Abstract
Background
The aim of this prospective observational study was to compare peri/post-operative outcomes of thoracic epidural analgesia (TEA) versus intrathecal morphine and fentanyl patient-controlled analgesia (ITM+fPCA) for patients undergoing a hepatic resection (HR).
Method
Patients undergoing elective, one-stage, open HR for benign and malignant liver lesions, receiving central neuraxial block as part of the anaesthetic, in a high-volume hepato-pancreato-biliary unit, were included in the study. The primary outcome measure was post-operative length of stay (LoS).
Results
A total of 73 patients (36 TEA and 37 ITM+fPCA) were included in the study. The median (IQR) post-operative LoS was 13 (11–15) and 11 (9–13) days in the TEA and ITM+fPCA groups, respectively (P = 0.011). There was significantly lower median intra-operative central venous pressure (P < 0.001) and blood loss (P = 0.017) in the TEA group, and a significant reduction in the time until mobilization (P < 0.001), post-operative intra-venous fluid/vasopressor requirement (P < 0.001/P = 0.004) in the ITM+fPCA group. Pain scores were lower at a clinically significant level 12 h post-operatively in the TEA group (P < 0.001); otherwise there were no differences out to day five. There were no differences in quality of recovery or postoperative morbidity/mortality between the two groups.
Conclusion
ITM+fPCA provides acceptable post-operative outcomes for HR, but may also increase the incidence of intra-operative blood loss in comparison to TEA.
Introduction
A hepatic resection (HR) offers a potential cure for patients with colorectal liver metastases with 5-year survival rates of approximately 30–40%1 and cure rates of 16% at 10 years.2 Advances in surgical technique and peri-operative care enable a safe resection of up to 70% of functional liver parenchyma, including multifocal metastases, and has an associated in-hospital mortality of 5% or less.3,4
The success of peri-operative care programmes is dependent on optimal analgesia, which can modulate the stress response to surgery and improve post-operative outcomes.5,6 Thoracic epidural analgesia (TEA) is often considered the gold standard analgesic modality for major intra-abdominal surgery as it provides better analgesia than the common alternative of intra-venous opioids and results in less respiratory complications.7,8 However, a disadvantage of TEA includes sympathetic block leading to hypotension in the post-operative period. This is often treated with excessive fluid administration that can lead to increases in blood product transfusion rates, delayed mobilization and increased hospital length of stay (LoS).9–12 TEA also has a high failure rate and requires greater resources including specialized nursing care.13,14 To date, TEA is the main mode of analgesia in most peri-operative care programmes for patients undergoing HR, including those utilizing enhanced recovery protocols.15–17
Intrathecal opioid has been reported as an alternative to TEA, offering equivalent analgesia, for patients undergoing HR.18,19 When intrathecal opioid has been compared with TEA in colorectal surgery, it has been shown to improve outcomes including decreased post-operative morbidity, reduced resource allocation and shorter LoS.20,21
The aim of this study was to determine differences in peri/post-operative outcomes of patients receiving TEA versus intrathecal morphine and fentanyl patient-controlled analgesia (ITM+fPCA) for patients undergoing HR.
Methods
A prospective, single centre, observational study was conducted for all patients undergoing HR in a high-volume hepato-pancreato-biliary (HPB) surgical unit (Royal Free London NHS Foundation Trust, University College, London), between August 2012 and June 2013. The study was approved by the local institutional review board.
Patients undergoing an elective, one-stage, open hepatic resection for benign and malignant liver lesions receiving central neuraxial blocks (CNB) as part of their anaesthetic were included in the study. Patients were excluded if they had pre-existing chronic pain, opioid dependence, pre-operative mechanical assistance for mobility, alternative laparotomy incisions other than inverted ‘L’ incisions, a laparoscopic/hand-assisted laparoscopic operation or extended post-operative ventilation beyond 12 h. A total of five consultant HPB surgeons and seven consultant anaesthetists were directly involved in the peri-operative care of the patients.
Peri-operative care
All CNBs were performed with the patient awake, before induction of general analgesia, in the sitting position and with an aseptic technique. Further information regarding the CNB technique/dosage and the remainder of the anaesthetic is provided in Supporting Information 1. Weaning of the epidurals in the TEA group was commenced on post-operative day (POD) three with an aim of cessation by POD four. In the intra-thecal morphine group, fPCA was commenced post-operatively at a bolus dose of 25 mcg with a 5-min lockout with an aim for cessation by POD four.
Patients in the TEA group who required intra-venous opioid to achieve satisfactory pain relief in the presence of a non-recoverable inadequate sensory block (assessed by the acute pain team or duty anaesthetist) were deemed to have ‘failed TEA’. The epidurals in these patients were stopped and analgesia was converted to fPCA or rescue intra-venous opioids.
In addition to CNB, all patients were given regular oral paracetamol (1 g) and tramadol (50 mg) in the post-operative period, at intervals of 6 h. Patients were also prescribed intra-venous ondansetron at a dose of 4 mg twice daily for anti-emesis. Those requiring additional anti-emetics were given cyclizine (50 mg), up to three times daily, on an as required basis.
After the surgery, all patients were transferred to the intensive care unit for a minimum of 12 h and then to a specialized HPB ward where they received standardized care (Supporting Information 2).
Operative technique
HR was performed using the Cavitron Ultrasonic Surgical Aspirator (CUSA; Valleylab, CO, USA) and Argon beam coagulation. For patients with malignant tumours, the transection plane was first determined by intra-operative ultrasonography. The inflow and outflow vessels supplying the sectors to be resected were divided by stapling or ligated with a running polypropylene suture. Inflow occlusion (Pringle manoeuvre) was used when deemed necessary to control on-going blood loss during parenchymal division at the discretion of the consultant surgeon. When utilized, inflow occlusion was used in periods of up to 20 min with a 3–5 min recovery. In some instances the raw surface of the liver remnant was sealed with TachoSil (Nycomed, Zurich, Switzerland).
Clinical outcomes
Patient demographics [age, gender, body mass index (BMI), American society of anaesthesiologists (ASA) score, predicted morbidity and mortality] and pre-operative variables (aetiology of liver disease, chemotherapy status, presence of liver cirrhosis and type of HR) were recorded for all patients. The predicted risk of morbidity and mortality was calculated using a risk prediction model based on the Portsmouth physiological and operative severity score for the enumeration of mortality and morbidity (P-POSSUM).22 The type of HR was determined according to the number of segments resected (minor < 3 or major >3).
The primary clinical outcome was post-operative LoS and was measured from the date and start time of surgery to the date and time of discharge. Patients were deemed to be medically fit for discharge when they met the following discharge criteria: good pain control with oral analgesia, resumption of a normal diet, independent mobility, normalizing liver function and no unresolved medical or surgical complications requiring further treatment.
Secondary clinical outcomes included intra-operative blood loss/packed red blood cell (PRBC) transfusion/Pringle manoeuvre outcomes/central venous pressure (CVP) measurements/operative time, post-operative intravenous fluids (IVF) requirement/blood product transfusion/vasopressor requirement, time delay until intake of fluids/intake of solids/removal of catheter/mobilisation/medically fit for discharge, cumulative opioid requirements, pain scores, morbidity, mortality and quality of recovery (QoR). Pain scores were measured using a visual analogue scale (VAS) ranging from 0 to 100 mm at 12-h intervals up to 96 h post-operatively.23 Cumulative opioid requirements were calculated for the first 4 post-operative days (total opioid administered was converted to equivalent fentanyl dose in micrograms). Surgical complications were graded up to 30 days post-operatively in accordance to the Clavien–Dindo classification.24 Patients were asked to complete a validated QoR questionnaire25 at a pre-assessment clinic, POD three and five.
Statistical analysis
Statistical analysis was performed using SPSS version 20 (SPSS, Chicago, IL, USA). A P-value of < 0.05 was used to define statistical significance. Mann–Whitney U-test and Chi-squared test were used to compare continuous and categorical variables, respectively.
Results
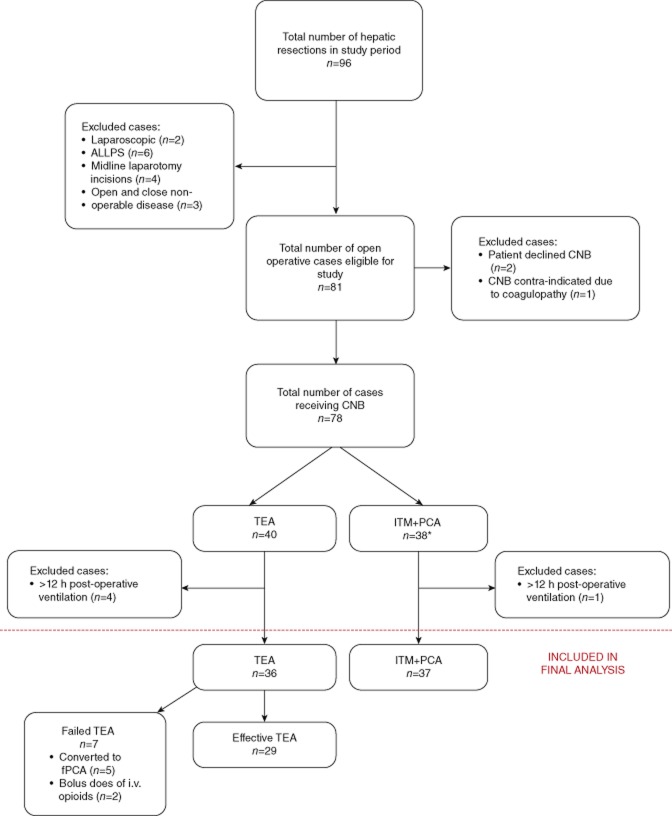
A total of 96 patients were identified in the study period. After application of the exclusion criteria, 73 patients (76%), consisting of 36 with TEA and 37 with ITM+fPCA were included in the final analysis (Fig. 1). No differences were observed in the ratio of patients receiving TEA versus ITM+fPCA in the first (1.3), second (0.9), third (1.1) and fourth (0.7) quartiles of the study time period (P = 0.823). There were also no differences observed in the ratio of patients receiving TEA versus ITM+fPCA by the seven anaesthetists (0.6, 0.8, 1.2, 0.9, 1.5, 0.8, 0.6, P = 0.918) and five surgeons (1, 0.6, 1, 0.9, 1.4, P = 0.867). Patient demographics and pre-operative variables are demonstrated in Table 1.
Figure 1.
Flow of patients throughout the study. *Including three patients who had initial failed attempts at placement of TEA. ALLPS; associating liver partition with portal vein ligation for staged hepatectomy; CNB, central neuraxial block; TEA, thoracic epidural analgesia; ITM+fPCA, intra-thecal morphine and fentanyl patient controlled analgesia; fPCA, fentanyl patient controlled analgesia
Table 1.
Patient demographics, pre-operative variables and intra-operative outcomes
| TEA | ITM+fPCA | Pc | |
|---|---|---|---|
| n = 36 | n = 37 | ||
| Age (years)a | 58 (50–64) | 61 (58–69) | 0.118d |
| Gender ratio (M : F) | 11:25 | 17:20 | 0.176 |
| Body mass index (kg/m2)a | 25.5 (23.2–27.3) | 24.5 (22.7–26.8) | 0.275d |
| ASA score | 0.788 | ||
| 1 | 2 | 2 | |
| 2 | 27 | 30 | |
| 3 | 7 | 5 | |
| 4 | 0 | 0 | |
| Predicted Morbidity-POSSUM (%)a | 43.4 (31.8–64.0) | 58.9 (31.8–66.5) | 0.282d |
| Predicted Mortality-POSSUM (%)a | 2.2 (1.2–4.3) | 2.3 (1.2–4.4) | 0.326d |
| Aetiology of liver disease | 0.221 | ||
| Colorectal liver metastases | 27 | 34 | |
| HCC | 5 | 2 | |
| Cholangiocarcinoma | 2 | 0 | |
| Benign tumour | 2 | 1 | |
| Neo-adjuvant chemotherapy | 12 | 14 | 0.688 |
| Cirrhosis | 4 | 6 | 0.526 |
| Type of resection | 0.665 | ||
| Minor | 26 | 25 | |
| Major | 10 | 12 | |
| Pringle manoeuvre | 13 | 19 | 0.180 |
| Pringle time (min)a | 12.0 (10.0–15.0) | 12 (11.5–15.0) | 0.779 |
| Intra-operative blood loss (ml) | 0.017 | ||
| ≤500 | 29 | 20 | |
| 501–1000 | 4 | 16 | |
| 1001–2000 | 2 | 1 | |
| >2000 | 1 | 0 | |
| Intra-operative transfusion of PRBC (SU)a,b | 0 (0-0)/0.7 (2.1) | 0 (0–2)/0.8 (1.0) | 0.066d |
| Intra-operative time (min)a | 266 (200–362) | 323 (254–401) | 0.062d |
Values are median (IQR).
Values are mean (SD) otherwise absolute numbers.
χ2 test, except
the Mann–Whitney U-test.
PRBC, packed red cells; SU, standard unit; HCC, hepatocellular carcinoma.
The median (IQR) post-operative LoS was 13 (11–15) and 11 (9–13) days in the TEA and ITM+fPCA groups, respectively (P = 0.011). After excluding patients with failed TEA (n = 7), the median (IQR) post-operative LoS remained the same at 13 (11–15) days (P = 0.015). The median (IQR) time until medically fit for discharge was 12 (10–14) and 10 (8–12) days in the TEA and ITM+fPCA groups, respectively (P = 0.007).
The trend of CVP at baseline, pre-resection and the median of multiple readings taken during the resection phase are demonstrated in Fig. 2. Post-operative variables and outcomes are shown in Table 2.
Figure 2.
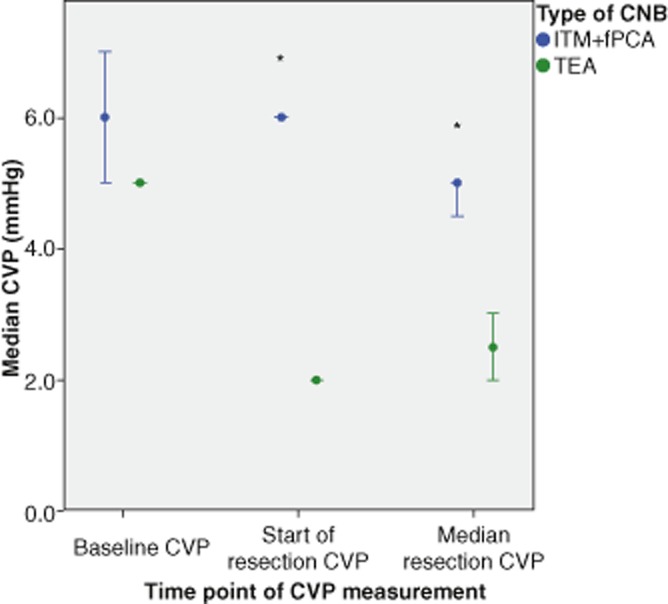
Comparison of intra-operative central venous pressure (CVP) measurements between thoracic epidural analgesia (TEA) and intrathecal morphine and fentanyl patient-controlled analgesia (ITM+fPCA). CVP measurements compared between types of central neuraxial block (CNB) at different time points using the Mann–Whitney U-test, *P < 0.001. Error bars indicate 95% confidence intervals
Table 2.
Post-operative outcomes
| TEA | ITM+fPCA | Pc | |
|---|---|---|---|
| n = 36 | n = 37 | ||
| Volume of fluid/blood products administered post-operatively (litres/SU) | |||
| Crystalloida | 4.0 (3.5–4.9) | 2.5 (2-0-2.5) | <0.001 |
| Colloida | 0.5 (0.5–0.9) | 0.5 (0–500) | 0.064 |
| Total IVFa | 4.5 (4.0–5.5) | 2.5 (2.3–3.0) | <0.001 |
| PRBCa,b | 0 (0–2)/0.9 (1.5) | 0 (0-0)/0.5 (1.3) | 0.089 |
| Other blood productsa,b | 0 (0-0)/0.1 (0.5) | 0 (0-0)/0 | 0.064 |
| Vasopressor requirement | 30 | 19 | 0.004d |
| Total time of post-operative inotropic requirements (h)a | 8 (6–10) | 5 (4–9) | 0.012 |
| Post-operative delay until tolerating clear fluids (h)a | 6 (3–11) | 8 (5–12) | 0.404 |
| Post-operative delay until tolerating solids (h)a | 31 (12–39) | 31 (24–39) | 0.942 |
| Post-operative time until removal of urinary catheter (h)a | 42 (32–72) | 39 (32–82) | 0.811 |
| Post-operative time until independent mobilisations (h)a | 49 (40–64) | 34 (28–45) | <0.001 |
Values are median (IQR).
Values are mean (SD), otherwise absolute numbers.
Mann–Whitney U-test, except
χ2 test.
IVF, intra-venous fluid; PRBC, packed red cells; SU, standard unit.
Morbidity and mortality
Post-operative morbidity outcomes of the TEA and ITM+fPCA groups are demonstrated in Table 3. There were no immediate or delayed complications of TEA or ITM placement and no evidence of neurological sequelae up to 30 days post-operatively. One patient in the TEA group required re-operation 4 h post-operatively for bleeding, and a further patient in the ITM+PCA group required closure of an abdominal wound dehiscence on POD 6. There were two episodes of respiratory depression requiring medical intervention, both in the TEA group, in patients who had ‘failed epidurals’ requiring rescue intravenous opioid. One patient required naloxone and the other supplemental oxygen for treatment. Two patients were readmitted to hospital, one in the TEA group for a superficial wound infection on POD 12, and the other in the ITM+fPCA group with a deep vein thrombosis on POD 14. They received routine treatment and had no long-term complications. There was no 30-day mortality in either group.
Table 3.
Post-operative morbidity
| TEA | ITM+fPCA | Pa | |
|---|---|---|---|
| n = 36 | n = 37 | ||
| Clavien–Dindo classification | 0.346 | ||
| 0 | 13 | 21 | |
| 1 | 12 | 4 | |
| 2 | 5 | 5 | |
| 3a | 3 | 3 | |
| 3b | 1 | 1 | |
| 4a | 1 | 2 | |
| 4b | 1 | 1 | |
| Specific complications | |||
| Respiratory | |||
| LRTI | 2 | 2 | 0.978 |
| Pleural effusion | 2 | 2 | 0.978 |
| VTE | 1 | 1 | 0.984 |
| Respiratory depression (req. medical intervention) | 2 | 0 | 0.146 |
| CVS | |||
| Myocardial infarction | 1 | 0 | 0.307 |
| Malignant arrhythmia | 1 | 0 | 0.307 |
| Renal | |||
| Renal impairment | 2 | 1 | 0.539 |
| Sepsis | 2 | 1 | 0.539 |
| Ileus | 1 | 2 | 0.572 |
| Surgical complications | |||
| Biloma/bile Leak | 1 | 2 | 0.572 |
| Postop. haemorrhage | 1 | 0 | 0.307 |
| Wound infection | 4 | 1 | 0.155 |
| Postop. liver failure | 0 | 1 | 0.321 |
χ2 test.
Values are absolute numbers. LRTI, lower respiratory tract infection; VTE, venous thrombo-embolism.
Visual analogue scale pain scores
Outcomes of VAS pain scores at varying time points for all patients included in the study, grouped as per initial mode of CNB, are demonstrated in Fig. 3. The median (IQR)/mean (SD) cumulative intra-venous opioid use in the first 4 PODs was 0 (0-0)/ 119 (263) and 500 (473–550)/ 532 (130) mcg of fentanyl in the TEA and ITM+fPCA group, respectively (P < 0.001).
Figure 3.
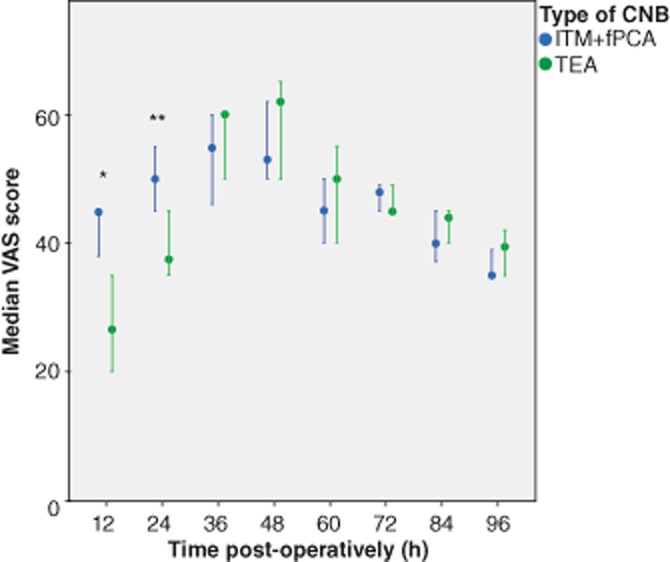
Visual analogue scale scores (VAS) of post-operative pain on coughing, comparing thoracic epidural analgesia (TEA) and intrathecal morphine and fentanyl patient-controlled analgesia (ITM+fPCA) for hepatic resection surgery. VAS scores compared between type of central neuraxial block (CNB) at different time points post-operatively, with the Mann–Whitney U-test *P < 0.001, **P = 0.010. Error bars indicate 95% confidence intervals
Quality of recovery scores
The median (IQR) QoR scores in the TEA versus ITM+fPCA group was 115 (109–138) vs. 113 (107–134) pre-operatively (P = 0.321), 98 (85–102) vs. 90 (83–102) on POD 3 (P = 0.482), and 91 (81–101) vs. 87 (82–98) on POD 5 (P = 0.343).
Discussion
This study has shown that patients receiving ITM+fPCA have a significantly shorter post-operative LoS than those receiving TEA. Patients receiving ITM+fPCA also had significantly less post-operative intra-venous fluid administration, vasopressor requirement and an earlier time to independent mobilization. Apart from 12 h post-operatively there were no clinically significant differences in pain scores between patients having ITM+fPCA or TEA. There were also no differences in post-operative morbidity scores, defined morbidities and quality of recovery. Patients receiving TEA did however have a shorter operative duration and a significantly lower intra-operative blood loss.
The shorter post-operative LoS in the ITM+fPCA group has also been previously demonstrated by two retrospective studies of HR showing reductions of 2.1 and 4.5 days in comparisons to patients receiving TEA.19,26 Reduced post-operative LoS may be accounted for by the significantly improved time until mobilization. However, the median post-operative LoS for patients in this study is higher than some modern day high-volume HPB centres, which report figures of between 3–6 days.15–17 These improved outcomes are associated with the use of enhanced recover after surgery (ERAS) programmes, which are currently being implemented in the study institution. The results of this study are therefore limited in the context of ERAS.
Patients having TEA had a shorter operating time then those having ITM+fPCA by a median of 57 min. There was also significantly more blood loss in the ITM+fPCA group, possibly suggesting more challenging and thus longer surgery. Although not statistically significant the ITM+fPCA group was also more likely to have hepatic inflow occlusion to reduce bleeding. An explanation for this may be the ability of TEA to reduce CVP and consequently decrease venous congestion and bleeding during the resection phase of the operation.27,28 This is supported by the findings of significantly lower CVP in the TEA group, at the start and during the resection phase of the operation.
Patients in the ITM+fPCA group received approximately 2 litres less intra-venous fluid than their TEA counterparts during the post-operative period. Although not statistically significant, the ITM+fPCA group also received less post-operative blood product transfusion. These findings are consistent with previous reports comparing intravenous fluid administration for patients having TEA to alternative methods of analgesia such ITM or PCA.11,19,20,26 Increased fluid administration is thought to be a response to the increased incidence of hypotension caused by the sympathetic blockade with TEA,11,26 which is also responsible for the increased post-operative vasopressor requirement within this group of patients.
There was a clinically significant difference in pain scores at 12 h post-operatively, favouring the TEA group. Although pain scores were also statistically lower 24 h post-operatively in the TEA group, there was only a mean difference of 9 mm. The minimum clinically significant change in patient pain severity measured with a 100-mm VAS is considered to be 10 mm.29 No differences in VAS were observed between 36 and 96 h. In spite of demonstrating a better pain profile in the first 12 h, the TEA group had similar outcomes after this interval, which was reflected in similar outcomes of patient experience, as demonstrated by comparable QoR scores.
The limitations of this study include the lack of randomization of patients into the defined interventional groups. Furthermore, the outcomes of this observational study are of a single centre, raising the question of outcome reproducibility. In spite of these limitations, the data collection was prospective in nature and conducted by a clinician not involved in post-operative patient care. All patients had standardized analgesia regimes and post-operative care pathways, reducing the introduction of bias from other confounding variables.
In conclusion, this study demonstrates that ITM+fPCA provides acceptable post-operative outcomes for HR and may be a feasible alternative to TEA, but may cause a comparative increase in the incidence of intra-operative blood loss.
Acknowledgments
The authors thank Miss P. Meale, peri-operative research nurse, University College London for assistance with the data collection and database production.
Conflicts of interest
None declared.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Supporting information 1 Central neuraxial block (CNB) and anaesthesia.
Supporting information 2 Post-operative care.
References
- 1.Adam R. Developing strategies for liver metastases from colorectal cancer. Semin Oncol. 2007;34(Suppl. 1):S7–11. doi: 10.1053/j.seminoncol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 3.Lee S-GS, Hwang SS. How I do it: assessment of hepatic functional reserve for indication of hepatic resection. J Hepatobiliary Pancreat Surg. 2005;12:38–43. doi: 10.1007/s00534-004-0949-9. [DOI] [PubMed] [Google Scholar]
- 4.Abdalla EK, Vauthey J-N, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehlet H. Multimodal approach to postoperative recovery. Curr Opin Crit Care. 2009;15:355–358. doi: 10.1097/MCC.0b013e32832fbbe7. [DOI] [PubMed] [Google Scholar]
- 6.Kehlet H, Holte K. Effect of postoperative analgesia on surgical outcome. Br J Anaesth. 2001;87:62–72. doi: 10.1093/bja/87.1.62. [DOI] [PubMed] [Google Scholar]
- 7.Wu CL, Cohen SR, Richman JM, Rowlingson AJ, Courpas GE, Cheung K, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology. 2005;103:1079–1088. doi: 10.1097/00000542-200511000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Rigg JRA, Jamrozik K, Myles PS, Silbert BS, Peyton PJ, Parsons RW, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359:1276–1282. doi: 10.1016/S0140-6736(02)08266-1. [DOI] [PubMed] [Google Scholar]
- 9.Page A, Rostad B, Staley CA, Levy JH, Park J, Goodman M, et al. Epidural Analgesia in Hepatic Resection. J Am Coll Surg. 2008;206:1184–1192. doi: 10.1016/j.jamcollsurg.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 10.Gustafsson UO, Hausel J, Thorell A, Ljungqvist O, Soop M, Nygren J, et al. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg. 2011;146:571–577. doi: 10.1001/archsurg.2010.309. [DOI] [PubMed] [Google Scholar]
- 11.Revie EJ, Massie LJ, McNally SJ, McKeown DW, Garden OJ, Wigmore SJ. Effectiveness of epidural analgesia following open liver resection. HPB. 2011;13:206–211. doi: 10.1111/j.1477-2574.2010.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzimas P, Prout J, Papadopoulos G, Mallett SV. Epidural anaesthesia and analgesia for liver resection. Anaesthesia. 2013;68:628–635. doi: 10.1111/anae.12191. [DOI] [PubMed] [Google Scholar]
- 13.McLeod G, Davies H, Munnoch N, Bannister J, MacRae W. Postoperative pain relief using thoracic epidural analgesia: outstanding success and disappointing failures. Anaesthesia. 2001;56:75–81. doi: 10.1046/j.1365-2044.2001.01763-7.x. [DOI] [PubMed] [Google Scholar]
- 14.Hermanides JJ, Hollmann MWM, Stevens MFM, Lirk PP. Failed epidural: causes and management. Br J Anaesth. 2012;109:144–154. doi: 10.1093/bja/aes214. [DOI] [PubMed] [Google Scholar]
- 15.Jones C, Kelliher L, Dickinson M, Riga A, Worthington T, Scott MJ, et al. Randomized clinical trial on enhanced recovery versus standard care following open liver resection. Br J Surg. 2013;100:1015–1024. doi: 10.1002/bjs.9165. [DOI] [PubMed] [Google Scholar]
- 16.van Dam RM, Hendry PO, Coolsen MME, Bemelmans MHA, Lassen K, Revhaug A, et al. Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg. 2008;95:969–975. doi: 10.1002/bjs.6227. [DOI] [PubMed] [Google Scholar]
- 17.Schultz NA, Larsen PN, Klarskov B, Plum LM, Frederiksen HJ, Christensen BM, et al. Evaluation of a fast-track programme for patients undergoing liver resection. Br J Surg. 2013;100:138–143. doi: 10.1002/bjs.8996. [DOI] [PubMed] [Google Scholar]
- 18.De Pietri L, Siniscalchi A, Reggiani A, Masetti M, Begliomini B, Gazzi M, et al. The use of intrathecal morphine for postoperative pain relief after liver resection: a comparison with epidural analgesia. Anesth Analg. 2006;102:1157–1163. doi: 10.1213/01.ane.0000198567.85040.ce. [DOI] [PubMed] [Google Scholar]
- 19.Koea JB, Young Y, Gunn K. Fast track liver resection: the effect of a comprehensive care package and analgesia with single dose intrathecal morphine with gabapentin or continuous epidural analgesia. HPB Surg. 2009;2009:1–8. doi: 10.1155/2009/271986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virlos I, Clements D, Beynon J, Ratnalikar V, Khot U. Short-term outcomes with intrathecal versus epidural analgesia in laparoscopic colorectal surgery. Br J Surg. 2010;97:1401–1406. doi: 10.1002/bjs.7127. [DOI] [PubMed] [Google Scholar]
- 21.Levy BF, Scott MJ, Fawcett W, Fry C, Rockall TA. Randomized clinical trial of epidural, spinal or patient-controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg. 2011;98:1068–1078. doi: 10.1002/bjs.7545. [DOI] [PubMed] [Google Scholar]
- 22.Prytherch DR, Whiteley MS, Higgins B, Weaver PC, Prout WG, Powell SJ. POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity. Br J Surg. 1998;85:1217–1220. doi: 10.1046/j.1365-2168.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- 23.Turk DC, Melzack R. Handbook of Pain Assessment. 3rd edn. New York: Guilford Press; 1957. pp. 1–206. [Google Scholar]
- 24.Clavien PA, Barkun J, de Oliveira ML, Vauthey J-N, Dindo D, Schulick RD, et al. The Clavien-Dindo Classification of Surgical Complications. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 25.Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology. 2013;118:1332–1340. doi: 10.1097/ALN.0b013e318289b84b. [DOI] [PubMed] [Google Scholar]
- 26.Sakowska M, Docherty E, Linscott D, Connor S. A change in practice from epidural to intrathecal morphine analgesia for hepato-pancreato-biliary surgery. World J Surg. 2009;33:1802–1808. doi: 10.1007/s00268-009-0131-2. [DOI] [PubMed] [Google Scholar]
- 27.Gurusamy KS, Li J, Vaughan J, Sharma D, Davidson BR. Cardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database Syst Rev. 2012;(5) doi: 10.1002/14651858.CD007338.pub3. CD007338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058–1060. doi: 10.1046/j.1365-2168.1998.00795.x. [DOI] [PubMed] [Google Scholar]
- 29.Bodian CAC, Freedman GG, Hossain SS, Eisenkraft JBJ, Beilin YY. The visual analog scale for pain: clinical significance in postoperative patients. Anesthesiology. 2001;95:1356–1361. doi: 10.1097/00000542-200112000-00013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information 1 Central neuraxial block (CNB) and anaesthesia.
Supporting information 2 Post-operative care.