Abstract
Objective
To assess whether loop electrosurgical excision procedure (LEEP) increases the risk for preterm birth before 37 weeks of gestation, and clarify whether the increased risk for preterm birth is attributable to the procedure itself or to risk factors associated with cervical dysplasia.
Data Sources
Two authors performed a search of the relevant data through February 2013 utilizing PubMed, Embase, Scopus, CENTRAL, and ClinicalTrials.gov.
Methods of Study Selection
We included observational studies that compared rates of preterm birth in women with prior LEEP to women with no history of cervical excision. Nineteen out of 559 identified studies met selection criteria.
Tabulation, Integration, and Results
We compared women with a history of LEEP to two unexposed groups without a history of cervical excision: 1) women with unknown or no history of cervical dysplasia; and 2) women with history of cervical dysplasia, but no cervical excision. The primary outcome was preterm birth before 37 weeks. Secondary outcomes were preterm birth before 34 weeks, spontaneous preterm birth, preterm premature rupture of membranes, and perinatal mortality. DerSimonian-Laird random effects models were used. We assessed heterogeneity between studies using the Q and I2 tests. Stratified analyses and meta-regression were performed to assess confounding. Nineteen studies were included, with a total of 6,589 patients with history of LEEP, and 1,415,015 without. Overall, LEEP was associated with an increased risk of preterm birth before 37 weeks (pooled RR 1.61, 95% CI 1.35–1.92). However, no increased risk was found when women with a history of LEEP were compared to women with a history cervical dysplasia but no cervical excision (pooled RR 1.08, 95% CI 0.88–1.33).
Conclusion
Women with history of LEEP have similar risk of preterm birth when compared to women with prior dysplasia, but no cervical excision. Common risk factors for both preterm birth and dysplasia likely explain findings of association between LEEP and preterm birth, but LEEP itself may not be an independent risk factor for preterm birth.
INTRODUCTION
In the United States, approximately 12% of all infants are born preterm (1). Preterm birth is a leading cause of neonatal morbidity and mortality. Prior cervical procedures, particularly excisional procedures used to diagnose and treat cervical dysplasia, are a commonly cited risk factor for preterm delivery (2). This is important because in the US alone, over 400,000 women are diagnosed with cervical dysplasia annually and the majority are among women of childbearing age (3).
Many prior studies have investigated the risk of preterm birth in women who have had one of the three primary methods of cervical excision, namely cold-knife conization, laser cone, or loop electrosurgical excision procedure (LEEP). These studies have yielded conflicting results as to the risk of preterm birth after cervical excisional procedures. A possible explanation is that they have used differing unexposed groups, have varying inclusion and exclusion criteria, and do not uniformly control for confounding factors. Meta-analysis has been used in the past to attempt to explore the variability of results and pool the available data (4–7). However after the most recent meta-analysis several well-performed studies have been published (7). Additionally, the most recent systematic reviews and meta-analyses combined results from all cervical excisional procedures, rather than focusing on LEEP, the most commonly performed type of procedure. This approach limits the application of the results to contemporary gynecologic practice.
An important consideration in estimating the risk of preterm birth after LEEP is whether the increased risk for preterm birth is attributable to the cervical excision procedure itself, or secondary to risk factors associated with cervical dysplasia. Establishing whether LEEP is a true risk factor for preterm birth is imperative to assist practitioners in counseling patients who present with dysplasia and in making optimal treatment decisions.
SOURCES
We performed a systematic review and meta-analysis based on a predesigned protocol. The protocol outlined the research question, populations, exposures, outcomes of interest, search strategies, study selection, exclusion criteria, methods of data abstraction and statistical analysis. All methods followed the guidelines set forth by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group (8).
Two authors (S.C. and H.F.) and a medical librarian trained in systematic reviews conducted a search of the existing literature through February 2013. We searched the databases using standard term indices to cover the concepts of ‘cervical dysplasia’, ‘preterm birth’, and ‘cervical excision’. The search model was created based on guidelines published in the Cochrane Handbook for Systematic Review of Interventions (9). We searched the databases PubMed, Embase, Scopus, CENTRAL (Cochrane Central Register of Controlled Trials), and ClinicalTrials.gov. Duplicate studies were removed and two of the authors (S.C. and H.F.) screened the remaining publications for relevance and fulfillment of predefined inclusion and exclusion criteria. We identified additional publications by hand-searching citation lists of the retrieved articles.
STUDY SELECTION
We included cohort and case-control studies that compared rates of preterm birth in women with prior LEEP to women who had no history of cervical excision. We excluded studies that compared preterm birth rates in the same group of women before and after LEEP and those without a defined comparison group. Because LEEP is the most commonly performed procedure for cervical dysplasia (10, 11), and the focus of our study, we excluded studies that reported only preterm birth rates following other types of cervical excisional procedures, such as cold knife conization or laser conization, and non-excisional therapies for cervical dysplasia. In addition, we excluded studies that combined women who had a prior LEEP with women with other types of excision as a single exposure group and did not report rates of preterm birth among women with prior LEEP separately. We also excluded case series, case reports, abstracts, unpublished data, expert opinions, studies that studied LEEP only in women who were pregnant at the time of the procedure, studies that included women who had a LEEP for invasive cancer, and non-English publications. When multiple studies examined the same cohort of women, we included the study that provided the most data on our primary and secondary outcomes.
Two authors (S.C. and H.F.) independently evaluated each study. Data abstracted included description of the unexposed group(s), identification of possible sources of bias that could affect the quality of the study, and rates of the outcomes.
The primary outcome was preterm birth prior to 37 weeks of gestation. Secondary outcome measures were preterm birth before 34 weeks of gestation, spontaneous preterm birth , preterm premature rupture of membranes (PROM), and perinatal mortality.
The exposure was a history of LEEP for treatment of cervical dysplasia. Two categories of unexposed were identified: 1) women with no or unknown history of cervical dysplasia; and 2) women with a history of cervical dysplasia but no cervical excisional procedure.
Differences in design, analysis and reporting among studies can be sources of significant statistical bias in meta-analyses (9, 12). Rather than using quality scoring systems which may be poorly discriminatory, we assessed study quality based on 3 factors we considered most likely to threaten study validity: 1) selection bias, 2) independence of outcome measures, and 3) data source quality. Risk of selection bias in each study was judged to be high or low based on the methods used to identify exposed and unexposed women. Independence of outcomes measures was defined by the pregnancy that was evaluated in the exposure group. Outcomes were considered independent if only the first pregnancy or first pregnancy >20 weeks after LEEP was included in analysis. Lastly, we classified data source quality as high if the study was prospective or used a database or registry that was validated or reported minimal missing data, while medical records, databases or registries of uncertain quality, and surveys were considered lower quality. Overall quality was assessed as higher if at least two of the three criteria were assessed as favorable.
Data was analyzed using Stata 12.0 with METAN software package (Stata-Corp, College Station, TX). Raw data was abstracted from each study and combined using the DerSimonian-Laird random-effects model which accounts for between and within study variance. Pooled relative risks (RR) with 95% confidence intervals were calculated for the primary and secondary outcomes if more than two studies reported the specific outcome. All outcomes evaluated were categorical. If a study included more than one unexposed group, the raw data were combined so that an overall rate of the outcomes was considered for analysis. Results were plotted graphically as forest plots.
We assessed statistical heterogeneity using Cochran’s Q (qualitative) and Higgins I2 (quantitative) tests (13). To take into account the low statistical power of tests of heterogeneity, we considered statistically significant heterogeneity as Cochran’s Q test with a p<0.1 or I2 >30%. Sources of heterogeneity were further explored by stratifying on individual variables. We also performed meta-regression to estimate how much of the heterogeneity was explained by covariates. We assessed publication bias graphically using funnel plots and statistically using the Harbord test (14). The Harbord test is a parametric test to estimate whether there is significant correlation between effects size and sample size, which supports the presence of publication bias.
RESULTS
The flow diagram of study identification for the meta-analysis is illustrated in Figure 1. A total of 559 potentially relevant publications were identified. After exclusion of duplications and studies not relevant to the topic of interest, 47 studies remained and were retrieved for detailed review. Studies were further eliminated for the following indications: meta-analyses, inclusion of cases of invasive cancer, use of an inappropriate unexposed group, no outcome of preterm birth before 37 weeks, and reporting data only after exposure to other forms of excision (laser or cold knife cone). Ultimately, nineteen publications remained after excluding duplicated cohorts and studies that evaluated multiple types of excision as the exposure and LEEP data could not be extracted independently. (15–33) Of the nineteen included studies, 16 were retrospective cohort, 2 were prospective cohort, and 1 was a case-control study. In total, the studies included 6,589 patients with a history of LEEP (exposed), and 1,415,015 without a history of LEEP (unexposed). Table 1 details the characteristics of the included studies, providing each study’s year of publication, country, study design, inclusion and exclusion criteria, unexposed group used, and number of subjects in the LEEP and unexposed groups.
Figure 1.
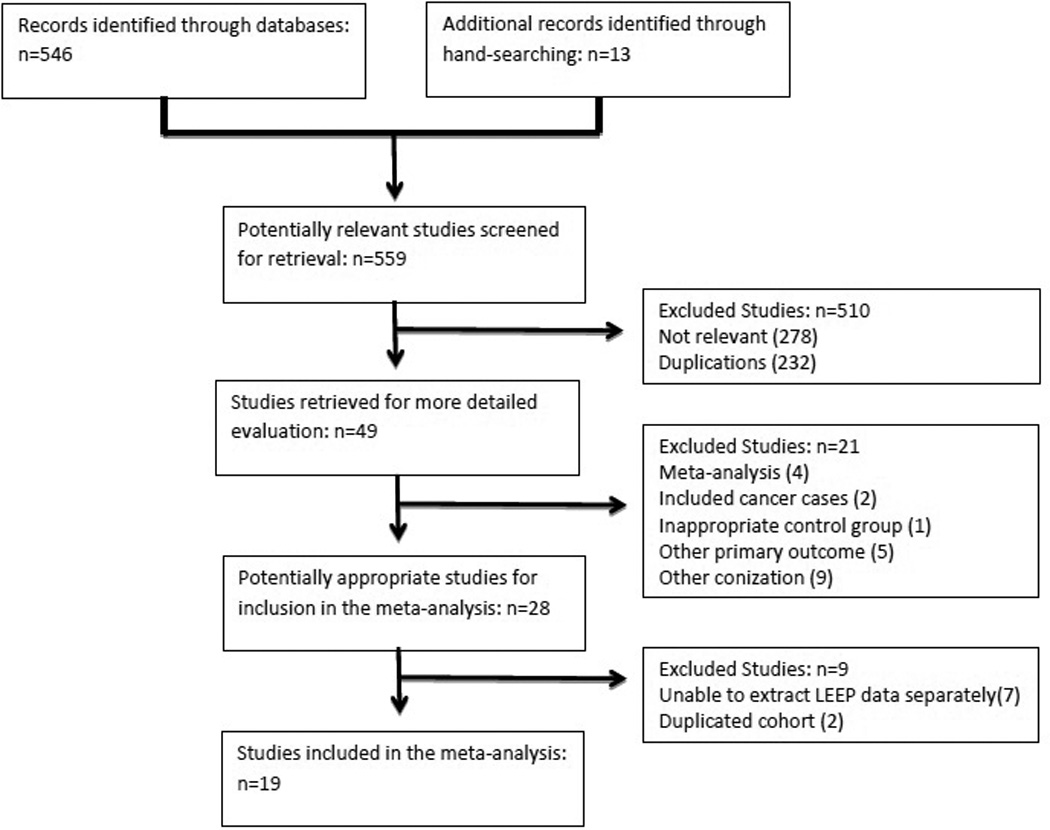
Flow diagram of studies in meta-analysis
Table 1.
Characteristics of Included Studies
| Study | Year | Country | Study Design | Inclusion Criteria |
Exclusion Criteria | LEEP (n) |
Unexposed |
|---|---|---|---|---|---|---|---|
| Haffenden | 1993 | UK | Retrospective cohort | Pregnancy post- LEEP |
Birth < 24 weeks | 152 | Unknown dysplasia hx, no prior tx N= 152 |
| Blomfield | 1993 | UK | Retrospective cohort | Pregnancy post- LEEP |
Birth < 20 weeks | 40 | Unknown dysplasia hx, no prior tx N= 80 |
| Braet | 1994 | UK | Retrospective cohort |
1st pregnancy post-LEEP |
Multiple gestation | 78 | Unknown dysplasia hx, no prior tx N= 78 |
| Cruickshank | 1995 | UK | Retrospective cohort |
1st pregnancy post-LEEP |
Birth < 20 weeks, multiple gestation | 149 | Unknown dysplasia hx, no prior tx N= 298 |
| Sadler | 2004 | New Zealand |
Retrospective cohort |
1st pregnancy post-LEEP |
Birth < 20 weeks, multiple gestation, LEEP during pregnancy |
278 | Prior dysplasia, no prior tx N=426 |
| Tan | 2004 | UK | Retrospective cohort |
1st pregnancy post-LEEP |
Age > 35 years | 119 | Unknown dysplasia hx, no prior tx N= 119 |
| Acharya | 2005 | Norway | Retrospective cohort |
1st pregnancy post-LEEP |
Age >45 years, birth <20 weeks | 79 | Unknown dysplasia hx, no prior tx N= 158 |
| Samson | 2005 | Canada | Retrospective cohort |
1st pregnancy post-LEEP |
Birth < 20 weeks, multiple gestation, prior PTB, indicated deliveries |
571 | Unknown dysplasia hx, no prior tx N=571 |
| Crane | 2006 | Canada | Prospective cohort | Pregnancy post- LEEP |
Multiple gestation | 75 | Unknown dysplasia hx, no prior tx N= 144 |
| Nohr | 2007 | Denmark | Retrospective cohort | Pregnancy post- LEEP |
Multiple gestation, indicated deliveries, stillbirth |
349 | Unknown dysplasia hx, no prior tx N= 14,567 |
| Himes | 2007 | USA | Retrospective cohort |
1st pregnancy post-LEEP |
Birth < 20 weeks, multiple gestation, anomalies, LEEP during pregnancy |
114 | Prior dysplasia, no prior tx N= 962 |
| Jakobsson | 2007 | Finland | Retrospective cohort | Pregnancy post- LEEP |
Age > 49 years, age < 15 years, multiple gestation |
2690 | Unknown dysplasia hx, no prior tx N= 1,056,855 |
| Ortoft | 2010 | Denmark | Retrospective cohort | Pregnancy post- LEEP |
Multiple gestation, indicated deliveries |
572 | No hx of dysplasia or prior tx N=72,899 |
| Werner | 2010 | USA | Retrospective cohort |
1st pregnancy post-LEEP |
Multiple gestation, LEEP during pregnancy |
511 | Unknown dysplasia hx, no prior tx N= 240,348 Prior dysplasia, no prior tx N= 842 |
| Andia | 2011 | Spain | Retrospective cohort | Pregnancy post- LEEP |
Multiple gestation | 189 | Unknown dysplasia hx, no prior tx N= 189 Prior dysplasia, no prior tx N= 189 |
| Lima | 2011 | Portugal | Retrospective cohort | Pregnancy post- LEEP |
None | 18 | Unknown dysplasia hx, no prior tx N= 58 |
| Poon | 2012 | UK | Case control | Pregnancy post- LEEP |
Multiple gestation, cerclage, anomalies, preterm PROM, contractions, progesterone, indicated deliveries |
473 | Unknown dysplasia hx, no prior tx N= 25,772 |
| Van Hentenryck | 2012 | Belgium | Retrospective cohort | Pregnancy post- LEEP |
None | 40 | Unk dysplasia hx, no prior tx N= 212 |
| Simoens | 2012 | Belgium | Prospective cohort | Pregnancy post – EEP |
Birth < 20 weeks, multiple gestation | 52 | No hx of dysplasia or prior tx N= 104 |
LEEP, loop electrosurgical excision procedure; hx, history; tx, treatment; PROM, premature rupture of membranes.
The results of the methodological quality assessment of each study are shown in Table 2. Based on evaluations in three categories, seven studies were classified as higher quality and twelve as lower quality. The higher quality studies all had low risk of selection bias, and most had independence of outcome measures. Conversely, of the studies categorized as lower quality, almost all had high risk of selection bias and lower quality of their data source.
Table 2.
Quality Assessment of Included Studies
| Study | Year | Selection Bias Risk |
Independence of Outcome Measures |
Data Source Quality |
Overall Study Quality |
|---|---|---|---|---|---|
| Haffenden | 1993 | High | Unknown | Low | Lower |
| Blomfield | 1993 | High | Unknown | Low | Lower |
| Braet | 1994 | High | Yes | Low | Lower |
| Cruickshank | 1995 | High | Yes | Low | Lower |
| Sadler | 2004 | Low | Yes | High | Higher |
| Tan | 2004 | High | Yes | Low | Lower |
| Acharya | 2005 | Low | Yes | Low | Higher |
| Samson | 2005 | Low | Yes | High | Higher |
| Crane | 2006 | High | No | High | Lower |
| Nohr | 2007 | High | No | Low | Lower |
| Himes | 2007 | Low | Yes | Low | Higher |
| Jakobsson | 2007 | Low | No | High | Higher |
| Ortoft | 2010 | Low | Unknown | Low | Lower |
| Werner | 2010 | Low | Yes | Low | Higher |
| Andia | 2011 | High | Unknown | Low | Lower |
| Lima | 2011 | High | Unknown | Low | Lower |
| Poon | 2012 | Low | Unknown | High | Higher |
| Van Hentenryck | 2012 | High | No | Low | Lower |
| Simoens | 2012 | High | No | Low | Lower |
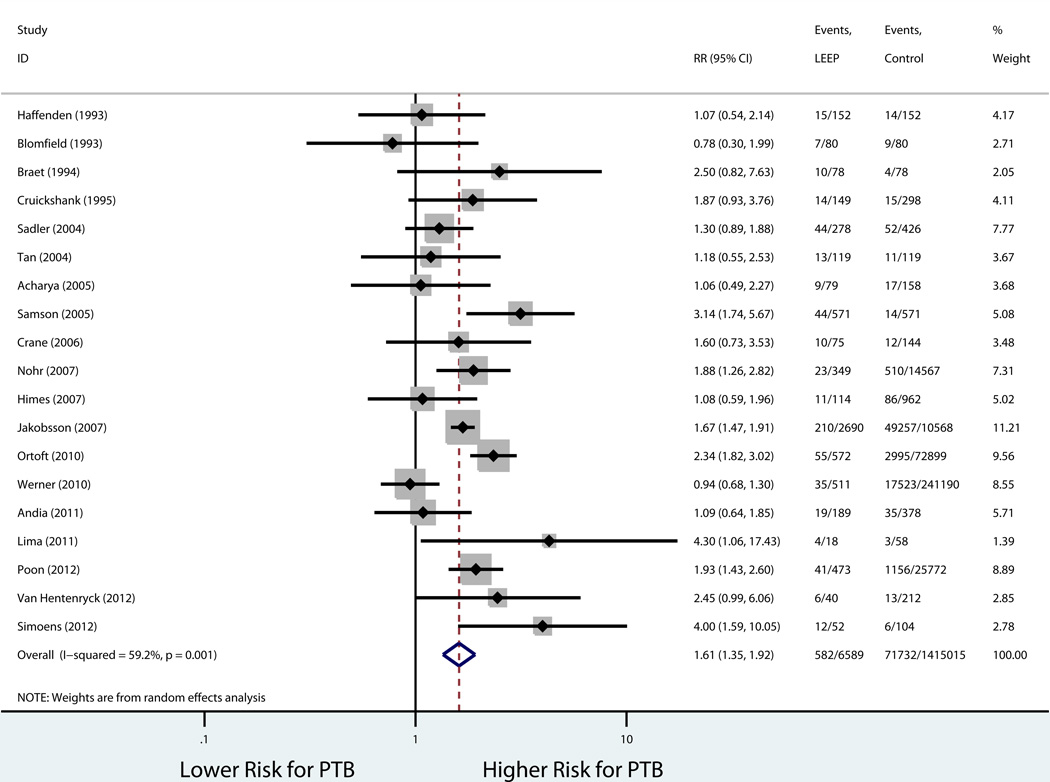
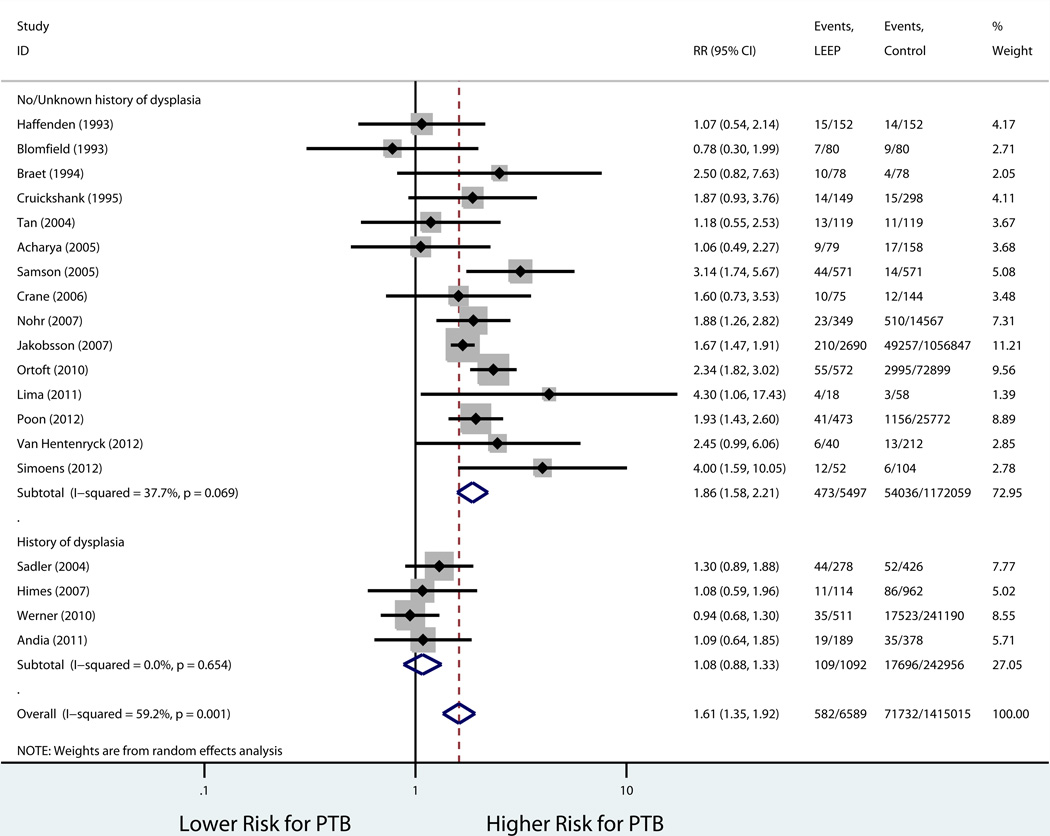
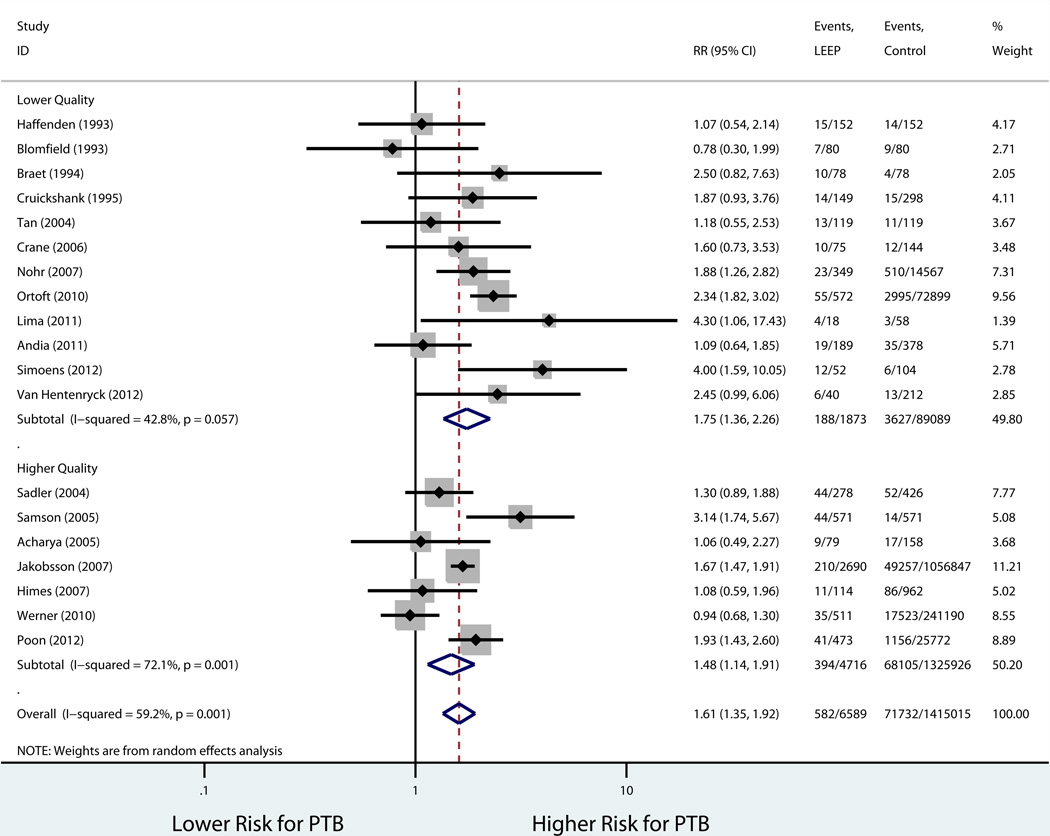
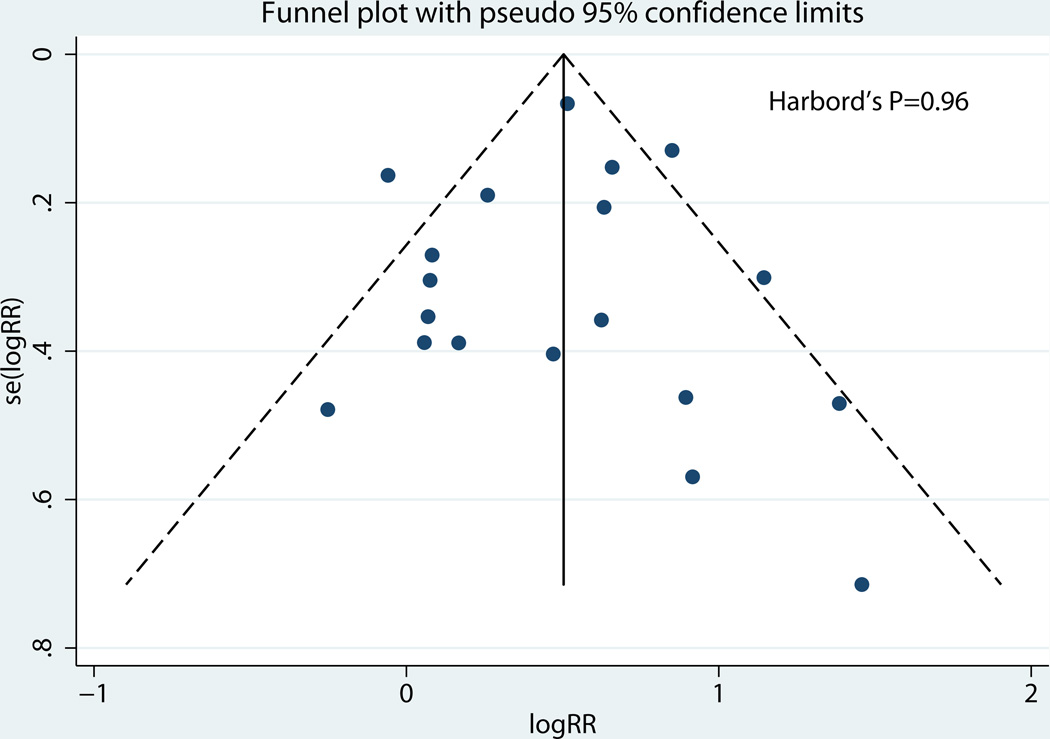
Table 3 shows the rates in the exposed and unexposed groups, pooled RR, 95% CI, and measures of heterogeneity for our primary outcome of preterm birth before 37 weeks, results of stratified analyses, and secondary outcomes. Consistent with our inclusion criteria for the meta-analysis, all studies reported preterm birth before 37 weeks of gestation as an outcome. Overall, LEEP was associated with a higher risk of preterm birth before 37 weeks (19 studies: 8.8% vs. 5.1%, pooled RR 1.61, 95% CI 1.35–1.92; Figure 2). There was evidence of statistical heterogeneity among studies (P=0.001, I2 = 59.2%). Sources of heterogeneity were explored using stratified analyses to evaluate the effect of the comparison group used and study quality. There was no statistically significant difference in the risk of preterm birth when the prior LEEP group was compared to unexposed women with a history cervical dysplasia but no cervical excision (4 studies: 10.0% vs. 7.2%, pooled RR 1.08, 95% CI 0.88–1.33; Figure 3). On the other hand, the association between LEEP and preterm birth persisted when the comparison group was women with either no history or unknown history of dysplasia (15 studies: 8.6% vs. 4.6%, pooled RR 1.86, 95% CI 1.58–2.21). In addition, when stratifying by study quality, the association between LEEP and preterm birth was lower for higher quality studies (7 studies: 8.4% vs. 5.1%, pooled RR 1.48, 95% CI 1.14–1.91) compared to lower quality studies (12 studies: 10.0% vs. 4.1%, pooled RR 1.75, 95% CI 1.36–2.26). Using meta-regression, comparison group type and study quality accounted for 83.8% of the heterogeneity between studies, leaving a non-significant residual heterogeneity of 28.9% (I2res= 28.9%, Adj R2 =83.8%). Importantly, there was no evidence of publication bias (Harbord’s P=0.96) (Figure 4).
Table 3.
Rates and Pooled Estimates for Primary Outcome, Stratified Analyses, and Secondary Analyses
| Outcome | No. of studies |
Exposed % (n outcome/n exposed) |
Unexposed % (n outcome/n unexposed) |
Pooled Relative Risk |
95% CI | Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|
| Cochran’s P value |
I2 | |||||||
| PTB <37 wks | 19 | 8.8% (582/6549) |
5.1% (71732/1415023) |
1.61 | 1.35–1.92 | 0.001 | 59.2% | |
| Stratified by study unexposed group |
||||||||
| No/unknown hx of dysplasia |
15 | 8.6% (473/5457) |
4.6% (54036/1172059) |
1.86 | 1.58–2.21 | 0.069 | 37.7% | |
| Hx of dysplasia | 4 | 10.0% (109/1092) |
7.2% (17696/242946) |
1.08 | 0.88–1.33 | 0.654 | 0.00% | |
| Stratified by Study Quality |
||||||||
| Lower | 12 | 10.3% (188/1833) |
4.1% (3627/89089) |
1.75 | 1.36–2.26 | 0.057 | 42.8% | |
| Higher | 7 | 8.4% (394/4716) |
5.1% (68105/1325926) |
1.48 | 1.14–1.91 | 0.001 | 72.1% | |
| Spontaneous PTB<37 wks |
8 | 6.8% (147/2175) |
3.4% (11684/342097) |
1.60 | 0.99–2.55 | <0.001 | 73.6% | |
| Preterm PROM | 6 | 5.1% (108/2102) |
2.5% (7940/314891) |
2.37 | 1.64–3.44 | 0.094 | 46.9% | |
| PTB<34 wks | 5 | 2.9% (48/1670) |
2.3% (6053/267889) |
2.21 | 1.33–3.67 | 0.157 | 39.6% | |
| Perinatal mortality | 7 | 1.0% (19/1925) |
0.8% (2496/315118) |
1.63 | 0.95–2.80 | 0.911 | 0.00% | |
CI, confidence interval; PTB, preterm birth; hx, history; PROM, premature rupture of membranes.
Figure 2.
Forest Plot LEEP and PTB <37 weeks
Figure 3.
Forest Plot LEEP and PTB stratified by unexposed group
Figure 4.
Forest plot LEEP and PTB stratified by study quality
We were able to isolate the secondary outcome of spontaneous preterm birth before 37 weeks in eight studies. There was significant heterogeneity between studies (P<0.001, I2= 73.6%). Notably, we found a similar magnitude of increase in the risk of spontaneous preterm birth before 37 weeks, although no longer statistically significant (8 studies: 6.8% vs. 3.4%, pooled RR 1.60, 95% CI 0.99–2.55). Meta-analysis of studies that reported on the outcome of preterm PROM, revealed an over 2-fold increased risk for preterm PROM amongst women with a history of LEEP (6 studies: 5.1 % vs. 2.5%, pooled RR 2.37, 95% CI 1.64–3.44). Women with a history of LEEP were also found to have a significantly increased risk for preterm birth before 34 weeks of gestation (5 studies: 2.9% vs. 2.3%, pooled RR 2.21, 95% CI 1.33–3.67), in studies that reported that outcome. Finally, the risk of perinatal mortality was elevated in women with history of LEEP, but not statistically significant (1.0% vs. 0.8%, pooled RR 1.63, 95% CI 0.95–2.80).
CONCLUSION
We found that whereas women with a history of LEEP are at increased risk for preterm birth before 37 weeks, the risk was not significantly different when compared to women with prior dysplasia, but no cervical excision. This suggests that the risk factors for dysplasia and preterm birth are shared, and that LEEP by itself may not be an independent risk factor for preterm birth.
While multiple prior meta-analyses have been performed investigating the risk of preterm birth in women with a history of a cervical excision procedure for dysplasia, our study offers several improvements over previous meta-analyses on the subject. (4–7) We included 6 new studies that have been published since the most recent meta-analysis. Notably, our analysis examined 2 types of comparison groups, enabling us to estimate whether LEEP itself or shared risk factors between cervical dysplasia and preterm birth explain the increased risk of preterm birth in women with a history of LEEP. The ability to stratify by multiple factors and perform meta-regression allowed us to account for heterogeneity between studies. Another strength of our study was our extensive search of the literature by 2 reviewers, including 5 databases, with the aid of a Master of Library and Information Science (MLIS) credentialed librarian, yielding a transparent and reproducible search strategy. In addition, by focusing solely on LEEP, instead of cervical excision procedures as a whole, we give an accurate risk assessment that can be applied to the most commonly used cervical excision procedure in contemporary practice. Lastly, our study differs from the most recent meta-analysis in providing risk estimates for multiple secondary outcomes, including spontaneous preterm birth.
Despite the strengths, the potential limitations of our study must be considered as well. Although an extensive search strategy was employed, the exclusion of non-English studies could have introduced possible selection bias. In order to meet our inclusion criteria of LEEP only, we excluded studies where we were unable to extract LEEP data separately. By excluding these studies, some data on LEEP and subsequent preterm birth was lost. In addition, like all meta-analyses, the quality of our findings is dependent on the quality of the primary studies included. It must be considered that many of the included studies were from countries in which the preterm birth rate is low compared to the United States. Therefore, the results may be different in countries with higher preterm birth rates. On the other hand, inclusion of studies from diverse countries increases the generalizability of our findings. Additionally, due to the smaller number of studies from which the secondary outcomes were drawn, it was not possible to stratify by different unexposed groups as performed for the primary outcome. Another consideration is that the unexposed groups were combined in the stratified analysis for Andia et al. and Werner et al. Therefore, some women with unknown history of dysplasia were included with women with history of dysplasia. However, combining these unexposed groups would serve to bias our results away from the null of no difference. This direction of any potential bias lends credence to the finding of no difference in the risk of preterm birth observed. Finally, it may be argued that the lack of significant difference in the risk of preterm birth when women with a history of LEEP are compared to those with a history of cervical dysplasia, but no excision, is due to lower statistical power. On the contrary, post hoc power analysis showed that the 1092 exposed and 242, 966 unexposed women provide >99% power to detect the 61% increased risk of preterm birth suggested by the overall pooled analysis.
In conclusion, results of this systematic review and meta-analysis of the current body of literature suggest that the notion that LEEP increases the risk of preterm birth needs to be re-evaluated. Our results indicate that the increased risk for preterm birth before 37 weeks in women with a history of LEEP may be related to shared risk factors rather than the cervical excision procedure itself. Larger studies with carefully selected comparison groups that are similar to women with a history of LEEP would further clarify the relationship between LEEP and preterm birth. Additionally, patient-level data could be utilized in a future review for a detailed investigation into individual risk factors for dysplasia and preterm birth. Currently, practitioners are urged to weigh the potential benefits of treating dysplasia with LEEP versus the risk to future pregnancies.(2) If our finding that LEEP is not an independent risk factor for preterm birth is confirmed, the risk and benefit discussion with patients regarding the option of LEEP or expectant management would be altered, thus ensuring optimal therapy without fear of increasing the risk of preterm birth.
Figure 5.
Funnel plot, Publication Bias
Acknowledgments
Dr. Conner and Dr. Frey are supported by the NICHD T32 grant (#22-3125-77026E) and the Washington University Institute of Clinical and Translational Sciences grant (#UL1TR000448).
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2008 period linked birth/infant death data set. National Vital Statistics Reports. 2012;60(5) [PubMed] [Google Scholar]
- 2.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121(4):829–846. doi: 10.1097/AOG.0b013e3182883a34. [DOI] [PubMed] [Google Scholar]
- 3.Henk HJ, Insinga RP, Singhal PK, Darkow T. Incidence and costs of cervical intraepithelial neoplasia in a US commercially insured population. J Low Genit Tract Dis. 2010;14(1):29–36. doi: 10.1097/LGT.0b013e3181ac05e9. [DOI] [PubMed] [Google Scholar]
- 4.Crane JM. Pregnancy outcome after loop electrosurgical excision procedure: a systematic review. Obstet Gynecol. 2003;102:1058–1062. doi: 10.1016/s0029-7844(03)00741-5. [DOI] [PubMed] [Google Scholar]
- 5.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–498. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 6.Arbyn M, Kyrgious M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284. doi: 10.1136/bmj.a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruinsma FJ, Quinn MA. The risk of PTB following treatment for precancerous changes in the cervix: a systematic review and meta-analysis. BJOG. 2011;118(9):1031–1041. doi: 10.1111/j.1471-0528.2011.02944.x. [DOI] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. 2011 Version 5.1.0. [Google Scholar]
- 10.Naumann RW, Bell MC, Alvarez RD, et al. LLETZ is an acceptable alternative to diagnostic cold-knife conization. Gynecol Oncol. 1994;55(2):224–228. doi: 10.1006/gyno.1994.1281. [DOI] [PubMed] [Google Scholar]
- 11.Kleinberg MJ, Straughn JM, Jr, Stringer JS, Partridge EE. A cost-effectiveness analysis of management strategies for cervical intraepithelial neoplasia grades 2 and 3. Am J Obstet Gynecol. 2003;188(5):1186–1188. doi: 10.1067/mob.2003.280. [DOI] [PubMed] [Google Scholar]
- 12.Egger M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 14.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 15.Haffenden DK, Bigrigg A, Codling BW. Pregnancy following large loop excision of the transformation zone. Br J Obstet Gynaecol. 1993;100:1059–1060. doi: 10.1111/j.1471-0528.1993.tb15153.x. [DOI] [PubMed] [Google Scholar]
- 16.Bloomfield PI, Buxton J, Dunn J, Luesley DM. Pregnancy outcome after large loop excision of the cervical transformation zone. Am J Obstet Gynecol. 1993;169(3):620–625. doi: 10.1016/0002-9378(93)90633-t. [DOI] [PubMed] [Google Scholar]
- 17.Braet PG, Peel JM, Fenton DW. A case controlled study of the outcome of pregnancy following loop diathermy excision of the transformation zone. Journal of Obstetrics and Gynaecology. 1994;14:79–82. [Google Scholar]
- 18.Cruickshank ME, Flannelly G, Campbell DM, Kitchener HC. Fertility and pregnancy outcome following large loop excision of the cervical transformation zone. Br J Obstet Gynaecol. 1995;102:467–470. doi: 10.1111/j.1471-0528.1995.tb11319.x. [DOI] [PubMed] [Google Scholar]
- 19.Sadler L, Saftlas A, Wang W, Exeter M, Whittaker J, McCowan L. Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA. 2004;291(17):2100–2106. doi: 10.1001/jama.291.17.2100. [DOI] [PubMed] [Google Scholar]
- 20.Tan L, Pepra E, Haloob RK. The outcome of pregnancy after large loop excision of the transformation zone of the cervix. Journal of Obstetrics and Gynaecology. 2004;24(1):25–27. doi: 10.1080/01443610310001620242. [DOI] [PubMed] [Google Scholar]
- 21.Acharya G, Kjeldberg I, Hansen SM, Sorheim N, Jacobsen BK, Maltau JM. Pregnancy outcome after loop electrosurgical excision procedure for the management of cervical intraepithelial neoplasia. Arch Gynecol Obstet. 2005;272:109–112. doi: 10.1007/s00404-005-0727-1. [DOI] [PubMed] [Google Scholar]
- 22.Samson SA, Bentley JR, Fahey TH, McKay DJ, Gill GH. The effect of loop electrosurgical excision procedure on future pregnancy outcome. Obstet Gynecol. 2005;105(2):325–332. doi: 10.1097/01.AOG.0000151991.09124.bb. [DOI] [PubMed] [Google Scholar]
- 23.Crane JMG, Delaney T, Hutchens D. Transvaginal Ultrasonography in the prediction of PTB after treatment for cervical intraepithelial neoplasia. Obstet Gynecol. 2006;107(1):37–44. doi: 10.1097/01.AOG.0000192169.44775.76. [DOI] [PubMed] [Google Scholar]
- 24.Nohr B, Tabor A, Frederiksen K, Kjaer SK. Loop electrosurgical excision of the cervix and the subsequent risk of preterm delivery. Acta Obstetricia et Gynecologica. 2007;86:596–603. doi: 10.1080/00016340701279145. [DOI] [PubMed] [Google Scholar]
- 25.Himes KP, Simhan HN. Time from cervical conization to pregnancy and PTB. Obstet Gynecol. 2007;109(2):314–319. doi: 10.1097/01.AOG.0000251497.55065.74. [DOI] [PubMed] [Google Scholar]
- 26.Jakobsson M, Gissler M, Sainio S, Paavonen J, Tapper AM. Preterm delivery after surgical treatment for cervical intraepithelial neoplasia. Obstet Gynecol. 2007;109(2):309–313. doi: 10.1097/01.AOG.0000253239.87040.23. [DOI] [PubMed] [Google Scholar]
- 27.Ortoft G, Henriksen TB, Hansen ES, Petersen LK. After conization of the cervix, the perinatal mortality as a result of preterm delivery increases in subsequent pregnancy. Br J Obstet Gynaecol. 2010;117:258–267. doi: 10.1111/j.1471-0528.2009.02438.x. [DOI] [PubMed] [Google Scholar]
- 28.Werner CL, Lo JY, Heffernan T, Griffith WF, McIntire DD, Leveno KJ. Loop electrosurgical excision procedure and risk of PTB. Obstet Gynecol. 2010;115(3):605–608. doi: 10.1097/AOG.0b013e3181d068a3. [DOI] [PubMed] [Google Scholar]
- 29.Andia D, Mozo de Rosales F, Villasante A, Rivero B, Diez J, Perez C. Pregnancy outcome in patients treated with cervical conization for cervical intraepithelial neoplasia. International Journal of Gynecology and Obstetrics. 2011;112:225–228. doi: 10.1016/j.ijgo.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Lima AF, Fancisco C, Julio C, Paula T, Vitorino A, Borrego J. Obstetric outcomes after treatment for cervical intraepithelial neoplasia: six years of experience. Journal of Lower Genital Tract Disease. 2011;15(4):276–279. doi: 10.1097/LGT.0b013e31821a6823. [DOI] [PubMed] [Google Scholar]
- 31.Poon LCY, Savvas M, Zamblera D, Skyfta E, Nicolaides KH. Large loop excision of transformation zone and cervical length in the prediction of spontaneous preterm delivery. Br J Obstet Gynaecol. 2012;119(6):692–698. doi: 10.1111/j.1471-0528.2011.03203.x. [DOI] [PubMed] [Google Scholar]
- 32.Van Hentenryck M, Noel JC, Simon P. Obstetric and neonatal outcome after surgical treatment of cervical dysplasia. Eur J Obstet Gynecol Reprod Bio. 2012;162(1):16–20. doi: 10.1016/j.ejogrb.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Simoens C, Goffin F, Simon P, barlow P, Antoine J, Foidart JM, Arbyn M. Adverse obstetrical outcomes after treatment of precancerous cervical lesion: a Belgian multicentre study. Br J Obstet Gynaecol. 2012;119(10):1247–1255. doi: 10.1111/j.1471-0528.2012.03429.x. [DOI] [PubMed] [Google Scholar]