Summary
Tissue stress and cell death result in inflammation even in the absence of pathogens. Such sterile inflammation is dependent on a cytosolic complex of proteins inside immune cells termed the inflammasome. This complex converts two groups of extracellular signals into an inflammatory response via activation of caspase-1 and secretion of IL-1β and IL-18. Group 1 signals are typically TOLL like receptor agonists and result in transcriptional upregulation of inflammasome components and pro-cytokines. Group 2 signals are diverse, ranging from uric acid to ATP, and lead to assembly and activation of the inflammasome complex. Inflammasome components are required for a wide range of acute and chronic pathologies, including experimental alcoholic and non-alcoholic steatohepatitis, and drug-induced liver injury. Collectively, group 1 and 2 signals, inflammasome components, and cytokine receptors provide a rich source of therapeutic targets. Many of the advances in the field have come from standard reductionist experiments. Progress in the understanding of complex human systems will, however, be dependent on novel strategies such as systems analysis, which analyze large data sets to provide new insights.
Introduction
The development of inflammation after tissue injury has been known since ancient times, and occurs in the absence of pathogens. Such sterile inflammation (SI) is pervasive in a wide range of pathologies, and is significant because it can increase the overall organ damage after the primary insult. Alcoholic and non-alcoholic steatohepatitis (ASH and NASH), and drug induced liver injury (DILI) have SI as an important component of liver damage. Such a generic recognition of a role for SI, and characterization of some of the cellular and cytokine components was complemented by a number of interconnecting developments. Firstly was the theoretical proposal that cell death results in the release and production of molecules which are not present in the extracellular environment during health (damage associated molecular patterns – DAMPs). The second was the identification of a range of DAMPs which possess a wide range of structures from true pattern molecules such a nuclear and mitochondrial DNA, to small molecules like ATP and large crystals like uric acid. The third was identification of the cell surface receptors and mechanisms activated by DAMPs, and the fourth was identification of the cytosolic machinery in innate immune cells which is activated by DAMP signals and has been termed the inflammasome. These discoveries overlapped with much of what was known about immune activation by pathogens, by pathogen associated molecular patterns (PAMPs), including the fact that many of the PAMP receptors such as TLRs are also activated by DAMPs.
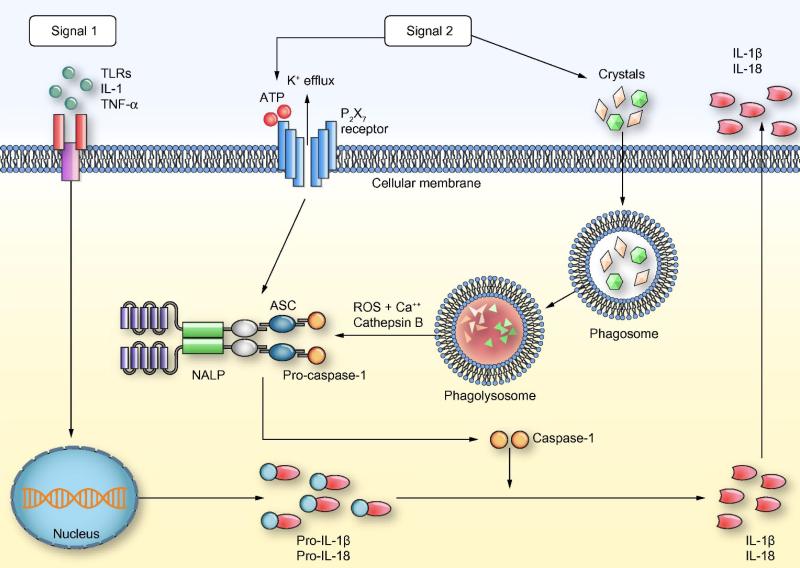
The current understanding of activation of inflammasome pathways in SI is shown in Fig. 1. Two broad types of signals are required in most cells for full activation of this pathway and production of the inflammatory cytokines IL-1β and IL-18. Signal 1 is delivered by a number of TLR ligands (Table 1) and results in transcriptional upregulation of pro-IL-1β pro-IL-18 and inflammasome components. A number of cytokine receptors share the signaling domain MyD88 used by most TLRs and can provide signal 1 allowing the possibility of a positive feedback loop. Signal two can be provided by a highly diverse range of molecules (Table 1) and result in assembly of the inflammasome machinery, which includes cytosolic proteins ASC (apoptosis-associated speck-like protein containing a CARD), NALP (NACHT, LRR, and PYD-containing protein) and caspase-1. Mitochondria likely form a central component proximal to inflammasome activation and integrate these diverse signals. The key step in inflammasome activation is cleavage and activation of caspase-1 which can subsequently cleave and activate the pro-cytokines pro-IL-1β and pro-IL-18. Both these cytokines are relatively proximal in the inflammatory cascade and result in the production of TNF-α and IFN-γ which can induce liver injury by a variety of mechanism.
Figure 1. Mechanisms of inflammasome activation.
Two types of signals are required for inflammasome activation and production of mature IL-1β and IL-18. Signal-1: This results in the production of pro-IL-1β and pro-IL-18 through interaction of various DAMPs/PAMPs and cytokines like TNF-α with TLRs and TNFR. Signal-2: This leads to inflammasome activation through multiple signaling pathways. MSU and other crystals result in the formation of phagolysosomes. Another pathway of inflammasome activation is via activation of the P2X7 receptor. The activation of inflammasome results in the cleavage and activation of the proteases caspase-1 which subsequently cleaves pro-IL-1β and pro-IL-18 to mature IL-1β and IL-18 that are secreted out of the cell. ASC, apoptosis-associated speck-like protein containing a CARD; ATP, adenosine triphosphate; DAMPs, disease associated molecular patterns; IL-1β, interleukin-1beta; IL-18, interleukin-18; MSU, monosodium urate; PAMPs, pathogen associated molecular patterns; ROS, reactive oxygen species; TLRs, toll like receptors; TNF-α, tumor necrosis factor-alpha; TNFR, tumor necrosis factor receptor.
Table 1.
Molecules required for sterile inflammatory [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11].
| Signal 1 | Signal 2 | Downstream pathways | |
|---|---|---|---|
| Alcoholic steatohepatitis | TLR4 [1] | ASC, caspase-1, IL1-R [2] | |
| Non-alcoholic steatohepatitis | TLR4 and 9 [1,3] | IL-1R [3] | |
| Ischemia reperfusion | TLR4 and 9 [4,5] | ||
| Drug induced liver Injury | TLR4 and 9 [6,7] | P2x7 [8], MSU [9] | ASC, caspase-1, IL-1R |
| Fibrosis | TLR4 and 9 [10,11] | IL1-R [3] |
The inflammasome and liver disease
The typical first line of investigation is to test experimental models of liver disease in mice genetically deficient (knockout, KO) in individual inflammasome components. This has been done for several, but not all, inflammasome components in experimental models of ASH, NASH, ischemia reperfusion (IR) and DILI. Such approaches have limitations but there is a broad consensus that many steps of the inflammasome pathways shown in Fig. 1 are necessary for the development of experimental ASH, NASH, IR and DILI (Table 1).
TLR4 and 9 are the ones most reported, and this may simply be because they are the most investigated.
Cell specific roles of inflammasome components
The functional roles of inflammasome components were initially identified in innate immune cells particularly macrophages and explain their rapid production of inflammatory mediators in response to pathogen and damage associated molecular patterns. In the liver, this suggests a major role for inflammasome pathways in Kupffer cells (KC), and this has been confirmed in a model of NASH where high levels of IL-1β production were observed in the KC fraction, and in vivo depletion of KC resulted in a significant decrease in IL-1β levels [3]. Supporting data for a major role for macrophages comes from the fact that baseline levels of inflammasome components were 20-fold higher in mononuclear cells than hepatocytes, and LPS only induced an increase in the mononuclear fraction [2]. This was further confirmed by depleting KC in vivo in models of alcoholic steatohepatitis, which resulted in a reduction in liver injury comparable to the total caspase-1 deficient animals. The signals from injured hepatocytes, which induce inflammasome activation in KC, are likely to include a number of the approximately 20 known DAMPs.
Detailed expression analysis of inflammasome components reveals prominent expression in KC and liver sinusoidal endothelial cells, moderate levels in periportal myofibroblasts and hepatic stellate cells, and virtually none in primary cultured hepatocytes [12]. Interestingly, challenge with LPS resulted in a time- and concentration-dependent expression of a number of inflammasome molecules in cultured hepatic stellate cells and hepatocytes. This makes it possible that at certain stages of disease states, inflammasome activation in parenchymal cells can have important biological roles, but this has not been fully explored.
Inhibition of signal 1
Due to the early identification of TLR4, it was an early target of antagonist development. The antagonist eritoran reduced acute liver injury and inflammation in a rat model of DILI mediated by galactosamine [13]. Eritoran has also been investigated in a large randomized clinical trial as therapy for sepsis, intravenous eritoran demonstrated a trend toward lower mortality in subjects with severe sepsis and high APACHE II scores (Table 2) [14]. Functional antagonists of TLR4 signaling include inhibitors of HMGB1, the purported major endogenous ligand in acute and chronic liver injury. These include glycyrrhizin, an inhibitor of HMGB1 expression, which has been shown to decrease inflammation in murine models of acute acetaminophen (APAP) injury and ischemia reperfusion injury [15]. Clinical validation of the efficacy of intravenous glycyrrhizin in liver injury has been explored in a randomized controlled trial of chronic HCV, where it was administered chronically and shown to significantly reduce serum ALT, but not HCV RNA levels [16]. More recently, intravenous glycyrrhizin has been shown to promote transaminase normalization in patients with acute autoimmune hepatitis though this prospective study did not have a steroid alone comparator arm to compare to the standard of care therapy [17]. Anti-HMGB1 blocking antibodies have been shown to protect from acetaminophen and concanvalin-induced acute liver injury, but have not been tested in humans [18] and [19].
Table 2.
| Agent | Inhibition of | Safety data |
|---|---|---|
| Signal 1 | ||
| Eritoran [14] | TLR4 (selective) | Yes |
| Glycyrrhizin [16,17] | HMGB1 | Yes |
| Ethyl pyruvate [23] | TLR4 | Yes |
| Melatonin [25] | TLR4 | Yes |
| Curcumin [28] | TLR4 | Yes |
| Anti-HMGB1 Ab | HMGB1 | No |
| IRS954 | TLR7 and 9 | No |
| Signal 2 | ||
| GS-9450 [36] | Caspase 1 | Yes |
| A438079 | P2X7 | No |
| Febuxostat [37] | Xanthine oxidase | Yes |
| Allopurinol [38] | Xanthine oxidase | Yes |
| Downstream signals | ||
| Anakinra [39] | IL-1R | Yes |
| Fostamatinib [40] | Syk | Yes |
Ethyl pyruvate supplementation has been shown to suppress TLR4 mediated pro-inflammatory responses and liver injury and inflammation in murine models of ischemia reperfusion injury, extrahepatic cholestasis, and alcohol-induced acute liver injury [20], [21] and [22]. The mechanism of this therapeutic benefit remains poorly defined. The only published clinical trial of ethyl pyruvate showed no therapeutic benefit in patients undergoing cardiopulmonary bypass [23]. Melatonin suppresses TLR4-mediated inflammation and is hepatoprotective in a murine LPS and galactosamine toxin model of liver injury [23] and [24]. In a human clinical trial in NASH patients, oral melatonin administered for 12 weeks significantly reduced serum AST and GGT in patients at 12-week follow-up relative to placebo [25].
Curcumin suppresses LPS mediated release of HMBG1 and presumably amplification of TLR4 mediated immune responses [26]. Curcumin protects the liver against injury and fibrogenesis caused by carbon tetrachloride in rats in part through suppression of inflammation [27]. Anti-inflammatory efficacy of oral curcumin has not been examined in human liver injury but has been recently investigated in the peri-operative period of coronary artery bypass graft, where it showed a significant reduction in in-hospital myocardial infarction as well as post-operative C-reactive protein levels [28]. TLR-7/9 antagonists, specifically the synthetic oligonucleotide IRS 954, has demonstrated great efficacy as a therapy in a murine model of acetaminophen-induced acute liver injury, reducing liver necrosis, hemorrhage, and inflammation [7].
Inhibition of signal 2
There are numerous small molecule antagonists of purinergic receptor P2X7 mediated pro-inflammatory signaling. Additionally, there are agents known to enzymatically deplete the P2X7 ligand ATP, specifically apyrase, as well as small molecular competitive inhibitors of the P2X7 ligand NAD, specifically etheno-NAD. The P2X7 small molecule antagonist A438079, apyrase, and etheno-NAD all demonstrate efficacy in mitigating liver injury and inflammation in a murine model of acetaminophen-induced acute liver injury [8]. Additionally, P2X7 receptor expression on lymphocytes is required for liver injury and inflammation in a murine model of autoimmune hepatitis induced by concanavalin A [29].
Uric acid crystals, similar to all other particulates, activate the Nlrp3 inflammasome. Pharmacologic antagonism of uric acid crystal deposition targets the enzyme xanthine oxidase, required for purine catabolism to uric acid. Allopurinol is an isomer of hypoxanthine that competitively inhibits purine catabolism through xanthine oxidase and has demonstrated clinical efficacy in lowering serum uric acid levels and decreasing the frequency of disease flares in gout. Allopurinol was dose-dependently protective of hepatocellular injury in a murine model of APAP-induced acute liver injury and warm ischemia reperfusion injury in rabbits [30]. Of note, allopurinol has antioxidant effects which may mediate hepatoprotection independent of alterations in uric acid metabolism. Febuxostat is an orally administered non-purine selective small molecule inhibitor of xanthine oxidase with demonstrated clinical efficacy in lowering serum uric acid. There are no published studies investigating the efficacy of febuxostat in liver injury, but febuxostat decreases kidney injury in a rat model of renal warm ischemia reperfusion injury [31]. This positive finding may be related to a reduction in oxidative stress mediated by metabolites generated by xanthine oxidase, though a role for the Nlrp3 inflammasome in mediating ischemia reperfusion injury has been recently defined [32].
Selective pharmacologic antagonism of caspase-1 has not been extensively investigated in experimental models of acute liver injury. The pan-caspase inhibitor IDN-6556 attenuated hepatic injury and fibrosis in a murine model of cholestatic liver injury mediated by bile duct ligation [33]. A double-blind placebo controlled trial of oral IDN-656 demonstrated efficacy in lowering serum aminotransferase levels in patients with chronic hepatitis C after two weeks of administration [34]. The selective caspase 1 inhibitor YVAD-CMK was found to be hepatoprotective in several murine models of immune and toxin-mediated liver injury, including LPS/galactosamine and Fas ligand injury [35]. More recently, a phase 2, randomized clinical trial investigating the oral caspase-1,8,9 inhibitor GS-9450 administration over 4 weeks, in patients with biopsy proven NASH, demonstrated significant reductions in serum aminotransferase [36].
Other inhibitors of inflammasome pathways
The recombinant human interleukin-1 receptor antagonist anakinra is hepatoprotective in rodent models of acute liver injury, including warm ischemia reperfusion injury and acetaminophen-induced acute liver injury [2]. To date, anakinra has not been investigated as therapy in clinical liver disease.
Syk tyrosine kinase is required for Nlrp3 inflammasome sensing of fungal and malarial antigens [41]. Recently, syk tyrosine kinase was identified as required for Nlrp3 inflammasome activation by foreign bodies [42]. The implications of this biology to sterile inflammation in the liver are yet to be determined but may have a role in hepatic fibrogenic responses to foreign materials such as talc or encysted parasites. To date, syk inhibitors have not been investigated in clinical or experimental liver disease. The oral syk inhibitor R788 (fostamatinib) did not demonstrate clinical efficacy with regard to primary end points in the treatment of rheumatoid arthritis in a randomized clinical trial [40].
Systems biology to model experimental data to predict clinical outcomes
Inflammasome-regulating and -driven processes can drive either self-sustaining inflammation or, alternatively, promote pathways that ultimately lead to fibrosis. These processes are dynamic, multi-dimensional, and multi-scale in nature. A reductionist, single-pathway study of the inflammasome would benefit from synthesis via systems approaches, including data-driven and mechanistic computational modeling. These approaches have been employed alone and in concert in order to study the acute inflammatory response in cells, in experimental animals and in humans, and have resulted in translational applications such as in silico clinical trials and individual-specific models [43], [44] and [45].
Though to date there have not been explicit computational models of the inflammasome, the role of IL-1 in inflammation has been modeled computationally [44] and [45]. Initial, unpublished in vitro studies from our group suggest that TLR-mediated inflammatory networks in macrophages drive a chemokine-mediated switching architecture that ultimately leads to inflammasome activation. A key hypothesis, derived from these and related studies, is that inflammation may be regulated via direct or indirect positive feedback loops that control switching behavior between beneficial and detrimental inflammatory responses.
Insights such as these may drive a future generation of inflammasome-targeted, personalized therapies. Such therapies are likely to be targeted at one or more of the aforementioned targets (e.g., TLR4, TLR7, TLR9, or the IL-1 receptor). The key to such therapies will be specificity to the receptor, the specific downstream signaling cascade(s), and the inflammatory mediators that are modulated by such therapies. Proceeding from models of TLR4 signaling of various degrees of complexity and model-derived insights on the ultimate roles of IL-1 in inflammation as applied to individual subjects, such computational models, would need to integrate the ever-increasing data on inflammasome biology [44], [45], [46], [47], [48] and [49]. We would envision a likely need for modulating different aspects of the intersecting TLR-NLR pathways with fine specificity, in a disease- (and possibly individual-) specific fashion. To do so, computational simulations could be employed both to design clinical trials and to predict the responses of individuals [43].
Conclusions
The data from KO animals suggests a role for inflammasome pathways in a number of liver disease models. Data from a broad range of diseases outside the liver suggests that this is a robust conclusion. In addition, data from release of DAMPs and success with antagonist strategies provides further support. DAMPs have also been detected in comparable human diseases and tissues. As with all newly discovered pathways, a strategy of customized small molecule inhibitors can be pursued. There is, however, further excitement in this field because agents such as TLR antagonists have already undergone clinical trials for sepsis and autoimmunity, and this has generated safety data. Other drugs are actually in clinical use, and include uricosuric agents such as febuxostat, and the IL-1R antagonist anakinra, which is currently in use to treat patients with rheumatoid arthritis. Novel approaches such as systems biology promise to allow a synthesis of the experimental data to provide a rational approach to selecting the best candidates to clinical trials in ASH, NASH, DILI and liver fibrosis.
Key points.
Cell death results in inflammation even in the absence of pathogens.
Sterile inflammation can increase organ damage.
The inflammasome is a cytosolic protein complex that is required for sterile inflammation.
Many liver diseases such as alcoholic steatohepatitis, non-alcoholic steatohepatitis, and drug-induced liver injury have sterile inflammation as a major component.
The above pathways have allowed for the development of novel therapies and the repositioning of older therapies.
Acknowledgments
Financial support
This work was supported by NIH K08DK092281 (R.H.), VA Merit award and NIH R01DK076674-01A2 (W.Z.M.), and by NIH UO1-DK-072146 (Y.V.).
Footnotes
Conflict of interest
The authors declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- 1.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 2.Petrasek J, Bala S, Csak T, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miura K, Kodama Y, Inokuchi S, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323 e7–334 e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsung A, Hoffman RA, Izuishi K, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 5.Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2010;51:621–632. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yohe HC, O'Hara KA, Hunt JA, et al. Involvement of Toll-like receptor 4 in acetaminophen hepatotoxicity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1269–G1279. doi: 10.1152/ajpgi.00239.2005. [DOI] [PubMed] [Google Scholar]
- 7.Imaeda AB, Watanabe A, Sohail MA, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoque R, Sohail MA, Salhanick S, et al. P2X7 receptor-mediated purinergic signaling promotes liver injury in acetaminophen hepatotoxicity in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1171–G1179. doi: 10.1152/ajpgi.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kono H, Chen CJ, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest. 2010;120:1939–1949. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe A, Hashmi A, Gomes DA, et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509–1518. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- 12.Boaru SG, Borkham-Kamphorst E, Tihaa L, Haas U, Weiskirchen R. Expression analysis of inflammasomes in experimental models of inflammatory and fibrotic liver disease. J Inflamm (Lond) 2012;9:49. doi: 10.1186/1476-9255-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitazawa T, Tsujimoto T, Kawaratani H, Fukui H. Therapeutic approach to regulate innate immune response by Toll-like receptor 4 antagonist E5564 in rats with d-galactosamine-induced acute severe liver injury. J Gastroenterol Hepatol. 2009;24:1089–1094. doi: 10.1111/j.1440-1746.2008.05770.x. [DOI] [PubMed] [Google Scholar]
- 14.Tidswell M, Tillis W, Larosa SP, et al. Phase 2 trial of eritoran tetrasodium (E5564), a toll-like receptor 4 antagonist, in patients with severe sepsis. Crit Care Med. 2010;38:72–83. doi: 10.1097/CCM.0b013e3181b07b78. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Sun R, Wei H, Tian Z. HMGB1-TLR4-IL-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: interaction of gammadelta T cells with macrophages. Hepatology. 2013;57:373–384. doi: 10.1002/hep.25982. [DOI] [PubMed] [Google Scholar]
- 16.van Rossum TG, Vulto AG, Hop WC, Schalm SW. Pharmacokinetics of intravenous glycyrrhizin after single and multiple doses in patients with chronic hepatitis C infection. Clin Ther. 1999;21:2080–2090. doi: 10.1016/s0149-2918(00)87239-2. [DOI] [PubMed] [Google Scholar]
- 17.Yasui S, Fujiwara K, Tawada A, Fukuda Y, Nakano M, Yokosuka O. Efficacy of intravenous glycyrrhizin in the early stage of acute onset autoimmune hepatitis. Dig Dis Sci. 2011;56:3638–3647. doi: 10.1007/s10620-011-1789-5. [DOI] [PubMed] [Google Scholar]
- 18.Yang R, Zhang S, Cotoia A, Oksala N, Zhu S, Tenhunen J. High mobility group B1 impairs hepatocyte regeneration in acetaminophen hepatotoxicity. BMC Gastroenterol. 2012;12:45. doi: 10.1186/1471-230X-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou RR, Liu HB, Peng JP, et al. High mobility group box chromosomal protein 1 in acute-on-chronic liver failure patients and mice with ConA-induced acute liver injury. Exp Mol Pathol. 2012;93:213–219. doi: 10.1016/j.yexmp.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Tsung A, Kaizu T, Nakao A, et al. Ethyl pyruvate ameliorates liver ischemia-reperfusion injury by decreasing hepatic necrosis and apoptosis. Transplantation. 2005;79:196–204. doi: 10.1097/01.tp.0000151681.07474.2e. [DOI] [PubMed] [Google Scholar]
- 21.Yang R, Uchiyama T, Watkins SK, Han X, Fink MP. Ethyl pyruvate reduces liver injury in a murine model of extrahepatic cholestasis. Shock. 2004;22:369–375. doi: 10.1097/01.shk.0000140659.71121.04. [DOI] [PubMed] [Google Scholar]
- 22.Yang R, Han X, Delude RL, Fink MP. Ethyl pyruvate ameliorates acute alcohol-induced liver injury and inflammation in mice. J Lab Clin Med. 2003;142:322–331. doi: 10.1016/S0022-2143(03)00138-0. [DOI] [PubMed] [Google Scholar]
- 23.Bennett-Guerrero E, Swaminathan M, Grigore AM, et al. A phase II multicenter double-blind placebo-controlled study of ethyl pyruvate in high-risk patients undergoing cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2009;23:324–329. doi: 10.1053/j.jvca.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Xu DX, Lv JW, Ning H, Wei W. Melatonin attenuates lipopolysaccharide (LPS)-induced apoptotic liver damage in d-galactosamine-sensitized mice. Toxicology. 2007;237:49–57. doi: 10.1016/j.tox.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Cichoz-Lach H, Celinski K, Konturek PC, Konturek SJ, Slomka M. The effects of l-tryptophan and melatonin on selected biochemical parameters in patients with steatohepatitis. J Physiol Pharmacol. 2010;61:577–580. [PubMed] [Google Scholar]
- 26.Kim DC, Lee W, Bae JS. Vascular anti-inflammatory effects of curcumin on HMGB1-mediated responses in vitro. Inflamm Res. 2011;60:1161–1168. doi: 10.1007/s00011-011-0381-y. [DOI] [PubMed] [Google Scholar]
- 27.Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73:399–409. doi: 10.1124/mol.107.039818. [DOI] [PubMed] [Google Scholar]
- 28.Wongcharoen W, Jai-Aue S, Phrommintikul A, et al. Effects of curcuminoids on frequency of acute myocardial infarction after coronary artery bypass grafting. Am J Cardiol. 2012;110:40–44. doi: 10.1016/j.amjcard.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 29.Kawamura H, Aswad F, Minagawa M, Govindarajan S, Dennert G. P2X7 receptors regulate NKT cells in autoimmune hepatitis. J Immunol. 2006;176:2152–2160. doi: 10.4049/jimmunol.176.4.2152. [DOI] [PubMed] [Google Scholar]
- 30.Taha MO, Simoes MJ, Noguerol EC, et al. Effects of allopurinol on ischemia and reperfusion in rabbit livers. Transplant Proc. 2009;41:820–823. doi: 10.1016/j.transproceed.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 31.Tsuda H, Kawada N, Kaimori JY, et al. Febuxostat suppressed renal ischemiareperfusion injury via reduced oxidative stress. Biochem Biophys Res Commun. 2012;427:266–272. doi: 10.1016/j.bbrc.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 32.Shigeoka AA, Mueller JL, Kambo A, et al. An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury. J Immunol. 2010;185:6277–6285. doi: 10.4049/jimmunol.1002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191–1196. doi: 10.1124/jpet.103.060129. [DOI] [PubMed] [Google Scholar]
- 34.Pockros PJ, Schiff ER, Shiffman ML, et al. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007;46:324–329. doi: 10.1002/hep.21664. [DOI] [PubMed] [Google Scholar]
- 35.Mignon A, Rouquet N, Fabre M, et al. LPS challenge in d-galactosamine-sensitized mice accounts for caspase-dependent fulminant hepatitis, not for septic shock. Am J Respir Crit Care Med. 1999;159:1308–1315. doi: 10.1164/ajrccm.159.4.9712012. [DOI] [PubMed] [Google Scholar]
- 36.Ratziu V, Sheikh MY, Sanyal AJ, et al. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology. 2012;55:419–428. doi: 10.1002/hep.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naoyuki K, Shin F, Toshikazu H, et al. Placebo-controlled double-blind dose-response study of the non-purine-selective xanthine oxidase inhibitor febuxostat (TMX-67) in patients with hyperuricemia (including gout patients) in japan: late phase 2 clinical study. J Clin Rheumatol. 2012;17:S35–S43. doi: 10.1097/RHU.0b013e31821d351d. [DOI] [PubMed] [Google Scholar]
- 38.Schumacher HR, Jr., Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540–1548. doi: 10.1002/art.24209. [DOI] [PubMed] [Google Scholar]
- 39.Opal SM, Fisher CJ, Jr., Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The interleukin-1 receptor antagonist sepsis investigator group. Crit Care Med. 1997;25:1115–1124. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Genovese MC, Kavanaugh A, Weinblatt ME, et al. An oral Syk kinase inhibitor in the treatment of rheumatoid arthritis: a three-month randomized, placebo-controlled, phase II study in patients with active rheumatoid arthritis that did not respond to biologic agents. Arthritis Rheum. 2011;63:337–345. doi: 10.1002/art.30114. [DOI] [PubMed] [Google Scholar]
- 41.Gross O, Poeck H, Bscheider M, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 42.Malik AF, Hoque R, Ouyang X, et al. Inflammasome components Asc and caspase-1 mediate biomaterial-induced inflammation and foreign body response. Proc Natl Acad Sci USA. 2011;108:20095–20100. doi: 10.1073/pnas.1105152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vodovotz Y, Csete M, Bartels J, Chang S, An G. Translational systems biology of inflammation. PLoS Comput Biol. 2008;4:e1000014. doi: 10.1371/journal.pcbi.1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li NY, Verdolini K, Clermont G, et al. A patient-specific in silico model of inflammation and healing tested in acute vocal fold injury. PLoS One. 2008;3:e2789. doi: 10.1371/journal.pone.0002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieman G, Brown D, Sarkar J, et al. A two-compartment mathematical model of endotoxin-induced inflammatory and physiologic alterations in swine. Crit Care Med. 2012;40:1052–1063. doi: 10.1097/CCM.0b013e31823e986a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riviere B, Epshteyn Y, Swigon D, Vodovotz Y. A simple mathematical model of signaling resulting from the binding of lipopolysaccharide with Toll-like receptor 4 demonstrates inherent preconditioning behavior. Math Biosci. 2009;217:19–26. doi: 10.1016/j.mbs.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An G. A model of TLR4 signaling and tolerance using a qualitative, particle-event-based method: introduction of spatially configured stochastic reaction chambers (SCSRC). Math Biosci. 2009;217:43–52. doi: 10.1016/j.mbs.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 48.An GC, Faeder JR. Detailed qualitative dynamic knowledge representation using a BioNetGen model of TLR-4 signaling and preconditioning. Math Biosci. 2009;217:53–63. doi: 10.1016/j.mbs.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vandenbon A, Teraguchi S, Akira S, Takeda K, Standley DM. Systems biology approaches to toll-like receptor signaling. Wiley Interdiscip Rev Syst Biol Med. 2012;4:497–507. doi: 10.1002/wsbm.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]