Abstract
Many organisms display phenotypic plasticity as adaptation to seasonal environmental fluctuations. Often, such seasonal responses entails plasticity of a whole suite of morphological and life-history traits that together contribute to the adaptive phenotypes in the alternative environments. While phenotypic plasticity in general is a well-studied phenomenon, little is known about the evolutionary fate of plastic responses if natural selection on plasticity is relaxed. Here, we study whether the presumed ancestral seasonal plasticity of the rainforest butterfly Bicyclus sanaos (Fabricius, 1793) is still retained despite the fact that this species inhabits an environmentally stable habitat. Being exposed to an atypical range of temperatures in the laboratory revealed hidden reaction norms for several traits, including wing pattern. In contrast, reproductive body allocation has lost the plastic response. In the savannah butterfly, B. anynana (Butler, 1879), these traits show strong developmental plasticity as an adaptation to the contrasting environments of its seasonal habitat and they are coordinated via a common developmental hormonal system. Our results for B. sanaos indicate that such integration of plastic traits – as a result of past selection on expressing a coordinated environmental response – can be broken when the optimal reaction norms for those traits diverge in a new environment.
Keywords: Bicyclus anynana, Bicyclus martius, Bicyclus sanaos, constraints, life-history evolution, phenotypic plasticity, reproductive investment, seasonality
Introduction
Phenotypic plasticity is the ability of a particular genotype to express different phenotypes in response to environmental variation (Schlichting and Pigliucci 1998; West-Eberhard 2003). Although not necessarily adaptive, many instances of adaptive phenotypic plasticity have been documented. In these cases, organisms expressing distinct phenotypes in alternative environments have their highest relative fitness in the environment in which they typically occur (see Stearns 1989; Beldade et al. 2011; Simpson et al. 2011). In seasonal habitats, phenotypic plasticity may evolve as a result of contrasting but predictable seasonal selection pressures, resulting in different morphologies and/or life-history strategies being expressed in each season (Shapiro 1976; Brakefield and Zwaan 2011).
It has rarely been studied what the evolutionary fate of plasticity would be in an ancestrally plastic species that no longer inhabits such a seasonal environment so that the traits are no longer exposed to environmental variation previously associated with their plasticity (Lahti et al. 2009; Snell-Rood et al. 2010; but see Aalberg Haugen et al. 2012). This could occur when a species adapted to a seasonally fluctuating habitat establishes itself in a new, aseasonal habitat, or when the environmental conditions within a habitat change such that it becomes less seasonal. In such circumstances, part of the previous phenotypic range of this trait is no longer expressed on a regular or predictable basis and therefore not exposed to natural selection. Such relaxed selection also applies to plasticity for the trait, that is, the ability to express other mean trait values in response to environmental variation.
The question whether relaxed selection will result in loss or retention of seasonal plasticity for a particular trait will depend on the interplay between benefits, costs (both direct and indirect via pleiotropy), and neutral processes such as mutation accumulation and genetic drift (Lahti et al. 2009). Retaining plasticity in a less seasonal environment might be favored through direct benefits if the environment still shows limited variability, but of a less regular nature. Indirect benefits might be due to pleiotropic effects of seasonal plasticity on correlated traits that are still under strong selection. This might be the case if genetic or developmental mechanisms regulating seasonal plasticity are also involved in other, nonseasonal environmental responses such as responses to diurnal temperature fluctuations. If there is no such pleiotropy, mutations in genes responsible for seasonal plasticity can accumulate, potentially, but not necessarily, leading to reduction or loss of the seasonal response (Lahti et al. 2009; Aalberg Haugen et al. 2012). Finally, plasticity, when under relaxed selection, might be reduced or lost due to two proposed types of costs of plasticity. First, there can be direct ‘production’ costs of expressing a particular trait, as in the case of energetic costs of inducible anti predatory defenses. Second, theoretical considerations suggest the existence of inherent costs associated with the capacity to produce different phenotypes under different conditions. The costs of this capacity presumably lie in the maintenance of sensory and regulatory systems needed to sense environmental conditions and translate them into phenotypic alterations. However, empirical studies suggest that such plasticity costs are generally weak, making it unclear whether they would play a large role in evolutionary loss of plasticity under relaxed selection (Callahan et al. 2008; van Buskirk and Steiner 2009; Auld et al. 2010). In general, empirical studies on the evolutionary consequences of relaxed selection on plasticity are relatively rare (Lahti et al. 2009; Snell-Rood et al. 2010; Schwander and Leimar 2011), especially in the context of plasticity as adaptation to seasonally fluctuating environments (but see Aalberg Haugen et al. 2012). Here, we study whether a phylogenetically widespread and likely ancestral capability to respond to seasonal environmental variation is still retained in a species that inhabits a less seasonal habitat, where natural selection on plastic responses is assumed to be relaxed.
Seasonal plasticity of wing pattern occurs frequently in the Nymphalidae butterfly family, for example in Melanitis leda (Brakefield and Larsen 1984), Junonia coenia (Rountree and Nijhout 1995), and Araschnia levana (Windig and Lammar 1999). Seasonal plasticity, often with discrete phenotypes in the wet and dry season, is well documented in the Subtribe Mycalesina (Satyrinae), which inhabit a multitude of habitats in the old world tropics (Brakefield and Reitsma 1991; Braby 1994; Brakefield and Frankino 2009). Particularly well studied among Mycalesina is the genus Bicyclus (Kirby, 1871), where seasonal plasticity in wing pattern is very common. The genus comprises of ca. 90 species distributed throughout sub-Saharan Africa, inhabiting savannah-woodland as well as rainforest habitats (Condamin 1973; Brakefield and Frankino 2009). In the dry season in seasonal habitats, adult Bicyclus butterflies typically express a cryptic wing pattern allowing them to rest undetected among the dried out vegetation. In the wet season, vegetation is green and abundant and the adults of the same species now instead express prominent concentric eyespots along the distal margin of their wings. These dry and wet season morphs are expressed by separate cohorts of butterflies, and the adult wing pattern is fixed at emergence. The eyespots are probably involved in deflecting vertebrate predator attacks away from the vulnerable body toward the margin of the wing (Lyytinen et al. 2003, 2004; Brakefield and Frankino 2009). Given that seasonal polyphenism is widespread throughout the whole Subfamily (Satyrinae), and that most species of Bicyclus (even those inhabiting evergreen forest) show signs of plasticity, this is likely the ancestral state for the group (Brakefield and Frankino 2009). The most studied species is Bicyclus anynana (Butler, 1879), a savannah-woodland butterfly distributed throughout East Africa (Brakefield et al. 2009). In addition to wing pattern, B. anynana adults of the wet and dry seasons differ markedly in their life-history strategies. In the field, adults spend the harsh dry season being relatively inactive and delay reproduction until the beginning of the wet season, when larval food plants reappear. The relatively short-lived adults of the wet season morph are more active and reproduce rapidly. Normally, two to three such generations occur in the wet season. In the laboratory, wet season females allocate relatively more mass to the abdomen (Oostra et al. 2011) and lay more (albeit smaller) eggs (Brakefield and Zwaan 2011).
At a proximate level, the major cue for the induction of adult dry or wet season phenotypes is the temperature experienced during the late larval and early pupal stages. At high temperatures, corresponding to wet season conditions in the field, B. anynana larvae develop into wet season adults, whereas low temperatures, indicative of an approaching dry season in the field, induce development of the dry season morph (see Oostra et al. 2011). Both adult wing pattern and life history are determined by developmental temperature, although life-history traits retain the ability to acclimatize over the course of several weeks when environmental conditions change during adult life (e.g., Fischer et al. 2003). Recently, we showed that Ecdysteroid hormones during the pupal stage play a functional role in regulating developmental plasticity of adult reproductive strategy, a role for this hormone that had already been established for wing pattern plasticity (Oostra et al. 2011b). This indicates that, at least in B. anynana, developmental plasticity of wing pattern and of life history share developmental-physiological mechanisms. Such developmental integration of both forms of plasticity may have been driven by the correlated fluctuations in selection pressures on both wing pattern and life history between the seasons.
To examine the evolutionary effects of relaxed selection on developmental plasticity of wing pattern and life history, we used Bicyclus sanaos (Hewitson, 1866) (see method for discussion about species name issues), a rainforest species that is only rarely exposed to dry season like conditions in the field. B. sanaos' closest relatives are all forest species, and show some level of wing pattern plasticity. However, only two of the related forest species can be classified as possibly seasonally polyphenic (B. istaris and B. sophrosyne). At the basal branching of the same clade of Bicyclus is one species group whose members are among the most seasonal savannah species of the whole genus (B. cottrelli and B. safitza) (Brakefield and Frankino 2009). The available phylogenetic data suggest that the ancestors of B. sanaos left the savannah habitat about 10 million years ago (Monteiro and Pierce 2001). This long-term lack of seasonal exposure to harsh dry season conditions likely reflects a situation of relaxed selection on plasticity.
The aim of this study was to investigate to what extent B. sanaos has retained the ability to express alternative phenotypes when exposed in the laboratory to a range of ‘seasonal’ temperatures not normally encountered in the field. Using a recently established laboratory population, we measured the thermal responses for a suite of traits that in B. anynana are plastic and involved in the seasonal adaptation. This was done on parallel cohorts of developing larvae, across a temperature range that in other Bicyclus species induce plasticity (Roskam and Brakefield 1996). The traits investigated included life-history traits, physiological traits, and wing patterns. We then compared thermal responses to those observed in a previous reaction norm experiment in B. anynana (Oostra et al. 2011). Our experiment allowed us to determine not only the thermal plasticity of each individual trait but also the extent to which these traits show an integrated response to the environment comparable to that in B. anynana. Performing a comparative study of plasticity responses using only two species is a limitation, but to our knowledge this is the first time a reaction norm study with detailed measurements of several traits has been conducted using nonmodel species of butterflies.
Materials and Methods
Bicyclus sanaos habitat and laboratory population
Bicyclus martius sanaos, in several previous publications called B. sanaos (Larsen 2003), is fairly common in African rainforests, but never found in open savannah habitats. Ongoing revisional work on the taxonomy of the whole genus Bicyclus shows that this subspecies of Bicyclus martius is indeed a valid species of its own (O. Brattström, H-L. Wang, K. Aduse-Poku, C. Löfstedt and P. M. Brakefield, unpubl. data). In this article, we are therefore using the name Bicyclus sanaos when referring to what is still currently officially named Bicyclus martius sanaos. The laboratory stock was established in the laboratory in Leiden from 60 gravid females collected in Ologbo Forest (N 6.02, E 5.55, 20 m.a.s.l.) in southern Nigeria. On this location, temperature varies very little throughout the year (25–28°C mean monthly temperature), but precipitation shows marked seasonality (30–450 mm per month; as measured at a weather station ca. 60 km away (N 5.52, E5.73; National Climatic Data Center). Despite the variation in precipitation, the soil remains wet during the whole dry season, the vegetation in the forest interior remains green and the humidity stays high throughout the year. Thus, larval food plants (i.e., grasses) are likely to be continuously available. Furthermore, B. sanaos adults of all ages can be observed at any given time, from recently eclosed individuals with no visible wing wear through to old individuals with extensive wing damage (O. Brattström, pers. obs.). This suggests that females breed throughout the year and show no seasonal suppression of reproductive activity. Occasionally, individuals with small ventral eyespots, resembling a typical Bicyclus dry season morph, are found in the field. However, the majority of individuals have large, wet season-like eyespots all year round, including during the dry season (O. Brattström, pers. obs.; Roskam and Brakefield 1999). This contrast strongly with the natural savannah habitat of B. anynana that shows a high annual variation in mean monthly temperature (17–25°C) and a marked seasonality in rainfall (0–290 mm per month). More importantly, most years will see at least three consecutive months with no rainfall at all meaning all available food plants for larvae will dry out for a prolonged time (National Climatic Data Center).
Overall, the butterfly rearing setup for the stock of B. sanaos in the laboratory was comparable to that used in B. anynana (see Brakefield et al. 2009), with a slightly higher temperature (28°C) and relative humidity (RH; 85%). The only major difference compared to B. anynana was the larval food plants. Adult females oviposited, and larvae grew, on young pot-grown Oplismenus sp., and Triticum sp. (wheat) plants (both Poaceae). Each generation, about 400 larvae were reared, of which between 40 to 60% normally survived through to adulthood. Females generally started ovipositing between 2 to 3 weeks after eclosion, and continued to do so at a relatively constant rate for several weeks. Adults regularly survived longer than 100 days after eclosion.
Experimental design and measurement of phenotypic responses
We assessed developmental plasticity in B. sanaos by rearing separate cohorts of larvae at three different temperatures and measuring phenotypic responses for life history and wing pattern traits. We collected eggs from the stock population and allowed the larvae to hatch on Triticum (wheat) plants. The freshly hatched larvae were collected on a daily basis and transferred in batches of 20 larvae onto separate one-week-old Triticum plants kept in individual net sleeves, which were each placed in one of three environmental climate chambers set at 19, 23, or 27°C (and 85% RH with a 12:12 L:D photoperiod). We placed a total of 10 such sleeves in each climate chamber, rearing 200 larvae per temperature. Plants were monitored daily and watered or replaced as necessary. Pre-pupae were collected daily and placed in Petri dishes to pupate. One-day-old pupae were weighed to the nearest 0.01 mg using a Sartorius Research RC 210D scale, and then placed in individual pots until eclosion. Larval and pupal development times were recorded in days. Subsequently, adult resting metabolic rate (RMR) was measured as the individual rate of CO2 respiration (mL·h−1) over a period of 20 min, at 27°C during the dark phase of the diurnal cycle (following Pijpe et al. 2007). Butterflies were then frozen at −20°C until further processing. Abdomens and thoraces (removing head, wings, antennae, and legs) were then dried to constant mass for 48 h at 60°C before being weighed separately. One ventral hindwing of each adult was imaged using a Leica M125 stereo microscope coupled to a Leica DFC495 digital camera. In B. sanaos, the basic wing pattern elements on the ventral wing surfaces are similar to those in B. anynana: a series of concentric eyespots along the distal margins of the fore and hind wing (Roskam and Brakefield 1996). We used ImageJ software v1.46 r (Abramoff et al. 2004) to measure three characteristics of the ventral wing pattern on the digital image of each hindwing: radius of the second eyespot (starting from anterior), radius of the fifth eyespot, and distance between the center of the second and the fifth eyespot (as a measurement of wing size). Eyespot radii were measured from the center of each eyespot's white focus to the most proximal point on the outer boundary of the golden ring.
Comparison with Bicyclus anynana
We compared the thermal responses in B. sanaos to those of B. anynana using data on developmental plasticity in B. anynana from a previous reaction norm experiment (Oostra et al. 2011). In this experiment, B. anynana larvae were also reared at 19, 23 and 27°C and phenotypic responses in the adults were measured exactly as for B. sanaos. The only differences between the experiments were the food plants (B. anynana larvae were fed Zea mays plants rather than Triticum; see Brakefield et al. 2009), and the use of a slightly lower humidity (70%) for B. anynana. We know that the choice of food plant species, and the quality of the plants used affects the development time and the absolute values of at least the eyespot patterns in the laboratory stock of B. anynana, but the shape of the reaction norm is unchanged (Kooi et al. 1996). We also know that the same is true when comparing thermal responses of B. anynana populations from different locations across its range (de Jong et al. 2010). When interpreting the results from our study it is therefore important to put the emphasis on the shape of the reaction norm rather than focusing on differences in absolute values (except very large differences).
Statistical analyses
Two-way ANOVAs were used to analyze the effect of developmental temperature and sex on each phenotypic trait of interest, initially fitting full models including temperature, sex, and their interaction as fixed factors and removing nonsignificant terms successively. Minimum adequate and full models are presented in Table 1 and Supplementary Table 1, respectively. Abdomen ratio was computed by dividing abdomen dry mass by total adult dry mass, and this measure was arcsine transformed prior to statistical analysis. RMR and eyespot size were first corrected for body or wing size prior to analysis in the two-way ANOVAs. This was done by first fitting separate linear regression models on each of those traits with adult dry mass (in the case of RMR), or wing size (in the case of eyespot size) as sole predictor variable. The residuals of each of these models were analyzed as dependent variables in the two-way ANOVAs. In all ANOVAs, the residuals were normally distributed as evaluated visually using Q-Q plots.
Table 1.
Minimum adequate models of the effect of developmental temperature and sex on a suite of phenotypic traits in Bicyclus sanaos and B. anynana, related to Figures 6. In one case where none of the fixed effects was significant (P < 0.05), the full model is shown (marked with an *). See Supplementary Table 1 for full models of all traits. The data for B. anynana have previously been used in another study Oostra et al. (2011)
| Species | Dependent variable | Fixed effects | F | df | P |
|---|---|---|---|---|---|
| B. sanaos | Total development time | Temperature | 356.69 | 2,202 | <0.00001 |
| B. anynana | Total development time | Sex | 46.49 | 1,830 | <0.00001 |
| Temperature | 3443.87 | 2,830 | <0.00001 | ||
| Sex × Temperature | 5.88 | 2,830 | 0.00292 | ||
| B. sanaos | Larval development time | Temperature | 201.54 | 2,202 | <0.00001 |
| B. anynana | Larval development time | Sex | 170.39 | 1,830 | <0.00001 |
| Temperature | 3999.71 | 2,830 | <0.00001 | ||
| Temperature × Sex | 15.26 | 2,830 | <0.00001 | ||
| B. sanaos | Pupal development time | Sex | 34.69 | 1,202 | <0.00001 |
| Temperature | 2224.42 | 2,202 | <0.00001 | ||
| B. anynana | Pupal development time | Sex | 16.12 | 1,832 | 0.00006 |
| Temperature | 780.42 | 2,832 | <0.00001 | ||
| B. sanaos* | Pupal mass | Sex | 0.05 | 1,200 | 0.8253 |
| Temperature | 0.32 | 2,200 | 0.7283 | ||
| Temperature × Sex | 0.09 | 2,200 | 0.9159 | ||
| B. anynana | Pupal mass | Sex | 1262.0 | 1,832 | <0.00001 |
| Temperature | 54.21 | 1,832 | <0.00001 | ||
| B. sanaos | Adult dry mass | Sex | 87.21 | 1,203 | <0.00001 |
| Temperature | 4.32 | 2,203 | 0.01458 | ||
| B. anynana | Adult dry mass | Sex | 3801.5 | 1,305 | <0.00001 |
| Temperature | 16.89 | 1,305 | <0.00001 | ||
| B. sanaos | Size-corrected RMR | Sex | 106.27 | 1,191 | <0.00001 |
| Temperature | 21.19 | 2,191 | <0.00001 | ||
| B. anynana | Size-corrected RMR | Temperature | 87.58 | 2,186 | <0.00001 |
| Temperature × Sex | 4.73 | 2,186 | 0.00332 | ||
| B. sanaos | Abdomen ratio (arcsine transformed) | Sex | 1122.0 | 1,205 | <0.00001 |
| B. anynana | Abdomen ratio (arcsine transformed) | Sex | 1299.71 | 1,303 | <0.00001 |
| Temperature | 20.842 | 2,303 | <0.00001 | ||
| Temperature × Sex | 26.27 | 2,303 | <0.00001 | ||
| B. sanaos | Size-corrected second eyespot radius | Sex | 12.23 | 1,187 | 0.00059 |
| Temperature | 34.98 | 2,187 | <0.00001 | ||
| B. anynana | Size-corrected second eyespot radius | Sex | 18.91 | 1,214 | 0.00002 |
| Temperature | 171.01 | 2,214 | <0.00001 | ||
| B. sanaos | Size-corrected fifth eyespot radius | Sex | 90.70 | 1,187 | <0.00001 |
| Temperature | 67.05 | 2,187 | <0.00001 | ||
| B. anynana | Size-corrected fifth eyespot radius | Sex | 4.75 | 1,212 | 0.03049 |
| Temperature | 170.43 | 2,212 | <0.00001 | ||
| Temperature × Sex | 3.97 | 2,212 | 0.02034 |
Mortality was compared between the three temperature treatments by analyzing egg to adult survival using Chi-squared tests. All analyzes were performed in the R statistical environment (R Development Core Team 2010).
Results
Low survival at lowest temperature
Larvae and pupae performed significantly worse at the lowest temperature. Egg to adult survival rate was 20% at 19°C, 42% at 23°C, and 41.5% at 27°C (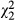 = 18.29, N = 207, P = 0.0001). Assuming an equal sex ratio among the hatching larvae, females had a slightly higher mortality across temperatures (
= 18.29, N = 207, P = 0.0001). Assuming an equal sex ratio among the hatching larvae, females had a slightly higher mortality across temperatures (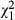 = 4.06, N = 207, P = 0.04). The total number of females eclosing was 89 and for the males 118.
= 4.06, N = 207, P = 0.04). The total number of females eclosing was 89 and for the males 118.
Development time and lack of protandry
Total egg to adult development time in B. sanaos was strongly affected by developmental temperature, as indicated by the shape of reaction norms and wide differences between extreme temperatures (Table 1; Fig. 1). Both females and males developed faster at higher temperatures but there was no evidence for protandry in B. sanaos. The sexes developed at the same rate and showed the same temperature response. This contrasts sharply with the protandry shown by B. anynana across all temperatures, especially at the higher temperatures (Fig. 1A). Overall, B. sanaos developed much slower than B. anynana. Examining larval and pupal development time separately revealed that the lack of protandry in B. sanaos originated in the larval stage. The average duration of the larval stage was equal for females and males, while in B. anynana male larvae develop significantly faster than female larvae (Fig. 1B). In contrast, during the pupal stage females of both species developed faster than males (Fig. 1C). The latter effect was small and did not affect total development time.
Figure 1.
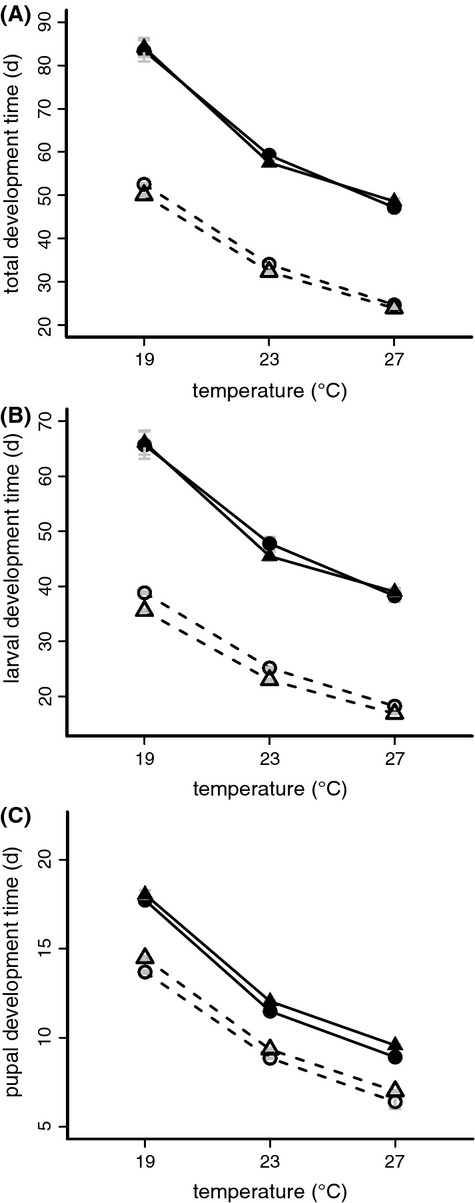
Development time for Bicyclus sanaos (solid lines) and B. anynana (dashed lines) shown as total (A) developmental time, and broken up in larval (B) and pupal (C) time. Both species are affected by temperature with slower development in cold conditions. In B. anynana, there is also strong protandry, with males (triangles) emerging before females (circles), a pattern not seen in B. sanaos. See Table 1 and Supplementary Table 1.
Plasticity and sexual dimorphism in body size
Despite large changes in development time in response to temperature, pupal mass of B. sanaos did not differ across developmental temperatures. Furthermore, there was no sexual dimorphism in this trait (Table 1; Fig. 2A). In contrast, adult dry mass showed a significant temperature response, with a larger size at lower temperatures, and females were consistently larger than males (Fig. 2B). In B. anynana, both pupal and adult dry masses are affected by developmental temperature (Fig. 2). Furthermore, female B. sanaos pupae developed to become larger adults than male pupae of similar mass. Thus, in B. sanaos, in contrast to B. anynana, temperature plasticity of body size and sexual size dimorphism are only detectable in adults, and, therefore, originate in the pupal stage. Although the same average weight is accumulated during the larval stage, male pupae lose more weight than female pupae during metamorphosis and end up as smaller adults. Pupae developed at higher temperatures lose more mass during the pupal stage than those developed at lower temperatures and do so in a shorter time period (cf. Fig. 1C).
Figure 2.
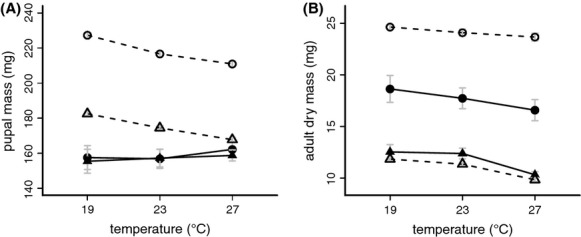
Pupal mass (A) and adult dry mass (B) in Bicyclus sanaos (solid lines) and B. anynana (dashed lines) as a function of developmental temperature for females (circles) and males (triangles). Both species produce smaller adults at warmer developmental temperatures, and females are consistently larger than males. For B. sanaos, the pupal mass is not affected by either rearing temperature or sex. See Table 1 and Supplementary Table 1.
Imprint of developmental temperature on adult RMR
Developmental temperature had a significant effect on mass-corrected adult RMR, with females and males reared at low temperatures expressing higher RMR as adult. Furthermore, across all temperatures males had a higher RMR than females (Table 1; Fig. 3A). Bicyclus anynana showed a similar temperature imprint with mass-corrected adult RMR being higher in individuals reared at lower temperatures. However, males only had higher RMR than females at 27°C (Fig. 3B).
Figure 3.
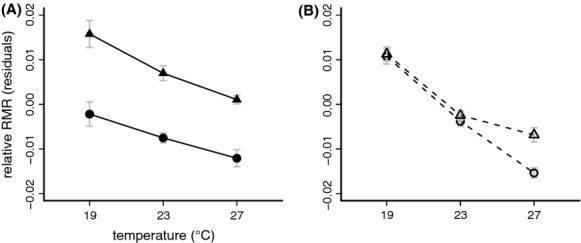
Mass-corrected adult RMR (ml CO2 hr−1; see Methods) in Bicyclus sanaos (A) and B. anynana (B) as a function of developmental temperature for females (circles) and males (triangles). The difference between the sexes is observed in B. sanaos is only present at 27 °C for B. anynana. See Table 1 and Supplementary Table 1.
Reduced plasticity in reproductive body allocation
We determined abdomen ratio (relative allocation of adult body mass to abdomen) as a measure of reproductive investment (cf. Oostra et al. 2011). There was no effect of temperature on abdomen ratio in B. sanaos (Table 1, Supplementary Table 1). In contrast, abdomen ratio in B. anynana was significantly affected by temperature, with relatively larger abdominal sizes at higher temperatures. The difference between the females of the two species is shown in Fig. 4. These data indicate a lack of developmental plasticity of allocation to abdomen in B. sanaos, contrasting with the high temperature sensitivity observed in B. anynana.
Figure 4.
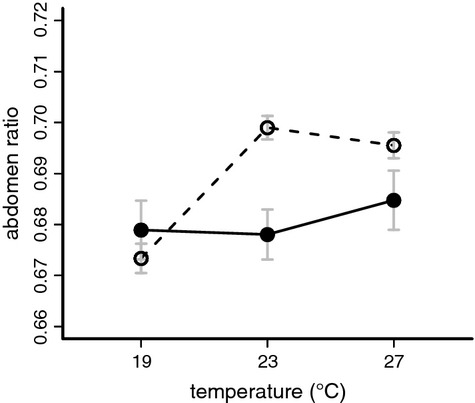
Abdomen ratio (abdomen dry mass divided by total dry mass) in Bicyclus sanaos (solid lines) and B. anynana (dashed lines) females as a function of developmental temperature. Females of B. anynana put more resources into abdominal tissue when developing at high temperatures, while no such effect is found for females of B. sanaos. See Table 1 and Supplementary Table 1.
Phenotypic plasticity of wing pattern
The ventral wing patterns of females and males of both species showed a marked response to developmental temperature. Both the sizes of the eyespots as well as coloration of the wing differed substantially between cohorts reared at different temperatures (Fig. 5). We quantified these differences for two eyespots and found that the size of the second (Fig. 6A and C) and fifth (Fig. 6B and D) eyespot on the ventral hind wing (corrected for wing size) was strongly affected by developmental temperature (Table 1). Both species had smaller eyespots when reared at lower temperatures and females generally had larger spots. The coefficients of variation (standard deviation divided by the mean per species per sex per temperature) were on average ca. 50% higher in B. sanaos compared to B. anynana, and at all temperatures variation was highest in B. sanaos in each sex (Fig. 6E and F). Taken together, we show that eyespot size is a phenotypically plastic trait in B. sanaos. It responds in the same direction and to the same extent to developmental temperature as in its seasonal congener B. anynana: a lower temperature during development induces the expression of smaller eyespots in adults. Although both species show plasticity in wing pattern, variation in eyespot size is consistently higher in B. sanaos compared to B. anynana. Data (Oostra et al 2014a) available from the Dryad Digital Repository.
Figure 5.
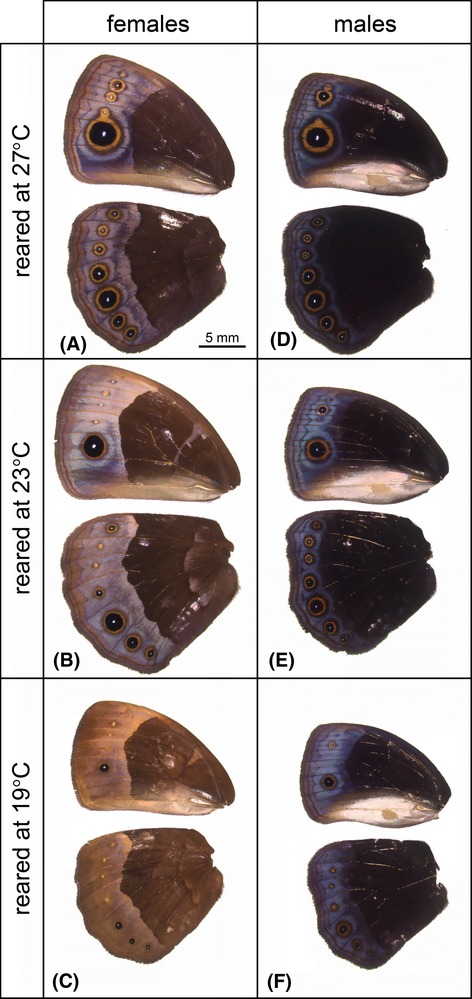
Wing patterns of adult Bicyclus sanaos reared at different temperatures as larvae. The ventral surfaces of fore, – and hindwings of representative B. sanaos females (left) and males (right) reared at 27 (A, D), 23 (B, E), or 19°C (C, F) are shown. More details of the quantitative phenotypic difference are shown in figure 6.
Figure 6.
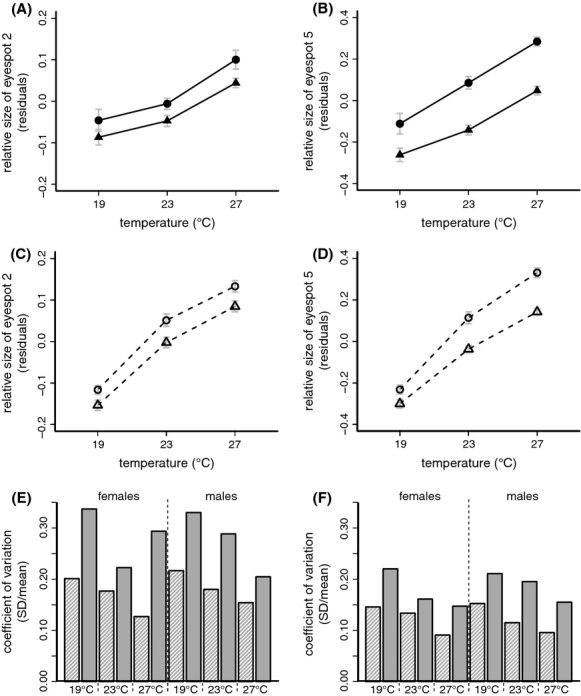
The relative size of two measured eyespots for Bicyclus sanaos (A, B) and B. anynana (C, D) on the ventral hindwing (mm; see Methods) as a function of developmental temperature, for females (circles) and males (triangles). Both species show a strong effect of temperature with smaller eyespots when reared in colder conditions. Females have consistently larger eyespots than males (See Table 1 and Supplementary Table 1). Investigating the coefficients of variation (standard deviation/mean) in the size of the second (E) and fifth (F) eyespot in B. sanaos (filled bars) and B. anynana (striped bars) in females and males at all three developmental temperatures (equal sample sizes for both species; see Methods) reveals higher variation at all temperatures for B. sanaos.
Discussion
The rainforest butterfly B. sanaos showed striking differences in thermal responses among traits that in B. anynana are all highly responsive to developmental temperature and are involved in seasonal adaptation. Most traits (development time, adult mass, RMR, and ventral eyespot size), responded readily to temperature, albeit not always in exactly the same way as in B. anynana (Figs. 3 and 6). The observed temperature plasticity in these traits was in stark contrast with the lack of response to temperature for relative abdomen size. In B. anynana, adults develop a relatively larger abdomen when reared under warm, wet season conditions, reflecting the higher early reproductive investment in this season (Oostra et al. 2011). However, in B. sanaos this was not the case: females showed no evidence of increased mass allocation to abdomen when reared in warm conditions, with the slope of thermal reaction norms not deviating from zero (Fig. 4). The same was true for the males. Thus, exposing B. sanaos in the laboratory to a range of temperatures the species not normally would be exposed to in nature reveals plasticity for some traits, but not all.
Mechanisms of life-history integration
In many animals, plastic traits are integrated into functional suites that covary in response to environmental cues (Schlichting and Pigliucci 1998; Pigliucci 2003; Brakefield and Zwaan 2011; Simpson et al. 2011). Shared endocrine regulation plays a key role in accomplishing such phenotypic integration of multiple traits and adjusting them in a coordinated and timely fashion (Denlinger 2002; Ketterson et al. 2009). In B. anynana, several seasonally plastic traits investigated in this study are controlled by a single hormonal system. In particular, Ecdysteroid hormones active during the pupal stage control pupal development time (Zijlstra et al. 2004) and mediate developmental plasticity of the ventral wing pattern (Koch et al. 1996; Brakefield et al. 1998), female relative abdomen size, and female reproductive strategy (Oostra et al. 2014b). Pupal development time and wing pattern show strong genetic and phenotypic correlations, due to sharing the same underlying hormonal regulator. At the same time, they also express substantial trait-independent genetic variation, and antagonistic selection targeting this variation is able to uncouple the two traits (Zijlstra et al. 2003, 2004). A similar scenario could explain the differences in plasticity between wing pattern and abdomen size as observed in B. sanaos, which in B. anynana both show plasticity and share a single hormonal regulator. These traits may have previously been hormonally coupled in the ancestral lineage of B. sanaos inhabiting the seasonal savannah. Divergent selective pressures for these traits in the less seasonal rainforest habitat (see below) in combination with trait-independent genetic variation may have resulted in their decoupling over evolutionary time. Hormonal manipulations in B. sanaos could reveal whether abdomen size has indeed lost its plasticity due to decoupling from environmentally sensitive hormonal regulation.
Adaptive value of body mass allocation
The allocation of body mass to the abdomen is an important determinant of female reproductive investment and early fecundity in many insects, including Lepidoptera (Boggs 1981; Jervis et al. 2005; Kivela et al. 2012). Any (plastic) reduction in female abdomen size would thus likely inflict a strong cost for species under time constraints (Brakefield et al. 2001). For B. anynana, the dry season in the savannah is a period of severely limited reproductive opportunities, due to lack of larval food plants (Brakefield and Zwaan 2011). Under such circumstances, selective pressures in the dry season likely drive the seasonally plastic reallocation of mass away from the abdomen, as observed in the laboratory (Saastamoinen et al. 2010). In contrast, the rainforest species B. sanaos does not experience a seasonal reduction in food availability, as green larval food plants continue to be abundant even at the end of the dry season. Therefore, it has the potential to breed continuously throughout the year, with overlapping generations. This would relax the need to invest larval-derived resources in a large abdomen already at eclosion in order to be able to start ovipositing very early in life (potentially at the expense of lifetime fecundity). Indeed, B. sanaos females in the laboratory start ovipositing late, continue to do so for a long time and live relatively long lives compared to B. anynana (pers. obs.). In addition, a relatively heavy abdomen could potentially constrain adult flight ability, increasing susceptibility to predation (cf. Srygley and Chai 1990). Together, this could explain why the ratio of abdomen to thorax mass at eclosion was more similar to that in B. anynana in the dry, not the wet season (Fig. 4). If B. sanaos females would still have the developmental machinery for reduced mass allocation to abdomen in response to temperature, occasional colder periods could inflict a serious cost on fecundity by further reducing an already decreased allocation to the abdomen. This loss of temperature sensitivity in abdominal allocation might therefore have evolved via selection for maintaining a stable abdomen size despite occasional temperature fluctuations.
Wing patterns and predation risk
Seasonal plasticity of eyespot size in Bicyclus butterflies is maintained by opposing forces of natural selection in the dry and wet season savannah environments (Brakefield and Frankino 2009). As a rainforest species from a constant, green environment, B. sanaos is not exposed regularly to a dry season where reduced eyespots would be beneficial, but is instead under constant selection favoring large, deflective eyespots. In absence of other major evolutionary forces, such a situation would presumably lead to reduced phenotypic plasticity in wing pattern. However, this is contrary to our current findings. One explanation for the evolutionary maintenance of eyespot size plasticity in B. sanaos could be that temperature sensitivity is still beneficial within the green rainforest environment. Higher temperatures permit higher flight activity (e.g., via increased metabolic rate; Niitepold et al. 2009) but this may carry enhanced predation risks (cf. Bonte et al. 2012). If large eyespots are more effective in deflecting predatory attacks than small ones, expressing large eyespots might permit higher levels of activity at higher temperatures. However, such temperature-dependent fitness consequences of variation in eyespot size within the rainforest (or during the wet season on the savannah) are probably very subtle, making it very difficult to test this hypothesis. Empirically establishing the selective advantage of wet season-like eyespots as deflectors of predator attacks has been notoriously difficult, contrasting with the firmly established strong selection for crypsis in the dry season (Brakefield and Frankino 2009).
If indeed there is no strong selection acting on wing pattern plasticity within the rainforest environment, constraints on evolutionary change toward reduced plasticity might be more important. In this hypothesis, the evolutionary retention of eyespot size plasticity in B. sanaos is best explained as a legacy of past selection in a seasonal environment. In B. anynana, it has been shown that a lack of temperature-independent genetic variation in eyespot size can constrain the evolution of reduced plasticity, at least in the short term. Artificial selection experiments targeting the slope of the reaction norm for wing pattern failed to produce lines with reduced plasticity. This was due to high, positive genetic correlations across temperatures (Wijngaarden and Brakefield 2001; Wijngaarden et al. 2002). The observed retention of wing pattern plasticity in B. sanaos indicates that such strong developmental constraints may also be relevant over longer evolutionary time scales, especially if selective benefits of wing pattern plasticity in the rainforest environment are weak. Although we did not measure genetic variation, phenotypic variation for eyespot size was substantially higher in B. sanaos compared to B. anynana, and more so at lower temperatures (Fig. 6E and F), consistent with the view of relaxed selection on eyespot size and/or increased mutational variation.
Protandry, sexual size dimorphism, and size plasticity
In seasonal insects, time constraints on development can promote the evolution of protandry, while continuous breeding opportunities with overlapping generations are associated with absence of protandry (Nylin et al. 1993; Blanckenhorn et al. 2007; Allen et al. 2011). Bicyclus sanaos, inhabiting an environment with limited seasonal variation in reproductive opportunities, showed no evidence of protandry: males did not develop faster than females at any temperature (Fig. 1A). This contrasts sharply with the significant protandry observed in a number of previous experiments in B. anynana (e.g., Oostra et al. 2011), which only has a limited time period in the wet season to reproduce. In this species, artificial selection experiments showed that strong genetic correlations in development time between the sexes preclude all but a small short-term evolutionary response to selection for increased or decreased protandry (Zwaan et al. 2008). This is likely the result of strong selection in the past on protandry, reducing sex-independent genetic variation for development time (Allen et al. 2011). The lack of protandry observed in B. sanaos suggests that such a genetic constraint can be broken over longer evolutionary time scales.
As in the majority of insects (Stillwell et al. 2010), B. sanaos adults showed female-biased sexual size dimorphism (Fig. 2B). However, this was not yet the case in the pupal stage (Fig. 2A), when males and females were equally large. This indicates that, in contrast to many insects (Allen et al. 2011), female and male B. sanaos larvae grow equally fast, reaching the same pupal mass at the same time (Fig. 1B). Subsequently, during the pupal stage, female pupae lose less mass and end up being larger as adult than males. The higher RMR observed in adult males compared to females (Fig. 3A) together with the longer pupal development time (Fig. 1C) suggests that pupal development consumes more resources in males compared to females.
A similar pattern was observed for thermal plasticity of pupal and adult mass: individuals reared at lower temperatures were larger as adult, but not as pupa (Fig. 2). The negative effect of developmental temperature on adult size observed in many ectotherms (Atkinson 1994) is usually explained by the effect of temperature on duration and rate of growth (Davidowitz and Nijhout 2004; Edgar 2006). However, B. sanaos larvae developing at low temperatures reached the same average pupal mass as those developing at high temperatures, despite the much longer larval developmental time (Fig. 1B). During the pupal stage, these pupae lost less mass than those that had developed at higher temperatures, ending up as larger adults. Thus, independent of its effects on larval growth, temperature can affect metamorphosis and utilization of larval-derived resources to the adult body.
A larval temperature signature of RMR
Previous studies in B. anynana (e.g., Pijpe et al. 2007) and other insects (Berrigan 1997; Le Lann et al. 2011) reported a negative effect of development temperature on adult RMR, suggesting a general mechanism for coping with lower temperatures rather than a specific seasonal adaptation. We observed the same general pattern in B. sanaos, but there are interesting differences between the sexes. In B. anynana, RMR is higher in males compared to females, but only at 27°C. This might relate to the higher reproductive activity in wet season conditions, when males are actively searching for females and fighting for territories. B. sanaos males have a higher RMR compared to females regardless of developmental temperature, suggesting that B. sanaos males do not alter breeding activity in response to temperature, similar to the lack of plasticity in female abdominal allocation (see above).
Conclusions
Exposing the aseasonal rainforest butterfly B. sanaos in the laboratory to a range of temperatures not normally encountered in the field revealed hidden reaction norms for several traits, including wing pattern and adult size. In contrast, allocation of adult mass to the abdomen (a proxy for early-life reproductive investment) was not affected by developmental temperatures. In the savannah butterfly B. anynana, these traits show developmental plasticity as an adaptation to the contrasting environments of its seasonal habitat. In that species, wing pattern and allocation to abdomen respond to developmental temperature via a common hormonal system active during pupal development. Our results for B. sanaos strongly suggest that such shared hormonal regulation does not preclude decoupling of temperature responses between traits over evolutionary time. The observed differences in plasticity with B. anynana are likely the result of long-term relaxed selection on plasticity over millions of years for B. sanaos. Other species from similar habitats as our two study species are likely to show similar responses. However, given that we have only been able to compare two species it could be possible that differences not directly related to species-specific selective regimes might influence our results. We can identify three such alternative explanations, but find them unlikely to cause the observed differences. First, the lack of plasticity in body allocation in B. sanaos (with a relatively small abdomen across treatments) might be attributable to wheat being a less efficient food source, so that the larvae struggle to reach anything but a small abdominal size at eclosion regardless of rearing temperature. Having reared around ten Bicyclus species from across Africa in the laboratory over the last years we have observed that maize in general is a much less suitable food plant for these species than wheat. While all reared species developed well on wheat, many showed high mortality when reared on maize (O. Brattström, unpubl. data). Second, the laboratory population of B. anynana has been bred in laboratory conditions for more than a hundred generations (Brakefield et al. 2009). This more stable environment may be interpreted as artificially relaxed selection on plasticity, but if that were the case its effect should be in the direction of more similarities between the species compared to their presumed natural state, which is contrary to our observed results. Third, the amount of available genetic variation within our two test populations could be quite different, given differences in founding population size and time since establishment in the laboratory. However, this cannot explain why B. sanaos should lose plasticity in one trait only (abdomen allocation) while retaining it in the others. Considering these factors we conclude that the results of our study reflect the natural thermal response of the wild populations of the two investigated species. We hypothesize that the loss of plasticity in abdomen allocation is the result of strong natural selection against temperature-induced fecundity reduction in the rainforest, combined with selection for more continuous breeding over a longer life span. For wing pattern, such selective forces are likely much weaker, resulting in retention of developmental plasticity. This implicates that costs of plasticity in Bicyclus butterflies mainly stem from mismatch costs, that is, expressing a suboptimal phenotype in a particular environment (Auld et al. 2010), which likely differ between phenotypically plastic traits. Thus, hormonal integration between plastic traits – as a result of past selection on expressing a coordinated environmental response – can be broken when the optimal reaction norms for those traits diverge in a new environment. Our understanding of the extent to which plasticity in wing pattern and in life history can evolve independently would greatly benefit from studying these traits systematically in a phylogenetic context. In general, we argue that comparing phylogenetically closely related species allows for both discerning patterns of adaptive evolution in the individual species and traits as well as to follow patterns of trait loss and their adaptive significance (Ellers et al. 2012).
Acknowledgments
The authors thank Mariël Lavrijsen and David Hallesleben for plant rearing, and Karl Gotthard and Erik van Bergen for providing helpful comments on this manuscript. VO and BJZ were financially supported by the European Union's FP6 (Network of Excellence LifeSpan FP6/036894) and FP7 Programme (IDEAL FP7/2007-2011/259679 to BJZ). PB and OB were financially supported by an ERC grant no 250325 (EMARES) to PB. The butterflies were initially brought to Leiden through grants to OB from the Wenner-Gren Foundation and the Helge Ax:son Johnson's Foundation.
Conflict of Interest
None declared
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Full models of the effect of developmental temperature, sex and their interaction on a suite of phenotypic traits in B. sanaos and B. anynana, related to Figures 1–4 & 6. See Table 1 in the main text for the minimum adequate models. The data for B. anynana have previously been used in another study (Oostra et al. 2011).
References
- Aalberg Haugen IM, Berger D, Gotthard K. The evolution of alternative developmental pathways: footprints of selection on life-history traits in a butterfly. J. Evol. Biol. 2012;25:1377–1388. doi: 10.1111/j.1420-9101.2012.02525.x. [DOI] [PubMed] [Google Scholar]
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- Allen CE, Zwaan BJ, Brakefield PM. Evolution of sexual dimorphism in the Lepidoptera. Annu. Rev. Entomol. 2011;56:445–464. doi: 10.1146/annurev-ento-120709-144828. [DOI] [PubMed] [Google Scholar]
- Atkinson D. Temperature and organism size—a biological law for ectotherms? Adv. Ecol. Res. 1994;25:1–58. [Google Scholar]
- Auld JR, Agrawal AA, Relyea RA. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 2010;277:503–511. doi: 10.1098/rspb.2009.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldade P, Mateus ARA, Keller RA. Evolution and molecular mechanisms of adaptive developmental plasticity. Mol. Ecol. 2011;20:1347–1363. doi: 10.1111/j.1365-294X.2011.05016.x. [DOI] [PubMed] [Google Scholar]
- Berrigan D. Acclimation of metabolic rate in response to developmental temperature in Drosophila melanogaster. J. Therm. Biol. 1997;22:213–218. [Google Scholar]
- Blanckenhorn WU, Dixon AFG, Fairbairn DJ, Foellmer MW, Gibert P, van der Linde K, et al. Proximate causes of Rensch's rule: does sexual size dimorphism in arthropods result from sex differences in development time? Am. Nat. 2007;169:245–257. doi: 10.1086/510597. [DOI] [PubMed] [Google Scholar]
- Boggs CL. Nutritional and life-history determinants of resource-allocation in holometabolous insects. Am. Nat. 1981;117:692–709. [Google Scholar]
- Bonte D, Van Dyck H, Bullock JM, Coulon A, Delgado M, Gibbs M, et al. Costs of dispersal. Biol. Rev. 2012;87:290–312. doi: 10.1111/j.1469-185X.2011.00201.x. [DOI] [PubMed] [Google Scholar]
- Braby MF. Phenotypic variation in adult Mycalesis Hubner (Lepidoptera: Nymphalidae: Satyrinae) from the Australian Wet-Dry tropics. J. Aust. Entomol. Soc. 1994;33:327–336. [Google Scholar]
- Brakefield PM. Polyphenisms in Lepidoptera: Multidisciplinary approaches to studies of evolution. In: Ananthakrishnan TN, Whitman DW, Frankino WA, editors. Phenotypic plasticity in insects. Mechanisms and consequences. Plymouth, UK: Science Publishers, Inc; 2009. pp. 121–152. [Google Scholar]
- Brakefield PM, Larsen TB. The evolutionary significance of dry and wet season forms in some tropical butterflies. Biol. J. Linn. Soc. 1984;22:1–12. [Google Scholar]
- Brakefield PM, Reitsma N. Phenotypic plasticity, seasonal climate and the population biology of Bicyclus butterflies (Satyridae) in Malawi. Ecol. Entomol. 1991;16:291–303. [Google Scholar]
- Brakefield PM, Zwaan BJ. Seasonal polyphenisms and environmentally-induced plasticity in the Lepidoptera - the coordinated evolution of many traits on multiple levels. In: Flatt T, Heyland A, editors. Mechanisms of life history evolution: the genetics and physiology of life history traits and trade-offs. Oxford, UK: Oxford Univ. Press; 2011. pp. 243–252. [Google Scholar]
- Brakefield PM, Kesbeke F, Koch PB. The regulation of phenotypic plasticity of eyespots in the butterfly Bicyclus anynana. Am. Nat. 1998;152:853–860. doi: 10.1086/286213. [DOI] [PubMed] [Google Scholar]
- Brakefield PM, El Filali E, Van der Laan R, Breuker CJ, Saccheri IJ, Zwaan BJ. Effective population size, reproductive success and sperm precedence in: the butterfly, Bicyclus anynana, in captivity. J. Evol. Biol. 2001;14:148–156. doi: 10.1046/j.1420-9101.2001.00248.x. [DOI] [PubMed] [Google Scholar]
- Brakefield PM, Beldade P, Zwaan BJ. The African butterfly Bicyclus anynana: a model for evolutionary genetics and evolutionary developmental biology. Cold Spring Harb. Protoc. 2009;1 doi: 10.1101/pdb.emo122. doi: 10.1101/pdb.emo122. [DOI] [PubMed] [Google Scholar]
- van Buskirk J, Steiner UK. The fitness costs of developmental canalization and plasticity. J. Evol. Biol. 2009;22:852–860. doi: 10.1111/j.1420-9101.2009.01685.x. [DOI] [PubMed] [Google Scholar]
- Callahan HS, Maughan H, Steiner UK. Phenotypic plasticity, costs of phenotypes, and costs of plasticity: toward an integrative view. In: Schlichting CD, Mousseau TA, editors. Year in evolutionary biology 2008. Wiley-Blackwell: Malden; 2008. pp. 44–66. [DOI] [PubMed] [Google Scholar]
- Condamin M. Monographie du genre Bicyclus (Lepidoptera Satyridae) Dakar: Ifan-Dakar; 1973. [Google Scholar]
- Davidowitz G, Nijhout HF. The physiological basis of reaction norms: the interaction among growth rate, the duration of growth and body size. Integr. Comp. Biol. 2004;44:443–449. doi: 10.1093/icb/44.6.443. [DOI] [PubMed] [Google Scholar]
- Denlinger DL. Regulation of diapause. Annu. Rev. Entomol. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- Edgar BA. How flies get their size: genetics meets physiology. Nat. Rev. Genet. 2006;7:907–916. doi: 10.1038/nrg1989. [DOI] [PubMed] [Google Scholar]
- Ellers J, Kiers ET, Currie CR, McDonald BR, Visser B. Ecological interactions drive evolutionary loss of traits. Ecol. Lett. 2012;15:1071–1082. doi: 10.1111/j.1461-0248.2012.01830.x. [DOI] [PubMed] [Google Scholar]
- Fischer K, Eenhoorn E, Bot AN, Brakefield PM, Zwaan BJ. Cooler butterflies lay larger eggs: developmental plasticity versus acclimation. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 2003;270:2051–2056. doi: 10.1098/rspb.2003.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jervis MA, Boggs CL, Ferns PN. Egg maturation strategy and its associated trade-offs: a synthesis focusing on Lepidoptera. Ecol. Entomol. 2005;30:359–375. [Google Scholar]
- de Jong MA, Kesbeke FMNH, Brakefield PM, Zwaan BJ. Geographic variation in thermal plasticity of life history and wing pattern in Bicyclus anynana. Clim. Res. 2010;43:91–102. [Google Scholar]
- Ketterson ED, Atwell JW, McGlothlin JW. Phenotypic integration and independence: Hormones, performance, and response to environmental change. Integr. Comp. Biol. 2009;49:365–379. doi: 10.1093/icb/icp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivela SM, Valimaki P, Carrasco D, Maenpaa MI, Manttari S. Geographic variation in resource allocation to the abdomen in geometrid moths. Naturwissenschaften. 2012;99:607–616. doi: 10.1007/s00114-012-0940-2. [DOI] [PubMed] [Google Scholar]
- Koch PB, Brakefield PM, Kesbeke F. Ecdysteroids control eyespot size and wing color pattern in the polyphenic butterfly Bicyclus anynana (Lepidoptera: Satyridae) J. Insect Physiol. 1996;42:223–230. [Google Scholar]
- Kooi RE, Brakefield PM, Rossie WEM-T. Effects of food plant on phenotypic plasticity in the tropical butterfly Bicyclus anynana. Entomol. Exp. Appl. 1996;80:149–151. [Google Scholar]
- Lahti DC, Johnson NA, Ajie BC, Otto SP, Hendry AP, Blumstein DT, et al. Relaxed selection in the wild. Trends Ecol. Evol. 2009;24:487–496. doi: 10.1016/j.tree.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Larsen TB. The validity and synonymy of the names Bicyclus martius Fabricius, 1793 and B. sanaos Hewitson, 1866 (Nymphalidae; Satyrinae) Entomol. Rec. J. Var. 2003;115:95–96. [Google Scholar]
- Le Lann C, Wardziak T, van Baaren J, van Alphen JJM. Thermal plasticity of metabolic rates linked to life-history traits and foraging behaviour in a parasitic wasp. Funct. Ecol. 2011;25:641–651. [Google Scholar]
- Lyytinen A, Brakefield PM, Mappes J. Significance of butterfly eyespots as an anti-predator device in ground-based and aerial attacks. Oikos. 2003;100:373–379. [Google Scholar]
- Lyytinen A, Brakefield PM, Lindstrom L, Mappes J. Does predation maintain eyespot plasticity in Bicyclus anynana. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 2004;271:279–283. doi: 10.1098/rspb.2003.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro A, Pierce NE. Phylogeny of Bicyclus (Lepidoptera: Nymphalidae) inferred from COI, COII, and EF-1a gene sequences. Mol. Phylogenet. Evol. 2001;18:264–281. doi: 10.1006/mpev.2000.0872. [DOI] [PubMed] [Google Scholar]
- National Climatic Data Center. 2014. Mean monthly temperature and precipitation data (average over 1900-1965) for Warri, Nigeria (5.52N, 5.73E; WMO station code: 65236), and Zomba, Malawi (15.40S, 35.30E; WMO station code: 67692.2 accessed on 23 April 20114 ( http://www.ncdc.noaa.gov/ghcnm/ via http://climexp.knmi.nl/getstations.cgi). National Oceanic and Atmospheric Administration, United States of America.
- Niitepold K, Smith AD, Osborne JL, Reynolds DR, Carreck NL, Martin AP, et al. Flight metabolic rate and Pgi genotype influence butterfly dispersal rate in the field. Ecology. 2009;90:2223–2232. doi: 10.1890/08-1498.1. [DOI] [PubMed] [Google Scholar]
- Nylin S, Wiklund C, Wickman PO, Garciabarros E. Absence of trade-offs between sexual size dimorphism and early male emergence in a butterfly. Ecology. 1993;74:1414–1427. [Google Scholar]
- Oostra V, de Jong MA, Invergo BM, Kesbeke F, Wende F, Brakefield PM, et al. Translating environmental gradients into discontinuous reaction norms via hormone signalling in a polyphenic butterfly. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 2011;278:789–797. doi: 10.1098/rspb.2010.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra V, Brakefield PM, Hiltemann Y, Zwaan BJ, Brattström O. Data from: On the fate of seasonally plastic traits in a rainforest butterfly under relaxed selection. Ecol: Evol; 2014a. . doi:10.5061/dryad.pt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra V, Mateus ARA, van den Burg KRL, Piessens T, van Eijk M, Brakefield PM, et al. Ecdysteroid hormones link the juvenile environment to alternative adult life histories in a seasonal insect. Am. Nat. 2014b doi: 10.1086/677260. (in press) [DOI] [PubMed] [Google Scholar]
- Pigliucci M. Phenotypic integration: studying the ecology and evolution of complex phenotypes. Ecol. Lett. 2003;6:265–272. [Google Scholar]
- Pijpe J, Brakefield PM, Zwaan BJ. Phenotypic plasticity of starvation resistance in the butterfly Bicyclus anynana. Evol. Ecol. 2007;21:589–600. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing (v. 2.11.1) Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- Roskam JC, Brakefield PM. A comparison of temperature-induced polyphenism in African Bicyclus butterflies from a seasonal savannah-rainforest ecotone. Evolution. 1996;50:2360–2372. doi: 10.1111/j.1558-5646.1996.tb03624.x. [DOI] [PubMed] [Google Scholar]
- Roskam JC, Brakefield PM. Seasonal polyphenism in Bicyclus (Lepidoptera: Satyridae) butterflies: different climates need different cues. Biol. J. Linn. Soc. 1999;66:345–356. [Google Scholar]
- Rountree DB, Nijhout HF. Hormonal control of a seasonal polyphenism in Precis coenia (Lepidoptera: Nymphalidae) J. Insect Physiol. 1995;41:987–992. [Google Scholar]
- Saastamoinen M, van der Sterren D, Vastenhout N, Zwaan BJ, Brakefield PM. Predicitive adaptive responses: condition-dependent impact of adult nutrition and flight in the tropical butterfly Bicyclus anynana. Am. Nat. 2010;176:686–698. doi: 10.1086/657038. [DOI] [PubMed] [Google Scholar]
- Schlichting C, Pigliucci M. Phenotypic evolution, a reaction norm perspective. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- Schwander T, Leimar O. Genes as leaders and followers in evolution. Trends Ecol. Evol. 2011;26:143–151. doi: 10.1016/j.tree.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Shapiro AM. Seasonal polyphenism. Evol. Biol. 1976;9:259–333. [Google Scholar]
- Simpson SJ, Sword GA, Lo N. Polyphenism in insects. Curr. Biol. 2011;21:R738–R749. doi: 10.1016/j.cub.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Snell-Rood EC, Van Dyken JD, Cruickshank T, Wade MJ, Moczek AP. Toward a population genetic framework of developmental evolution: the costs, limits, and consequences of phenotypic plasticity. BioEssays. 2010;32:71–81. doi: 10.1002/bies.200900132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srygley RB, Chai P. Flight morphology of Neotropical butterflies - palatability and distribution of mass to the thorax and abdomen. Oecologia. 1990;84:491–499. doi: 10.1007/BF00328165. [DOI] [PubMed] [Google Scholar]
- Stearns SC. The evolutionary significance of phenotypic plasticity - phenotypic sources of variation among organisms can be described by developmental switches and reaction norms. Bioscience. 1989;39:436–445. [Google Scholar]
- Stillwell RC, Blanckenhorn WU, Teder T, Davidowitz G, Fox CW. Sex differences in phenotypic plasticity affect variation in sexual size dimorphism in insects: from physiology to evolution. Annu. Rev. Entomol. 2010;55:227–245. doi: 10.1146/annurev-ento-112408-085500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. New York, NY: Oxford Univ. Press; 2003. [Google Scholar]
- Wijngaarden PJ, Brakefield PM. Lack of response to artificial selection on the slope of reaction norms for seasonal polyphenism in the butterfly Bicyclus anynana. Heredity. 2001;4:410–420. doi: 10.1046/j.1365-2540.2001.00933.x. [DOI] [PubMed] [Google Scholar]
- Wijngaarden PJ, Koch PB, Brakefield PM. Artificial selection on the shape of reaction norms for eyespot size in the butterfly Bicyclus anynana: direct and correlated responses. J. Evol. Biol. 2002;15:290–300. [Google Scholar]
- Windig JJ, Lammar P. Evolutionary genetics of seasonal polyphenism in the map butterfly Araschnia levana (Nymphalidae: Lepidoptera) Evol. Ecol. Res. 1999;1:875–894. [Google Scholar]
- Zijlstra WG, Steigenga MJ, Brakefield PM, Zwaan BJ. Simultaneous selection on two fitness-related traits in the butterfly Bicyclus anynana. Evolution. 2003;57:1852–1862. doi: 10.1111/j.0014-3820.2003.tb00593.x. [DOI] [PubMed] [Google Scholar]
- Zijlstra WG, Steigenga J, Koch PB, Zwaan BJ, Brakefield PM. Butterfly selected lines explore the hormonal basis of interactions between life histories and morphology. Am. Nat. 2004;163:E76–E87. doi: 10.1086/383595. [DOI] [PubMed] [Google Scholar]
- Zwaan BJ, Zijlstra WG, Keller M, Pijpe J, Brakefield PM. Potential constraints on evolution: sexual dimorphism and the problem of protandry in the butterfly Bicyclus anynana. J. Genet. 2008;87:395–405. doi: 10.1007/s12041-008-0062-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Full models of the effect of developmental temperature, sex and their interaction on a suite of phenotypic traits in B. sanaos and B. anynana, related to Figures 1–4 & 6. See Table 1 in the main text for the minimum adequate models. The data for B. anynana have previously been used in another study (Oostra et al. 2011).


