Abstract
Retinal degenerations represent a heterogeneous group of disorders affecting the function of the retina. The frequency of retinitis pigmentosa (RP) is 1/3500 worldwide, however, in northern Sweden it is 1/2000 due to limited migration and a ‘founder’ effect. In this study we identified genetic mechanisms underlying autosomal dominant and recessive RP present in northern Sweden. Several novel mutations unique for this region were found. In an autosomal recessive form of RP, Bothnia dystrophy caused by mutations in the RLBP1 gene, bi-allelic mutations R234W, M226K and compound heterozygosity, M226K+R234W was detected.
In dominant form of RP mapped to 19q13.42 a 59 kb genomic deletion including the PRPF31 and three other genes was found.
These data provide additional information on the molecular mechanisms of RP evolvement and in the future might be useful in development of therapeutic strategies. Identification of the disease-causing mutations allowed introducing molecular genetic testing of the patients and their families into the clinical practice.
29.1 Introduction
Retinitis pigmentosa (RP) is a group of inherited retinal disorders with a considerable genetic variation. Typical signs of the disease are night blindness and progressive loss of the peripheral visual field, characteristic pigment deposition in the retina, attenuation of the retinal blood vessels, and optic disc pallor.
A form of autosomal dominant RP (adRP) in patients with night blindness in the first and second decade, progressive visual field loss in later life, along with asymptomatic individuals though having an affected parent and an affected child was mapped to 19q13.4 (Al-Maghtheh et al., 1994) (RP11, MIM 600138). A putative human ortholog of yeast pre m-RNA splicing factor, PRPF31 was reported as a disease causing gene (Vithana et al., 2001). To date 42 PRPF31 mutations are listed in the Human Genome Mutation Database and among these only 9 are missense, while the rest are deletions, insertions, indels and splicing mutations (http://www.hgmd.cf.ac.uk/ac/all.php). The majority of the mutations would result in truncated proteins due to exon skipping and premature stop codons (Vithana et al., 2001; Martinez-Gimeno et al., 2003; Sato et al. 2005; Sullivan et al., 2006), therefore, haploinsuffiency was suggested as a mechanism of RP11 evolvement (Vithana et al., 2001).
A rather large group of patients with a variant of autosomal recessive RP (arRP), Bothnia dystrophy (BD) (MIM 180090) has been identified in northern Sweden (Burstedt et al. 1999; 2001). The phenotype is characterized by night blindness in early childhood, retinitis punctata albescens (RPA) in young adulthood and a progressive macular and peripheral retinal degeneration (Burstedt et al. 2001).
The disease was reported to be associated with a bi-allelic c.700C>T mutation in the RLBP1 gene (p.R234W). The majority of the reported cases carried RLBP1 mutations in homozygous state although compound heterozygotes have also been described (http://www.hgmd.cf.ac.uk/ac/all.php). Sequence changes involving single nucleotides are not the only type of mutation affecting the RLBP1 gene. In a patient with RPA a large homozygous deletion was recently described (Humbert et al. 2006).
In our inventory work on retinal dystrophies in northern Sweden, we found among patients with a BD phenotype homozygotes and heterozygotes for the c.700C>T mutation. The patients heterozygous for the c.700C>T mutation appeared to be carriers of the second mutation in the RLBP1 gene, c.677T>A.
Thus, in this study we report a novel genomic deletion including almost the entire PRPF31 gene in two families with adRP linked to 19q13.42 and compound heterozygosity in the RLBP1 gene in arRP of Bothnia type.
29.2 Materials and Methods
29.2.1 Patients and Ophthalmologic Examinations
Patients residing in the four counties of northern Sweden with a population of 880,000 were included in this study. All of them had a history of night blindness and a clinical diagnosis of either arRP or adRP. The study followed the tenets of the Declaration of Helsinki, and consent was obtained from all individuals. Standard ophthalmologic examination included fundus photography and visual field testing. Dark adaptation tests and full-field ERGs were performed in selected cases.
29.2.2 Molecular Genetic Analysis
DNA was extracted from peripheral blood and used for linkage analysis with the ABI PRISM Linkage Mapping set version 2.5 (Applied Biosystems) as described elsewhere (Köhn et al. 2007). Sequence analysis of coding exons and adjacent intronic sequences of candidate genes was performed as described by Köhn et al. (2007). The products of the sequencing reactions obtained with Big Dye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) were run on a 3730 xl DNA analyzer (Applied Biosystems).
DNA from two BD patients carrying the RLBP1 c.700C>T mutation on one allele were subjected to microarray genotyping designed and manufactured according to the APEX technology (Pastinen et al. 1997). (http://www.asperbio.com). Genetic testing was performed using the arRP array for both patients and in addition patient 223:3 was analysed with the autosomal dominant retinitis pigmentosa (adRP) array taking into account that only one mutation was detected in the BD patients. The arRP array included testing for 501 known mutations in 16 genes. adRP panel comprised 347 mutations in 13 genes (information about testing is available on http://www.asperophthalmics.com).
MLPA was done according to Sullivan et al. (2006) with a set of probes designed by this group in combination with the Retinitis Pigmentosa Kit (MRC Holland, http://www.mrc-holland.com/pages/indexpag.html). Additionally, we used VSTM1 probe with following sequences forward 5′ – GGGTTCCCTAAGGGTTGGAccttcacggacctgaagcctaaggatgctgggag; reverse 5′ – gtacttttgtgcctacaagacaacagcctcccatgagtggTCTAGATTGGATCTTGCTGGCAC (DNA Technology, Denmark). The collected raw data were analysed with ABI Prism GeneMapper Software v3.0 (Applied Biosystems). The ratio of 1.0 indicates the presence of two alleles (normal diploid) and 0.5 or 1.5 suggests either deletion or duplication of the target sequence, respectively. The breakpoint region was defined using long range PCR across the deletion with forward primer VSTM F1 5′– GATAGAGGAGGTTTTGCTCTGAC and reverse primer PRPF31 13R 5′ – CGGACCCTGCAGAAGCAGAGCGTCGTAT. PCR product was cloned into pGEM-T Easy (Promega) vector and positive clones were sequenced. Allele specific PCR on genomic DNA was done as described elsewhere using specific primers for both mutant and wild type alleles (BP-F 5′ –TGAAAGAGAGAAGGGGCTCA, BP-R 5′ – GTGGCCTCGTTTACCTGTGT, cDNA PRPF31 12F 5′ – ATCGAGGAGGACGCCT).
29.3 Results and Discussion
29.3.1 adRP
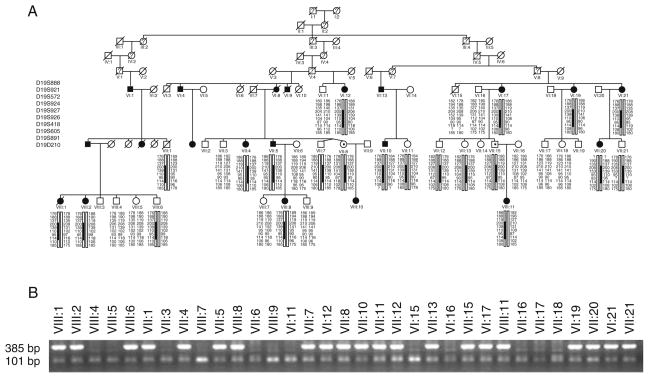
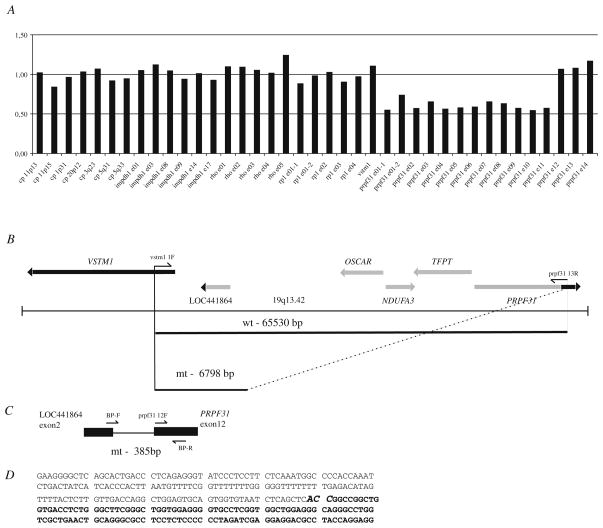
In one of two families with adRP at least 2 individuals were obligate carriers of the mutation since they had both an affected parent and offspring (VII:8, VII:15) (Fig. 29.1). Significant LOD scores with a maximum of 7.58 at the marker D19S926 at 19q13.42 were revealed in the region spanning nearly 1.77 Mb. The reconstructed haplotypes in both families confirmed segregation of adRP with markers D19S924, D19S927, D19S926, D19S418 and D19S605. Since no disease causing mutations were detected by PCR-based methods in RDH13, SYT5, PPP1R12C, PRKCG, CACNG6, 7, 8 and PRPF31genes we considered testing for large genomic deletions using MLPA examining PRPF31, RHO, RP1, and IMPDH1 genes. A large genomic deletion including TFPT, NDUFA3, OSCAR genes and 11 exons of the PRPF31 gene was detected (Fig. 29.2). Based on the MLPA results indicating a normal ratio for the VSTM1 probe and probes for exons 12–14 of the PRPF31, we applied a long range PCR with VSTM1 and exon 13 PRPF31 primers (Fig. 29.2b) and obtained a ~7 kb PCR fragment. Sequencing of this product revealed a deletion of 58,733 nucleotides with breakpoints in intron 11 of the PRPF31 gene and in LOC441864 (ref|NT_011109.15|Hs19_11266), similar to osteoclast-associated receptor isoform 5 (Fig. 29.2b–d). Allele specific PCR with primers set shown at Fig. 29.2c revealed presence of the mutation in affected individuals, obligatory carriers and also several asymptomatic members (Fig. 29.1b). None of 20 simplex adRP cases or 94 healthy controls (188 control chromosomes) from the matched population demonstrated the mutant allele (data not shown).
Fig. 29.1.
Haplotype analysis (a) and segregation of the mutation (b) in the family 78. a – filled symbols indicate affected individuals, while empty symbols indicate unaffected. Symbols with represent an asymptomatic gene carrier. Only disease haplotypes shared by affected individuals in both families are boxed. b – allele-specific PCR where a band of 385 bp indicates the mutant allele and a band of 101 bp indicates presence of the internal PCR control, as a result of wild type and mutant allele’s amplification
Fig. 29.2.
Genomic deletion including PRPF31 gene in adRP. a – a deletion detected by MLPA in VII:20 from family 078. a P235 Retinitis Pigmentosa kit (MRC Holland) along with the VSTM1 probe was applied. The graph indicates presence of one gene copy with probes for exons 1–11 in the PRPF31 gene (ratio is ~0.5). Two gene copies are present with the probes for IMPDH1, RHO, RP1 and for exons 12–14 in the PRPF31 (ratio is ~1.0). b – schematic representation of the genomic region 19q13.42 in proximity to PRPF31. PCR primers VSTM1 1F and PRPF31 13R were applied to amplify across the deleted region (the estimated size of a wild type allele is 65,530 bp and can not be amplified by PCR). PCR fragment of ~7 kb (mutant allele) was obtained by long range PCR, subcloned into pGEM-T Easy vector and sequenced. c – localisation of allele specific primers. Primers sequences and allele specific PCR are described in Materials and Methods and Results and Discussion. PCR with allele-specific primers BP-F and BP-R primers used for segregation analysis (Figs. 29.1B and 29.2B) resulted in 385 bp fragment representing a mutant allele. d – a partial sequence across the deletion. Nucleotide sequence belonging to the predicted gene ‘similar to osteoclast-associated receptor isoform 5’ and intron 11 in the PRPF31 gene is shown. ACC at breakpoint shown in bold can be part of either LOC441864 or PRPF31
PRPF31 codes for a protein needed for splicing in all cell types although its pathologic effect is seen only in rod photoreceptors, causing adRP with incomplete penetrance. PRPF31 is a 61 kDa protein, part of the U4/U6-U5 tri-snRNP complex (Makarova et al. 2002). The proposed mechanism of adRP (RP11) evolvement is haploinsufficiency rather than a dominant negative effect (Vithana et al. 2001). Identification of the large genomic deletion with almost entire loss of PRPF31 gene in this study provides additional evidence for haploinsuffciency as the mechanism in adRP pathogenesis.
Molecular methods used for mutation detection are mainly based on PCR and, therefore, large genomic rearrangements are easily missed. The deletion encompassing almost 59 kb of genomic sequence includes three genes additional to PRPF31 and breakpoints occur in intron 11 of PRPF31 and within the predicted gene, LOC441864, annotated as ‘similar to osteoclast-associated receptor isoform 5’. A number of Alu-repeats in PRPF31 introns can prone to internal unequal recombination resulting in a deletion; however the exact mechanism is not known.
Due to the size of the deletion we could expect a severe phenotype in our families as reported previously (Abu-Safieh et al. 2006). However, among our patients there were individuals with quite preserved visual fields and recordable ERGs at their 50 s. No additional symptoms associated with the genetic defects in NDUFA3, TFPT or OSCAR was observed. In conclusion, identification of such large deletion involving the PRPF31 gene reveals additional evidence that haploinsufficency is a molecular mechanism of evolvement of adRP with incomplete penetrance. Identification of deletion breakpoint provides an important tool for molecular testing and genetic counselling of these patients.
29.3.2 Bothnia Dystrophy
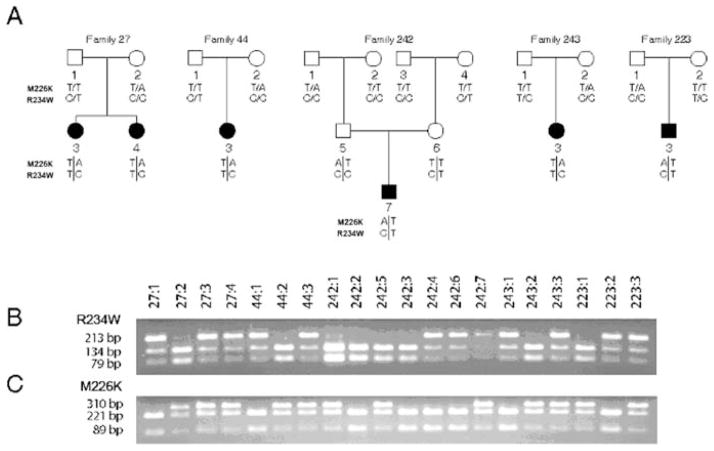
67 out of 121 individuals affected with arRP were homozygous and 10 were heterozygous for the c.700C>T mutation in the RLBP1. Simultaneous evaluation of 501 mutations known as a cause of arRP performed by arrayed APEX technology in two BD patients (027:4 and 223:3, Fig. 29.3a) revealed besides the one known to us RLBP1 c.700C>T (p.R234W) a second mutation, c.677T>A, resulting in p.M226K. Segregation analysis in five tested families showed that c.700C>T and c.677T>A were allelic and the patients were compound heterozygotes, [677T>A]+[700C>T] (Fig. 29.3). None of BD patients homozygous for c.700C>T carried c.677T>A mutation. Allele frequency of the c.677T>A mutation was 1 in 233. Two homozygotes for the RLBP1 c.677T>A mutation were found in RP population from northern Sweden.
Fig. 29.3.
Allelic c.677T>A and c.700C>T mutations in families to probands diagnosed with arRP of Bothnia dystrophy. Detection of both mutations was done by PCR-RFLP. a. Pedigree charts of 5 families where filled symbols indicate affected individuals, while empty symbols indicate unaffected. b. c.700C>T mutation abolishes a MspI restriction site and results in one fragment of 213 bp. c. c.677T>A mutation abolishes a NspI restriction site and results in one fragment of 310 bp
Testing of a BD patient (223:3) with the adRP panel which included 347 known mutations in 13 genes resulted in detection of only one sequence change, c.40C>T in exon 1 of carbonic anhydrase, CAIV (p.R14W). The p.R14W mutation was reported as a cause of adRP, RP17 (Rebello et al. 2004; Yang et al. 2005). Testing of all compound RLBP1 heterozygotes for the presence of c.40C>T revealed absence of the CAIV c.40C>T in nine carriers of RLBP1 c.[677T>A]+[700C>T] while its presence was detected in 223:3 and his unaffected mother (223:2). 6 carriers of CAIV c.40C>T were detected among 143 healthy blood donors (data not shown).
29.4 Conclusions
The prevalence of nonsyndromic RP is approximately 1/2000 in Västerbotten County in the northern part of Sweden. This can be explained by ‘founder’ effect, isolation of population in small villages and low migration rate. To date, at least 40 causative genes and loci have been identified in nonsyndromic RP but one can expect that only a limited number of mutated genes causes retinal degenerations in such populations like the northern Swedish.
We identified a large group of patients with similar clinical appearance and an identical underlying genetic defect. 67 patients have a biallelic mutation in the RLBP1 gene, c.700C>T (p.R234W). Molecular testing in 10 patients with a phenotype similar to BD revealed two mutations, c.700C>T and c.677T>A. Based on allele frequency of c.700C>T (3/200) and c.677T>A (1/233) in a control population we expect that c.700C>T was the first mutation to appear in northern Sweden. Analysis of all ten c.[677T>A]+[700C>T] BD patients and 143 healthy control individuals for the p.R14W mutation in CAIV revealed the presence of this sequence variant in 4% of the population from northern Sweden. The phenotype of the RLBP1 compound heterozygote carrying also CAIV p.R14W could not be distinguished from the other BD patients and no signs of retinal degenerative changes were detected in his 61 year-old mother carrying the same sequence variant.
In summary, the high frequency of arRP observed in northern Sweden is due to the presence of two mutations in the RLBP1 gene, c.677T>A and c.700C>T. The patients are either homozygotes or compound heterozygotes. All 79 patients originating from Västerbotten County, with a population of 257,000 inhabitants presented the BD-like phenotype. Bothnia dystrophy is caused by loss of CRALBP function due to changed physical features and impaired activity of retinoid binding. The CAIV p.R14W known as a cause of RP17 found in one of BD patients is not pathogenic in population of northern Sweden.
Furthermore, a novel mutation unique in patients of Swedish origin, a large genomic deletion resulting in almost entire loss of PRPF31and three additional genes was identified as the cause of adRP with reduced penetrance. Identification of the deletion breakpoints allowed development of a simple tool for molecular testing of this genetic subtype of adRP.
References
- Abu-Safieh L, Vithana EN, Mantel I, et al. A large deletion in the adRP gene PRPF31: evidence that haploinsufficiency is the cause of disease. Mol Vis. 2006;12:384–388. [PubMed] [Google Scholar]
- Al-Maghtheh M, Inglehearn CF, Keen TJ, et al. Identification of a sixth locus for autosomal dominant retinitis pigmentosa on chromosome 19. Hum Mol Genet. 1994;3:351–354. doi: 10.1093/hmg/3.2.351. [DOI] [PubMed] [Google Scholar]
- Burstedt MSI, Forsman-Semb K, Golovleva I, et al. Ocular phenotype of Bothnia dystrophy, an autosomal recessive retinitis pigmentosa associated with an P.R234W mutation in the RLBP1 gene. Arch Ophthalmol. 2001;119:260–267. [PubMed] [Google Scholar]
- Burstedt MSI, Sandgren O, Holmgren G, et al. Bothnia dystrophy caused by mutation in the cellular retinaldehyde-binding protein gene (RLBP1) on chromosome 15q26. Invest Ophthalmol Visl Sci. 1999;40:995–1000. [PubMed] [Google Scholar]
- Humbert G, Delettre C, Senechal A, et al. Homozygous deletion related to Alu repeats in RLBP1 causes retinitis punctata albescens. Invest Ophthalmol Vis Sci. 2006;47:4719–4724. doi: 10.1167/iovs.05-1488. [DOI] [PubMed] [Google Scholar]
- Köhn L, Kadzhaev K, Burstedt MSI, et al. Mutation in PYK2-binding domain of the PITPNM3 causes autosomal dominant cone dystrophy (CORD5) Eur J Hum Genet. 2007;15:664–671. doi: 10.1038/sj.ejhg.5201817. [DOI] [PubMed] [Google Scholar]
- Makarova OV, Makarov EM, Liu S, et al. Protein 61 K, encoded by a gene (PRPF31) linked to autosomal dominant retinitis pigmentosa, is required for U4/U6*U5 tri-snRNP formation and pre-mRNA splicing. EMBO J. 2002;21:1148–1157. doi: 10.1093/emboj/21.5.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gimeno M, Gamundi MJ, Hernan I, et al. Mutations in the pre-mRNA splicing-factor genes PRPF3, PRPF8, and PRPF31 in Spanish families with autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2003;44:2171–2177. doi: 10.1167/iovs.02-0871. [DOI] [PubMed] [Google Scholar]
- Pastinen T, Kurg A, Metspalu A, et al. Minisequencing: a specific tool for DNA analysis and diagnostics on oligonucleotide arrays. Genome Res. 1997;7:606–614. doi: 10.1101/gr.7.6.606. [DOI] [PubMed] [Google Scholar]
- Rebello G, Ramesar R, Vorster A, et al. Apoptosis-inducing signal sequence mutation in carbonic anhydrase IV identified in patients with the RP17 form of retinitis pigmentosa. Proc Natl Acad Sci USA. 2004;101:6617–6622. doi: 10.1073/pnas.0401529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Wada Y, Itabashi T, Nakamura M, Kawamura M, Tamai M. Mutations in the pre-mRNA splicing gene, PRPF31, in Japanese families with autosomal dominant retinitis pigmentosa. Am J Ophthalmol. 2005;140:537–540. doi: 10.1016/j.ajo.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Sullivan LS, Bowne SJ, Seaman CR, et al. Genomic rearrangements of the PRPF31 gene account for 2.5% of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2006;47:4579–4588. doi: 10.1167/iovs.06-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vithana EN, Abu-Safieh L, Allen MJ, et al. A human homolog of yeast pre-mRNA splicing gene, PRPF31, underlies autosomal dominant retinitis pigmentosa on chromosome 19q13.4 (RP11) Mol Cell. 2001;8:375–381. doi: 10.1016/s1097-2765(01)00305-7. [DOI] [PubMed] [Google Scholar]
- Yang Z, Alvarez BV, Chakarova C, et al. Mutant carbonic anhydrase 4 impairs pH regulation and causes retinal photoreceptor degeneration. Hum Mol Genet. 2005;14:255–265. doi: 10.1093/hmg/ddi023. [DOI] [PubMed] [Google Scholar]