Abstract
Background
Despite the increasing annual incidence of hepatocellular carcinoma (HCC) in the USA, now estimated at 2.7 cases per 100 000 population, only a small proportion of patients receive treatment and 5-year survival rates range from 9% to 17%.
Objectives
The present study examines the effects of multimodal treatment on survival in a mixed-stage HCC cohort, focusing on the impact of radical therapy in patients with Barcelona Clinic Liver Cancer (BCLC) stage B disease.
Methods
A retrospective review of the medical records of 254 patients considered for HCC treatment between 2003 and 2011 at a large tertiary referral centre was conducted.
Results
A total of 195 (76.8%) patients were treated with a median of two liver-directed interventions. Median survival time was 16 months. In proportional hazards analysis, radiofrequency ablation (RFA) and resection were associated with significantly improved 1- and 5-year survival among patients with BCLC stage 0–A disease. In patients with BCLC stage B disease, RFA conferred a survival benefit at 1 year and resection was associated with significantly improved survival at 5 years.
Conclusions
As one of few studies to track the complete course of sequential HCC therapies, the findings of the present study suggest that HCC patients with intermediate-stage (BCLC stage B) disease may benefit from aggressive interventions not currently included in societal guidelines.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality worldwide; approximately 750 000 new cases are diagnosed and nearly as many attributable deaths occur per year.1 In the USA, annual incidence increased by approximately 3.5% per year from 2001 to 2006, and is now 2.7 per 100 000 persons. This rate is expected to rise through the next decade.2,3 In the USA, the major aetiologic agents are chronic hepatitis C virus (HCV) or hepatitis B virus (HBV) infection.2 Excessive alcohol consumption and non-alcoholic steatohepatitis are also implicated, either as amplifiers of the effects of viral hepatitis or as independent risk factors.4,5 The United States Department of Veterans Affairs (VA) has been disproportionately affected by HCC, largely because of the high incidence of chronic HCV infection among VA patients (5.4%) compared with that in the general US population (1.8%). Currently, 173 000 VA patients are known to be chronically infected with HCV.6 Over the past decade, the VA has seen a five-fold increase in HCC cases.6–8
In its earlier stages, HCC can be cured with aggressive therapies, but the optimal treatment strategy for patients in the intermediate stages of HCC has not been well established.3,9,10 Although treatment at any stage of HCC has been associated with survival benefits, only a small proportion of HCC patients receive any treatment over the course of their disease.3,11,12 Among US patients diagnosed with HCC between 1998 and 2008, 76% received no reported intervention. Although different treatment modalities are often employed sequentially, many studies report only on the first HCC therapy used and thus fail to capture the entire treatment experience.12–16 Other studies focus only on liver transplantation, the most curative intervention.17,18 Transplantation opportunities in HCC patients are limited by advanced stage at diagnosis, comorbid conditions, inadequate social support and constrained resources. There is a paucity of literature examining treatment patterns and outcomes in HCC patients in typical practice settings in which transplantation is not frequently employed.
Current societal guidelines recommend that only patients in the earliest stages of HCC should receive potentially curative HCC therapies, which typically include liver transplantation, hepatic resection and radiofrequency ablation (RFA).19,20 There is increasing evidence, however, in support of the extended use of some or all of these treatments in selected patients with intermediate-stage HCC.21–23 This retrospective medical records review was conducted to examine multimodal HCC treatment in a large tertiary referral veterans hospital. The study demonstrates that patients with both early- and intermediate-stage HCC can achieve a significant survival benefit from potentially curative interventions that are often reserved for early-stage patients.
Materials and methods
Ethics statement
This study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki and in a manner consistent with good clinical practice and applicable regulatory requirements.24 A waiver of consent for the retrospective review of records for this specific study of HCC patient outcomes was obtained from the University of California San Francisco Institutional Review Board and approved by the San Francisco VA Medical Center (SFVAMC) Research and Development Committee.
Patient population and data collection
The study cohort included all previously untreated patients referred to SFVAMC for consideration for HCC treatment between April 2003 and April 2011. Patients in whom HCC was confirmed by histology, cytology or cross-sectional imaging, such as computed tomography (CT) or magnetic resonance imaging (MRI), were included. Those with equivocal cases of HCC or with heterogeneous tumour cells (i.e. HCC and cholangiocarcinoma) were excluded. The study end date was 28 February 2012.
Patient demographic characteristics and baseline clinical risk factors, including results of laboratory studies and tumour staging, were those recorded in the medical record on the date closest to the index visit. Infection with HBV, HCV or human immunodeficiency virus (HIV) was confirmed using standard criteria. Alcohol abuse was defined as a longstanding history of excessive alcohol consumption (more than three standard drinks per day) or a history of alcohol-related medical complications.25 Raw Model of End-stage Liver Disease (MELD) scores (i.e. without consideration for HCC exception points) and Child–Pugh classes were calculated from the medical record data according to standard methods.26–28 Using this information, tumours were staged by the investigators according to the Barcelona Clinic Liver Cancer (BCLC) classification system, which is widely accepted.20,29
Treatment dates and modalities included those from the index visit to the study end date. Treatment decisions were made at multidisciplinary disease management conferences attended by hepatologists, surgeons, radiologists and medical oncologists, as guided by the National Comprehensive Cancer Network and the American Association for the Study of Liver Disease.19,20,29 In brief, surgical resection was considered for single tumours without vascular invasion or distant disease in the presence of adequate liver function. Radiofrequency ablation was most often used for single tumours of 3–5 cm in size in a favourable location in patients who did not fulfil the resection criteria. Other liver-directed therapies, henceforth termed ‘locoregional therapies’ (LRTs), include transarterial (chemo)embolization [TA(C)E], microwave ablation (MWA) and percutaneous ethanol injection (PEI). Locoregional therapies were used to achieve initial control of tumours prior to radical therapy or solely as palliative treatment. Transarterial (chemo)embolization was most often used in patients with several small tumours or impaired liver function. Percutaneous ethanol injection was generally only used for tumours of <3 cm in size.
Further interventions (other than liver transplant) were performed most commonly for recurrent HCC, although some lesions required serial therapies. Repeat contrast CT or MRI was obtained 1–3 months after each treatment, and then serially at 3–6-month intervals. Guided by the consensus in the literature, patients were grouped into one of five treatment groups in declining order of efficacy, based on the most efficacious treatment they had ever received: (i) liver transplantation; (ii) surgical resection; (iii) RFA; (iv) LRT, and (v) no procedural intervention. (Patients who received chemotherapy only and those who received no treatment were combined for analytic purposes, but are described separately in Table 3.)
Table 3.
Treatment modalities in patients with hepatocellular carcinoma by Barcelona Clinic Liver Cancer (BCLC) stage at the index visit
| Characteristics | BCLC stage | Total (n = 254) | P-valued | ||||
|---|---|---|---|---|---|---|---|
| 0 (n = 8) | A (n = 76) | B (n = 105) | C (n = 48) | D (n = 17) | |||
| Number of treatment modalities used, median (range) (n = 192) | 2 (1–3) | 2 (1–11) | 2 (1–9) | 1 (1–5) | 2 (1–4) | 2 (1–11) | |
| All treatment modalities received, n (%)a | |||||||
| Transplant | 2 (25%) | 8 (10.5%) | 1 (1.0%) | 0 | 0 | 11 (4.3%) | 0.0009 |
| Surgical resection | 3 (37.5%) | 23 (32.9%) | 21 (20.0%) | 3 (6.3%) | 1 (5.9%) | 53 (20.9%) | 0.001 |
| RFA | 4 (50.0%) | 30 (39.5%) | 23 (21.9%) | 2 (4.2%) | 1 (5.9%) | 60 (23.6%) | <0.0001 |
| MWA | 0 | 3 (3.9%) | 5 (4.8%) | 0 | 0 | 8 (3.1%) | 0.63 |
| TACE | 2 (25%) | 13 (17.1%) | 23 (21.0%) | 8 (16.7%) | 1 (5.9%) | 47 (18.1%) | 0.55 |
| TAE | 3 (37.5%) | 33 (43.4%) | 51 (48.6%) | 15 (31.3%) | 4 (23.5%) | 106 (41.7%) | 0.16 |
| PEI | 1 (12.5%) | 11 (14.5%) | 23 (21.9%) | 3 (6.3%) | 3 (17.6%) | 41 (16.1%) | 0.15 |
| Chemotherapy | 2 (25.5%) | 11 (14.4%) | 16 (15.3%) | 12 (25.0%) | 0 | 41 (16.1%) | 0.49 |
| Never treated | 0 | 4 (5.3%) | 16 (15.2%) | 20 (41.7%) | 11 (64.7%) | 51 (20.1%) | <0.0001 |
| First treatment modality received, n (%) | |||||||
| Transplant | 0 | 0 | 0 | 0 | 0 | 0 | <0.0001 |
| Surgical resection | 3 (37.5%) | 23 (30.3%) | 17 (16.2%) | 2 (4.2%) | 1 (5.9%) | 46 (18.1%) | |
| RFA | 4 (50.0%) | 25 (32.9%) | 13 (12.4%) | 1 (2.1%) | 0 | 43 (16.9%) | |
| Locoregional therapyb | 1 (12.5%) | 23 (30.2%) | 58 (55.2%) | 19 (39.5%) | 5 (29.4%) | 106 (41.7%) | |
| Chemotherapy or none | 0 | 5 (6.6%) | 17 (16.2%) | 26 (54.2%) | 11 (64.7%) | 59 (23.2%) | |
| Most potentially efficacious treatment received, n (%) | |||||||
| Transplant | 2 (25.0%) | 8 (10.5%) | 1 (1.0%) | 0 | 0 | 11 (4.3%) | <0.0001 |
| Surgical resection | 3 (37.5%) | 24 (31.6%) | 21 (20.0%) | 3 (6.3%) | 1 (5.9%) | 52 (20.5%) | |
| RFA | 2 (25.0%) | 20 (26.3%) | 20 (19.0%) | 2 (4.2%) | 1 (5.9%) | 45 (17.7%) | |
| Locoregional therapyb | 1 (12.5%) | 19 (25.0%) | 46 (43.8%) | 17 (35.4%) | 4 (23.5%) | 87 (34.3%) | |
| Chemotherapy or nonec | 0 | 5 (6.6%) | 17 (16.2%) | 26 (54.2%) | 11 (64.7%) | 59 (23.2%) | |
The percentages in this section of the table exceed the number/percentages of patients listed at the head of each column as patients may have received multiple treatments.
Locoregional therapy includes: PEI, MWA, TA(C)E.
Eight patients received chemotherapy.
P-values were calculated from Fisher's exact test or chi-squared tests for categorical variables; continuous variables were rank-transformed.
RFA, radiofrequency ablation; LRT, locoregional therapy; MWA, microwave ablation; TAE, transarterial embolization; TACE, transarterial chemoembolization; PEI, percutaneous ethanol injection.
Major outcomes
The major outcomes explored in this study were: (i) median survival time; (ii) overall survival by BCLC stage at the index visit, and (iii) 1-year and 5-year hazard ratios (HRs) for survival, stratified by BCLC stage (0–A and B), for the most efficacious treatment modality used.
Data sources
Study data were abstracted from the VA's comprehensive electronic medical record system by two investigators, using a standardized search algorithm. Deaths were obtained from the medical record and confirmed by cross-referencing with the Social Security Death Index to ensure completeness. Data validation was conducted by a third investigator on a random sample of patient charts to confirm accuracy.
Statistical analysis
Chi-squared tests were used for categorical data analyses and analysis of variance (anova) was used to assess differences in mean values of continuous variables. To obtain P-values, Fisher's exact test was used to correct for small sample size, and continuous variables were rank-transformed. Cox proportional hazards models and Kaplan–Meier curves were generated for survival analyses. Survival time was measured from the date of the index visit to the date of death or confirmed follow-up at 1 year and 5 years. Statistical analyses were performed using sas Version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
A total of 254 patients with newly diagnosed HCC were evaluated at SFVAMC for consideration of treatment between April 2003 and April 2011. All patients were classified by BCLC stage at the index visit. The baseline demographic and clinical characteristics of the cohort are presented in Table 1. All patients were followed through to the end of the study period or to the date of death. The mean ± standard deviation (SD) age at the index visit was 61.8 ± 8.5 years. As expected in a veteran population, all but two patients were men. Notably, 80.0% of patients had chronic HCV infection and 62.6% had histories of alcohol abuse. Only 2.4% were identified as having HBV-related HCC.
Table 1.
Characteristics of the study population by Barcelona Clinic Liver Cancer (BCLC) stage of hepatocellular carcinoma (HCC) at the index visit
| Characteristics | BCLC stage | Total (n = 254) | P-valuec | ||||
|---|---|---|---|---|---|---|---|
| 0 (n = 8) | A (n = 76) | B n = 105) | C (n = 48) | D (n = 17) | |||
| Age, years, mean ± SD | 58.7 ± 3.8 | 60.3 ± 7.7 | 63.5 ± 9.4 | 62.3 ± 8.1 | 59.1 ± 7.1 | 61.8 ± 8.5 | 0.06 |
| Male, n (%) | 8 (100%) | 74 (97.4%) | 105 (100%) | 48 (100%) | 17 (100%) | 252 (99.2%) | 0.13 |
| BMI, kg/m2, mean ± SD | 27.6 ± 4.6 | 28.9 ± 5.6 | 28.6 ± 5.8 | 27.9 ± 5.0 | 28.4 ± 7.1 | 28.5 ± 5.6 | 0.94 |
| Ethnicity, n (%) | |||||||
| White | 3 (37.5%) | 43 (56.6%) | 56 (53.3%) | 31 (64.6%) | 9 (52.9%) | 142 (55.9%) | 0.91 |
| African-American | 3 (37.5%) | 18 (23.7%) | 25 (23.8%) | 7 (14.6%) | 4 (23.5%) | 57 (22.4%) | |
| Hispanic | 2 (25.0%) | 11 (14.5%) | 16 (15.2%) | 6 (12.6%) | 2 (11.8%) | 37 (14.6%) | |
| Unknown | 0 | 4 (5.3%) | 8 (7.6%) | 4 (8.3%) | 2 (11.8%) | 18 (7.1%) | |
| Risk factors for HCC, n (%) | |||||||
| Chronic hepatitis C | 8 (100%) | 65 (85.5%) | 78 (74.3%) | 39 (81.3%) | 13 (76.5%) | 203 (80.0%) | 0.24 |
| Chronic hepatitis B | 1 (12.5%) | 4 (5.3%) | 1 (1.0%) | 0 | 0 | 6 (2.4%) | 0.08 |
| Alcohol abuse | 2 (25.0%) | 49 (64.5%) | 63 (60.0%) | 31 (64.6%) | 14 (82.4%) | 159 (62.6%) | 0.09 |
| HIV | 0 | 0 | 2 (1.9%) | 0 | 0 | 2 (0.8%) | 0.75 |
| Other liver disease | 1 (12.5%) | 1 (1.3%) | 1 (1.0%) | 0 | 3 (17.6%) | 6 (2.4%) | 0.002 |
| Laboratory values, median | |||||||
| Platelet count, ×103/μl | 130.5 | 108.5 | 128.0 | 134.5 | 128.0 | 122.0 | 0.19 |
| ALP, U/l | 99.0 | 94.5 | 114.0 | 130.5 | 124.0 | 114.0 | 0.0001 |
| AST, U/l | 93.5 | 85.5 | 68.0 | 90.0 | 104.0 | 80.0 | 0.01 |
| Total bilirubin, mg/dl | 1.0 | 1.1 | 1.2 | 1.2 | 3.8 | 1.2 | <0.0001 |
| INR | 1.0 | 1.1 | 1.1 | 1.1 | 1.5 | 1.1 | <0.0001 |
| Albumin, g/dl | 3.6 | 3.4 | 3.3 | 3.2 | 2.3 | 3.3 | <0.0001 |
| Creatinine, mg/dl | 0.9 | 0.9 | 1.0 | 1.0 | 0.9 | 1.0 | 0.03 |
| α-Fetoprotein, median, ng/ml | 17.7 | 24.6 | 34.3 | 1006.9 | 19.6 | 31.2 | <0.0001 |
| ≤200, n (%) | 8 (100%) | 67 (88.2%) | 76 (72.4%) | 19 (39.6%) | 13 (76.5%) | 183 (72.1%) | <0.0001 |
| 201–400, n (%) | 0 | 4 (5.3%) | 3 (2.9%) | 1 (2.1%) | 0 | 8 (3.2%) | |
| >400, n (%) | 0 | 3 (4.0%) | 25 (23.8%) | 23 (47.9%) | 3 (17.7%) | 54 (21.3%) | |
| MELD score, mediana | 7.5 | 9.2 | 9.8 | 9.9 | 16.4 | 9.7 | <0.0001 |
| Low, < 15, n (%) | 225 (88.6%) | 5 (29.4%) | 45 (93.8%) | 97 (92.4%) | 70 (92.1%) | 8 (100%) | <0.0001 |
| Moderate–high, ≥15, n (%) | 29 (11.4%) | 12 (70.6%) | 3 (6.3%) | 8 (7.6%) | 6 (7.9%) | 0 | |
| Child–Pugh class, n (%)b | |||||||
| A | 7 (87.5%) | 45 (59.2%) | 55 (52.4%) | 29 (60.4%) | 0 | 136 (53.5%) | <0.0001 |
| B | 1 (12.5%) | 31 (40.8%) | 50 (47.6%) | 18 (37.5%) | 1 (5.9%) | 101 (39.8%) | |
| C | 0 | 0 | 0 | 0 | 16 (94.1%) | 16 (6.3%) | |
MELD scores were used to assess the severity of chronic liver disease.27
The Child–Pugh system evaluates the severity of liver disease and prognosticate outcome. Patients are divided into classes A to C, with class C indicating the worst prognosis.26,28
P-values were calculated from Fisher's exact test or chi-squared tests for categorical variables and anova for continuous variables. Continuous variables were rank-transformed as appropriate.
SD, standard deviation; BMI, body mass index; HCC, hepatocellular carcinoma; HIV, human immunodeficiency virus; AST, aspartate aminotransferase; ALP, alkaline phosphatase; INR, international normalized ratio; MELD, Model for End-Stage Liver Disease.
Raw MELD score, alpha-fetoprotein (AFP) level and Child–Pugh class were predictably higher in more advanced BCLC stages (P < 0.0001 for each marker) (Table 1). Only 6.3% of patients were classified as being of Child–Pugh class C status, but significant proportions of early- and intermediate-stage patients had Child–Pugh class B liver disease at presentation (40.8% and 47.6% of subjects with BCLC stage A and B disease, respectively). Data on the number and size of HCC lesions at the index visit are summarized in Table 2.
Table 2.
Tumour characteristics in patients with hepatocellular carcinoma by Barcelona Clinic Liver Cancer (BCLC) stage at the index visit
| Tumour characteristics | BCLC stage | Total (n = 254) | P-valuea | ||||
|---|---|---|---|---|---|---|---|
| 0 (n = 8) | A (n = 76) | B (n = 105) | C (n = 48) | D (n = 17) | |||
| Number of lesions, n (%) | |||||||
| 1 | 8 (100%) | 49 (64.5%) | 31 (29.5%) | 24 (50.0%) | 7 (41.2%) | 119 (46.9%) | <0.0001 |
| 2 | 0 | 15 (19.7%) | 27 (25.7%) | 4 (8.3%) | 4 (23.5%) | 50 (19.7%) | |
| 3 | 0 | 11 (14.5%) | 19 (18.1%) | 3 (6.3%) | 2 (11.8%) | 36 (14.2%) | |
| 4 or 5 | 0 | 0 | 11 (10.5%) | 3 (6.3%) | 0 | 14 (5.5%) | |
| >5 | 0 | 0 | 17 (16.2%) | 14 (29.2%) | 4 (23.5%) | 35 (13.8%) | |
| Largest tumour size, median, cm | 1.5 | 2.7 | 5.0 | 6.9 | 4.6 | 4 | <0.0001 |
| <3 cm, n (%) | 7 (87.5%) | 57 (75%) | 14 (13.3%) | 5 (10.4%) | 6 (35.3%) | 89 (35.0%) | <0.0001 |
| 3–5 cm, n (%) | 1 (12.5%) | 18 (23.7%) | 41 (39.1%) | 10 (20.8%) | 3 (17.7%) | 73 (28.7%) | |
| >5 cm, n (%) | 0 | 1 (1.3%) | 50 (47.6%) | 33 (68.8%) | 8 (47.1%) | 92 (36.2%) | |
P-values were calculated using Fisher's exact test or chi-squared tests for categorical variables; continuous variables were rank-transformed.
Among the 195 patients who were given liver-directed therapy, the median number of treatments received was two (range: one to 11) (Table 3). Eleven (4.3%) patients received transplants, of whom 10 had BCLC stage 0–A and one had BCLC stage B disease at baseline. Of the 53 surgical resections performed, 71.7% were segmental or multi-segmental, 17.0% were hemi-hepatectomies and 11.3% were extended hemi-hepatectomies. A total of 28.0% of these procedures were completed laparoscopically (data not shown). Sixty RFAs were performed, representing 23.6% of all procedures. Locoregional therapies represented the most frequently used treatment type: 202 such procedures were performed. A total of 51 (20.1%) patients received no treatment and eight (3.1%) received only chemotherapy.
With regard to the first treatment modality used, 65.4% of patients with BCLC stage 0–A disease received either surgical resection (30.9%) or RFA (34.5%), compared with 28.6% of those with BCLC stage B disease, the majority (55.2%) of whom received LRT first (P < 0.0001). The first treatment received was not necessarily the most potentially curative; 24 (12.7%) of the 189 patients who underwent more than one treatment modality received a more aggressive form of treatment subsequent to their first. Twelve patients who received LRT first later underwent RFA or liver resection (data not shown).
Patients who presented with lower baseline MELD scores and BCLC status were significantly more likely to undergo radical therapy. Overall, 40.4% of patients with MELD scores of < 15 underwent liver resection or RFA, whereas 20.7% of those with baseline MELD scores of ≥15 did so. Mean ± SD baseline MELD scores were 9.51 ± 3.62 in patients undergoing at least one resection or RFA treatment and 11.32 ± 4.17 in patients who did not receive these treatments (P < 0.0001). Overall, 58.0% of patients with BCLC stage 0–A disease and 39.0% of those with BCLC stage B disease received these treatments, whereas only 10.8% of those with BCLC stage C or D disease at baseline did so (P < 0.0001).
Patients submitted to the most potentially efficacious therapies (Table 3), in declining order, included: 11 (4.3%) patients submitted to liver transplant, 52 (20.5%) patients submitted to surgical resection, 45 (17.7%) patients submitted to RFA, and 87 (34.3%) patients submitted to LRT. Not surprisingly, a statistically significantly higher proportion of patients with stage 0–A disease received transplant, resection or RFA compared with those with BCLC stage B or C–D disease (P < 0.0001).
Barcelona Clinic Liver Cancer stage-specific survival data are presented in Table 4. The median length of survival across the cohort was 16 months, and ranged from 23.5 months in patients with BCLC stage 0 disease to 3 months in those with stage D disease (P < 0.0001). Overall, 63.2% of patients survived 1 year and 11.6% survived 5 years. All patients with BCLC stage 0 disease and 83.3% of those with stage A disease were alive at 1 year, whereas only 29.2% of patients with stage C and 29.4% of those with stage D disease remained alive (P < 0.0001). At 5 years, 33.3% and 25.5% of patients with BCLC stage 0 and A disease, respectively, remained alive, whereas only 9.4% of patients with BCLC stage B, none with stage C and only one with stage D disease remained alive (P < 0.0004). Kaplan–Meier curves for survival at 1 year and 5 years, stratified by index BCLC stage, are presented in Fig. S1 (online).
Table 4.
Median survival time and percentage survival at 1, 2, 3 and 5 years in patients with hepatocellular carcinoma by Barcelona Clinic Liver Cancer (BCLC) stage at the index visit
| Characteristics | BCLC stage | Total (n = 254) | P-valuea | ||||
|---|---|---|---|---|---|---|---|
| 0 (n = 8) | A (n = 76) | B (n = 105) | C (n = 48) | D (n = 17) | |||
| Median survival, months | 23.5 | 19.5 | 18.0 | 50 | 3.0 | 16.0 | <0.0001 |
| Overall survival, % | |||||||
| 1-year (n = 247)b | 100% | 83.3% | 67.6% | 29.2% | 29.4% | 63.2% | <0.0001 |
| 2-year (n = 223)b | 57.1% | 57.6% | 40.9% | 14.9% | 17.6% | 38.6% | <0.0001 |
| 3-year (n = 213)b | 57.1% | 42.6% | 23.3% | 8.9% | 11.8% | 25.4% | 0.0004 |
| 5-year (n = 199)b | 33.3% | 25.5% | 9.4% | 0% | 6.3% | 11.6% | 0.001 |
P-values were calculated using Fisher's exact test or chi-squared tests for categorical variables; continuous variables were rank-transformed.
For each time period, the number used to calculate percentage survival includes patients who died during follow-up and those remaining alive and not lost to follow-up at the end of the designated time period.
The 11 transplant recipients ranged in age from 49 years to 66 years at the index visit. Two had BCLC stage 0 disease, eight had stage A disease and one had stage B disease. Each received at least one intervention prior to transplantation and eight received two or more pre-transplant treatments. The mean length of waiting time from the initial diagnosis of HCC to liver transplantation was 24 months (range: 11–62 months). The median length of follow-up between the initial visit and either death or the study end date was 65 months (data not shown). Two transplant patients died prior to the study cut-off date. The other nine patients remained alive without evidence of recurrent HCC. Reasons why patients with BCLC stage 0–A disease did not undergo liver transplantation included advanced age, medical comorbidity, patient preference and lack of social support. In addition, some patients remained on waitlists for transplant at the study end date.
The results of proportional hazards analysis examining the survival effects of the most curative treatments in non-transplanted early-stage (BCLC stage 0–A) and intermediate-stage (BCLC stage B) patients are presented in Table 5. Among patients with BCLC stage 0–A disease, RFA and resection were associated with significantly improved 1-year survival compared with no treatment [for death: HR = 0.13, 95% confidence interval (CI) 0.02–0.78, and HR = 0.19, 95% CI 0.04–0.88, respectively]. At 5 years, the hazard for death was markedly reduced in patients with BCLC stage 0–A disease in receipt of any form of therapy compared with those without treatment (LRT: HR = 0.11, 95% CI 0.02–0.58; RFA: HR = 0.08, 95% CI 0.02–0.40; resection: HR = 0.05, 95% CI 0.01–0.26).
Table 5.
Proportional hazards analyses at 1 year and 5 years in non-transplanted hepatocellular carcinoma patients with Barcelona Clinic Liver Cancer (BCLC) stage 0–A and B disease, by most efficacious treatment
| HRs comparing most efficacious treatment with no treatment at 1 year and 5 years | ||||
|---|---|---|---|---|
| Treatment modality | 1-year HRc (95% CI) | P-valued | 5-year HRc (95% CI) | P-valued |
| BCLC stage 0–A (n = 73a) | ||||
| Chemotherapy or no treatment | Reference | Reference | ||
| Locoregional therapyb | 0.24 (0.05–1.23) | 0.9 | 0.11 (0.02–0.56) | 0.002 |
| Radiofrequency ablation | 0.13 (0.02–0.78) | 0.03 | 0.08 (0.02–0.40) | 0.008 |
| Resection | 0.19 (0.04–0.88) | 0.03 | 0.05 (0.01–0.26) | 0.0003 |
| BCLC stage B (n = 104a) | ||||
| Chemotherapy or no treatment | Reference | Reference | ||
| Locoregional therapyb | 0.57 (0.26–1.27) | 0.17 | 0.82 (0.44–1.51) | 0.52 |
| Radiofrequency ablation | 0.10 (0.02–0.47) | 0.003 | 0.54 (0.27–1.10) | 0.09 |
| Resection | 0.23 (0.06–0.85) | 0.03 | 0.27 (0.12–0.60) | 0.002 |
| HRs at 1 year and 5 years comparing most efficacious treatment with LRT in non-transplanted patients with BCLC stage B diseasea | ||||
|---|---|---|---|---|
| Treatment modality | 1-year HRc (95% CI) | P-valued | 5-year HRc (95% CI) | P-valued |
| Locoregional therapyb | Reference | Reference | ||
| Radiofrequency ablation | 0.17 (0.04–0.74) | 0.02 | 0.63 (0.34–1.11) | 0.13 |
| Resection | 0.38 (0.11–1.33) | 0.13 | 0.31 (0.14–0.66) | 0.002 |
Transplant patients (10 BCLC stage 0–A and one BCLC stage B) were excluded from these analyses.
LRTs include microwave ablation, transarterial embolization, transarterial chemoembolization and percutaneous ethanol injection.
Age-adjusted HRs were calculated using Cox proportional hazards modelling in sas Version 9.2.
P-values were calculated using Fisher's exact test or chi-squared tests for categorical variables; continuous variables were rank-transformed.
HR, hazards ratio; 95% CI, 95% confidence interval; LRT, locoregional therapy.
Among non-transplanted patients with BCLC stage B disease, RFA and resection reduced the hazard for death at 1 year in comparison with no treatment (HR = 0.10, 95% CI 0.02–0.47 and HR = 0.23, 95% CI 0.06–0.85, respectively) (Table 5). At 5 years, only resection yielded a significant benefit (HR = 0.27, 95% CI 0.12–0.80), whereas RFA was associated with a marginally reduced hazard for death (HR = 0.54, 95% CI 0.27–1.10). A comparison of the effects of resection or RFA with those of LRT in patients with BCLC stage B disease showed RFA to confer a survival benefit at 1 year (HR = 0.17, 95% CI 0.04–0.74) and resection to be associated with improved survival at 5 years (HR = 0.31, 95% CI 0.14–0.66). These results are illustrated in 1- and 5-year Kaplan–Meier survival curves for patients with BCLC stage B disease stratified by three treatment modalities (Fig. 1).
Figure 1.
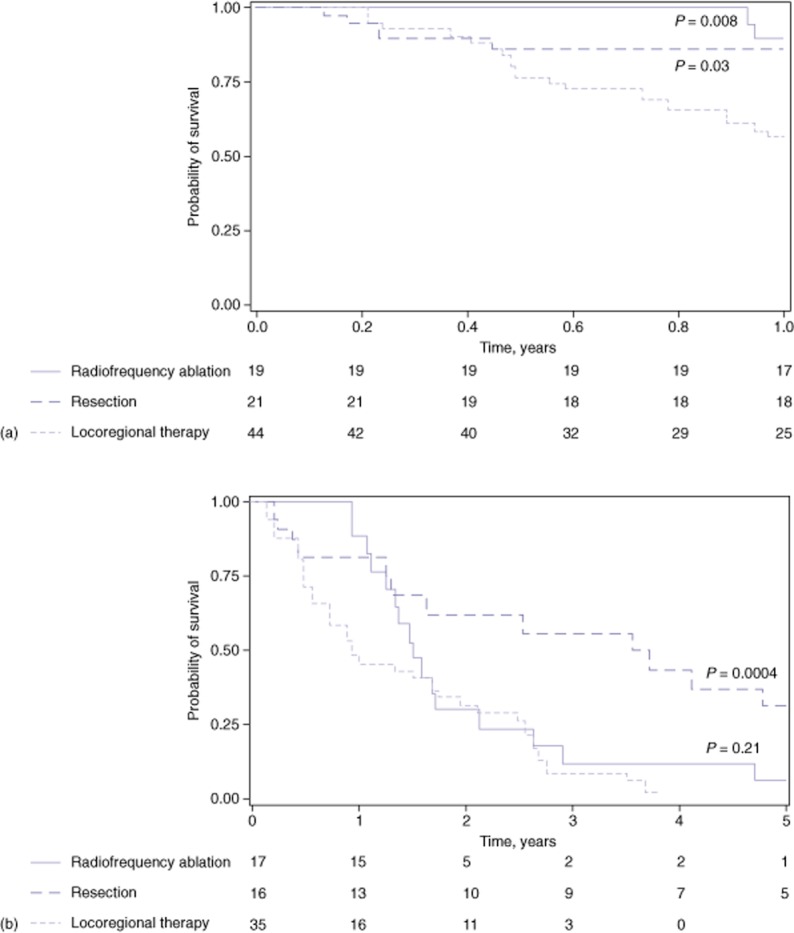
Probability of remaining alive at (a) 1 year and (b) 5 years by most efficacious treatment modality received in hepatocellular carcinoma patients with Barcelona Clinic Liver Cancer (BCLC) stage B disease. (a) Kaplan–Meier curves indicate that the use of aggressive therapy confers a statistically significant survival benefit compared with the use of locoregional therapies (LRTs) at 1 year among patients with BCLC stage B disease. (b) Patients who underwent hepatic resection achieved a statistically significant increase in the probability of remaining alive at 5 years compared with those undergoing LRTs
Discussion
This study describes treatment and survival experiences in a mixed-stage cohort of HCC patients at a large tertiary care VA medical centre. Overall, 75.1% of patients evaluated for treatment had early- or intermediate-stage disease (33.8% had BCLC stage 0–A disease and 41.3% had BCLC stage B disease). These findings contrast favourably with recent reports from population-based Surveillance, Epidemiology and End Results (SEER) and VA patient registries, which indicated that 45% and 29% of HCC patients, respectively, had BCLC stage 0–B disease at diagnosis.7,12,20 The larger proportion of earlier-stage patients seen at the study centre reflects the vigilant approach to the surveillance of at-risk patients at this hospital and its affiliates, but also is a function of the cohort's inclusion of only patients referred for HCC treatment. Liver transplantation, the treatment of choice for HCC patients, was performed in only a small minority of cases and had a limited impact on overall survival. Only 11 (4.3%) patients underwent transplant, 10 of whom had BCLC stage 0–A disease, comprising 11.9% of those presenting within the Milan criteria.17 Not surprisingly, these proportions correspond almost exactly with those reported by Davila et al. in their recent VA HCV registry-based review, in which they found liver transplant rates of 11.6% in patients with BCLC stage A disease and 3.2% in HCC patients overall.7 A complex combination of factors, including age, comorbidities, substance use and organ shortage, contribute to the low rate of transplantation among VA patients. The present experience is reflective of those at other interventional centres at which the underutilization of transplant in HCC patients has been noted.21 There is a worldwide debate on whether liver transplantation is a practical therapeutic option for HCC, given its increasing incidence and the relative shortage of organs. The low number of transplants performed in health care settings such as that of the present study, at which comprehensive care and treatment are available, reflect these concerns. Persistent obstacles to transplantation have motivated this centre's active pursuit of alternative curative treatments for early- and intermediate-stage HCC.
Whereas few patients in the present cohort underwent liver transplantation, the majority received some form of liver-directed therapy during follow-up, including 93.4% of patients with BCLC stage 0–A disease, 83.8% of those with stage B, and 56.9% of those with stage C–D disease. This centre's aggressive use of these interventions contrasts with reports from SEER, Medicare and VA studies, in which only 30–34% of HCC patients are reported to have received any form of liver-directed treatment.7,12 Despite the present aggressive approach, only 11.6% of the cohort survived ≥5 years, a finding that aligns with the 5-year survival rates of 17.9% and 8.6% reported in recent studies.30,31 Longterm survival was essentially confined to patients who underwent liver transplantation or curative resection.
Resection and RFA are widely appreciated as effective treatment strategies, either as first-line treatment or as part of a multimodal approach.32 In the present study, only 15 of the 24 patients with BCLC stage A disease who underwent resection had been followed for 5 years by the study end date. Of those, four (26.7%) patients survived ≥5 years. These disappointing outcomes are attributable in part to the high proportion of patients with BCLC stage A disease and Child–Pugh class B status at presentation or with unfavourable tumour characteristics, resulting in a poor prognosis. In several cases, the surgeons at the present centre resected lesions of >5 cm in size if they were located in favourable locations. In a retrospective study of patients from six hospitals in three countries, investigators found a 5-year survival rate of 66% following resection in Child–Pugh class A patients with a single nodule measuring up to 5 cm.33 An Italian study of resection of tumours of <3 cm in size found a 5-year survival rate of 53%.34 The outcomes reported by these studies are comparable with the benchmark 5-year survival rates of 65–75% achieved after liver transplantation in patients within the Milan criteria.17,34,35 Radiofrequency ablation alone can also achieve benefits. A recent review of eight non-randomized studies comparing RFA with resection for HCC found comparable overall 5-year survival rates (41–77% for RFA; 54–80% for resection).36 Although no statistically significant difference in survival was detected, resected patients had significantly lower rates of recurrence and higher rates of disease-free survival. The current findings also demonstrate that RFA diminishes the risk for death in patients with stage 0–A disease, and support previously reported evidence that both surgical resection and RFA are potentially beneficial in BCLC stage 0–A HCC.
The optimal therapy in BCLC stage B disease has not been empirically defined. Typically, these patients are not considered candidates for liver transplantation, surgical resection or RFA because they are thought to be too medically compromised to tolerate these procedures.17,20,37 A recent assessment of HCC patients in the VA system, however, found that 42% of 265 patients with BCLC stage B disease received some form of therapy, including liver transplant (3%), resection (9%), ablation (12%), TACE (25%) and systemic chemotherapy (6%).7 Among the present BCLC stage B group, 20.0% underwent surgical resection and 19.0% submitted to RFA as their most potentially efficacious therapy. One patient with BCLC stage B disease received a liver transplant. Compared with LRT, RFA afforded a survival benefit at 1 year and surgical resection improved 5-year survival among those with BCLC stage B disease. These results support recent findings of favourable outcomes in BCLC stage B disease of aggressive therapy with either resection or liver transplantation.21,33 Thus, the present authors conclude that potentially curative procedures can be safely administered to selected patients with BCLC stage B disease. The current findings are in accordance with the proposed sub-classification of BCLC stage B, made by participants in a recent consensus conference, to facilitate curative treatment when feasible.38
In summary, the present study is one of the first to describe in detail the treatment experiences of an entire clinical cohort undergoing serial curative and palliative treatments for HCC over time. The total, first and most curative treatments received are presented in order to provide a dynamic view of the study centre's multimodal approach. The most potentially curative treatment was not always the first treatment used, even among non-transplanted patients. Moreover, the present study suggests an evolving treatment paradigm for patients with intermediate-stage (BCLC stage B) HCC, who may benefit from the selective application of potentially curative therapies that are not typically recommended by current societal guidelines.
Acknowledgments
The authors would like to acknowledge Amit Arunkumar, bs, for assistance with data collection, and Francis Yao, md, Norah Terrault, md, Marion Peters, md, Bilal Hameed, md, Jennifer Lai, md, Mandana Khalili, md, and James Ostroff, md, for their expert guidance, and Kenneth R. McQuaid, md, for his support and encouragement. The authors would also like to express their gratitude to the patients in this study, who gave them the opportunity to study hepatocellular carcinoma through their experiences and to contribute to the advancement of medical care.
Conflicts of interest
None declared.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's website:
Figure S1. Probability in hepatocellular carcinoma patients of remaining alive at (a) 1 year (n = 247 patients) and (b) 5 years (n = 199 patients) by Barcelona Clinic Liver Cancer (BCLC) stage at the index visit. Kaplan–Meier curves reveal that both patients with early-stage disease (BCLC stage 0–A) and patients with intermediate-stage disease (BCLC stage B) achieved a statistically significant improvement in survival at both 1 year and 5 years compared with patients with advanced-stage disease (BCLC stage C–D) (P < 0.0001 for each paired comparison, respectively).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Hepatocellular carcinoma – United States, 2001–2006. MMWR Morb Mortal Wkly Rep. 2010;59:517–520. [PubMed] [Google Scholar]
- 3.Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992–2008. Hepatology. 2010;55:476–482. doi: 10.1002/hep.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127:1372–1380. doi: 10.1053/j.gastro.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Center for Quality Management in Public Health. 2010. The State of Care for Veterans with Chronic Hepatitis C. Palo Alto, CA: Public Health Strategic Health Care Group, US Department of Veterans Affairs, Health CfQMiP.
- 7.Davila JA, Kramer JR, Duan Z, Richardson PA, Tyson GL, Sada YH, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and non-patient factors. Hepatology. 2013;57:1858–1868. doi: 10.1002/hep.26287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominitz JA, Boyko EJ, Koepsell TD, Heagerty PJ, Maynard C, Sporleder JL, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centres. Hepatology. 2005;41:88–96. doi: 10.1002/hep.20502. [DOI] [PubMed] [Google Scholar]
- 9.Lurje G, Lesurtel M, Clavien PA. Multimodal treatment strategies in patients undergoing surgery for hepatocellular carcinoma. Dig Dis. 2013;31:112–117. doi: 10.1159/000347205. [DOI] [PubMed] [Google Scholar]
- 10.Belghiti J. Resection and liver transplantation for HCC. J Gastroenterol. 2009;44(Suppl. 19):132–135. doi: 10.1007/s00535-008-2250-1. [DOI] [PubMed] [Google Scholar]
- 11.Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154:85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 12.Shah SA, Smith JK, Li Y, Ng SC, Carroll JE, Tseng JF. Underutilization of therapy for hepatocellular carcinoma in the Medicare population. Cancer. 2011;117:1019–1026. doi: 10.1002/cncr.25683. [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM. Evidence-based medicine in the treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17(Suppl. 3):428–433. doi: 10.1046/j.1440-1746.17.s3.40.x. [DOI] [PubMed] [Google Scholar]
- 14.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 15.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Longterm survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yopp AC, Subramanian M, Jain MK, Mansour JC, Schwarz RE, Balch GC, et al. Presentation, treatment, and clinical outcomes of patients with hepatocellular carcinoma, with and without human immunodeficiency virus infection. Clin Gastroenterol Hepatol. 2012;10:1284–1290. doi: 10.1016/j.cgh.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–700. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 18.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumour size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 19.Benson AB, 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–391. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Avola D, Inarrairaegui M, Pardo F, Rotellar F, Marti P, Bilbao JI, et al. Prognosis of hepatocellular carcinoma in relation to treatment across BCLC stages. Ann Surg Oncol. 2011;18:1964–1971. doi: 10.1245/s10434-011-1551-4. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Won HJ, Shin YM, Kim SH, Yoon HK, Sung KB, et al. Medium-sized (3.1–5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol. 2011;18:1624–1629. doi: 10.1245/s10434-011-1673-8. [DOI] [PubMed] [Google Scholar]
- 23.Piscaglia F, Bolondi L. The intermediate hepatocellular carcinoma stage: should treatment be expanded? Dig Liver Dis. 2010;42(Suppl. 3):258–263. doi: 10.1016/S1590-8658(10)60514-2. [DOI] [PubMed] [Google Scholar]
- 24.Bruce-Chwatt LJ. Declaration of Helsinki. Recommendations Guiding Doctors in Clinical Research. WHO Chron. 1965;19:31–32. [PubMed] [Google Scholar]
- 25.Schuckit MA. Alcohol-use disorders. Lancet. 2009;373:492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- 26.Child CGI, editor. The Liver and Portal Hypertension. Vol. 1 of Major Problems in Clinical Surgery. Philadelphia, PA: W B Saunders; 1964. [PubMed] [Google Scholar]
- 27.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 28.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 29.Chang TT, Sawhney R, Monto A, Davoren JB, Kirkland JG, Stewart L, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB. 2008;10:405–411. doi: 10.1080/13651820802356572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathur AK, Osborne NH, Lynch RJ, Ghaferi AA, Dimick JB, Sonnenday CJ. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 2010;145:1158–1163. doi: 10.1001/archsurg.2010.272. [DOI] [PubMed] [Google Scholar]
- 31.Berrino F, De Angelis R, Sant M, Rosso S, Bielska-Lasota M, Coebergh JW, et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995–99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773–783. doi: 10.1016/S1470-2045(07)70245-0. [DOI] [PubMed] [Google Scholar]
- 32.Colombo M, Raoul JL, Lencioni R, Galle PR, Zucman-Rossi J, Banares R, et al. Multidisciplinary strategies to improve treatment outcomes in hepatocellular carcinoma: a European perspective. Eur J Gastroenterol Hepatol. 2013;25:639–651. doi: 10.1097/MEG.0b013e32835e33bb. [DOI] [PubMed] [Google Scholar]
- 33.Silva MF, Sapisochin G, Strasser SI, Hewa-Geeganage S, Chen J, Wigg AJ, et al. Liver resection and transplantation offer similar 5-year survival for Child–Pugh–Turcotte A HCC patients with a single nodule up to 5 cm: a multicentre, exploratory analysis. Eur J Surg Oncol. 2013;39:386–395. doi: 10.1016/j.ejso.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Giuliante F, Ardito F, Pinna AD, Sarno G, Giulini SM, Ercolani G, et al. Liver resection for hepatocellular carcinoma ≤3 cm: results of an Italian multicentre study on 588 patients. J Am Coll Surg. 2012;215:244–254. doi: 10.1016/j.jamcollsurg.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Germani G, Gurusamy K, Garcovich M, Toso C, Fede G, Hemming A, et al. Which matters most: number of tumours, size of the largest tumour, or total tumour volume? Liver Transpl. 2011;17(Suppl. 2):58–66. doi: 10.1002/lt.22336. [DOI] [PubMed] [Google Scholar]
- 36.Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2011;98:1210–1224. doi: 10.1002/bjs.7669. [DOI] [PubMed] [Google Scholar]
- 37.Sherman M, Burak K, Maroun J, Metrakos P, Knox JJ, Myers RP, et al. Multidisciplinary Canadian consensus recommendations for the management and treatment of hepatocellular carcinoma. Curr Oncol. 2011;18:228–240. doi: 10.3747/co.v18i5.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, et al. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Probability in hepatocellular carcinoma patients of remaining alive at (a) 1 year (n = 247 patients) and (b) 5 years (n = 199 patients) by Barcelona Clinic Liver Cancer (BCLC) stage at the index visit. Kaplan–Meier curves reveal that both patients with early-stage disease (BCLC stage 0–A) and patients with intermediate-stage disease (BCLC stage B) achieved a statistically significant improvement in survival at both 1 year and 5 years compared with patients with advanced-stage disease (BCLC stage C–D) (P < 0.0001 for each paired comparison, respectively).


