Abstract
Wolbachia is the most prevalent symbiont described in arthropods to date. Wolbachia can manipulate host reproduction, provide nutrition to insect hosts and protect insect hosts from pathogenic viruses. So far, 13 supergroups of Wolbachia have been identified. The whitefly Bemisia tabaci is a complex containing more than 28 morphologically indistinguishable cryptic species. Some cryptic species of this complex are invasive. In this study, we report a comprehensive survey of Wolbachia in B. tabaci and its relative B. afer from 1658 insects representing 54 populations across 13 provinces of China and one state of Australia. Based on the results of PCR or sequencing of the 16S rRNA gene, the overall rates of Wolbachia infection were 79.6% and 0.96% in the indigenous and invasive Bemisia whiteflies, respectively. We detected a new Wolbachia supergroup by sequencing five molecular marker genes including 16S rRNA, groEL, gltA, hcpA, and fbpA genes. Data showed that many protein-coding genes have limitations in detecting and classifying newly identified Wolbachia supergroups and thus raise a challenge to the known Wolbachia MLST standard analysis system. Besides, the other Wolbachia strains detected from whiteflies were clustered into supergroup B. Phylogenetic trees of whitefly mitochondrial cytochrome oxidase subunit I and Wolbachia multiple sequencing typing genes were not congruent. In addition, Wolbachia was also detected outside the special bacteriocytes in two cryptic species by fluorescence in situ hybridization, indicating the horizontal transmission of Wolbachia. Our results indicate that members of Wolbachia are far from well explored.
Keywords: Bemisia tabaci, FISH, horizontal transmission, multilocus sequence typing, vertical transmission, whiteflies, Wolbachia
Introduction
Wolbachia are rickettsial endosymbiotic bacteria in the class Alphaproteobacteria. Wolbachia bacteria are considered the most widespread endosymbionts in animals as they are found in all major classes of arthropods and some nematodes (Jeyaprakash and Hoy 2000; Werren and Windsor 2000; Duron et al. 2008; Russell et al. 2012). A meta-analysis suggests that the proportion of Wolbachia infection in insect species in the terrestrial world is about 40% (Zug and Hammerstein 2012).
In some host species, the successful maintenance and spread of Wolbachia is mainly achieved by the induction of cytoplasmic incompatibility to produce more female offspring, thus enhancing its maternal transmission (Stouthamer et al. 1999). In addition, manipulation of reproduction by Wolbachia includes feminizing genetic males, causing parthenogenesis, and killing male progenies (Stouthamer et al. 1999; Werren et al. 2008). Recent studies found that Wolbachia benefits insect hosts by providing essential nutrition (Hosokawa et al. 2010), enhancing host stem cell's proliferation (Fast et al. 2011), and protecting insect from pathogenic RNA viruses (Hedges et al. 2008).
The genus Wolbachia is highly divergent and has so far been divided into 13 supergroups (A-N, except for G which is a combination of A and B) (Lo et al. 2002, 2007; Baldo and Werren 2007; Haegeman et al. 2009; Ros et al. 2009; Augustinos et al. 2011). Wolbachia supergroups are characterized mainly with molecular markers such as rrs (16S rRNA), ftsZ (cell division protein), gltA (Citrate synthase), groEL (Chaperonin GroEL) and wsp (Wolbachia surface protein) genes (O'Neill et al. 1992; Zhou et al. 1998; Werren and Windsor 2000; Casiraghi et al. 2005). Wolbachia genotyping is inferred mainly from multi locus sequence typing (MLST) genes (gatB, coxA, hcpA, fbpA, and ftsZ genes) and amino acid sequences of the four hypervariable regions (HVRs) of WSP protein (Baldo et al. 2005, 2006).
Bemisia tabaci (Hemiptera: Aleyrodidae) is a complex containing more than 28 morphologically indistinguishable cryptic species (De Barro et al. 2011; Hu et al. 2011). Through millions of years of evolution, the various cryptic species of this complex show a clear geographic pattern of distribution around the globe (Boykin et al. 2007, 2013; De Barro et al. 2011). However, with the development of modern transport, whiteflies have been transferred frequently among different continents (Naranjo et al. 2010). During the last twenty years, two cryptic species of the B. tabaci complex, Middle East-Asia Minor 1 (formerly known as the B “biotype,” hereafter MEAM1) and Mediterranean (formerly known as the Q “biotype,” hereafter MED) have invaded many regions of the world (Dalton 2006; Hu et al. 2011). They have caused serious damages to local agriculture through direct plant sap sucking and transmission of plant pathogenic viruses (Oliveira et al. 2001). What is more, the rapid invasion of MEAM1 and MED has caused the replacement of many indigenous cryptic species of the B. tabaci complex (Liu et al. 2007; Hu et al. 2011; Muñiz et al. 2011; Rao et al. 2011). These events provide us a unique opportunity for studying the evolution and transmission of Wolbachia among different B. tabaci cryptic species, which were geographically isolated in history but have become sympatric recently.
Previous studies have investigated the diversity of Wolbachia in the B. tabaci species complex (Nirgianaki et al. 2003; Chiel et al. 2007; Gueguen et al. 2010; Chu et al. 2011; Pan et al. 2012; Singh et al. 2012; Bing et al. 2013a). However, most of these reports focused on the two invasive cryptic species MEAM1 and MED and only used one to three marker genes in the investigation, and the distribution of Wolbachia in most indigenous B. tabaci cryptic species remains largely unknown. In this study, we examined the distribution of Wolbachia in B. afer and 10 cryptic species of the B. tabaci species complex collected from 13 provinces of China and one state of Australia. We report: (1) the prevalence of Wolbachia in B. afer and B. tabaci; (2) the discovery of a probably new Wolbachia (supergroup O) in whiteflies by sequencing of rrs gene and four protein-coding genes (fbpA, hcpA, gltA, and groEL); (3) the diversity and phylogenetic status of Wolbachia strains within these whiteflies; and (4) evidence for horizontal transfer of Wolbachia among B. tabaci cryptic species.
Materials and Methods
Whitefly collection and DNA extraction
Bemisia specimens were collected from 13 provinces of China and one state of Australia. Details for collection (geographical locations, host plants, and dates) of those populations are summarized in Fig. 1 and Table A1. Whiteflies collected from the same locality and host plant were considered as one population. Whitefly samples were initially immersed in 95% ethanol after collection and subsequently kept at −20°C until DNA extraction. Total whitefly DNA was extracted from individual adult specimens according to the method of DeBarro and Driver (1997). The quality of the DNA samples was confirmed by PCR amplification of a 0.8 kb fragment of whitefly mitochondrial cytochrome oxidase I (mtCOI) gene using the primers C1-J-2195 and L2-N-3014 (Table A2). Cryptic species of B. tabaci were first identified based on the polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) method described by Qin et al. (2013), and the sex of whiteflies was identified through genital morphology. A total of 1658 whitefly DNA samples were positive for PCR amplification using the mtCOI primers, indicating satisfactory quality of the DNA templates.
Figure 1.
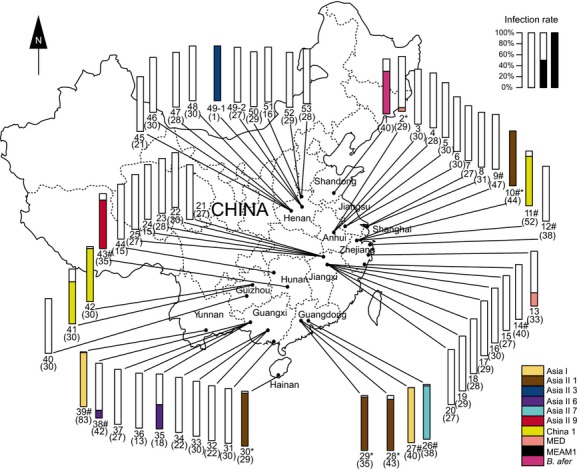
Localities of sampling and infection frequencies of Wolbachia in 53 Chinese populations of Bemisia. About 30 individuals from each population were subjected to diagnostic PCR analysis. Whiteflies collected from the same host plant in the same locality were considered as one population. The Arabic numerals correspond to populations numbered in Table A1. Figures in parentheses indicate the numbers of individuals sampled from each of the populations. Different colors represent B. afer and different cryptic species of the B. tabaci complex. The “#” signs indicate the laboratory lines that had been maintained on cotton since collection, and the “*” signs indicate the 5 populations that are positive for Wolbachia supergroup O (referred to in Table 3 and Fig. 2).
Diagnostic screening of Wolbachia
The presence of Wolbachia was screened based on the amplification of a 0.6 kb fragment with the Wolbachia rrs primers (Table A2). Standard PCR analyses were performed using 2×EasyTaq PCR SuperMix (TransGen, Beijing, China) in a PTC-200 Thermocycler (Bio-Rad, Hercules, CA). PCR procedures were an initial step of 94°C for 3 min, followed by 32 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 45 s and a final step of 72°C for 10 min. Amplified DNA products were electrophoresed on agarose gels and stained with GelRed (Biotium, San Francisco, CA). To verify PCR results, amplified bands (especially uncertain ones) were purified by AxyPrep DNA gel extraction kit (Axygen, Silicon Valley, CA) and cloned into the pGEM-T vector (Promega, Madison, WI). Plasmids containing the DNA inserts of expected sizes were confirmed by PCR and sequenced in an ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA). Sequencing results were then checked by Blast in NCBI nr database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Only those individuals, which were blasted to expected products of the specific primers, were considered to be infected. All PCRs included a negative control (sterile water instead of DNA) and a positive control (DNA of China 1 whitefly). For Wolbachia-positive populations, Bemisia species were further identified by phylogenetic analysis of the mtCOI gene (Fig. A3). Wolbachia infection rates between whitefly sexes were statistically tested using the Fisher's exact test. Statistical significance of the infection rates among different B. afer and B. tabaci cryptic species was calculated using the χ2 test and corrected by the Bonferroni procedure. All statistical analyses were performed using the Data Processing System (DPS) software (Tang and Zhang 2013).
Sequencing and typing of Wolbachia
As the phylogenetic analysis of the 0.6 kb rrs sequences indicated a possible new supergroup of Wolbachia, we amplified the rrs gene from whitefly populations and introduced an endonuclease VspI (AT/TAAT) (Fisher Scientific, Pittsburgh, PA) to digest the target bands, to investigate the infection prevalence of the new supergroup of Wolbachia. Several whitefly individuals were then randomly selected for sequencing confirmation of PCR-RFLP results. The groEL, wsp, and MLST (gatB, coxA, hcpA, fbpA, and ftsZ) genes from every combination of host species and rrs genotype were amplified by TransTaq-T DNA Polymerase (TransGen), cloned into pEASY-T1 vectors (TransGen) and sequenced on ABI 3730 DNA analyzer (Applied Biosystems).
In addition, to confirm the finding of the new Wolbachia supergroup, nearly a complete rrs sequence (1417 bp) and a partial gltA sequence (659 bp) were amplified from one Wolbachia new supergroup singular-infected population. PCR amplifications, DNA cloning and sequencing procedures were accomplished as described previously. The cycling procedures were the same as described earlier with changes on the annealing temperature for different primers. Primer sequences and annealing temperatures of rrs, gltA, groEL, wsp, and the MLST genes were listed in Table A2.
Sequences of MLST and wsp genes were manually trimmed in line with the template provided in Wolbachia MLST website and compared with sequences in the Wolbachia MLST database (http://pubmlst.org/wolbachia/). Novel sequences were submitted to the database curators as new alleles. Each unique combination of five MLST sequences was designated a strain type (ST) number in the Wolbachia MLST database (Baldo et al. 2006). Previously published sequences from other whiteflies were added to the data set to increase the power of statistical comparisons. All newly obtained allele numbers and ST numbers in this study are summarized in Table 1.
Table 1.
GenBank accession numbers of Wolbachia sequences and allele profiles of Wolbachia-positive whitefly populations
| Pop. | Strain | Species | rrs | groEL | ST | gatB | coxA | hcpA | ftsZ | fbpA | wsp | HRV1 | HRV2 | HRV3 | HRV4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | wBa_1 | Bemisia afer | KF454753 | KF452533 | 382 | KF452586 (105) | KF452567 (11) | KF454725 (220) | KF452573 (11) | KF454737 (162) | KF465800 (670) | (231) | (265) | (143) | (23) |
| 2 | wBt_2 | MED | KF454762 | – | – | – | – | – | KF454744 (386) | KF465817 (668) | (230) | (264) | (3) | (23) | |
| 10 | wBt_10 | Asia II 1 | KF454771 | KF452543 | 391 | KF452588 (207) | KF452561 (88) | KF454726 (234) | KF452576 (170) | KF454746 (390) | |||||
| 11 | wBt_11 | China 1 | JF795502 | KF452544 | 379 | KF452591 (105) | KF452563 (88) | KF454731 (13) | KF452578 (170) | KF454752 (9) | KF465816 (669) | (2) | (17) | (3) | (2) |
| 13 | wBt_13 | MED | KF454764 | – | – | – | KF454719 (236) | KF452575 (181) | KF454745 (387) | KF465805 (671) | (232) | (17) | (3) | (2) | |
| 26 | wBt_26 | Asia II 7 | JF795503 | KF452536 | 378 | KF452590 (105) | KF452562 (88) | KF454730 (106) | KF452577 (7) | KF454747 (387) | KF465815 (665) | (78) | (88) | (90) | (2) |
| 27 | wBt_27 | Asia I | KF454755 | KF452540 | 385 | KF452594 (210) | KF452565 (88) | KF454721 (106) | KF452580 (7) | KF454751 (387) | KF465810 (163) | (78) | (88) | (90) | (23) |
| 28-1 | wBt_28-1 | Asia II 1 | KF454768 | KF452534 | 392 | KF452596 (207) | KF452560 (88) | KF454727 (230) | KF452581 (170) | KF454734(392) | KF465806 (669) | (2) | (17) | (3) | (2) |
| 28-2 | wBt_28-2 | Asia II 1 | KF454769 | KF465814 | – | KF452558 (88) | KF454728 (231) | – | KF454733 (390) | – | |||||
| 29-1 | wBt_29-1 | Asia II 1 | KF454758 | KF452535 | 390 | KF452595 (207) | KF452559 (88) | KF454729 (232) | KF452582 (170) | KF454738 (9) | KF465807 (669) | (2) | (17) | (3) | (2) |
| 29-2 | wBt_29-2 | Asia II 1 | KF454757 | KF452547 | – | – | – | – | – | – | |||||
| 30-1 | wBt_30-1 | Asia II 1 | KF454759 | KF452538 | 389 | KF452600 (208) | KF452551 (88) | KF454715 (106) | KF452584 (170) | KF454735 (393) | KF465808 (669) | (2) | (17) | (3) | (2) |
| 30-2 | wBt_30-2 | Asia II 1 | KF454760 | KF452539 | – | KF452552 (88) | KF454716 (13) | KF454736 (391) | KF465809 (669) | (2) | (17) | (3) | (2) | ||
| 35 | wBt_35 | Asia II 6 | KF454761 | KF452601 | 393 | KF452599 (216) | KF452553 (88) | KF454722 (106) | KF452570 (7) | KF454741 (387) | KF465818 (163) | (78) | (88) | (90) | (23) |
| 38 | wBt_38 | Asia II 6 | KF454767 | KF452537 | 394 | KF452589 (207) | KF452550 (88) | KF471409 (235) | KF452569 (170) | KF454740 (9) | KF465812 (669) | (2) | (17) | (3) | (2) |
| 39 | wBt_39 | Asia I | KF454766 | KF452541 | 395 | KF452587 (207) | KF452556 (88) | KF454718 (106) | KF452568 (182) | KF454750 (387) | KF465811 (672) | (2) | (17) | (261) | (2) |
| 41 | wBt_41 | China 1 | KF454765 | KF452548 | 377 | KF452597 (207) | KF452557 (88) | KF454724 (13) | KF452572 (170) | KF454743 (9) | KF465803 (669) | (2) | (17) | (3) | (2) |
| 42 | wBt_42 | China 1 | KF454770 | KF452546 | 383 | KF452598 (209) | KF452554 (88) | KF454723 (13) | KF452571 (170) | KF454742 (9) | KF465802 (669) | (2) | (17) | (3) | (2) |
| 43 | wBt_43 | Asia II 9 | KF454756 | KF452542 | 384 | KF452593 (207) | KF452566 (88) | KF454720 (13) | KF452583 (170) | KF454748 (386) | KF465813 (160) | (2) | (17) | (3) | (23) |
| 49-1 | wBt_49-1 | Asia II 3 | KF454763 | KF452549 | 396 | KF452585 (207) | KF452555 (88) | KF454717 (228) | KF452574 (180) | KF454739 (386) | KF465804 (160) | (2) | (17) | (3) | (23) |
| 54 | wBt_54 | Australia | KF454754 | KF452545 | 380 | KF452592 (207) | KF452564 (88) | KF454732 (221) | KF452579 (170) | KF454749 (9) | KF465801 (160) | (2) | (17) | (3) | (23) |
ST numbers and allele profile numbers in bold represent new sequence data generated from this study.
Molecular phylogenetic analysis
Phylogenetic analyses were constructed using (1) Wolbachia sequences of rrs, gltA, groEL, MLST, and wsp genes of different supergroups from various hosts; and (2) whitefly mtCOI sequences. All sequences used in this study were edited and aligned manually using Clustal W (ver. 1.6) (Thompson et al. 1994) in MEGA (ver. 5.10) (Tamura et al. 2011). The Gblocks program (ver. 0.91b) (Castresana 2000) was used to remove poorly aligned positions and to obtain nonambiguous sequence alignments. The best-fit evolutionary model for the sequence data was determined using hierarchical likelihood ratio tests and Akaike information criterion with the program jModelTest (ver. 0.1.1) (Posada 2008). Phylogenetic trees were constructed with the Bayesian inference using MrBayes (ver 3.1.2) (Ronquist and Huelsenbeck 2003). For these gene data, 5 million generations were run; 50,000 trees were obtained, and the first 25% trees were discarded as burn-in. The resulting phylogenetic trees were visualized in TreeView (ver. 1.6.6) (Page 1996). The comparison between the phylogeny of whitefly mtCOI and Wolbachia concatenate MLST data sets was constructed with Dendroscope (ver. 3.2.8) (Huson and Scornavacca 2012). The new Wolbachia rrs, gltA, groEL, wsp, and MLST gene sequences and whitefly mtCOI gene sequences have been deposited in the GenBank database (Table 1, Fig. A3 and Table A4).
The pairwise genetic divergence of different Wolbachia supergroups was calculated using the Kimura 2-parameter method (Kimura 1980) in MEGA (ver. 5.10) (Tamura et al. 2011). Because recombination of sequences has potentially disruptive influences on phylogenetic-based molecular evolution analyses (Martin et al. 2011), alignments of individual and concatenated genes were checked for significant levels of recombination using the Phi test (Bruen et al. 2006) in SplitsTree4 under default conditions (Huson and Bryant 2006). When recombination was tested to be significant, a phylogenetic network framework was constructed based on uncorrected P distances using the Neighbor-net method (Bryant and Moulton 2004) implemented in SplitsTree4 (ver. 4.13.1) (Huson and Bryant 2006).
FISH
Localization of Portiera and Wolbachia was studied in nymphs and adults of B. tabaci Asia II 1 (Pop. 10) and Asia II 9 (Pop. 43) using fluorescence labeled probes specifically targeting the rrs genes of these bacteria. We followed the previous protocols for the FISH experiments (Bing et al. 2013b). Briefly, specimens were collected directly into Carnoy's fixative and fixed overnight. After fixation, the samples were hybridized overnight in hybridization buffer (20 mM Tris-HCl, pH 8.0, 0.9 M NaCl, 0.01% sodium dodecyl sulfate, 30% deionized formamide) containing 10 pmol of fluorescent probes. The probe, BTP1-Cy3 (5′-Cy3- TGTCAGTGTCAGCCCAGAAG-3′), was used to target rrs gene of Portiera (Gottlieb et al. 2006). A new probe, Wolb-1-488 (5′- Alexa Fluor 488- TAATATAGGCTCATCTAATAGCAA -3′), was designed to target rrs gene of Wolbachia. The specificity of the detection was first checked by “probe match” in RDP 10 (update to May 14, 2013) (Cole et al. 2009) and BLAST in nr database of NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and then confirmed using the following controls: a no probe control and Wolbachia-free whiteflies (samples of the B. tabaci MED species and MEAM1 species). Stained samples were wholly mounted and viewed under a Zeiss LSM780 confocal microscope.
Results
Prevalence of Wolbachia in Bemisia species
Bemisia tabaci has a wide distribution in China. In this study, samples of Bemisia were obtained from 24 localities of 13 provinces of this country (Fig. 1). In all, these samples represent one population of B. afer and 52 populations of 9 cryptic species of the B. tabaci complex from China (Table A1). In addition, one sample of an indigenous species of the B. tabaci complex was obtained from Australia (Table A1).
Of the 1658 individuals examined, rrs PCR assays indicated that the infection rates of Wolbachia varied among species, and even among populations of a given species, ranging from 0% to 100% (Fig. 1; Table 2, Chi-square test, P < 0.0001), but did not differ between sexes in each of the populations that were tested statistically (Table A1). The incidence of Wolbachia infection in indigenous whiteflies (79.61%, n = 618) was significantly higher than that in invasive whiteflies (MEAM1 and MED, 0.96%, n = 1040; Fisher's exact 2-tailed test, P < 0.0001). The infection rate of Wolbachia also varied among different indigenous species of the B. tabaci complex. For instance, 88.4% of China 1 whiteflies were positive for Wolbachia while so were only 2.1% of Asia II 3 whiteflies (Table 2). The rate of Wolbachia infection in B. afer was 77.5% (Table 2).
Table 2.
Rates of Wolbachia infection in Bemisia afer and cryptic species of the B. tabaci complex
| Whitefly species | n | No. of localities | Infection rate (%)1 |
|---|---|---|---|
| B. afer | 40 | 1 | 77.5 a |
| Asia I | 123 | 2 | 99.2 b |
| Asia II 1 | 151 | 4 | 96.7 bc |
| Asia II 3 | 48 | 2 | 2.1 de |
| Asia II 6 | 60 | 2 | 23.3 d |
| Asia II 7 | 38 | 1 | 97.4 ab |
| Asia II 9 | 35 | 1 | 88.6 ab |
| China 1 | 112 | 3 | 88.4 ac |
| Australia | 11 | 1 | 100.0 ab |
| MEAM1 | 334 | 12 | 0.0 e |
| MED | 706 | 26 | 1.4 e |
Figures followed by different letters differ significantly (adjusted P < 0.05, using Bonferroni corrections).
Diversity of Wolbachia infections
For those Wolbachia-positive populations, 2–3 individuals were further analyzed by sequencing Wolbachia rrs gene (592 bp) and performing Bayesian phylogenetic analysis. Most of whitefly Wolbachia rrs sequences were clustered into the supergroup B (Fig. 2). However, several Wolbachia rrs sequences obtained from four Asia II 1 populations (wBt_10, wBt_28-2, wBt_29-2, wBt_30-2) and one MED population (wBt_2) formed a strict and robust monophyletic clade (Fig. 2).
Figure 2.
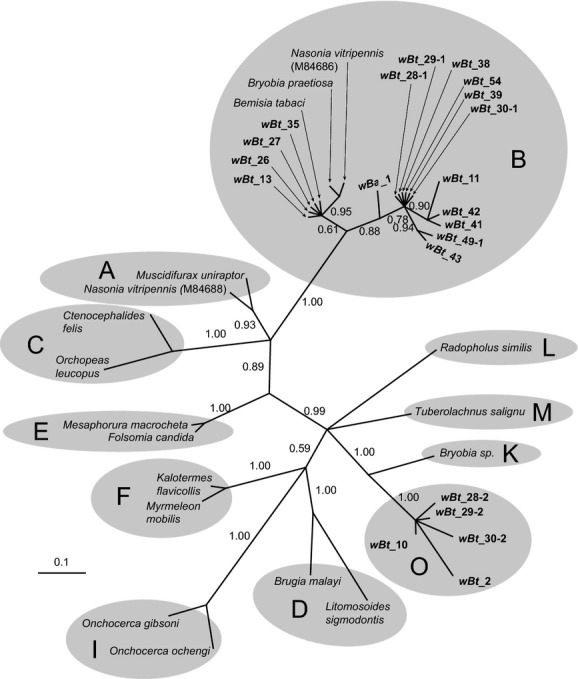
Phylogeny of the Wolbachia identified from Bemisia afer and cryptic species of the B. tabaci complex based on bacterial rrs gene sequences (592 sites). Wolbachia strains are characterized by the names of their host species. The tree was constructed using a TPM1uf + G substitution model for Bayesian analysis. Bayesian posterior probabilities are shown on the branches. Sequences obtained in this study are shown in bold. The bar indicates a branch length of 0.1 substitutions/site. The sequence names and GenBank accession numbers are listed in Tables A4 and A6.
Identification of Wolbachia supergroup O
Further Bayesian phylogenetic analysis on a nearly complete rrs sequence (1317 bp) of the strange Wolbachia (wBt_10) revealed that this Wolbachia differed widely from other known Wolbachia (Fig. A5). The divergence of rrs between wBt_10 and the supergroup M is 2.52%, which is the smallest of that between wBt_10 and all previously reported Wolbachia supergroups (A to N) (Table A6). In view of these apparent differences, we proposed to name these Wolbachia as supergroup O temporarily.
Our results thus showed the presence of two genetically distant Wolbachia supergroups in whiteflies. To clarify the detailed infection prevalence of Wolbachia O in Bemisia whiteflies, PCR-RFLP was introduced to digest all positive rrs PCR products by the restriction enzymes VspI. No VspI restriction site was found in rrs sequences of Wolbachia supergroup O, whereas rrs amplicons from Wolbachia supergroup B could be digested into multiple bands by VspI (Fig. A7). Whiteflies in populations 2 (MED species) and 10 (Asia II 1 species) are infected singly by Wolbachia O, whereas populations 28, 29, and 30 (all three are Asia II 1 species) are infected by both Wolbachia O and Wolbachia B (Table 3).
Table 3.
Infection frequencies of the Wolbachia O in five populations of the Bemisia tabaci complex
| Single infection (%) | ||||||
|---|---|---|---|---|---|---|
| Pop. no. | Cryptic species | n1 | % without Wolbachia infection | O | B | Double infection (%) |
| 2 | MED | 29 | 93.1 | 6.9 | ||
| 10 | Asia II 1 | 44 | 100 | |||
| 28 | Asia II 1 | 43 | 6.9 | 14.0 | 62.8 | 16.3 |
| 29 | Asia II 1 | 35 | 2.9 | 5.7 | 65.7 | 25.7 |
| 30 | Asia II 1 | 29 | 3.4 | 24.1 | 3.4 | 69.0 |
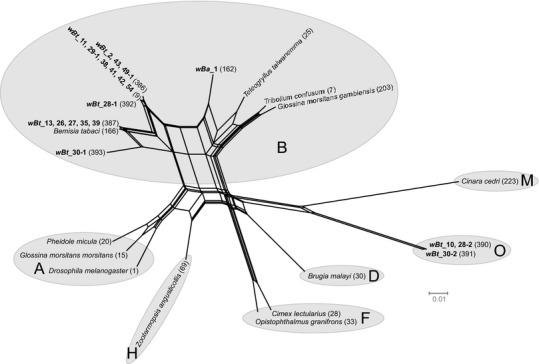
At least one of the eight tested protein-coding genes (gltA, groEL, MLST (gatB, coxA, hcpA, fbpA, ftsZ), and wsp genes) was successfully amplified and sequenced for all Wolbachia-infected populations in this study. Both neighbor-net analysis of fbpA, gltA, hcpA gene and Bayesian interference of groEL gene supported the existence of the Wolbachia supergroup O (wBt_10) (Figs. 3 and 4, and Figs. A10 and A12). However, it should be noted that the results of phylogenetic analyses with different genes were not always consistent. In particular, analysis of fbpA, groEL, and hcpA gene clustered some strange Wolbachia, such as wBt_29-2 and wBt_30-2, which were identified as O by rrs gene, into supergroup B (Fig. 4, and Figs. A10 and A12).
Figure 3.
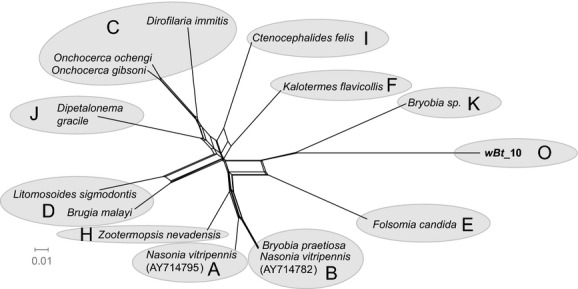
Phylogenetic position of the Wolbachia identified from the putative species Asia II 1 of the Bemisia tabaci complex based on bacterial gltA gene sequences (636 sites) using the Neighbor-net method. Each edge (or a set of parallel edges) corresponds to a split in the data set and has a length equal to the weight of the split. The sequence obtained in this study is shown in bold. The bar indicates a branch length of 0.1 substitutions/site. The names and sequence GenBank accession numbers are listed in Table A4.
Figure 4.
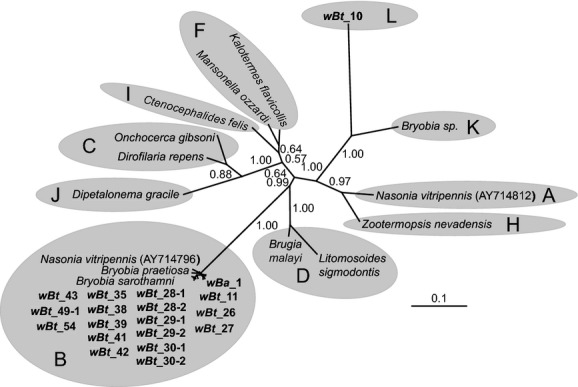
Phylogenetic position of the Wolbachia identified from Bemisia afer and B. tabaci putative species based on bacterial groEL gene sequences (491 sites). Wolbachia strains are characterized by the names of their host species. The tree was constructed using a GTR + G substitution model for Bayesian analysis. Bayesian posterior probabilities are shown on the branches. The sequence obtained in this study is shown in bold. The bar indicates a branch length of 0.1 substitutions/site. The names and sequence GenBank accession numbers are listed in Table 1 and Table A4.
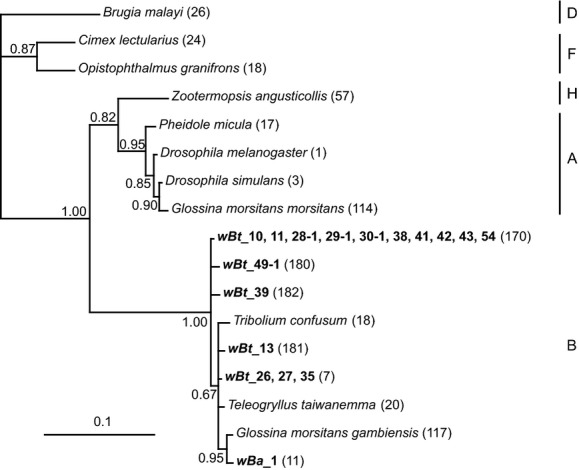
Sixteen STs were identified in whiteflies from this study, and all of them are new to the MLST database (Table 1). Though efforts were made, some PCRs failed when amplifying the MLST and wsp genes from supergroup O-infected whiteflies (Table 1). As a result, sequences from supergroup O-infected whiteflies were excluded from phylogenetic analysis of the concatenated MLST sequences. Respective Bayesian interference of separate gatB, coxA, ftsZ, and wsp genes showed that all Wolbachia detected in whiteflies belonged to supergroup B (Fig. 5, and Figs. A8, A9 and A11). Neighbor-net analysis clustered the majority of Wolbachia into supergroup B except for wBt_10 (Figs. A10 and A12). The hcpA genes from wBt_10 and fbpA from wBt_10 and wBt_28-2 formed a separate branch that differs distinctly from all known reference sequences.
Figure 5.
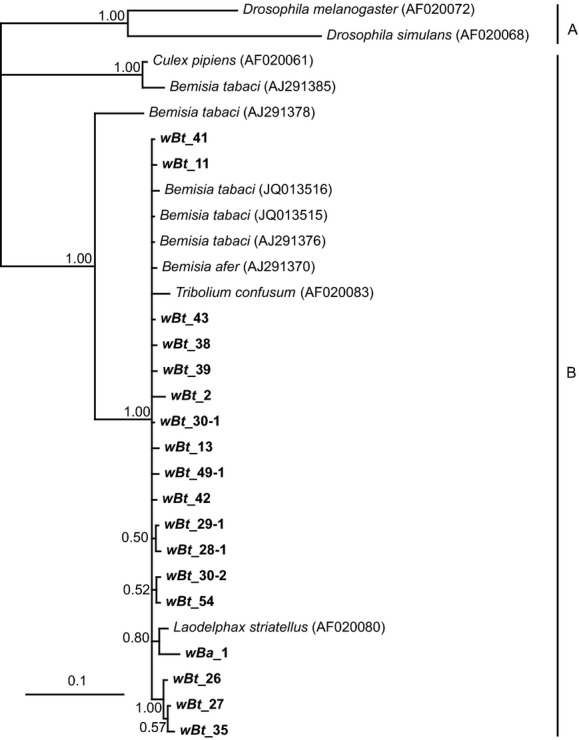
Phylogenetic position of the Wolbachia identified from Bemisia afer and B. tabaci putative species based on bacterial wsp gene sequences (512 sites). Wolbachia strains are characterized by the names of their host species. The two Drosophila wsp sequences are the outgroups. The tree was constructed using a TIM3 + G substitution model for Bayesian analysis. Bayesian posterior probabilities are shown on the branches. The sequence obtained in this study is shown in bold. The bar indicates a branch length of 0.1 substitutions/site. The names and sequence GenBank accession numbers are listed in parentheses and Table 1.
Co-divergence between the divergence of Wolbachia supergroup B and whitefly species
The codivergence of Bemisia and Wolbachia supergroup B was assessed by studying the sequences of partial mtCOI gene and Wolbachia MLST genes. For those Wolbachia identified from B. tabaci, very poor congruence was found between the phylogenies of mtCOI and concatenated MLST genes (Fig. 6). The topology of MLST tree differs obviously from that of mtCOI. Whiteflies belonging to the same cryptic species harbored distant Wolbachia strains. For instance, two populations (wBt_35 and wBt_38) of Wolbachia identified from Asia II 6 are clustered in different phylogenetic groups.
Figure 6.
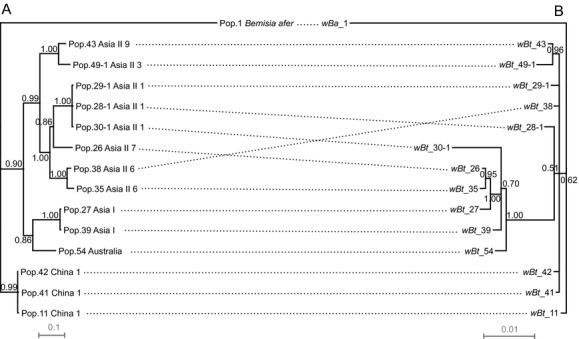
Comparisons of Bemisia and Wolbachia phylogenies. A, the whitefly phylogeny constructed based on Bayesian analysis of mtCOI sequences (817 bp) as shown in Fig. A3 using TIM3 + I + G model. B, the Wolbachia phylogeny constructed based on Bayesian analysis of concatenated sequences of MLST genes (2079 bp) as shown in Table 1 using GTR + I + G model. Bayesian posterior probabilities are shown on the branches. Dashed lines connect hosts to their respective Wolbachia strains. The scale bar is in units of substitutions/site.
Localization of Wolbachia in Bemisia tabaci
The FISH of bacteria revealed that Portiera was seen exclusively in the bacteriocytes of whiteflies. In the tested nymphs, Wolbachia was strictly located in the bacteriocytes among the abundant Portiera (Fig. 7). Nevertheless, in the adults, Wolbachia was detected both outside and inside the bacteriocytes (Fig. 7). Signals of Wolbachia shown at the anterior pole of the oocytes of female adults indicate its vertical transmission (arrows marked in Fig. 7 D & H).
Figure 7.
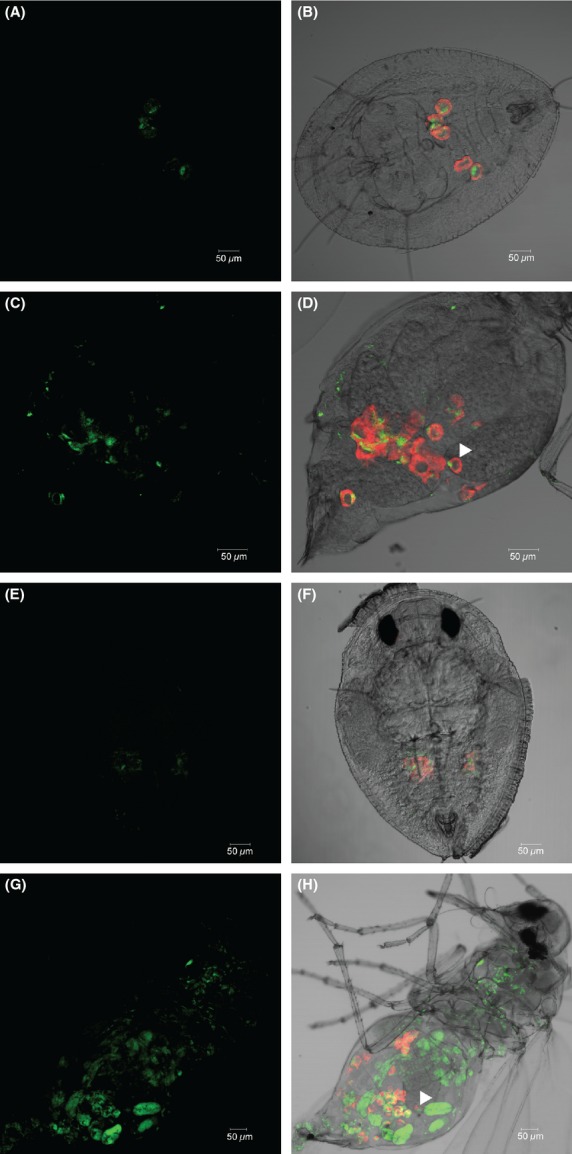
Whole-mount FISH of Bemisia tabaci nymphs and female adults using a Portiera-specific probe (red) and a Wolbachia-specific probe (green). Upper column, Asia II 1 nymph and female adult; lower column, Asia II 9 nymph and female adult. A, C, E, G: Wolbachia channel on a dark-field channel. B, D, F, H: Overlay of Portiera and Wolbachia channels on a bright-field channel. White triangles in D and H indicate anterior poles of the oocytes. Signals on legs, joints, and wings are chitin autofluoresence.
Discussion
Wolbachia is widely distributed among invertebrates and is considered as the most prevalent symbiont identified so far. Though several research groups have investigated the prevalence of Wolbachia in some cryptic species of the B. tabaci complex, our study represents the first comprehensive analysis of Wolbachia infection among both invasive and indigenous cryptic species of the B. tabaci complex in Asia. In addition, compared with previous investigations, we used five more molecular markers in our analyses.
Prevalence of Wolbachia varies between invasive and indigenous whiteflies
In this study, Wolbachia infection rates in five (Asia I, Asia II 1, Asia II 7, Asia II 9, and China 1) of the seven Chinese indigenous species reached over 70%. In contrast, Wolbachia infection rate in the MED populations from China was only 1.4% (10/706), and no infection (0/334) was detected in all MEAM1 populations from this country. The low rates of Wolbachia infection in MEAM1 and MED agree with those observed in most previous studies. For example, in populations of MEAM1 and MED from Europe and Western Africa, infection rates of Wolbachia varied from 0–8.3% and 0–33% (Nirgianaki et al. 2003; Chiel et al. 2007; Gueguen et al. 2010; Skaljac et al. 2010; Chu et al. 2011; Thierry et al. 2011; GnankinÉ et al. 2013). And in populations of MEAM1 and MED from China, the rates of Wolbachia infection were 0.2% (1/456) and 0% (0/1149), respectively (Pan et al. 2012). As a whole, our data indicate a high variability of prevalence of Wolbachia between cryptic species of the B. tabaci complex. In our sampling, we obtained adequate numbers of whitefly individuals for five (Asia II 1, Asia II 6, China 1, MEAM1, and MED) of the 11 whitefly species from both laboratory and field. The data indicate that the frequencies of Wolbachia infection between laboratory and field populations in each of the five species appeared similar (Table A1). Thus, the laboratory rearing seemed to have exerted little effects on the frequencies of Wolbachia infection in these whitefly species. Until now, factors underlying the high variability of Wolbachia infection between the whitefly species are virtually unknown but certainly warrant future investigations.
In contrast to a previous study that reports absence of Wolbachia infection in B. afer populations from China (Chu et al. 2010), the rate of Wolbachia infection in the B. afer population examined in the current study reached 77.5%. Phylogenetic analysis of rrs, groEL, MLST, and wsp genes showed that the Wolbachia detected from B. afer belongs to supergroup B, which agrees with the report of Nirgianaki et al. (2003).
Identification of a novel Wolbachia supergroup O
Preliminary Bayesian phylogenetic analysis based on rrs gene sequences strongly supports the existence of one strange monophyletic group compared with the other Wolbachia identified in whiteflies. The rrs sequences from five of the whitefly populations (wBt_2, wBt_10, wBt_28-2, wBt_29-2, and wBt_30-2) were clustered into group O. Average distance among those strange rrs sequences (592 bp) are 0.48%. The divergence of rrs between wBt_10 and all previously described Wolbachia supergroups (A to N) is higher than the 2% distance, a level of divergence that may merit the establishment of a new supergroup (Stouthamer et al. 1993; Augustinos et al. 2011). What is more, independent Bayesian analysis of rrs and groEL gene sequences and Neighbor-net analysis of gltA, hcpA, and fbpA gene sequences confirmed the distinct phylogenetic position of wBt_10 from the other supergroups. Based on the evidence, we propose the strange Wolbachia group as a new supergroup – Supergroup O.
All previously known Wolbachia in Bemisia tabaci belong to supergroup B
Except for the five supergroup O strains, phylogenetic analysis of eight molecular markers (rrs, groEL, gatB, coxA, hcpA, fbpA, ftsZ, and wsp genes) showed that all the Wolbachia strains detected from Chinese whiteflies as well as one strain from the Australia species belong to supergroup B. This is consistent with previous diversity studies on Bemisia and Trialeurodes whiteflies (Nirgianaki et al. 2003; Sintupachee et al. 2006; Gueguen et al. 2010; Singh et al. 2012; Tsagkarakou et al. 2012).
The protein-coding genes are limited in Wolbachia diversity investigation
At the early stage of Wolbachia research, the identification of Wolbachia strains was inferred based on the rrs gene (O'Neill et al. 1992; Stouthamer et al. 1993; Dumler and Walker 2005). As the research progressed, the rrs gene was found too conserved for further analysis of the Wolbachia genus. Subsequently, additional protein-coding genes (gltA, groEL, ftsZ, and wsp genes) were developed for infection and evolutionary analysis of Wolbachia (Werren et al. 1995b; Zhou et al. 1998; Lo et al. 2002, 2007; Casiraghi et al. 2005). Baldo et al. (2006) developed a standard MLST-based system (gatB, coxA, hcpA, ftsZ, and fbpA) for genotyping and strain classification of Wolbachia infections. However, with more exploration of Wolbachia diversity, conflict results occurred among these different markers (Augustinos et al. 2011). In this study, the presence of supergroup O was confirmed by rrs and four protein-coding genes (fbpA, gltA, groEL, and hcp genes) (Figs. 2–4, and Figs. A10 and A12). Whereas phylogenetic analysis of several protein-coding genes (coxA, groEL, gatB, ftsZ, and wsp) clustered many Wolbachia O strains into supergroup B (Figs. 4 and 5, and Figs. A9, A8 and A11). Similar phenomena have been noticed in previous studies. For example, even though the supergroup M and N have been identified as new groups of Wolbachia by rrs gene clustering, Augustinos et al. (2011) found that several popular protein-coding sequences such as gltA, groEL, and MLST genes clustered some individuals of those new groups into the old supergroup B. Besides, failures of amplifying MLST and wsp genes in many Wolbachia O-infected whiteflies (Table 1) indicated protein-coding genes may not be sufficient for investigating the diversity of Wolbachia in B. tabaci. The failure of amplification of ftsZ and wsp genes were also observed in Wolbachia-infected aphids (Augustinos et al. 2011). Consequently, it seems clear that phylogenetic analysis merely using protein-coding genes may underestimate the diversity of Wolbachia.
That inadequacy of protein-coding genes for analyzing the diversity of Wolbachia may be explained by: (1) primers of protein-coding genes are designed based on the earliest known Wolbachia (mostly A and B); and (2) different protein-coding genes suffer different selective pressure and thus have different evolutionary patterns. The rrs gene sequence is more conserved than wsp gene and the results of amplification are more stable compared with that of wsp or ftsZ genes which often produces unexpected bands (not target-size bands or not the gene of Wolbachia). In fact, no single pair of primers can ensure detection of all Wolbachia specifically among various samples (SimÕEs et al. 2011). In view of the limitation of the various primers, we suggest that infection data obtained by any of these genes should be confirmed by vector cloning and sequencing of all representative bands.
Wolbachia in Bemisia tabaci are transmitted horizontally
Our FISH data indicate that Wolbachia can be vertically transmitted in whiteflies (Fig. 7), a result in agreement with that of a previous report (Gottlieb et al. 2008). In addition, our FISH data show the distribution of Wolbachia outside of bacteriocytes of the whitefly adults and thus also indicate potential horizontal transmission of Wolbachia. Not surprisingly, incongruence was found between the phylogeny of Bemisia mtCOI sequences and that of Wolbachia supergroup B based on concatenated MLST sequences (Fig. 6). In addition, in several cases, a population of a given whitefly species harbored divergent Wolbachia strains (e.g., Population of 28 in Table A1). As speculated by a rate of rrs gene divergence of 1–2% per 50 million years in bacterial endosymbionts (Moran et al. 1993; Ochman et al. 1999), the divergence between supergroup B and supergroup O probably started more than 120 million years ago. While the divergence date of B. tabaci complex was speculated to start about 50 million years ago, much more recent than that of supergroup B and O Wolbachia (Boykin et al. 2013). The double infection of Wolbachia supergroups B and O in the same population indicates horizontal transmission of Wolbachia. Horizontal transmission of Wolbachia has often been speculated based on phylogenetic analysis (Werren et al. 1995a; Sintupachee et al. 2006; Stahlhut et al. 2010; Schuler et al. 2013; Zhang et al. 2013). Wolbachia has also been reported from other whitefly genera such as Trialeurodes and some parasitoids (Raychoudhury et al. 2009; Cass et al. 2014), and this diversity of distribution may also hint horizontal transmission. Sintupachee et al. (2006) hypothesized that the horizontal transmission of Wolbachia from whiteflies to other arthropods may occur through plants, because whiteflies could feed on plants without ruining plant cells. Caspi-Fluger et al. (2011) presented a case study of horizontal transmission of Rickttesia in whiteflies via plants. Though we are yet unable to speculate on the origin of Wolbachia in whiteflies, we suggest that horizontal transmission of Wolbachia in whiteflies via plants warrants investigation especially as this bacterium has been detected outside of the bacteriocytes in the insect hosts.
Conclusion
We conducted a comprehensive screening for Wolbachia in whiteflies, and the findings have broadened substantially the host spectrum of Wolbachia and revealed a new supergroup of Wolbachia in whiteflies. Our study also shows the limitations of protein-coding genes as molecular markers for Wolbachia investigation. Both specific and efficient molecular markers are needed for intensive surveys of Wolbachia. Wolbachia are transmitted vertically and horizontally in whiteflies. Clarifying the Wolbachia strains of whiteflies and their biological functions may provide novel clues for the development of efficient control technologies against invasive whiteflies and whitefly-transmitted plant viruses.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (Project number 31321063 and 31390421). We thank Qiong Rao and Yun-Lin Su for comments on the manuscript, Qi-Yi Tang for help in doing the statistical analysis, Amanda Avery for assistance in submitting Wolbachia sequences to the Wolbachia MLST Databases, and Paul De Barro for providing whitefly samples of the “Australia” species.
Appendix
Table A1.
Details of screen of Bemisia afer and B. tabaci cryptic species for Wolbachia. Rates of infections do not differ between sexes in each of the populations analyzed with Fisher's exact test (Populations with fewer than 10 individuals were excluded from the analysis)
| Sample size | Infection rate (%)2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Whitefly species | Location | Latitude | Longitude | Collection date | Host plant (family)1 | F | M | UN | Females | Males | Overall |
| 1 | Bemisia afer | Linyi, Shandong, China | 35°47′N | 118°37′E | July 2012 | Broussonetia papyrifera (1) | 24 | 16 | – | 87.50 | 62.50 | 77.50 |
| Bemisia tabaci | ||||||||||||
| 23 | MED | Hefei, Anhui, China | 31°95′N | 117°48′E | October 2009 | Solanum melongena (2) | 21 | 8 | – | 9.52 | 0.00 | 6.90 |
| 3 | MED | Hefei, Anhui, China | 31°95′N | 117°48′E | October 2009 | Solanum lycopersicum (2) | 17 | 13 | – | 0.00 | 0.00 | 0.00 |
| 4 | MEAM1 | Hefei, Anhui, China | 31°92′N | 117°14′E | October 2009 | Salvia splendens (3) | 21 | 7 | – | 0.00 | 0.00 | 0.00 |
| 5 | MED | Nanjing, Jiangsu, China | 32°02′N | 118°54′E | September 2009 | Solanum melongena (2) | 25 | 5 | – | 0.00 | 0.00 | 0.00 |
| 6 | MED | Nanjing, Jiangsu, China | 32°02′N | 118°54′E | September 2009 | Brassica oleracea var. capitata (4) | 15 | 15 | – | 0.00 | 0.00 | 0.00 |
| 7 | MEAM1 | Chongmingdao, Shanghai, China | 31°50′N | 121°80′E | November 2009 | Brassica oleracea var. capitata (4) | 16 | 11 | – | 0.00 | 0.00 | 0.00 |
| 8 | MED | Chongmingdao, Shanghai, China | 31°50′N | 121°80′E | November 2009 | Capsicum annuum (2) | 21 | 10 | – | 0.00 | 0.00 | 0.00 |
| 94 | Asia II 3 | Hangzhou, Zhejiang, China | 30°23′N | 120°18′E | April 2009 | Glycine max (5) | 26 | 21 | – | 0.00 | 0.00 | 0.00 |
| 103,4 | Asia II 1 | Hangzhou, Zhejiang, China | 29°27′N | 119°18′E | October 2010 | Gossypium hirsutum (6) | 29 | 15 | – | 100.00 | 100.00 | 100.00 |
| 114 | China 1 | Hangzhou, Zhejiang, China | 30°23′N | 120°18′E | November 2009 | Solanum lycopersicum (2) | 23 | 29 | – | 100.00 | 82.76 | 90.38 |
| 124 | MED | Ningbo, Zhejiang, China | 29°48′N | 121°35′E | June 2009 | Capsicum annuum (2) | 22 | 16 | – | 0.00 | 0.00 | 0.00 |
| 13 | MED | Taizhou, Zhejiang, China | 28°30′N | 121°34′E | October 2012 | Cucurbita moschata (7) | 22 | 11 | – | 31.82 | 9.09 | 24.24 |
| 144 | MEAM1 | Wenzhou, Zhejiang, China | 27°47′N | 120°39′E | September 2008 | Solanum melongena (2) | 20 | 20 | – | 0.00 | 0.00 | 0.00 |
| 15 | MED | Nanchang, Jiangxi, China | 28°72′N | 115°91′E | October 2009 | Cucurbita moschata (7) | 16 | 11 | – | 0.00 | 0.00 | 0.00 |
| 16 | MED | Nanchang, Jiangxi, China | 28°72′N | 115°91′E | October 2009 | Ipomoea batatas (8) | 16 | 14 | – | 0.00 | 0.00 | 0.00 |
| 17 | MED | Nanchang, Jiangxi, China | 28°72′N | 115°91′E | October 2009 | Brassica campestris ssp. Pekinensis (4) | 21 | 8 | – | 0.00 | 0.00 | 0.00 |
| 18 | MED | Nanchang, Jiangxi, China | 28°72′N | 115°91′E | October 2009 | Humulus scandens (9) | 16 | 12 | – | 0.00 | 0.00 | 0.00 |
| 19 | MED | Nanchang, Jiangxi, China | 28°29′N | 116°01′E | October 2009 | Citrullus lanatus (7) | 13 | 16 | – | 0.00 | 0.00 | 0.00 |
| 20 | MED | Nanchang, Jiangxi, China | 28°29′N | 116°01′E | October 2009 | Ipomoea batatas (8) | 20 | 7 | – | 0.00 | 0.00 | 0.00 |
| 21 | MED | Jiujiang, Jiangxi, China | 29°76′N | 115°79′E | October 2009 | Capsicum annuum (2) | 16 | 11 | – | 0.00 | 0.00 | 0.00 |
| 22 | MED | Jiujiang, Jiangxi, China | 29°76′N | 115°79′E | October 2009 | Ipomoea batatas (8) | 24 | 6 | – | 0.00 | 0.00 | 0.00 |
| 23 | MED | Jiujiang, Jiangxi, China | 29°76′N | 115°79′E | October 2009 | Cucumis sativus (7) | 20 | 8 | – | 0.00 | 0.00 | 0.00 |
| 24 | MED | Jiujiang, Jiangxi, China | 29°76′N | 115°79′E | October 2009 | Solanum melongena (2) | 13 | 2 | – | 0.00 | 0.00 | 0.00 |
| 25 | MED | Jiujiang, Jiangxi, China | 29°76′N | 115°79′E | October 2009 | Phaseolus vulgaris (5) | 16 | 11 | – | 0.00 | 0.00 | 0.00 |
| 264 | Asia II 7 | Guangzhou, Guangdong, China | 23°09′N | 113°21′E | October 2007 | Gossypium hirsutum (6) | 30 | 8 | – | 100.00 | 87.50 | 97.37 |
| 274 | Asia I | Zhaoqing, Guangdong, China | 23°56′N | 112°1′E | November 2010 | Ipomoea batatas (8) | 20 | 20 | – | 100.00 | 100.00 | 100.00 |
| 283 | Asia II 1 | Zhaoqing, Guangdong, China | 23°56′N | 112°1′E | August 2012 | Arachis hypogaea (5) | 31 | 12 | – | 96.77 | 83.33 | 93.02 |
| 293 | Asia II 1 | Zhaoqing, Guangdong, China | 23°56′N | 112°1′E | August 2012 | Ipomoea batatas (8) | 32 | 3 | – | 96.88 | 100.00 | 97.14 |
| 303 | Asia II 1 | Sanya, Hainan, China | 18°24′N | 109°42′E | August 2009 | Ipomoea batatas (8) | 24 | 5 | – | 100.00 | 80.00 | 96.55 |
| 31 | MEAM1 | Nanning, Guangxi, China | 22°38′N | 108°23′E | August 2009 | Vigna unguiculata (5) | 17 | 13 | – | 0.00 | 0.00 | 0.00 |
| 32 | MEAM1 | Nanning, Guangxi, China | 22°38′N | 108°23′E | August 2009 | Gossypium hirsutum (6) | 22 | 0 | – | 0.00 | – | 0.00 |
| 33 | MEAM1 | Nanning, Guangxi, China | 22°38′N | 108°23′E | August 2009 | Cucumis sativus (7) | 17 | 13 | – | 0.00 | 0.00 | 0.00 |
| 34 | MED | Beihai, Guangxi, China | 21°29′N | 109°09′E | August 2009 | Ipomoea batatas (8) | 10 | 12 | – | 0.00 | 0.00 | 0.00 |
| 35 | Asia II 6 | Baise, Guangxi, China | 22°94′N | 108°54′E | August 2009 | Luffa cylindrica (7) | 15 | 3 | – | 53.33 | 0.00 | 44.44 |
| 36 | MEAM1 | Baise, Guangxi, China | 23°52′N | 106°37′E | August 2009 | Vigna unguiculata (5) | 4 | 9 | – | 0.00 | 0.00 | 0.00 |
| 37 | MEAM1 | Baise, Guangxi, China | 23°45′N | 106°47′E | August 2009 | Benincasa hispida (7) | 15 | 12 | – | 0.00 | 0.00 | 0.00 |
| 384 | Asia II 6 | Baise, Guangxi, China | 22°94′N | 108°54′E | November 2011 | Ipomoea batatas (8) | 36 | 6 | – | 13.89 | 16.67 | 14.29 |
| 394 | Asia I | Honghe, Yunnan, China | 24°38′N | 103°46′E | November 2011 | Ipomoea batatas (8) | 46 | 37 | – | 97.83 | 100.00 | 98.80 |
| 40 | MED | Guiyang, Guizhou, China | 26°40′N | 106°67′E | July 2009 | Glechoma longituba (3) | 9 | 21 | – | 0.00 | 0.00 | 0.00 |
| 41 | China 1 | Zunyi, Guizhou, China | 27°39′N | 107°70′E | July 2009 | Solanum melongena (2) | 24 | 6 | – | 75.00 | 83.33 | 76.67 |
| 42 | China 1 | Zunyi, Guizhou, China | 27°39′N | 107°70′E | July 2009 | Ipomoea batatas (8) | 23 | 7 | – | 100.00 | 85.71 | 96.67 |
| 434 | Asia II 9 | Shaoyang, Hunan, China | 26°59′N | 111°16′E | October 2011 | Ipomoea batatas (8) | 19 | 16 | – | 89.47 | 87.50 | 88.57 |
| 44 | MED | Jishou, Hunan, China | 28°18′N | 109°38′E | September 2009 | Raphanus sativus (4) | 7 | 8 | – | 0.00 | 0.00 | 0.00 |
| 45 | MED | Luoyang, Henan, China | 34°46′N | 112°46′E | September 2009 | Ipomoea batatas (8) | 16 | 5 | – | 0.00 | 0.00 | 0.00 |
| 46 | MEAM1 | Luoyang, Henan, China | 34°66′N | 112°51′E | September 2009 | Gossypium hirsutum (6) | 15 | 15 | – | 0.00 | 0.00 | 0.00 |
| 47 | MEAM1 | Luoyang, Henan, China | 34°59′N | 112°58′E | September 2009 | Solanum melongena (2) | 20 | 8 | – | 0.00 | 0.00 | 0.00 |
| 48 | MEAM1 | Luoyang, Henan, China | 34°59′N | 112°58′E | September 2009 | Cucumis sativus (7) | 26 | 4 | – | 0.00 | 0.00 | 0.00 |
| 49-1 | Asia II 3 | Zhengzhou, Henan, China | 34°78′N | 113°66′E | September 2009 | Solanum melongena (2) | 0 | 1 | – | – | 100.00 | 100.00 |
| 49-2 | MED | Zhengzhou, Henan, China | 34°78′N | 113°66′E | September 2009 | Solanum melongena (2) | 18 | 9 | – | 0.00 | 0.00 | 0.00 |
| 50 | MEAM1 | Zhengzhou, Henan, China | 34°78′N | 113°66′E | September 2009 | Cucumis sativus (7) | 27 | 2 | – | 0.00 | 0.00 | 0.00 |
| 51 | MED | Xinxiang, Henan, China | 35°47′N | 113°75′E | September 2009 | Phaseolus vulgaris (5) | 12 | 4 | – | 0.00 | 0.00 | 0.00 |
| 52 | MED | Xinxiang, Henan, China | 35°47′N | 113°75′E | September 2009 | Raphanus sativus (4) | 26 | 3 | – | 0.00 | 0.00 | 0.00 |
| 53 | MED | Xinxiang, Henan, China | 35°47′N | 113°75′E | September 2009 | Brassica campestris ssp. Pekinensis (4) | 20 | 8 | – | 0.00 | 0.00 | 0.00 |
| 54 | Australia | Bundaberg, Queensland, Australia | 24°48′S | 152°27′E | – | Euphorbia cyathophora (10) | – | – | 11 | – | – | 100.00 |
F, female adult; M, male adult; UN, unknown sex; –, not ascertained.
In all 22 species of host plants from 10 families, figures in parentheses indicate the names of the families: (1), Moraceae; (2), Solanaceae, (3), Lamiaceae, (4), Cruciferae, (5), Fabaceae, (6), Malvaceae, (7), Cucurbitaceae, (8), Convolvulaceae, (9), Cannabaceae, (10), Euphorbiaceae.
Infection rates of Wolbachia detected by diagnostic PCR of rrs gene.
Populations for the detection of Wolbachia supergroup O (Table 3).
Populations maintained in the laboratory on cotton since collection.
Table A2.
List of the primers used for screening and sequencing
| Gene | Hypothetical product | Primer name | Primer sequences (5′-3′) | Tm | Product size | Reference |
|---|---|---|---|---|---|---|
| Bemisia spp. | ||||||
| mtCOI | Mitochondrial cytochrome oxidase subunit I | COI-F-C1-J-2195: | TTGATTTTTTGGTCATCCAGAAGT | 54°C | 759 bp | Frohlich et al. (1999) |
| COI-R-TL2-N-3014: | TCCAATGCACTAATCTGCCATATTA | |||||
| Universal bacteria | ||||||
| rrs | Ribosomal RNA 16S | 27F: | AGAGTTTGATCMTGGCTCAG | 50°C | 1417 bp | Weisburg et al. (1991) |
| 1494R: | CTACGGCTACCTTGTTACGA | |||||
| Wolbachia spp. | ||||||
| rrs | Ribosomal RNA 16S | Wol-16S-F: | CGGGGGAAAAATTTATTGCT | 55°C | 589 bp | Heddi et al. (1999) |
| Wol-16S-R: | AGCTGTAATACAGAAAGTAAA | |||||
| gatB | Glutamyl-tRNA(Gln) amidotransferase, subunit B | gatB_F1: | GAKTTAAAYCGYGCAGGBGTT | 54°C | 471 bp | Baldo et al. (2006) |
| gatB_R1: | TGGYAAYTCRGGYAAAGATGA | |||||
| coxA | Cytochrome coxidase, subunit I | coxA_F1: | TTGGRGCRATYAACTTTATAG | 54°C | 487 bp | Baldo et al. (2006) |
| coxA_R1: | CTAAAGACTTTKACRCCAGT | |||||
| hcpA | Conserved hypothetical protein | hcpA_F1: | GAAATARCAGTTGCTGCAAA | 54°C | 515 bp | Baldo et al. (2006) |
| hcpA_R1: | GAAAGTYRAGCAAGYTCTG | |||||
| ftsZ | Cell division protein | ftsZ_F1: | ATYATGGARCATATAAARGATAG | 54°C | 524 bp | Baldo et al. (2006) |
| ftsZ_R1: | TCRAGYAATGGATTRGATAT | |||||
| fbpA | Fructose-bisphosphate aldolase | fbpA_F1: | GCTGCTCCRCTTGGYWTGAT | 59°C | 509 bp | Baldo et al. (2006) |
| fbpA_R1: | CCRCCAGARAAAAYYACTATTC | |||||
| wsp | Outer surface protein | wsp_F1: | GTCCAATARSTGATGARGAAAC | 59°C | 546 bp | Baldo et al. (2006) |
| wsp_R1: | CYGCACCAAYAGYRCTRTAAA | |||||
| groEL | Chaperonin GroEL | groEL-F: | CAACRGTRGSRRYAACTGCDGG | 54°C | 491 bp | Ros et al. (2009) |
| groEL-R: | GATADCCRCGRTCAAAYTGC | |||||
| gltA | Citrate synthase | WgltAF1: | TACGATCCAGGGTTTGTTTCTAC | 54°C | 659 bp | Casiraghi et al. (2005) |
| WgltARev2: | CATTTCATACCACTGGGC | |||||
Figure A3.
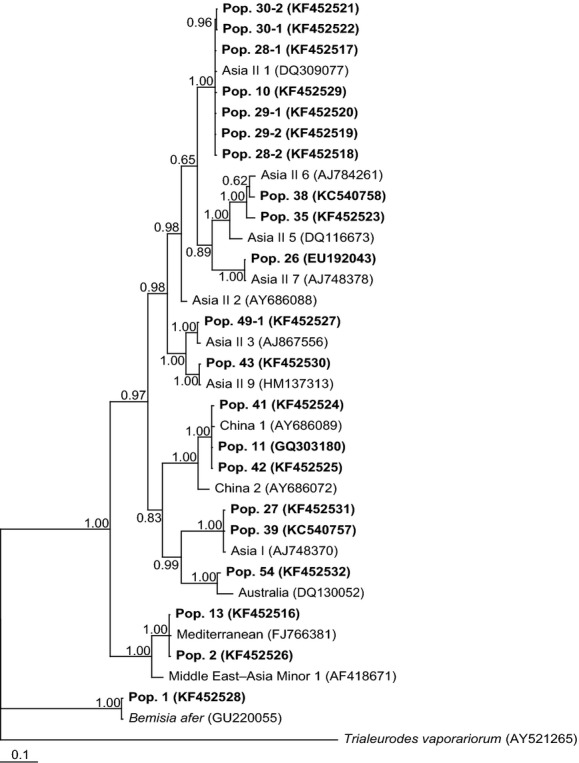
Phylogenetic analysis of the Bemisia spp. based on whitefly mtCOI gene sequences (657 sites). Trialeurodes vaporariorum is used as out group. Reference sequences are obtained from the CSIRO data access portal (De Barro and Boykin 2013). The tree was constructed using a TIM3 + I + G substitution model for Bayesian analysis. Bayesian posterior probabilities are indicated at nodes. The sequences obtained in this study are shown in bold. The bar indicates a branch length of 0.1 substitutions/site. The sequence GenBank accession numbers are shown in parentheses.
Table A4.
Taxonomic details of Wolbachia hosts and the GenBank accession numbers of sequences included in the analysis
| Phylum | Class | Order | Host species | 16S rRNA gene | gltA | groEL | Supergroup |
|---|---|---|---|---|---|---|---|
| Arthropoda | Insecta | Hymenoptera | Muscidifurax uniraptor | L02882 | – | – | A |
| Arthropoda | Insecta | Hymenoptera | Nasonia vitripennis | M84688 | AY714795 | AY714812 | A |
| Arthropoda | Prostigmata | Acarina | Bryobia sarothamni | EU499315 | – | EU499330 | B |
| Arthropoda | Prostigmata | Acarina | Bryobia praetiosa | EU499317 | EU499327 | EU499332 | B |
| Arthropoda | Insecta | Hymenoptera | Nasonia vitripennis | M84686 | AY714782 | AY714796 | B |
| Arthropoda | Insecta | Hemiptera | Bemisia tabaci | JN204507 | – | – | B |
| Nematoda | Secernentea | Spirurida | Onchocerca ochengi | AJ010276 | AJ609640 | – | C |
| Nematoda | Secernentea | Spirurida | Onchocerca gibsoni | AJ276499 | AJ609639 | AJ609652 | C |
| Nematoda | Secernentea | Spirurida | Dirofilaria repens | AJ276500 | – | AJ609653 | C |
| Nematoda | Secernentea | Spirurida | Dirofilaria immitis | Z49261 | AJ609641 | – | C |
| Nematoda | Chromadorea | Spirurida | Brugia malayi | AF051145 | AJ609643 | AE017321 | D |
| Nematoda | Secernentea | Spirurida | Litomosoides sigmodontis | AF069068 | AJ609645 | AF409113 | D |
| Arthropoda | Collembola | Collembola | Folsomia candida | AF179630 | AJ609649 | – | E |
| Arthropoda | Ellipura | Collembola | Mesaphorura macrocheta | AJ422184 | – | – | E |
| Arthropoda | Insecta | Neuroptera | Myrmeleon mobilis | DQ068882 | – | – | F |
| Arthropoda | Insecta | Isoptera | Kalotermes flavicollis | Y11377 | AJ609651 | AJ609660 | F |
| Nematoda | Secernentea | Spirurida | Mansonella ozzardi | AJ279034 | – | AJ609657 | F |
| Arthropoda | Insecta | Isoptera | Zootermopsis nevadensis | AY764280 | AY764282 | AY764277 | H |
| Arthropoda | Insecta | Siphonaptera | Ctenocephalides felis | AY335923 | AJ609650 | AJ609659 | I |
| Arthropoda | Insecta | Siphonaptera | Orchopeas leucopus | AY335924 | – | – | I |
| Nematoda | Secernentea | Spirurida | Dipetalonema gracile | AJ548802 | AJ609648 | AJ609658 | J |
| Arthropoda | Arachnida | Prostigmata | Bryobia sp. | EU499316 | EU499326 | EU499331 | K |
| Nematoda | Phasmida | Tylenchida | Radopholus similis | EU833482 | – | EU833484 | L |
| Arthropoda | Insecta | Hemiptera | Tuberolachnus salignu | JN384085 | – | – | M |
| Arthropoda | Insecta | Hemiptera | Aphis sp. | JN384091 | M | ||
| Arthropoda | Insecta | Hemiptera | Cinara cedri | – | – | JN384053 | M |
| Arthropoda | Insecta | Hemiptera | Toxoptera aurantii | JN384094 | – | – | N |
| Arthropoda | Insecta | Hemiptera | Toxoptera aurantii | JN384095 | – | – | N |
| Arthropoda | Insecta | Hemiptera | Bemisia tabaci | KF454771 | KF587270 | KF452543 | O |
Figure A5.
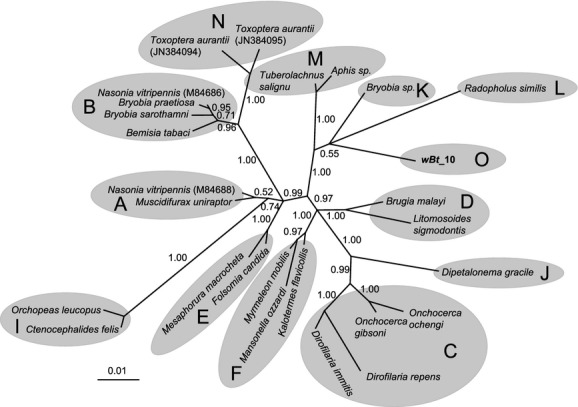
Phylogenetic position of the Wolbachia identified from Bemisia tabaci putative species Asia II 1 (wBt_10) based on bacterial rrs gene sequences (1317 sites). Wolbachia strains are characterized by the names of their host species. The tree was constructed using a HKY + G substitution model for Bayesian analysis. Bayesian posterior probabilities are shown on the branches. The sequence obtained in this study is shown in bold. The bar indicates a branch length of 0.01 substitutions/site. The names and sequence GenBank accession numbers are listed in Tables A4 and A6.
Table A6.
Divergence between the Wolbachia detected in the putative species Asia II 1 of the B. tabaci complex (wBt_10) and other supergroups based on 16S rRNA gene sequences
| Host species | Supergroup | Divergence (%) | GenBank accession no. |
|---|---|---|---|
| Muscidifurax uniraptor | A | 3.73 | L02882 |
| Nasonia vitripennis | A | 3.64 | M84688 |
| Bryobia sarothamni | B | 4.98 | EU499315 |
| Bryobia praetiosa | B | 4.98 | EU499317 |
| Bemisia tabaci | B | 5.18 | JN204507 |
| Nasonia vitripennis | B | 4.83 | M84686 |
| Onchocerca ochengi | C | 5.61 | AJ010276 |
| Onchocerca gibsoni | C | 5.44 | AJ276499 |
| Dirofilaria repens | C | 5.99 | AJ276500 |
| Dirofilaria immitis | C | 5.63 | Z49261 |
| Brugia malayi | D | 4.59 | AF051145 |
| Litomosoides sigmodontis | D | 5.15 | AF069068 |
| Folsomia candida | E | 3.65 | AF179630 |
| Mesaphorura macrocheta | E | 4.04 | AJ422184 |
| Mansonella ozzardi | F | 4.95 | AJ279034 |
| Myrmeleon mobilis | F | 3.90 | DQ068882 |
| Kalotermes flavicollis | F | 3.70 | Y11377 |
| Zootermopsis nevadensis | H | 4.41 | AY764280 |
| Ctenocephalides felis | I | 7.22 | AY335923 |
| Orchopeas leucopus | I | 7.48 | AY335924 |
| Dipetalonema gracile | J | 5.59 | AJ548802 |
| Bryobia sp. | K | 3.25 | EU499316 |
| Radopholus similis. | L | 4.79 | EU833482 |
| Tuberolachnus salignu | M | 2.52 | JN384085 |
| Aphis sp. | M | 2.59 | JN384091 |
| Toxoptera aurantii | N | 4.50 | JN384094 |
| Toxoptera aurantii | N | 4.80 | JN384095 |
Figure A7.
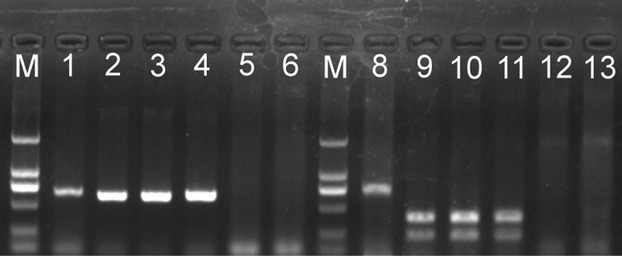
RFLP pattern of PCR products of rrs gene of the Wolbachia supergroup O and B in Bemisia tabaci corresponding to VspI (AseI) digestion. The different profiles were obtained from individuals representing different Wolbachia in B. tabaci. The bands shown on the bottom are primer dimers. Lane 1, undigested Wolbachia O obtained by PCR from Asia II 1; lane 2–4, undigested Wolbachia B obtained by PCR from Asia II 7, Asia I and China 1, respectively; lane 5–6, Wolbachia PCR amplified production from MEAM1 and MED as controls; lane 8, digested Wolbachia O obtained by PCR from Asia II 1; lane 9–11, Wolbachia B obtained by PCR from Asia II 7, Asia I and China 1, respectively; lane 12–13, Wolbachia PCR amplified production from MEAM1 and MED as controls; lane M, DNA size markers (100, 250, 500, 750, 1000, and 2000 bp from bottom to top).
Figure A8.
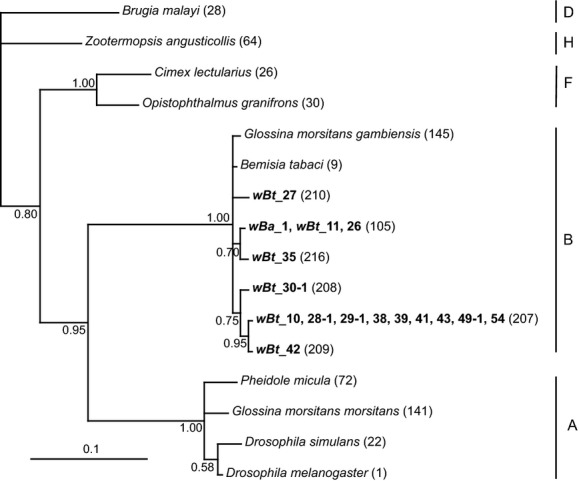
Phylogenetic position of the Wolbachia identified from Bemisia afer and B. tabaci putative species based on bacterial gatB gene sequences (369 sites). Wolbachia strains are characterized by the names of their host species and allele numbers from the MLST database. The tree was constructed using a TPM2uf + G substitution model for Bayesian analysis. Bayesian posterior probabilities are shown on the branches. The sequences obtained in this study are shown in bold. The bar indicates a branch length of 0.1 substitutions/site.
Figure A9.

Phylogenetic position of the Wolbachia identified from Bemisia afer and B. tabaci putative species based on bacterial coxA gene sequences (402 sites). Wolbachia strains are characterized by the names of their host species and allele numbers from the MLST database. The tree was constructed using a TIM1 + G substitution model for Bayesian analysis. Bayesian posterior probabilities are shown on the branches. The sequences obtained in this study are shown in bold. The bar indicates a branch length of 0.1 substitutions/site.
Figure A10.
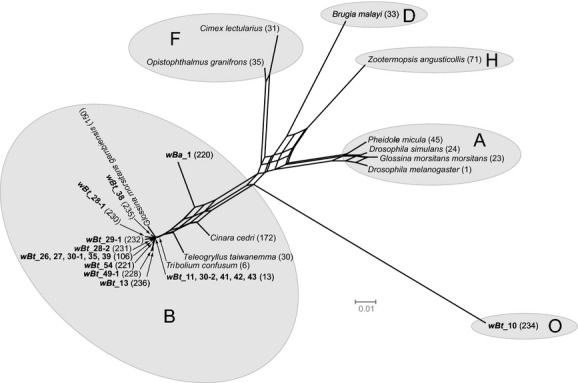
Phylogenetic position of the Wolbachia identified from Bemisia afer and B. tabaci putative species based on bacterial hcpA gene sequences (444 sites) using the Neighbor-net method. Each edge (or a set of parallel edges) corresponds to a split in the data set and has length equal to the weight of the split. The sequence obtained in this study is shown in bold. MLST Database allele numbers of hcpA sequences are shown in parenthesis. The bar indicates a branch length of 0.01 substitutions/site.
Figure A11.
Phylogenetic position of the Wolbachia identified from Bemisia afer and B. tabaci putative species based on bacterial ftsZ gene sequences (435 sites). Wolbachia strains are characterized by the names of their host species and allele numbers from the MLST database. The tree was constructed using a TrN + I substitution model for Bayesian analysis. Bayesian posterior probabilities are shown on the branches. The sequences obtained in this study are shown in bold. The bar indicates a branch length of 0.1 substitutions/site.
Figure A12.
Phylogenetic position of the Wolbachia identified from Bemisia afer and B. tabaci putative species based on bacterial fbpA gene sequences (429 sites) using the Neighbor-net method. Each edge (or a set of parallel edges) corresponds to a split in the data set and has length equal to the weight of the split. The sequences obtained in this study are shown in bold. MLST Database allele numbers of fbpA sequences are shown in parenthesis. The bar indicates a branch length of 0.01 substitutions/site.
Figure A13.
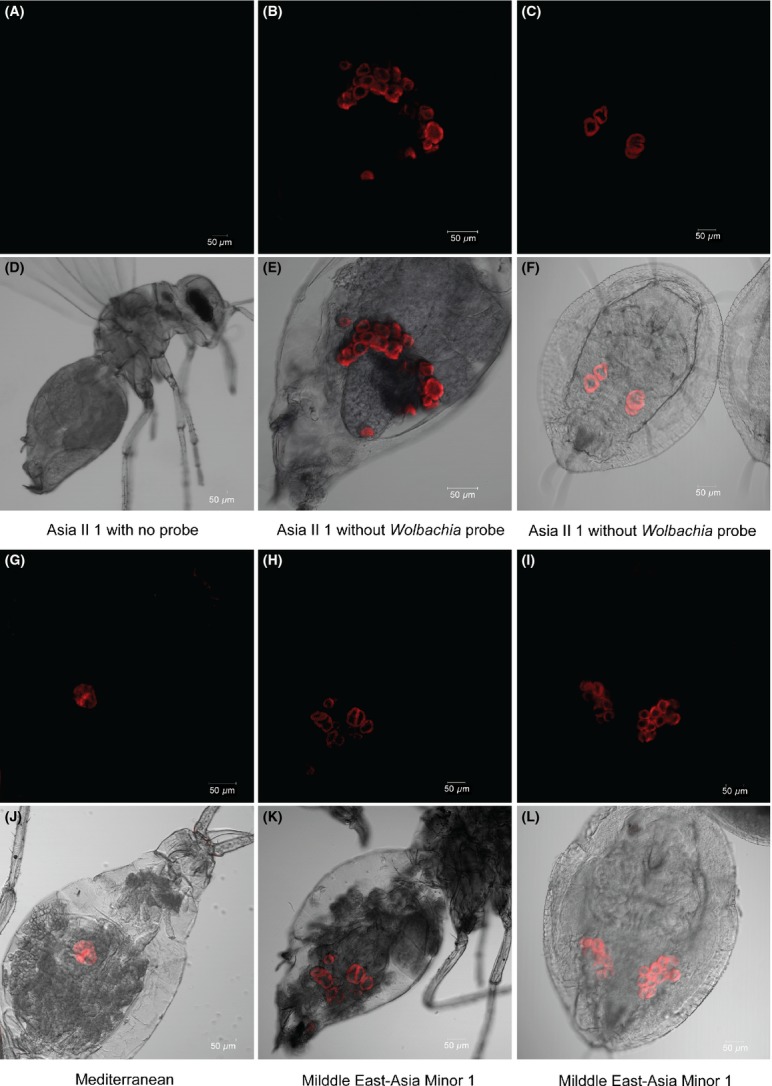
FISH controls. No probe control: Asia II 1; No Wolbachia probe control: Asia II 1; Wolbachia-free whiteflies control: Mediterranean (harboring Portiera and Hamiltonella) and Middle East-Asia Minor 1 (harboring Portiera, Hamiltonella and Rickettsia; Bing et al. 2013a). A–C, G–I: Overlay of channels of Portiera (red) and Wolbachia (green); D–F, J–L: Overlay of channels of Portiera (red), Wolbachia (green) and white light.
Conflict of Interest
None declared.
References
- Augustinos AA, Santos-Garcia D, Dionyssopoulou E, Moreira M, Papapanagiotou A, Scarvelakis M, et al. Detection and characterization of Wolbachia infections in natural populations of aphids: is the hidden diversity fully unraveled? PLoS ONE. 2011;6:e28695. doi: 10.1371/journal.pone.0028695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo L, Werren JH. Revisiting Wolbachia supergroup typing based on WSP: spurious lineages and discordance with MLST. Curr. Microbiol. 2007;55:81–87. doi: 10.1007/s00284-007-0055-8. [DOI] [PubMed] [Google Scholar]
- Baldo L, Lo N, Werren JH. Mosaic nature of the Wolbachia surface protein. J. Bacteriol. 2005;187:5406–5418. doi: 10.1128/JB.187.15.5406-5418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo L, Dunning Hotopp JC, Jolley KA, Bordenstein SR, Biber SA, Choudhury RR, et al. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 2006;72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing XL, Ruan YM, Rao Q, Wang XW, Liu SS. Diversity of secondary endosymbionts among different putative species of the whitefly Bemisia tabaci. Insect Sci. 2013a;20:194–206. doi: 10.1111/j.1744-7917.2012.01522.x. [DOI] [PubMed] [Google Scholar]
- Bing XL, Yang J, Zchori-Fein E, Wang XW, Liu SS. Characterization of a newly discovered symbiont in the whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) Appl. Environ. Microbiol. 2013b;79:569–575. doi: 10.1128/AEM.03030-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boykin LM, Shatters RG, Rosell RC, McKenzie CL, Bagnall RA, De Barro P, et al. Global relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) revealed using Bayesian analysis of mitochondrial COI DNA sequences. Mol. Phylogenet. Evol. 2007;44:1306–1319. doi: 10.1016/j.ympev.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Boykin L, Bell C, Evans G, Small I, De Barro P. Is agriculture driving the diversification of the Bemisia tabaci species complex (Hemiptera: Sternorrhyncha: Aleyrodidae)?: dating, diversification and biogeographic evidence revealed. BMC Evol. Biol. 2013;13:228. doi: 10.1186/1471-2148-13-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruen TC, Philippe H, Bryant D. A simple and robust statistical test for detecting the presence of recombination. Genetics. 2006;172:2665–2681. doi: 10.1534/genetics.105.048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D, Moulton V. Neighbor-net: an agglomerative method for the construction of phylogenetic networks. Mol. Biol. Evol. 2004;21:255–265. doi: 10.1093/molbev/msh018. [DOI] [PubMed] [Google Scholar]
- Casiraghi M, Bordenstein SR, Baldo L, Lo N, Beninati T, Wernegreen JJ, et al. Phylogeny of Wolbachia pipientis based on gltA, groEL and ftsZ gene sequences: clustering of arthropod and nematode symbionts in the F supergroup, and evidence for further diversity in the Wolbachia tree. Microbiology. 2005;151:4015–4022. doi: 10.1099/mic.0.28313-0. [DOI] [PubMed] [Google Scholar]
- Caspi-Fluger A, Inbar M, Mozes-Daube N, Katzir N, Portnoy V, Belausov E, et al. Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proc. R. Soc. B. Biol. Sci. 2011;279:1791–1796. doi: 10.1098/rspb.2011.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass BN, Mozes-Daube N, Iasur-Kruh L, Bondy EC, Kelly SE, Hunter MS, et al. Bacterial endosymbionts in field-collected samples of Trialeurodes sp. nr. abutiloneus (Hemiptera: Aleyrodidae) Res. Microbiol. 2014;165:77–81. doi: 10.1016/j.resmic.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- Chiel E, Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Katzir N, Inbar M, et al. Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci. Bull. Entomol. Res. 2007;97:407–413. doi: 10.1017/S0007485307005159. [DOI] [PubMed] [Google Scholar]
- Chu D, Liu G, Wan F, Tao Y, Gill RJ. Phylogenetic analysis and rapid identification of the whitefly, Bemisia afer, in China. J. Insect Sci. 2010;10:1–9. doi: 10.1673/031.010.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D, Gao C, De Barro P, Zhang Y, Wan F, Khan I. Further insights into the strange role of bacterial endosymbionts in whitefly, Bemisia tabaci: comparison of secondary symbionts from biotypes B and Q in China. Bull. Entomol. Res. 2011;101:477–486. doi: 10.1017/S0007485311000083. [DOI] [PubMed] [Google Scholar]
- Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton R. Whitefly infestations: the Christmas invasion. Nature. 2006;443:898–900. doi: 10.1038/443898a. [DOI] [PubMed] [Google Scholar]
- De Barro PJ, Boykin LM. Global Bemisia dataset release version 31 Dec 2012. v1. CSIRO; 2013. Data Collection. 10.4225/08/50EB54B6F1042. [Google Scholar]
- De Barro PJ, Liu SS, Boykin LM, Dinsdale AB. Bemisia tabaci: a statement of species status. Annu. Rev. Entomol. 2011;56:1–19. doi: 10.1146/annurev-ento-112408-085504. [DOI] [PubMed] [Google Scholar]
- DeBarro PJ, Driver F. Use of RAPD PCR to distinguish the B biotype from other biotypes of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) Aust. J. Entomol. 1997;36:149–152. [Google Scholar]
- Dumler JS, Walker DH. Order II. Rickettsiales Gieszczykiewicz 1939, 25 AL emend. Dumler, Barbet, Bekker, Dasch, Palmer, Ray, Rikihisa and Rurangirwa 2001, 2156. In: Brenner DJ, Krieg NR, Staley JT, editors. Bergey's manual of systematic bacteriology. New York, NY: Springer; 2005. pp. 96–160. [Google Scholar]
- Duron O, Bouchon D, Boutin S, Bellamy L, Zhou LQ, Engelstadter J, et al. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008;6:27. doi: 10.1186/1741-7007-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast EM, Toomey ME, Panaram K, Desjardins D, Kolaczyk ED, Frydman HM. Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science. 2011;334:990–992. doi: 10.1126/science.1209609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich DR, Torres-Jerez I, Bedford ID, Markham PG, Brown JK. A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers. Mol. Ecol. 1999;8:1683–1691. doi: 10.1046/j.1365-294x.1999.00754.x. [DOI] [PubMed] [Google Scholar]
- Gnankiné O, Mouton L, Henri H, Terraz G, Houndeté T, Martin T, et al. Distribution of Bemisia tabaci (Homoptera: Aleyrodidae) biotypes and their associated symbiotic bacteria on host plants in West Africa. Insect Conserv. Divers. 2013;6:411–421. [Google Scholar]
- Gottlieb Y, Ghanim M, Chiel E, Gerling D, Portnoy V, Steinberg S, et al. Identification and localization of a Rickettsia sp in Bemisia tabaci (Homoptera: Aleyrodidae) Appl. Environ. Microbiol. 2006;72:3646–3652. doi: 10.1128/AEM.72.5.3646-3652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb Y, Ghanim M, Gueguen G, Kontsedalov S, Vavre F, Fleury F, et al. Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies. FASEB J. 2008;22:2591–2599. doi: 10.1096/fj.07-101162. [DOI] [PubMed] [Google Scholar]
- Gueguen G, Vavre F, Gnankine O, Peterschmitt M, Charif D, Chiel E, et al. Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Mol. Ecol. 2010;19:4365–4378. doi: 10.1111/j.1365-294X.2010.04775.x. [DOI] [PubMed] [Google Scholar]
- Haegeman A, Vanholme B, Jacob J, Vandekerckhove TTM, Claeys M, Borgonie G, et al. An endosymbiotic bacterium in a plant-parasitic nematode: member of a new Wolbachia supergroup. Int. J. Parasitol. 2009;39:1045–1054. doi: 10.1016/j.ijpara.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Heddi A, Grenier AM, Khatchadourian C, Charles H, Nardon P. Four intracellular genomes direct weevil biology: nuclear, mitochondrial, principal endosymbiont, and Wolbachia. Proc. Natl Acad. Sci. USA. 1999;96:6814–6819. doi: 10.1073/pnas.96.12.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LM, Brownlie JC, O' Neill SL, Johnson KN. Wolbachia and virus protection in insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Koga R, Kikuchi Y, Meng X-Y, Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl Acad. Sci. USA. 2010;107:769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, De Barro P, Zhao H, Wang J, Nardi F, Liu SS. An extensive field survey combined with a phylogenetic analysis reveals rapid and widespread invasion of two alien whiteflies in China. PLoS ONE. 2011;6:e16061. doi: 10.1371/journal.pone.0016061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006;23:254. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Huson DH, Scornavacca C. Dendroscope 3: an interactive tool for rooted phylogenetic trees and networks. Syst. Biol. 2012;61:1061–1067. doi: 10.1093/sysbio/sys062. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash A, Hoy MA. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 2000;9:393–405. doi: 10.1046/j.1365-2583.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Liu SS, De Barro PJ, Xu J, Luan JB, Zang LS, Ruan YM, et al. Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science. 2007;318:1769–1772. doi: 10.1126/science.1149887. [DOI] [PubMed] [Google Scholar]
- Lo N, Casiraghi M, Salati E, Bazzocchi C, Bandi C. How many Wolbachia supergroups exist? Mol. Biol. Evol. 2002;19:341–346. doi: 10.1093/oxfordjournals.molbev.a004087. [DOI] [PubMed] [Google Scholar]
- Lo N, Paraskevopoulos C, Bourtzis K, O'Neill S, Werren J, Bordenstein S, et al. Taxonomic status of the intracellular bacterium Wolbachia pipientis. Int. J. Syst. Evol. Microbiol. 2007;57:654. doi: 10.1099/ijs.0.64515-0. [DOI] [PubMed] [Google Scholar]
- Martin DP, Lemey P, Posada D. Analysing recombination in nucleotide sequences. Mol. Ecol. Resour. 2011;11:943–955. doi: 10.1111/j.1755-0998.2011.03026.x. [DOI] [PubMed] [Google Scholar]
- Moran NA, Munson MA, Baumann P, Ishikawa H. A molecular clock in endosymbiotic bacteria is calibrated using the insect hosts. Proc. R. Soc. Lond. B Biol. Sci. 1993;253:167–171. [Google Scholar]
- Muniz Y, Granier M, Caruth C, Umaharan P, Marchal C, Pavis C, et al. Extensive settlement of the invasive MEAM1 population of Bemisia tabaci (Hemiptera: Aleyrodidae) in the Caribbean and rare detection of indigenous populations. Environ. Entomol. 2011;40:989–998. doi: 10.1603/EN11129. [DOI] [PubMed] [Google Scholar]
- Naranjo SE, Castle SJ, Barro PJ, Liu SS. Population dynamics, demography, dispersal and spread of Bemisia tabaci. In: Stansly PA, Naranjo SE, editors. Bemisia: Bionomics and management of a global pest. New York, NY: Springer; 2010. pp. 185–226. [Google Scholar]
- Nirgianaki A, Banks GK, Frohlich DR, Veneti Z, Braig HR, Miller TA, et al. Wolbachia infections of the whitefly Bemisia tabaci. Curr. Microbiol. 2003;47:93–101. doi: 10.1007/s00284-002-3969-1. [DOI] [PubMed] [Google Scholar]
- Ochman H, Elwyn S, Moran NA. Calibrating bacterial evolution. Proc. Natl Acad. Sci. USA. 1999;96:12638–12643. doi: 10.1073/pnas.96.22.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MRV, Henneberry TJ, Anderson P. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 2001;20:709–723. [Google Scholar]
- O'Neill SL, Giordano R, Colbert AM, Karr TL, Robertson HM. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl Acad. Sci. USA. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RDM. Tree View: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Pan H, Li X, Ge D, Wang S, Wu Q, Xie W, et al. Factors affecting population dynamics of maternally transmitted endosymbionts in Bemisia tabaci. PLoS ONE. 2012;7:e30760. doi: 10.1371/journal.pone.0030760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Qin L, Wang J, Bing XL, Liu SS. Identification of nine cryptic species of Bemisia tabaci (Hemiptera: Aleyrodidae) from China by using the mtCOI PCR-RFLP technique. Acta Entomol. Sin. 2013;56:186–194. [Google Scholar]
- Rao Q, Luo C, Zhang H, Guo X, Devine GJ. Distribution and dynamics of Bemisia tabaci invasive biotypes in central China. Bull. Entomol. Res. 2011;101:81–88. doi: 10.1017/S0007485310000428. [DOI] [PubMed] [Google Scholar]
- Raychoudhury R, Baldo L, Oliveira DC, Werren JH. Modes of acquisition of Wolbachia: horizontal transfer, hybrid introgression, and codivergence in the Nasonia species complex. Evolution. 2009;63:165–183. doi: 10.1111/j.1558-5646.2008.00533.x. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Ros VID, Fleming VM, Feil EJ, Breeuwer JAJ. How diverse is the genus Wolbachia? multiple-gene sequencing reveals a putatively new Wolbachia supergroup recovered from spider mites (Acari: Tetranychidae) Appl. Environ. Microbiol. 2009;75:1036–1043. doi: 10.1128/AEM.01109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Funaro CF, Giraldo YM, Goldman-Huertas B, Suh D, Kronauer DJC, et al. A veritable menagerie of heritable bacteria from ants, butterflies, and beyond: broad molecular surveys and a systematic review. PLoS ONE. 2012;7:e51027. doi: 10.1371/journal.pone.0051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler H, Bertheau C, Egan SP, Feder JL, Riegler M, Schlick-Steiner BC, et al. Evidence for a recent horizontal transmission and spatial spread of Wolbachia from endemic Rhagoletis cerasi (Diptera: Tephritidae) to invasive Rhagoletis cingulata in Europe. Mol. Ecol. 2013;22:4101–4111. doi: 10.1111/mec.12362. [DOI] [PubMed] [Google Scholar]
- SimÕEs PM, Mialdea G, Reiss D, Sagot MF, Charlat S. Wolbachia detection: an assessment of standard PCR Protocols. Mol. Ecol. Resour. 2011;11:567–572. doi: 10.1111/j.1755-0998.2010.02955.x. [DOI] [PubMed] [Google Scholar]
- Singh ST, Priya NG, Kumar J, Rana VS, Ellango R, Joshi A, et al. Diversity and phylogenetic analysis of endosymbiotic bacteria from field caught Bemisia tabaci from different locations of North India based on 16S rDNA library screening. Infect. Genet. Evol. 2012;12:411–419. doi: 10.1016/j.meegid.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Sintupachee S, Milne JR, Poonchaisri S, Baimai V, Kittayapong P. Closely related Wolbachia strains within the pumpkin arthropod community and the potential for horizontal transmission via the plant. Microb. Ecol. 2006;51:294–301. doi: 10.1007/s00248-006-9036-x. [DOI] [PubMed] [Google Scholar]
- Skaljac M, Zanic K, Ban SG, Kontsedalov S, Ghanim M. Co-infection and localization of secondary symbionts in two whitefly species. BMC Microbiol. 2010;10:142. doi: 10.1186/1471-2180-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut JK, Desjardins CA, lark ME, Baldo L, Russell JA, Werren JH, et al. The mushroom habitat as an ecological arena for global exchange of Wolbachia. Mol. Ecol. 2010;19:1940–1952. doi: 10.1111/j.1365-294X.2010.04572.x. [DOI] [PubMed] [Google Scholar]
- Stouthamer R, Breeuwer JAJ, Luck RF, Werren JH. Molecular identification of microorganisms associated with parthenogenesis. Nature. 1993;361:66–68. doi: 10.1038/361066a0. [DOI] [PubMed] [Google Scholar]
- Stouthamer R, Breeuwer J, Hurst G. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- Tamuram K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QY, Zhang CX. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013;20:254–260. doi: 10.1111/j.1744-7917.2012.01519.x. [DOI] [PubMed] [Google Scholar]
- Thierry M, Becker N, Hajri A, Reynaud B, Lett JM, Delatte H. Symbiont diversity and non-random hybridization among indigenous (Ms) and invasive (B) biotypes of Bemisia tabaci. Mol. Ecol. 2011;20:2172–2187. doi: 10.1111/j.1365-294X.2011.05087.x. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsagkarakou A, Mouton L, Kristoffersen JB, Dokianakis E, Grispou M, Bourtzis K. Population genetic structure and secondary endosymbionts of Q Bemisia tabaci (Hemiptera: Aleyrodidae) from Greece. Bull. Entomol. Res. 2012;102:353–365. doi: 10.1017/S0007485311000757. [DOI] [PubMed] [Google Scholar]
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH, Windsor DM. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc. R. Soc. Lond. B Biol. Sci. 2000;267:1277–1285. doi: 10.1098/rspb.2000.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH, Windsor D, Guo L. Distribution of Wolbachia among neotropical arthropods. Proc. R. Soc. Lond. B Biol. Sci. 1995a;262:197–204. [Google Scholar]
- Werren JH, Zhang W, Guo LR. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc. R. Soc. Lond. B Biol. Sci. 1995b;261:55–63. doi: 10.1098/rspb.1995.0117. [DOI] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- Zhang YK, Zhang KJ, Sun JT, Yang XM, Ge C, Hong XY. Diversity of Wolbachia in natural populations of spider mites (genus Tetranychus): evidence for complex infection history and disequilibrium distribution. Microb. Ecol. 2013;65:731–739. doi: 10.1007/s00248-013-0198-z. [DOI] [PubMed] [Google Scholar]
- Zhou W, Rousset F, O'Neill S. Phylogeny and PCR–based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. B Biol. Sci. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug R, Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE. 2012;7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]


