v. matamizol and i.v. paracetamol | X | X (100%) | X | X i.v. PCA and paracetamol, then ibuprofen | X Epidural followed by gabapentin, celocoxib and paracetamol | X Epidural removed PoD 3 then NSAIDS | X |
| Early feeding | X (within 4 h in 92%) | X | X | X (100%) | X | X (supplement drink also) | X | X | X |
| Early mobilization | X | X | X | X (100%) | X | X (100%) | X | X | X |
IDC, indwelling urinary catheter; IVI, intravenous infusion; NGT, nasogastric tube; NSAIDs, non-steroidal anti-inflammatory drugs; PCA, patient-controlled analgesia; PoD, postoperative day; VTE, venous thromboembolism.
Complications
All nine studies assessed complication rates.13–21 Median overall complication rates were 25.0% (range: 11.5–46.4%) in ERAS patients, and 31.0% (range: 11.8–46.2%) in conventional care patients. However, Ni et al.17 observed a significantly reduced overall complication rate in the ERAS group (Table 3), and a meta-analysis of overall complication rates in the two RCTs16,17 shows that significantly fewer complications occurred after ERAS surgery [I2 = 0%; odds ratio (OR): 0.49, 95% confidence interval (CI) 0.28–0.84; P = 0.01]. Both Jones et al.16 and Ni et al.17 reported significantly fewer non-surgical complications in the ERAS arms of their studies [7.0% versus 27.0% (P = 0.02) in Jones et al.16; 12.5% versus 25.0% (P = 0.04) in Ni et al.17], but showed no statistically significant difference in the occurrence of liver-specific complications [15.0% in ERAS patients and 11.0% in conventional care patients (P = 0.612) in Jones et al.16; 17.5% in ERAS patients and 21.0% in conventional care patients (P = 0.55) in Ni et al.17)] (Tables S1 and S2, online). Mortality rates were low and were similar in both groups (Table 3).
Table 3.
Outcome data reported in studies of the impact of enhanced recovery after surgery (ERAS) programmes
| Design | Arm A | Arm B | LoS, A, days, median (range) | LoS, B, days, median (range) | Functional recovery, A, days, median (range) | |
|---|---|---|---|---|---|---|
| van Dam et al13 | Case–control | ERAS (n = 61) | Conventional care (n = 100) | 6.0 (3–82) (P < 0.01) | 8.0 (4–68) | NA |
| Sànchez-Pérez et al14 | Case–control | Laparoscopic ERAS (n = 26) | Laparoscopic conventional care (n = 17) | 2.5 (1–39) ns | 3 (1–22) ns | NA |
| Stoot et al15 | Case–control | Laparoscopic ERAS (n = 13) | Laparoscopic conventional care (n = 13) | 5.0 (3–10) ns | 7.0 (3–12) ns | 3 (1–7) (P = 0.044) |
| Jones et al16 | RCT | ERAS (n = 46) | Conventional care (n = 45) | 4 (IQR 3–5) (P < 0.01) | 7 (IQR 6–8) | 3 (IQR 3–4) (P < 0.001) |
| Ni et al17 | RCT | ERAS (n = 80) | Conventional care (n = 80) | Mean 6.9 (±2.8) (P = 0.018) | Mean 8.0 (±3.7) | Mean 5.2 (±2.3) (P = 0.0004) |
| Mackay & O'Dwyer18 | Prospective cohort | ERAS (n = 12) | – | 4 (2–7) days | – | NA |
| Schultz et al19 | Prospective cohort | ERAS (n = 100) | – | 5 days (minor open); 6 days (major open) | – | NA |
| Lin et al20 | Case–control | ERAS (n = 56) | Conventional care (n = 61) | 7 (3–26) (P < 0.01) | 11 (4–37) | NA |
| Conner et al21 | Retrospective review | ERAS (n = 128) | – | 4 (2–111) | – | NA |
| Functional recovery, B, days, median (range) | Readmissions A | Readmissions B | Complications A | Complications B | Mortality | |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| van Dam et al13 | NA | 8 (13) ns | 10 (10) ns | 25 (41) ns | 31 (31) ns | A = 0 B = 2 (2) ns |
| Sànchez-Pérez et al14 | NA | 1 (3.8) ns | 1 (5.8) ns | 3 (11.5) ns | 2 (11.8) ns | A = 0 B = 0 |
| Stoot et al15 | 5 (2–8) | 0 ns | 0 ns | 2 (15) ns | 2 (15) ns | A = 0 B = 0 |
| Jones et al16 | 6 (IQR 6–7) | 2 (4) ns | 0 ns | 8 (17) ns | 14 (31) ns | A = 1 (2) B = 1 (2) ns |
| Ni et al17 | Mean 6.7 (±2.9) | NA | NA | 24 (30) (P = 0.03) | 37 (46) | A = 0 B = 0 ns |
| Mackay & O'Dwyer18 | – | NA | – | 3 (25) | – | A = 0 |
| Schultz et al19 | – | 6 (6) | – | 25 (25) | – | A = 1 (1) |
| Lin et al20 | NA | 4 (7.1) ns | 2 (3.3) ns | 26 (46.4) ns | 27 (44.3%) ns | A = 1 (2) B = 1 (2) ns |
| Conner et al21 | – | 14 (11) | – | 34 (26.6) | – | A = 2 (1.6) |
C, conventional care; IQR, interquartile range; HCC, hepatocellular carcinoma; LoS, length of stay; NA, not assessed; ns, no statistically significant difference; SD, standard deviation. Statistically significant results are highlighted in bold.
Length of stay
The median LoS reported by the studies was 5.0 days (range: 2.5–7.0 days) in ERAS patients and 7.5 days (range: 3.0–11.0 days) in non-ERAS patients. The three cohort studies reported a median LoS of 4.0 days18,21 and 5.0 days.19 All four studies that compared ERAS with conventional management in open liver surgery showed a significantly reduced LoS in the ERAS groups.13,16,17,19 Neither of the two laparoscopic studies14,15 identified a reduced LoS. However, Stoot et al.15 reported reduced time to achieve functional recovery. Functional recovery was reported by only three studies,15–17 all of which showed a reduced time to recovery following ERAS care. Five13,14,16,19,20 of the nine trials reported on readmission rates, but observed no significant differences (Table 3).
Adherence
Three of the studies reported rates of adherence to the protocol.13,16,18 Jones et al.16 reported 100% adherence in all 19 ERAS categories except the early removal of the indwelling urinary catheter (IDC). Mackay and O'Dwyer18 reported prolonged use of i.v. fluid administration beyond the first postoperative day. Rates of intra-abdominal drain insertion and reduced feeding were also reported (Table 2).
Parameters of recovery
Only three14,16,17 of the trials reported on the achievement of individual recovery milestones. Time to flatus was significantly reduced in the ERAS groups. Time to establishment of oral diet and time to independent mobilization were either comparable or improved in the ERAS groups when reported (Table S3, online).
Discussion
This review investigated the effects of ERAS protocols on recovery following liver resection. Three previous reviews11,22,23 have been performed in this area, and have concluded that safety and feasibility were satisfactory and that a reduced LoS does not result in increased morbidity or mortality. However, these reviews11,22,23 included studies other than those concerned purely with ERAS versus conventional care, did not report any RCTs and reviewed only two studies comparing ERAS with conventional care after open surgery. Since these reviews11,22,23 were released, five studies have been published, including two RCTs. Therefore, in light of this new evidence, it is important to review the current recommendations.
The present review was limited because the small number of RCTs prevents any meaningful meta-analysis. The majority of studies were observational, which reduces the power of the review and prevents optimal quantitative comparison. However, all trials were procedure-specific and compared ERAS with conventional recovery protocols and thus this review represents the current best available evidence.
In concordance with the previous reviews on this subject,11,22,23 the current review observed that LoS is reduced by ERAS programmes, a result seen in all of the individual studies in open liver resection. However, by contrast with the previous reviews, and in line with the colorectal literature,1 complication rates in hepatic surgery can also be reduced by ERAS protocols: a meta-analysis of both published RCTs16,17 shows a significant reduction in overall complication rates.
This reduction was not repeated in the non-RCT studies. This may be related to study design and power. However, it is noteworthy that the study conducted by Ni et al.17 featured the youngest population of all the studies and both RCTs16,17 included relatively fitter populations. Advanced age and American Society of Anesthesiologists (ASA) class are both independent predictors of morbidity following abdominal surgery24 and it is possible that the younger and fitter populations of these studies16,17 progressed better in an enhanced recovery protocol. Furthermore, Jones et al.16 employed an ERAS programme incorporating 19 components – more than in any other trial – and compliance with the protocol was exceptionally high, a key consideration in the conduct of ERAS programmes.25
Adherence was poorly reported: only three trials commented on this aspect. The main areas of reported poor compliance were i.v. fluid restriction, IDC removal and early mobility. Within the literature on ERAS programmes in the context of colorectal surgery, compliance is often not recorded or may be as low as 5%.25 Higher rates of compliance are associated with reduced LoS and reduced compliance is associated with high readmission rates.25 Hence adherence is clearly an area which has potential for improvement in ERAS protocols following liver surgery.
Although the rates of general complications were observed to have been reduced in the two RCTs, no difference in liver-specific surgical complications was observed. Liver resection offers a unique set of postoperative circumstances as a result of the process of liver regeneration, the anatomical complexity of biliary drainage and intraoperative vascular inflow control, and the transient impairment of liver function following resection. It is therefore not surprising that an ERAS approach does not reduce surgical complications in such patients.
Whereas ERAS protocols focus on pre- and postoperative considerations, liver surgery provides an opportunity to optimize intraoperative care. The minimizing of blood loss should reduce liver-specific surgical complications.26–28 Raised central venous pressure (CVP) has been shown to be associated with intraoperative blood loss during liver resection.29 Six of the nine trials covered in this review included a care component based on the reduction of intraoperative fluid, but only two13,21 commented on titration of i.v. fluid according to CVP. Jones et al.16 used goal-directed fluid therapy guided by LiDCO cardiac output monitor to prevent fluid overload, although they did this in the early postoperative period. It would appear that ERAS protocols in liver surgery should incorporate both intraoperative and postoperative components to maximize their gains.
Areas that were not explored by the studies covered in this review included the use of a thoracic epidural. Although a thoracic epidural is recommended in ERAS in the context of colorectal surgery,30 its use has been questioned in liver surgery.31 There is evidence suggesting that epidurals may impair recovery in liver surgery, and that alternative methods of analgesia should be considered.32,33
Furthermore, a small liver remnant may be a contraindication to the administration of paracetamol.34 Paracetamol is routinely utilized as the backbone of analgesic regimens,35 but in major hepatic resections it is often withheld for fear of inducing liver damage, which increases opiate requirements. At present, further evaluation of analgesia in liver surgery within the context of an ERAS programme is required to establish optimal practice.
This review has highlighted the benefits of the application of enhanced recovery principles following liver surgery. However, the evidence supporting these principles stems from the literature on colorectal surgery. Resectional liver surgery comes with its own set of unique conditions which must be acknowledged when attempting to optimize the outcomes of patients following liver surgery. In order to maximize the potential benefit of such programmes, future research should aim to establish perioperative care plans specific to liver surgery and should accommodate the unique requirements of this operation.
Length of stay is not an ideal outcome by which to judge the success of an ERAS programme because the factors that make patients able to or keen to leave hospital are many.36 Functional recovery was infrequently assessed in the present studies, which offered only modest reporting of recovery milestones. When recovery milestones were reported, ERAS protocols resulted in either parity or some improvement in these outcomes. Both recovery milestones and functional recovery have been suggested as more meaningful than simple LoS in the assessment of the success of an enhanced recovery protocol11 and should represent the measurement of success in future programmes.
In summary, the evidence investigating ERAS following liver surgery is limited and only two RCTS have been conducted. However, postoperative LoS is reduced in the context of ERAS in comparison with that in conventional care. Medical complication rates seem to be reduced, although surgical morbidity remains high and is as yet unaffected by ERAS protocols following liver surgery. Future research should concentrate on perioperative care components specific to liver surgery, such as optimal analgesic regimens and intraoperative manipulations to reduce blood loss, rather than simply transferring components from the literature on colorectal surgery.
Conflicts of interest
None declared.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's website:
Table S1. Surgical complication rates.
Table S2. General complication rates.
Table S3. Parameters of recovery.
References
- 1.Kehlet H, Wilmore D. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189–198. doi: 10.1097/SLA.0b013e31817f2c1a. [DOI] [PubMed] [Google Scholar]
- 2.Scott N, McDonald D, Campbell J, Smith R, Carey A, Johnston I, et al. The use of enhanced recovery after surgery (ERAS) principles in Scottish orthopaedic units – an implementation and follow-up at 1 year, 2010–2011: a report from the Muculoskeletal Audit, Scotland. Arch Orthop Trauma Surg. 2013;133:117–124. doi: 10.1007/s00402-012-1619-z. [DOI] [PubMed] [Google Scholar]
- 3.Lv D, Wang X, Shi G. Perioperative enhanced recovery programmes for gynaecological cancer patients. Cochrane Database Syst Rev. 2010;(6) doi: 10.1002/14651858.CD008239.pub2. CD008239. [DOI] [PubMed] [Google Scholar]
- 4.Arsalani-Zadaeh R, ElFadl D, Yassin N, MacFie J. Evidence-based review of enhancing postoperative recovery after breast surgery. Br J Surg. 2011;98:181–196. doi: 10.1002/bjs.7331. [DOI] [PubMed] [Google Scholar]
- 5.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606–617. doi: 10.1093/bja/78.5.606. [DOI] [PubMed] [Google Scholar]
- 6.Holte K, Kehlet H. Epidural anaesthesia and analgesia – effects on surgical stress response and implications for postoperative nutrition. Clin Nutr. 2002;21:199–206. doi: 10.1054/clnu.2001.0514. [DOI] [PubMed] [Google Scholar]
- 7.Grade M, Quintel M, Ghadimi M. Standard perioperative management in gastrointestinal surgery. Langenbecks Arch Surg. 2011;396:591–606. doi: 10.1007/s00423-011-0782-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palavecino M, Kishi Y, Chun Y, Brown D, Gottumukkala V, Lichtiger B, et al. Two-surgeon technique of parenchymal transection contributes to reduced transfusion rate in patients undergoing major hepatectomy: analysis of 1557 consecutive liver resections. Surgery. 2010;147:40–48. doi: 10.1016/j.surg.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Virani S, Michaelson J, Hutter M, Lancaster R, Washaw A, Henderson W, et al. Morbidity and mortality after liver resections: results of the patient safety in surgery study. J Am Coll Surg. 2007;204:1248–1292. doi: 10.1016/j.jamcollsurg.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coolsen M, Wong-Lun-Hing E, van Dam R, van der Wilt A, Slim K, Lassen K, et al. A systematic review of outcomes in patients undergoing liver surgery in an enhanced recovery after surgery pathways. HPB. 2013;15:245–251. doi: 10.1111/j.1477-2574.2012.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downs S, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomized and non-randomized studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dam R, Hendry P, Coolsen M, Bemelmans H, Lassen K, Revhaug A, et al. on behalf of the Enhanced Recovery After Surgery (ERAS) Group Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg. 2008;95:969–975. doi: 10.1002/bjs.6227. [DOI] [PubMed] [Google Scholar]
- 14.Sànchez-Pérez B, Aranda-Narvaez J, Suarez-Munoz M, Del Fresno M, Fernandez-Aguilar J, Perez-Daga J, et al. Fast-track programme in laparoscopic liver surgery: theory of fact. World J Gastrointest Surg. 2012;4:246–250. doi: 10.4240/wjgs.v4.i11.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoot J, van Dam R, Busch O, van Hillegersberg R, De Boer M, Olde Damink S, et al. on behalf of the Enhanced Recovery After Surgery (ERAS) Group The effect of multimodal fast-track programme on outcomes in laparoscopic liver surgery: a multicentre pilot study. HPB. 2009;11:140–144. doi: 10.1111/j.1477-2574.2009.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones C, Kelliher L, Dickinson M, Riga A, Worthington T, Scott M, et al. Randomized clinical trial on enhanced recovery versus standard care following open liver resection. Br J Surg. 2013;100:1015–1024. doi: 10.1002/bjs.9165. [DOI] [PubMed] [Google Scholar]
- 17.Ni C, Yang Y, Chang Y, Cai H, Xu B, Yang F, et al. Fast-track surgery improves postoperative recovery in patients undergoing partial hepatectomy for primary liver cancer: a prospective randomized controlled trial. Eur J Surg Oncol. 2013;39:542–547. doi: 10.1016/j.ejso.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 18.MacKay G, O'Dwyer P. Early discharge following liver resection for colorectal metastases. Scott Med J. 2008;53:22–24. doi: 10.1258/rsmsmj.53.2.22. [DOI] [PubMed] [Google Scholar]
- 19.Schultz N, Larsen P, Klarskov B, Plum L, Frederiksen H, Christensen B, et al. Evaluation of a fast-track programme for patients undergoing liver resection. Br J Surg. 2013;100:138–143. doi: 10.1002/bjs.8996. [DOI] [PubMed] [Google Scholar]
- 20.Lin D, Li X, Ye Q, Lin F, Li L, Zhang Q. Implementation of a fast-track clinical pathway decreases postoperative length of stay and hospital charges for liver resection. Cell Biochem Biophys. 2011;61:413–419. doi: 10.1007/s12013-011-9203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connor S, Cross A, Sakowska M, Linscott D, Woods J. Effects of introducing an enhanced recovery after surgery programme for patients undergoing open hepatic resection. HPB. 2013;15:294–301. doi: 10.1111/j.1477-2574.2012.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall T, Dennison A, Bilku D, Metcalfe M, Garcea G. Enhanced recovery programmes in hepatobiliary and pancreatic surgery: a systemic review. Ann R Coll Surg Engl. 2012;94:318–326. doi: 10.1308/003588412X13171221592410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spelt L, Ansari D, Sturesson C, Tingstedt B, Andersson R. Fast-track programmes for hepatopancreatic resections: where do we stand? HPB. 2011;13:833–838. doi: 10.1111/j.1477-2574.2011.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feroci F, Lenzi E, Baraghini M, Vannucchi A, Cantafio S, Scatizzi M. Fast-track surgery in real life: how patient factors influence outcomes and compliance with an enhanced recovery clinical pathway after colorectal surgery. Surg Laparosc Endosc Percutan Tech. 2013;23:259–265. doi: 10.1097/SLE.0b013e31828ba16f. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed J, Khan S, Lim M, Chandrasekekaran T, MacFie J. Enhanced recovery after surgery protocols – compliance and variations in practice during routine colorectal surgery. Col Dis. 2011;14:1045–1051. doi: 10.1111/j.1463-1318.2011.02856.x. [DOI] [PubMed] [Google Scholar]
- 26.Hammond J, Guha I, Beckingham I, Lobo D. Prediction, prevention and management of postresection liver failure. Br J Surg. 2011;98:1188–1200. doi: 10.1002/bjs.7630. [DOI] [PubMed] [Google Scholar]
- 27.Zimmitti G, Roses R, Andreou A, Shindoh J, Curley S, Aloia T, et al. Greater complexity of liver surgery is not associated with an increased incidence of liver-related complications except for bile leak: an experience with 2628 consecutive resections. J Gastrointest Surg. 2013;17:57–64. doi: 10.1007/s11605-012-2000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poon R, Fan S, Lo C, Liu C, Lam C, Yuen W, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases. Analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–710. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNally S, Revie E, Massie L, McKeown D, Parks R, Garden O, et al. Factors in perioperative care that determine blood loss in liver surgery. HPB. 2012;14:236–241. doi: 10.1111/j.1477-2574.2011.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lassen K, Soop M, Nygren J, Cox P, Hendry P, Spies C, et al. for the Enhanced Recovery After Surgery (ERAS) Group Consensus review of optimal perioperative care in colorectal surgery. Arch Surg. 2009;144:961–969. doi: 10.1001/archsurg.2009.170. [DOI] [PubMed] [Google Scholar]
- 31.Tzimas P, Prout J, Papadopolous G, Mallett S. Epidural anaesthesia and analgesia for liver resection. Anaesthesia. 2013;68:628–635. doi: 10.1111/anae.12191. [DOI] [PubMed] [Google Scholar]
- 32.Koea J, Young Y, Gunn K. Fast track liver resection: the effect of a comprehensive care package and analgesia with single dose intrathecal morphine with gabapentin or continuous epidural analgesia. HPB Surg. 2009;2009(2009):271986. doi: 10.1155/2009/271986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Revie E, McKeown D, Wilson J, Garden O, Wigmore S. Randomized clinical trial of local infiltration plus patient-controlled opiate analgesia vs. epidural analgesia following liver resection surgery. HPB. 2012;14:611–618. doi: 10.1111/j.1477-2574.2012.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galinski M, Delhotal-Landes B, Lockey D, Rouaud J, Bah S, Bossard A, et al. Reduction of paracetamol metabolism after hepatic resection. Pharmacology. 2006;77:161–165. doi: 10.1159/000094459. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann H, Kettelhack C. Fast-track surgery – conditions and challenges in postsurgical treatment: a review of elements of translational research in enhanced recovery after surgery. Eur Surg Res. 2012;49:24–34. doi: 10.1159/000339859. [DOI] [PubMed] [Google Scholar]
- 36.Maessen J, Dejong C, Kessels A, von Myenfeldt M. Length of stay: an inappropriate readout of the success of enhanced recovery programmes. World J Surg. 2008;32:971–997. doi: 10.1007/s00268-007-9404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Surgical complication rates.
Table S2. General complication rates.
Table S3. Parameters of recovery.


