Abstract
The zebrafish genome encodes two slc4a1 genes, one expressed in erythroid tissues and the other in the HR (H+-ATPase-rich) type of embryonic skin ionocytes, and two slc4a2 genes, one in proximal pronephric duct and the other in several extrarenal tissues of the embryo. We now report cDNA cloning and functional characterization of zebrafish slc4a3/ae3 gene products. The single ae3 gene on chromosome 9 generates at least two low-abundance ae3 transcripts differing only in their 5′-untranslated regions and encoding a single definitive Ae3 polypeptide of 1170 amino acids. The 7 kb upstream of the apparent initiator Met in ae3 exon 3 comprises multiple diverse, mobile repeat elements which disrupt and appear to truncate the Ae3 N-terminal amino acid sequence that would otherwise align with brain Ae3 of other species. Embryonic ae3 mRNA expression was detected by whole mount in situ hybridization only in fin buds at 24–72 hpf, but was detectable by RT-PCR across a range of embryonic and adult tissues. Epitope-tagged Ae3 polypeptide was expressed at or near the surface of Xenopus oocytes, and mediated low rates of DIDS-sensitive 36Cl−/Cl− exchange in influx and efflux assays. As previously reported for Ae2 polypeptides, 36Cl− transport by Ae3 was inhibited by both extracellular and intracellular acidic pH, and stimulated by alkaline pH. However, zebrafish Ae3 differed from Ae2 polypeptides in its insensitivity to NH4Cl and to hypertonicity. We conclude that multiple repeat elements have disrupted the 5′-end of the zebrafish ae3 gene, associated with N-terminal truncation of the protein and reduced anion transport activity.
Keywords: Chloride-bicarbonate exchanger, Acid–base regulation, in situ hybridization, Xenopus oocyte, Repeat elements
Introduction
The Na+-independent electroneutral anion exchangers of the SLC4 gene family mediate HCO3 − extrusion and Cl− uptake across cellular plasma membranes. These processes contribute to control of cell volume and intracellular pH, and to stabilization of resting membrane potential through regulation of cytoplasmic [Cl−]. Mutations in the human SLC4A1 gene cause hereditary spherocytic and stomatocytic anemias and distal renal tubular acidosis [2, 3]. Engineered genetic ablation of the mouse Slc4a2 gene leads to runting and death before weaning [12] with osteopetrosis [10] and reduced gastrointestinal epithelial secretion [13]. A missense polymorphism in the human SLC4A3 gene is associated with increased seizure susceptibility [37]. The Slc4a3−/− mouse exhibits late-onset retinal degeneration [4] in addition to seizure susceptibility [16], altered respiratory control [21] and susceptibility to cardiac dysfunction precipitated by coexisting deficiency of either Nkcc1 [25] or α-tropomyosin [1].
Na+-independent SLC4 anion exchangers are expressed widely among chordates. Genome duplication with subsequent sporadic gene loss through teleost evolution has led to the presence in fish of two divergent and differentially expressed paralogous copies (ohnologs) of ~70 % of teleost protein-coding genes [27]. The freshwater zebrafish, Danio rerio, thus expresses two slc4a1 genes, slc4a1a (retsina) in hematopoietic tissue [24], and slc4a1b (persephone) in skin ionocytes [19] and lateral line hair cells [15] Zebrafish also express two slc4a2 genes, slc4a2a in proximal pronephric duct [20, 23, 28], and slc4a2b in various extrarenal tissues [29]. However, the zebrafish slc4a3 gene was for many years unrecognized. Since chordates also express slc4a3 genes, we set out to find and study zebrafish Slc4a3 and its gene products.
Here we report that zebrafish slc4a3 is represented by a single gene generating at least two transcripts, both of which encode the same 1170 aa polypeptide. The exon-intron organization of the Ae3 gene resembles that of mammalian AE3 from exon 3 and 3′-ward. However, the zebrafish genomic regions corresponding to those upstream of exon 3 in mammalian Ae3 genes have been infiltrated by multiple repeat elements of differing length and sequence that disrupt the transcribed 5′-noncoding regions of the gene, resulting in an open reading frame N-terminally truncated compared to cloned mammalian and predicted fish AE3 polypeptides. Slc4a3 mRNA was detected by reverse transcription polymerase chain reaction (RT-PCR) in whole embryo and in adult fish. However, in situ hybridization detected low levels of slc4a3 mRNA only in embryonic fin buds, but not in embryonic brain, eye, or heart, sites of prominent mammalian AE3 expression. Xenopus laevis oocytes injected with zebrafish Ae3 cRNA expressed low levels of regulated anion exchange activity.
After completion of this work, the most recent Zv9 zebrafish genome predicted an ae3 cDNA sequence (XM_002662268) similar to that reported here.
Methods
Materials
Na36Cl was from PerkinElmer (Waltham, CA). Restriction enzymes were from New England Biolabs (Beverly, MA). EXPAND High-fidelity PCR System and T4 DNA ligase were from Roche Diagnostics (Indianapolis, IN). 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS) was from Calbiochem (La Jolla, CA). Other chemicals (of reagent grade) were from Sigma (St. Louis, MO) or Fluka (Milwaukee, WI).
Solutions
MBS consisted of (in mM) 88 NaCl, 1 KCl, 2.4 NaHCO3, 0.82 MgSO4, 0.33 Ca(NO3)2, 0.41 CaCl2, and 10 HEPES (pH 7.40). ND-96 consisted of (in mM) 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES (pH 7.40 and pH 8.50). For ND-96 at pH 5 Na HEPES was replaced by equimolar MES. In Cl−-free or partially Cl−-substituted solutions, NaCl was replaced mole-for-mole with Na cyclamate. Cl− salts of K+, Ca2+, and Mg2+ were substituted on an equimolar basis with the corresponding gluconate salts as needed. Addition to flux media of the weak acid sodium butyrate (40 mM) was in equimolar substitution for Na cyclamate. Bath addition of NH4Cl (26 mM) was in equimolar substitution for NaCl.
Sequencing of zebrafish Ae3 cDNA
The initial tBLASTN search for AE3-like sequences in the zebrafish genome (Zv6) was conducted with human bAE3 polypeptide (NP_005061) as input. The search yielded three adjacent but non-overlapping genomic clones (CAAK03024772.1, CAAK03024773.1 a n d CAAK03024774.1) encoding amino acid sequence homologous to human bAE3 aa 380–1227. Forward primers F1, F2, and F3, and reverse primers R1, R2, and R3 were designed to test possible transcription of this AE3-like sequence in zebrafish (Supplemental Table 1) Total RNA was purified from freshly collected zebrafish embryos or adult zebrafish tissues using the RNAeasy mini kit (Qiagen) and quantified by Nano-Drop. 1 μg total RNA was used for first strand cDNA synthesis (Retroscript kit, Ambion). RT-PCR products of the expected size (36–38 cycles hot start PCR with Expand High Fidelity Enzyme, Roche) were purified from 1 % agarose gel and and directly sequenced. Analysis of overlapping PCR products confirmed that the amplified fragments encoded a continuous AE3-like open reading frame. Reverse primers 3R1 and 3R2 (Supplemental Table 1) from the apparent 3′-UTR (based on CAAK03024774.1) were designed to amplify and sequence the remaining C-terminal coding region of the zebrafish Ae3-like ORF, which was shown to continue uninterrupted through a C-terminal Val corresponding to human bAE3 C-terminal Val1232.
Lambda-ZAP cDNA libraries from zebrafish epiboly stage, 15–19 hpf embryo, and adult whole fish and kidney were used for 5′-RACE extension of the zebrafish AE3-like sequence. Semi-nested PCR amplifications were performed using T7 or T3 vector-embedded primers and reverse primers R4 and R5. Guided by analysis of the amplified sequences, a second round of semi-nested PCR amplifications was carried out with the more 5′-ward reverse primers R6 and R7 (Supplemental Table 1). The 5′-extension reached nucleotide G207 (KF771000, our deposited AE3-like sequence), corresponding to mid-exon 3 of human bAE3. The extended zebrafish AE3-like sequence aligned well with much of the human bAE3 amino acid sequence (NP_005061). Although 5′-RACE extension failed to yield the 5′-end of putative exon 3 or upstream sequence containing a putative initiator Met corresponding to that in human bAE3 exon 2, the 5′-RACE sequence obtained did allow identification of several Zv7 sequences encompassing the complete putative exon 3 (CAAK04038951.1). Additional clones verified sequences for putative exon 4 (CAAK04038952.1), exon 6 (CAAK04038955.1), exon 7 (CAAK04038956.1) and exon 8 (CAAK04038957.1).
Human bAE3 exon 3 and putative zebrafish exon 3 (CAAK04038951.1) aligned well between human codons Glu72 to Val31 (Fig. 1a). However, whereas human bAE3 exon 3 continues 5′-ward until codon Gln17, zebrafish exon 3 appears to extend 5′-ward only to the codon corresponding to human Val31. Further 5′-extension of zebrafish exon 3 would include an in-frame terminator codon in putative intron 2. Two further rounds of semi-nested 5′-RACE were repeated with 5′-shifted reverse oligo R8 and either of the nested oligos R9 or R10, without success (Supplemental Table 1). Thus, the zebrafish Ae3 initiator Met resides in exon 3 at a position corresponding to human bAE3 Pro62, or alternatively, it resides upstream of this site in a region in which the exon-intron organization of the zebrafish slc4a3 gene differs considerably from that of its mammalian (and teleost) orthologs.
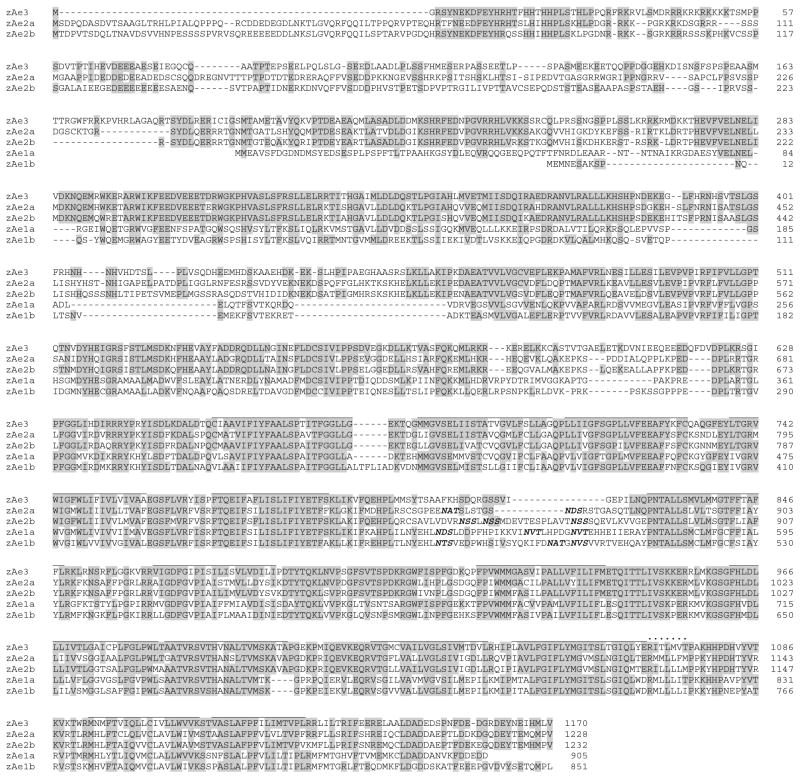
Fig. 1.
Amino acid sequence alignment of zebrafish Ae3 with those encoded by the duplicated Ae2 and Ae1 genes of zebrafish. Multiple sequence alignment was by COBALT (NCBI). Sequences and their Genbank numbers are as follows: Ae3 (AHC12990), Ae2a (NP_001032314), Ae2b (NP_001107912), Ae1a (NP_938152), and Ae1b (ACN32214). Ae3 amino acid residues conserved in all family members are highlighted in gray. Amino acid residue numbers are at right. Putative transmembrane domains are overlined. Consensus N-glycosylation sites are in bold italics
Sequence corresponding to exon 2 of human bAE3 was not found in the zebrafish genome version then available by tBLASTN or BLASTN. Therefore, in searching for additional upstream sequence of zebrafish ae3 we analyzed potential ORFs in contig CAAK04038951 (later replaced by FP243360). Within this ~7 kb upstream region, eight sites encoding putative N-terminal polypeptide fragments of suitable length (20–40 AA, based on other AE3 sequences) were arbitrarily selected for testing by 2 rounds of semi-nested RT-PCR with 24 hpf embrytonic cDNA. Forward oligos designed to encompass or precede each of the eight potential initiator Met residues were used for a first round of hot start PCR (38 cycles) with reverse oligo R11, spanning the exon 5/6 junction to guarantee discrimination of cDNA from genomic DNA products (Supplemental Table 1). None of these amplifications produced sufficient PCR products for direct sequencing. 1 μl volumes of 1st round PCR products were tested in a second round of (25 cycles) semi-nested PCR with (nominally shared) exon 3 forward primer F6 and reverse oligo R11. Two second-round reactions gave rise to products of expected length, and direct sequencing confirmed these two cDNAs as products of the zebrafish ae3 gene.
To confirm this upstream extension for these two products, forward primers MDMV.F3 and MSLG.F2 were used with reverse oligo R11 for a successful second round of seminested PCR (25 cycles). Downstream forward primers MDMV.F4, MDMV.F5, MSLG.F3, and MSLG.F4, in concert with reverse primers R11, R7, or R5, also generated zebrafish ae3 amplimers (one round of RT-PCR, 38 cycles) from 24 hpf embryo cDNA or adult head cDNA. Amplimer sequencing confirmed two zebrafish ae3 splice variant transcripts with different 5′-UTRs, both encoding the same polypeptide with its initiator Met in Ex3 (AHC12990). The two zebrafish ae3 variant cDNAs have been submitted as GeneBank KF771000 (Var 1, corresponding to the MDMV primer amplifications, or ae3-tv1) and KF771001 (Var 2, corresponding to the MSLG primer amplifications, or ae3-tv2).
Cloning of zebrafish Ae3 cDNA for functional expression
Since both splice variants of zebrafish ae3 shared the exon 3 initiator Met codon, forward primer F6 (from the common 5′-UTR of exon 3) and reverse primer 3R1 (3′-UTR; Supplemental Table 1) were used to generate full-length cDNAs from 24 hpf embryo cDNA with 36 cycles of hot start PCR (Expand High Fidelity Enzyme). PCR products were separated on 1 % agarose gel, and a band of expected length was purified and cloned into pCRII T/A-cloning vector (Invitrogen). The several clones sequenced each contained several individual PCR-introduced mutations, identified by comparison to the sequences of pooled PCR products. A full-length, missense mutation-free zAe3 cDNA was reconstructed and cloned into vector pcDNA3X (Invitrogen’s pCDNA3 modified by addition of the X. laevis β-globin 5′-UTR between HindIII and KpnI sites, and addition of β-globin 3′-UTR between XhoI and XbaI sites). An HA tag (followed by the flexible bridge sequence GASG) was added to the N-terminal Met, all preceded by the Kozak sequence (CAAGCGCGCGCG) from mouse SLC12A4/KCC1 (AF121118) used previously for successful oocyte expression of multiple heterologous gene products [35].
Primers TF and 3R3 (Supplemental Table 1) were used to generate a 796 bp PCR product of unique sequence (Zv7) from the zAe3 3′-UTR. This PCR product subcloned into pCRII was used as template for synthesis of anti-sense and sense probes for in situ hybridization.
cRNA expression in Xenopus oocytes
Capped zebrafish ae3 cRNA was synthesized at 17 °C from XbaI-linearized plasmid DNA-template with the Megascript T7 kit (Life Technologies), purified with the RNeasy mini-kit (Qiagen, Germantown, MD), and quantitated by Nanodrop spectrometer (ThermoFisher, Waltham, MA). Synthesis of high quality cRNA in sufficient yield required doubling the supplier-recommended concentrations of GTP and cap and lowering the reaction temperature. cRNA quality was verified by formaldehyde gel electrophoresis.
Mature female Xenopus laevis frogs (Dept. of Systems Biology, Harvard Medical School) were subjected to partial ovariectomy under hypothermic tricaine anesthesia following protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Beth Israel Deaconess Medical Center. Stage V-VI oocytes were prepared by overnight incubation of ovarian fragments in MBS with 1.5 mg/ml collagenase B (Alfa Aesar, Ward Hills, MA), followed by a 20 min rinse in Ca2+-free MBS, with subsequent manual selection and defolliculation as needed. Oocytes were injected on the same day with cRNA (50 ng as indicated), and maintained 72 hrs at 17.5 °C in MBS containing 10 μg/mL gentamicin until used for experiments. As uninjected and water-injected oocytes did not differ in anion transport at 72 hrs post-injection (not shown), uninjected oocytes were used as controls for the current experiments.
Isotopic influx experiments
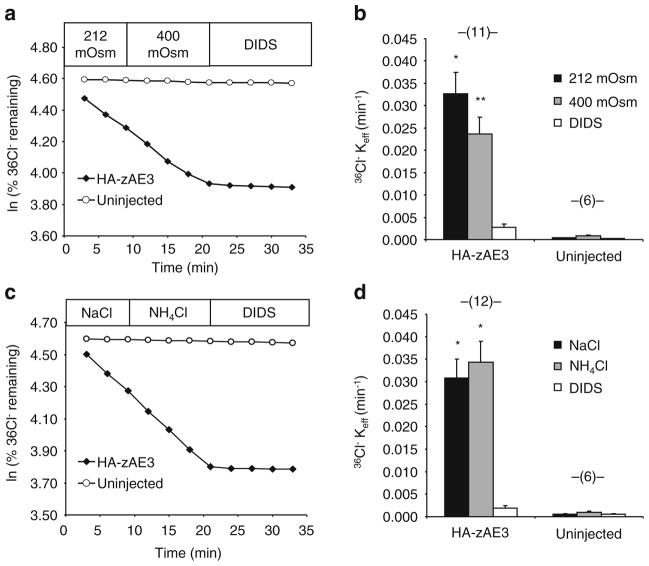
Unidirectional 36Cl− influx studies were carried out for periods of 30 min in 148 μL ND-96 and 2 μL of carrier-free 260 mM Na36Cl− (0.25 μCi), resulting in total bath [Cl−] of 103.6 mM. In experiments testing the effect of NH4 + on 36Cl− influx, ND-96 was substituted with ND-70 plus 26 mM NH4Cl. In experiments testing the effect of hypertonicity on 36Cl− influx, ND-96 (212 mOsm) was supplemented with mannitol to achieve a calculated osmolarity of 400 mOsm, maintaining constant [Cl−]. In all 36Cl− influx experiments the carrier solution was supplemented with 10 μM bumetanide to impair Cl− influx by native oocyte NKCC1. Influx experiments were terminated with four washes in ice-cold isotonic Na cyclamate solution. Washed oocytes were individually lysed in 150 μL 2 % sodium dodecyl sulfate (SDS). Triplicate 10 μL aliquots of the influx solution were used to calculate specific acitivity of radiolabeled substrate anions. Oocyte anion uptake was calculated from cpm values of washed oocytes and from bath specific activity.
Isotopic efflux experiments
For unidirectional 36Cl− efflux studies, individual oocytes were injected with 50 nl of 260 mM Na36Cl (6.25 nCi). Following a 5–10 min recovery period in Cl−-free solution (cyclamate, pH 5 for the pHo experiment), the efflux assay was initiated by transfer of individual oocytes to 6 ml borosilicate glass tubes, each containing 1 ml efflux solution. At intervals of 3 min, 0.95 ml of this efflux solution was removed for scintillation counting and replaced with an equal volume of fresh efflux solution. Following completion of the assay with a final efflux period either in Cl−-free cyclamate solution or in the presence of the inhibitor DIDS (200 μM), each oocyte was lysed in 150 μL of 2 % SDS. Samples were counted for 2 min periods or until the magnitude of 2SD was <5 % of the sample mean cpm value.
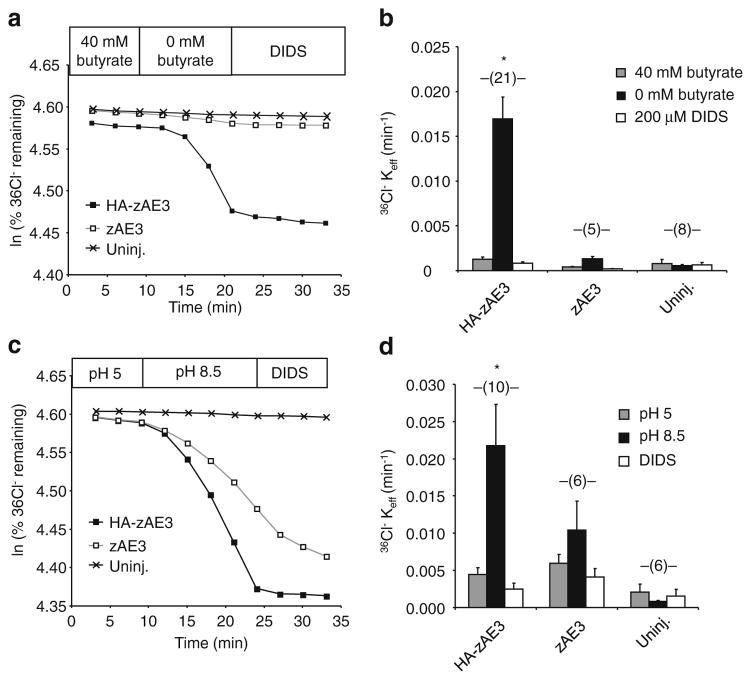
In experiments testing effects of intracellular acidification on Cl− efflux, ND96 was substituted with ND56 containing 40 mM Na butyrate to acidify oocyte pHi. to~6.7 [31]. Removal of bath butyrate with substitution by Na cyclamate to maintain constant bath [Cl−] rapidly realkalinized pHi back towards initial resting pHi (t1/2=6 min) while pHo remained constant. 40 mM butyrate neither inihibits nor serves as substrate of AE2 [31]. pHo sensitivity of zfAE3-mediated 36Cl− efflux at nearly constant pHi was measured by sequential exposure of individual oocytes to ND-96 at pHo values of 5.0 and 8.5, followed by addition of DIDS (200 μM) at pHo 8.5.
Efflux data was plotted as the natural logarithm (ln) of the quantity (% cpm remaining in the oocyte) vs. time. Efflux rate constants for 36Cl− were measured from linear fits to data from the last three time points sampled within each experimental period. For each experiment, uninjected oocytes from the same frog were subjected to parallel measurements with cRNA-injected oocytes. Oocytes with <15 % of injected 36Cl− remaining at the end of the assay were excluded from analysis.
Confocal immunofluorescence microscopy
Oocytes were injected with 50 ng cRNA encoding zebrafish Ae3 bearing the HA epitope (YPYDVPDYA) at the N-terminus, untagged zebrafish Ae3, or HA-tagged guinea pig Slc26a3 [35]. Uninjected oocytes and oocytes injected with cRNA were incubated at 17.5 °C for 72 hrs, fixed at 4 °C for 30 min in PBS containing 1.5 % paraformaldehyde (PFA), then washed three times in PBS supplemented with 0.002 % sodium azide. After a 5 min treatment with 1 % SDS in 1× PBS, the fixed, permeabilized oocytes were blocked with PBS containing 1 % bovine serum albumin and 0.05% saponin for 1 hr at 4 °C. Oocytes were then incubated overnight at 4 °C with rabbit monoclonal anti-HA peptide (dilution 1:1600; Cell Signaling, Danvers, MA), washed 3 times with 1 % PBS-BSA, then incubated for 2 h with Cy3-conjugated secondary donkey anti-rabbit Ig (dilution 1:1600; Jackson Immunochemicals, West Grove, PA), and again thoroughly washed in PBS-BSA. Oocytes were aligned in uniform orientation along a plexiglass groove and imaged in sequence through the 10× objective of a Zeiss LSM510 laser scanning confocal microscope, using the 543-nm laser line at 512X512 resolution, at constant filter, gain, and pinhole settings.
Polypeptide abundance at or near each oocyte’s surface was estimated by quantitation of specific fluorescence intensity (FI) at the periphery of one quadrant of an equatorial focal plane (Image J v. 1.38, National Institutes of Health). Mean FI background of uninjected oocytes was subtracted from each oocyte’s FI value. Normalized means and standard errors were calculated for each group. Median intensity images were selected from each group for presentation.
Whole mount in situ hybridization
Whole mount in situ hybridization on wild type zebrafish embryos at 24-, 36-, and 72-hours post-fertilization (hpf) were performed with sense and anti-sense strand cRNA as previously described [5]. Melanin pigmentation was chemically inhibited in zebrafish embryos older than 24 hpf by treatment with 0.003 % 1-phenyl-2-thiourea (Sigma) prior to fixation in 4 % para-formaldehyde. Results were indistinguishable with albino embryos processed in the absence of 1-phenyl-2-thiourea.
Statistics
Data are reported as mean±SEM. Flux data were compared by Student’s paired t-test (MS Excel) or, as appropriate, by ANOVA with Tukey’s post-hoc analysis (SigmaPlot 11.0). P <0.05 was taken as significant.
Results
cDNA sequence of zebrafish ae3
The cloned zebrafish ae3 cDNA encodes a single open reading frame of 1170 amino acids. The putative zebrafish Ae3 polypeptide shares 63 % amino acid identity with human bAE3 and 62 % identity with skate Ae3. Sequence identities are 60 % with mouse AE2a, 59 % with skate Ae2; 57 % with zebrafish Ae2a and 56 % with zebrafish Ae2b (Fig. 1). The deduced Ae3 protein sequence (of the full-length clone chosen for functional expression studies) differed substantially from NCBI predicted sequence XP_700692 available at the time of cloning. However, our sequence agrees with current NCBI predicted RefSeq XP_002662314 except at Thr344Ser (C1306G) and Asp431Glu (T1568A), likely reflecting polymorphic differences among individual zebrafish. Additional SNPs observed during sequencing of Ae3 cDNAs from several different zebrafish tissues representing pools from multiple individuals included (numbering and trailing nucleotide as in KF771000, leading nucleotide as in RefSeq XP_002662314): T302C, A458G, T515C, A527G, C699T (encoding cSNP Pro142Ser), G788A, T1043A, C1172T, A1196G, T1538C, T1850C, G2174A, A2921C, G2972A, C3038T, G3137A, C3425G, T3683C, C3798A, A3899C, A4038C, C4213A, G4229T, T4312C, G4332T, A4349G, and A4394C. (Underlined SNPs are those in which trailing nucleotide is present in KF771000, the coding region of which was used for functional expression).
The C-terminal transmembrane domain of zebrafish Ae3 protein (zAe3) is generally similar in length, sequence, and secondary structure to other Ae3 polypeptides (Supplemental Figures 1 and 2). The stilbene disulfonate binding motif KLIKVF is present at the ecto-end of transmembrane span 5, conserving the isothiocyanate-reactive K residues present also in Ae3 of tilapia Oreochromis niloticus and of pufferfish Takifugu ruprides, but absent from Ae3 of skate Raja erinacea (Supplemental Figure 2). However, the ecto 5–6 loop of the zAe3 transmembrane domain is shorter than, and lacks the single consensus N-glycosylation site of mammalian and skate Ae3 proteins. The absence of a consensus ecto-loop N-glycosylation site is shared with the predicted Ae3 sequences from tilapia and fugu.
The zAe3 transmembrane domain lacks the Cys residue at the start of the C-terminal cytoplasmic tail in the human (C1194) and rat proteins (C1189), and has one Cys residue (C79) which is absent from human or rat AE3. However, 3 of the 4 human AE3 transmembrane domain Cys residues are conserved in zAe3, including that corresponding to palmitoylated endofacial C843 of human AE1 [9, 18]. The majority of transmembrane domain residues contributing to the pH-sensitivity of mouse AE2 [32–34] are conserved in zAe3. Additional conserved zAe3 transmembrane domain residues include K850 (corresponding to human AE1 K590, the inhibitory binding site of phenyl-isothiocyanate and difluoronitrobenzene), E941 (corresponding to human AE1 E681, the inhibitory binding site of Woodward’s reagent K, and the H+ binding site for H+/sulfate cotransport), H994 (corresponding to human AE1 H734, the inhibitory diethylpyrocarbonate binding site), K1003 (corresponding to human AE1 K743, an endofacial low ionic strength tryptic site), and K1111 (corresponding to human AE1 K851, the H2-DIDS isothiocyanate crosslinking site). Conserved zAe3 Tyr1163 corresponds to the human AE1 src family phosphorylation site Y904 in the C-terminal cytoplasmic tail [7] (sites summarized in Tables 54–1 and 54–2 in [36]).
The far N-terminal cytoplasmic domain of zAe3 is truncated compared to other brain-type AE3 (bAE3) polypeptides. However, the 35 cytoplasmic domain residues 103–137 of zAe3 align with only 11 residues in human and rat AE3, with 3 gaps in the alignment. In addition to sharing 2 Cys residues in positions conserved in human or rat AE3, the zAe3 cytoplasmic domain has 4 Cys residues absent from corresponding positions in human and rat AE3. Only one of the two Cys residues (C201, C317) participating in intermolecular disulfide crosslinking of the human AE1 N-terminal cytoplasmic domains is conserved as zAe3 C556. Residues 42–52 of the zAe3 N-terminal cytoplasmic domain share with Ae3 of tilapia, fugu, and skate a stretch of 11 consecutive Arg/Lys (Supplemental Figure 2) considerably more basic than the corresponding shorter basic stretch in human and rat AE3 (Supplemental Figure 1). In contrast, zAe3 residues 68–76 are much less acidic than the 11 consecutive Glu/Asp residues in corresponding regions of zAe2a, zAe2b (Fig. 1) and of human and rat AE3 (Supplemental Figure 1). The functional significance of these highly charged regions remains unclear. Residues which, in human tetrameric AE1, contribute to the dimer-dimer interface are conserved in zAe3 [39], as are residues corresponding to the flexible link between the crystallized portion of human AE1’s N-terminal cytoplasic domain and its transmembrane domain.
Exon-intron organization of the zebrafish ae3 gene
The single zebrafish ae3 gene resides on chromosome 9, entirely within intron 1 of the hdac4 gene, with the same coding strand, and with unknown regulatory implications. The weak zebrafish-human synteny for ae3 is discontinuous and delocalized in contrast to the strong, locally ordered synteny for zebrafish ae1a [24] and ae2a [28], and the moderate synteny for ae2b [29]. Although human chr2q36 genes located near the human SLC4A3/AE3 gene can be found on zebrafish chromosome 9, their zebrafish orthologs are physically far removed from the zebrafish slc4a3/ae3 gene. Conversely, the human HDAC4 gene is 20 Mb removed and on the opposite strand from the human SLC4A3 gene).
Supplemental Table 2 compares the exon-intron organization of the zebrafish ae3 gene (deduced by alignment of our cloned cDNAs with Zv9 genomic clones FP243360.6 and FP103057.6) with that of the mouse Ae3 gene. All intronexon junctions are of consensus sequence except for the exon 14/Intron 14 acceptor splice junction GC dinucleotide. The early ae3 introns of the zebrafish gene are thousands of nt in length, much longer than in the mouse or (not shown) the rat, in which the only intron of comparable length is the 1350 nt intron 6 encompassing cardiac Ae3 alternate exon C1. However, the 20 kb intron 6 of zebrafish ae3 contains no sequence with detectable similarity to mammalian exon C1, as judged by tBLASTn. Mouse and rat ae3 introns 3, 5, 7, 8, and 9 are hundreds of nt in length, with other introns shorter still. The 3′-end of zebrafish exon 3 aligns well with exon 3 of mouse, rat, and human. However, the 5′-end of zebrafish exon 3 does not extend as far 5′-ward as exon 3 of mammalian AE3s.
RT-PCR experiments revealed two 5′-variant zebrafish ae3 transcripts each encoding the same 1170 aa polypeptide with initiator Met in exon 3 (in acceptable Kozak context) that aligns with Pro62 in brain AE3 of human, mouse, and rat (Fig. 2). The ~7 kb upstream of zebrafish ae3 exon 3 includes at least one region of sequence identifiably related to the N-terminal codons of mammalian brain AE3 proteins. However, exhaustive cloning and PCR efforts failed to reveal any additional candidate in-frame initiator Met codons within this upstream region. These results suggest that the AE3-like exon 3 sequence upstream of the initiator Met contributes to the 5′-UTR of zebrafish Ae3 mRNA.
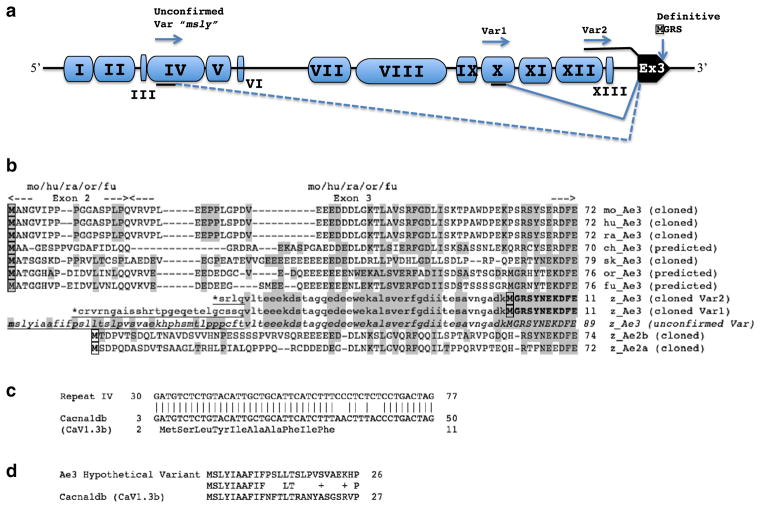
Fig. 2.
Repeat element insertions at the 5′-end of the zebrafish ae3 gene. a. Schematic of the ~7 kb region encompassing the 5′-end of the zebrafish ae3 gene and upstream sequence. Blue pods labeled with black roman numerals represent repeat elements of distinct sequence; the black pod is ae3 Exon 3. The number of imperfect copies (nt identities up to 90 %) detected in the zebrafish Zv9 genome for each (otherwise unannotated) repeat type (nt numbering from FP243360) was 107 copies of repeat I (2660–2947); 159 copies of repeat II (2946–3545); 100 copies of repeat III (3635–3727); 181 copies of repeat IV (3739–4392); 111 copies of repeat V (4393–4545); 103 copies of repeat VI (4585–4655); 189 copies of repeat VII (5706–6266); 153 copies of repeat VIII (6366–7467); 106 copies of repeat IX (7652–7859); 36 copies of repeat X (7875–8228); 122 copies of repeat XI (8230–8535); 496 copies of repeat XII (8550–9185); 116 copies of repeat XIII (9188–9284). The MSLY Met codon of hypothetical transcript Var3 is located at nt 3760–2 within repeat IV (black underline), and is predicted to be spliced to Ex3 (dashed splice line). The 5′-most extent of validated transcript Var1 within repeat X encodes an in-frame termination codon (*) in an exonic sequence (black underline) spliced to Ex3 (solid splice line). The 5′-most extent of validated transcript Var2 is within repeat XII, and the transcript is continuous (black overline) through the rest of putative ae3 intron 2 (including repeat XIII) into Ex3. Transcript variants 1 and 2 both encode the definitive (confirmed) initiator Met in Ex3 (boxed) starting the polypeptide presented in Figs. 1 and 2. Inter-repeat distances are not to scale. b. Aligned N-terminal Ae3 amino acid sequences (definitive or predicted, as indicated) from zebrafish, mouse, human, rat, chicken, skate, fugu, and tilapia, and from zebrafish Ae2a and Ae2b. The exon 2–3 junction shown at top applies to mouse, rat, and human sequences, and to the predicted sequences of tilapia and fugu. Highlighted in gray are zebrafish Ae3 residues shared in all or some of the other N-terminal sequences. Lowercase residues indicate potential zebrafish Ae3 Ex3 coding sequences confirmed by RT-PCR. The underlined lowercase residues (N-terminally marked with *), RT-PCR proven exonic sequences upstream of zebrafish Ex3, encode in-frame terminator codons and therefore represent 5′-UTR. The third instance of underlined lowercase residues (in italics) is encoded upstream of Exon 3 by a hypothetical exonic sequence remotely homologous to authentic or predicted N-terminal protein coding regions in ae3 genes of tilapia (Oreochromis niloticus; or, XP_003443372), skate (sk, CAD61187), fugu (fu, XP_003962174) chicken (ch, XP_003641688), as well as of mouse (mo, NP_033234), rat (ra, NP_058745), and human (hu, NP_005061). c. Aligned nucleotide sequences of Repeat IV from the zebrafish ae3 5′-upstream region and the N-terminal open reading frame of zebrafish cacna1db (AY528225) encoding the pore subunit of an L-type Ca2+ channel. d. Aligned N-terminal amino acid sequences encoded by hypothetical zebrafish ae3 transcript variant “msly” and by the zebrafish cacna1db gene encoding the Cav1.3b pore-forming subunit of the L-type voltage-gated Ca2+ channel (AAS20587). The shared N-terminal sequences are both encoded by exonized variants of repeat IV
We sought upstream ORF candidates in the zebrafish ae3 gene that could encode the (by analogy with other AE3 genes) apparently missing coding regions upstream of exon 3 and exon 2. Among these was a potential ORF ~6 kb upstream of exon 3 beginning with aa sequence MSLY (Fig. 2b) in a plausible Kozak context (not shown). This hypothetical zebrafish polypeptide without apparent N-terminal truncation would align moderately well with the N-terminal sequences of other AE3 polypeptides. However, attempts to amplify the sequence directly or by two-round semi-nested PCR, using either immediately downstream nested primer MSLY.F2 or the exon 3 downstream nested primer F6, failed to detect the predicted cDNA.
The ~7 kb genomic sequence upstream of zebrafish ae3 exon 3 also revealed integrated single copies of 13 different mobile repeat elements ranging in length form 70 bp to 1.1 kb (Fig. 2a). All 13 of these repeat elements are dispersed throughout the Zv9 zebrafish genome. 12 of the 13 are distributed in more than 100 imperfect copies of up to 91 % nucleotide identity, with nearly 500 copies of repeat element XII.
The repeat elements were analyzed by RepeatMasker (Insititute for Systems Biology; http://www.repeatmasker.org/). Repeat elements I, III, and X have no annotation in Zv9. Repeat elements II and XI are LTR repeats. Element II is of the Hobo-Activator Charlie class, whereas Element XI is a Copia2-LTR repeat of the Gypsy/DIRSI class, without an identified retroviral element. Repeat elements IV and V are DNA-type repeats of the TcMar-Tc1-IS630-Pogo family. Elements VI, VII, IX, XII, and XIII are DNA-type repeats of undefined family type. Repeat element VIII is a DNA-type concatemer of a hATT repeat linked to a hAT-Ac Hobo Activator repeat. Element VII also contains two, independent 10 nt palindromes, while element VIII contains an imperfect palindromic repeat of 55 nt in length. Element XII is bracketed by an imperfect palindromic repeat of 77 nt and four short stretches of uniform trinucleotide repeats.
Repeat element IV encodes the candidate upstream initiator Met of the hypothetical predicted zebrafish Ae3 polypeptide that could not be validated by RT-PCR. Interestingly, a closely related variant of repeat element IV has also inserted into the zebrafish cacna1db gene, one of two zebrafish Cav1.3 genes encoding pore subunits of L-type Ca2+ channels [30]. Despite nucleotide sequence drift among repeat IV variants (Fig. 2c), the polypeptide sequence encoded by the repeat IV elements of the two genes shows complete identity across 10 N-terminal amino acid residues of the Cacna1db polypeptide and the hypothetical but unconfirmed Ae3 exon upstream of exon 3 (Fig. 2d). However, this sequence is absent from the N-termini of the 70 % identical Cav1.3 polypeptides from human and from the tilapia O. niloticus.
Whole mount in situ hybridization analysis
Whole mount in situ hybridization revealed low-level but specific ae3 mRNA expression in developing fin bud at 36 and 72 hpf. The signal was not evident upon hybridization with sense strand probe (Fig. 3). ae3 mRNA expression was undetected through 96 hpf in brain, retina, or heart, mammalian sites of expression. However, ae3 mRNA was detected as a short amplimer by RT-PCR in adult zebrafish brain, eye, gill, kidney, heart, intestine, muscle, and ovary [19]. The low expression level of zebrafish Ae3 mRNA in embryonic tissue as evidenced by in situ hybridization paralleled the high cycle number required for robust RT-PCR amplification in the current study.
Fig. 3.
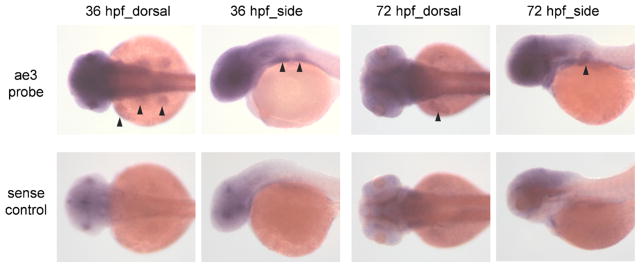
In situ hybridization of zAe3 mRNA in early development. In situ hybridization reveals specific Ae3 mRNA expression in fin buds of 36 and 72 hpf embryos (arrowheads, upper panels) that was not detected using Ae3 sense strand control probe (lower panels). Fin bud expression of Ae3 mRNA persisted at 96 hpf (not shown). Specific in situ hybridization signals were not detected in brain, heart or retina
HA-zAe3 at or near the oocyte surface exhibits low-levels of DIDS-sensitive 36Cl−/Cl− exchange activity
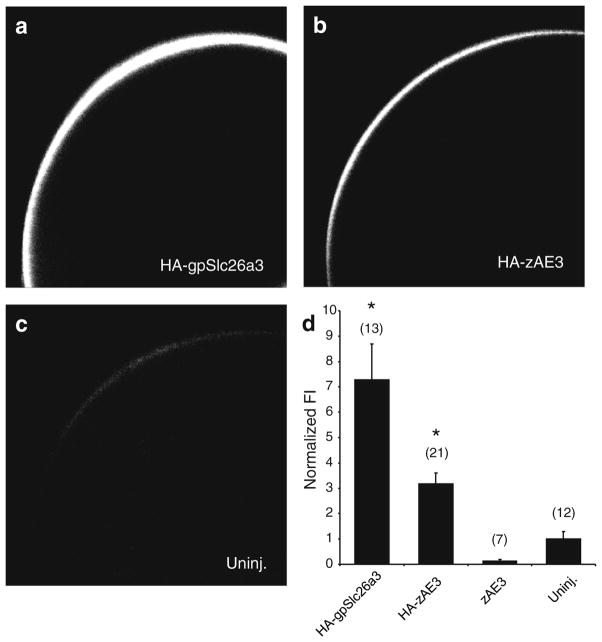
HA-zAe3 was expressed at or near the surface of Xenopus oocytes as judged by whole mount confocal immunofluorescence microscopy (Fig. 4) as compared to uninjected oocytes and to HA-SLC26A3 from guinea pig (positive control, [35]). zAe3 cRNA-injected oocytes did not exhibit 36Cl− influx at levels significantly higher than for uninjected oocytes (Fig. 5a). However, oocytes injected with cRNA encoding HA-zAe3 showed reproducibly increased 36Cl− influx to levels approaching those seen with rat cardiac AE3 (Fig. 5a). Therefore, subsequent functional characterization was carried out with zebrafish HA-zAe3 except as indicated.
Fig. 4.
HA-zAe3 is expressed at or near the oocyte surface. Xenopus oocytes (diameter 1–1.3 mm) expressing HA-tagged zebrafish Ae3 show significantly more protein surface expression than uninjected oocytes. Confocal immunofluorescence images of representative median intensity showing uninjected oocytes (Uninj.) or oocytes previously injected with cRNA encoding HA-tagged zAe3 (50 ng, HA-zAE3), untagged zAe3 (50 ng, zAE3) or HA-tagged guinea pig Slc26a3 (50 ng, HA-gpSlc26a3). The bottom right panel shows mean normalized fluorescence intensities for (n) oocytes. * p<0.05 vs. zAe3 and uninjected
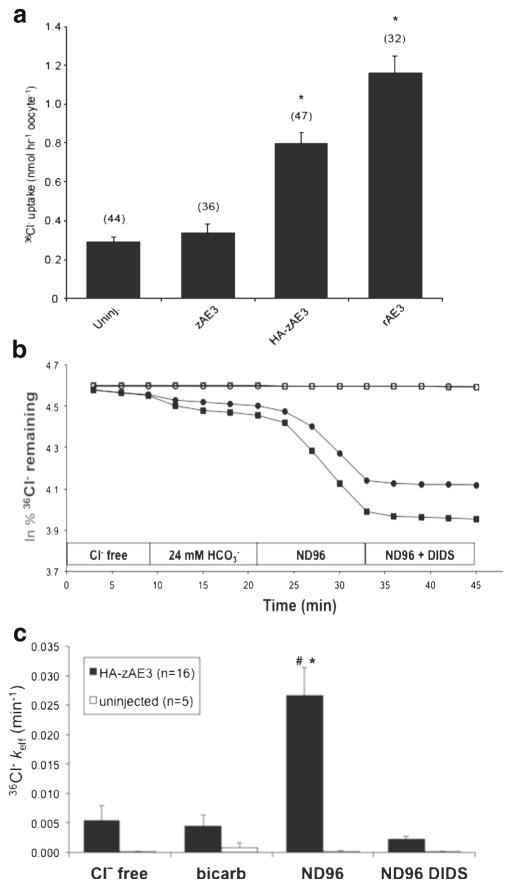
Fig. 5.
HA-tagged zAe3 mediates DIDS-sensitive Cl−/Cl− exchange. a. Mean 36Cl− influx into (n) uninjected (Uninj.) oocytes or oocytes previously injected with 50 ng of cRNA encoding zAe3, its HA-tagged variant (HA-zAe3), or rat Ae3 (rAe3). *, p<0.05 vs. zAe3 and uninjected. b. 36Cl− efflux from two representative uninjected oocytes from different frogs (open symbols, upper traces) and from two oocytes previously injected with cRNA encoding HA-zAe3 (filled symbols, lower traces) during sequential exposure to 96 mM Na cyclamate (Cl−-free), 72 mM Na cyclamate containing 24 mM NaHCO3 − (bicarb), 96 mM NaCl (ND-96), and ND-96 containing 200 μM DIDS. c. Efflux rate constants from 16 oocytes expressing HA-zAe3 and 5 uninjected oocytes subjected to the protocol shown in panel B. *, p<0.01 vs. uninjected in ND-96 (t-test); #, p<0.05 vs HA-zAe3-expressing oocytes in other bath conditions (ANOVA)
Cl− transport mediated by HA-zAe3 represented DIDS-sensitive Cl−/Cl− exchange as indicated by trans-anion dependence of 36Cl− efflux (Fig. 5b/c). The ability of HA-zAe3 to mediate Cl−/HCO3 − exchange (36Cl− efflux into 24 mM HCO3 −), although evident in some individual oocytes (Fig. 5b), was not reproducibly evident among multiple oocytes (Fig. 5c)
HA-zAe3 is inhibited by acidic pHi and by acidic pHo
HA-zAe3-associated Cl−/Cl− exchange activity was suppressed by pre-equilibration with 40 mM butyrate, known to elicit intracellular acidification of oocytes to pHi ~6.7 [31], but bath replacement of butyrate with cyclamate (at constant extracellular [Cl−]) restored DIDS-sensitive Cl−/Cl− exchange activity. Again, without the HA-tag, zAe3 lacked detectable activity in this assay (Fig. 6a/b). Since mouse AE2 and zebrafish Ae2a and Ae2b [28, 29] exhibit independent regulation by pHi and by pHo the response of HA-zAe3 to varied pHo was tested. As shown in Fig. 6c/d, DIDS-sensitive 36Cl−/Cl− exchange mediated by HA-zAe3 was suppressed at pHo of 5.0, but greatly activated at pHo 8.5. Even at this alkaline pHo, stimulation of 36Cl− efflux from oocytes expressing untagged zAe3 did not achieve statistical significance (Fig. 6c/d).
Fig. 6.
HA-zAe3-mediated Cl−/Cl− exchange is regulated by intracellular and by extracellular pH. a. 36Cl− efflux from a representative uninjected oocyte and from oocytes previously injected with 50 ng cRNA encoding zAe3 or HA-zAe3, during sequential exposure to ND-56 containing first 40 mM Na butyrate, then 40 mM Na cyclamate (0 butyrate), and finally 40 mM Na cyclamate plus 200 μM DIDS. b. Mean data for (n) oocytes subjected to the protocol in panel A. *, p<0.05 vs. zAe3 and uninjected. c. 36Cl− efflux from a representative uninjected oocyte and from oocytes previously injected with cRNA encoding zAe3 and HA-zAe3, during sequential exposure to ND-96 first at pH 5.0, then at pH 8.5, and finally at pH 8.5 in the presence of 200 μM DIDS. d. Mean data for (n) oocytes subjected to the protocol in panel C. *, p<0.05 vs. zAe3 and uninjected
HA-zAe3-mediated Cl−/Cl− exchange not stimulated by hypertonicity or NH4+
Since mouse AE2 and zebrafish Ae2a and Ae2b [28, 29] polypeptides are activated by hypertonicity and NH4+, HA-zAe3 was tested for responsiveness to these stimulatory conditions. However, as shown in Fig. 7, HA-zAe3 was insensitive to elevation of bath osmolarity from 212 to 400 mosM, and was also insensitive to exposure to 26 mM NH4+.
Fig. 7.
HA-zAe3-mediated 36Cl− efflux is insensitive to hypertonicity or NH4 +. a. 36Cl− efflux from a representative uninjected oocyte and from an oocyte previously injected with cRNA encoding HA-zAe3, during sequential exposure first to isotonic bath (212 mosM), then to hypertonic bath (400 mosM), and finally to hypertonic bath containing 200 μM DIDS. b. Mean data for (n) oocytes subjected to the protocol in panel A. *, p<0.05 and **, p<0.01 vs. uninjected. c. 36Cl− efflux from a representative uninjected oocyte and from an oocyte previously injected with cRNA encoding HA-zAe3, during sequential exposure first to 96 mM NaCl, then to 70 mM NaCl plus 26 mM NH4Cl, and finally to the same solution containing 200 μM DIDS. d. Mean data for (n) oocytes subjected to the protocol in panel C. *, p<0.05 vs. DIDS, and vs. corresponding uninjected oocytes
Discussion
Zebrafish ae3 is a single gene
Zebrafish slc4a3/Ae3 is encoded by a single slc4a3 gene, in contrast to the paired ae1a and ae1b genes, and the paired ae2a and ae2b genes. No additional ae3-like gene was recognized in the Zv9 genome, although a second ae3 gene likely existed for some time following the latest teleost genome duplication before it’s subsequent evolutionary loss. Single ae3 genes are also predicted in fugu (Takifugu ruprides) and in tilapia (Oreochromis niloticus). However, whereas tilapia harbors two predicted ae1 genes and two predicted ae2 genes, the much smaller fugu genome is predicted to contain only one gene each of ae1 and ae2. Thus, in multiple teleost species, the ae3 gene lacks identifiable ohnologs (paired genes arising from the teleost genomic duplication). Zebrafish ae3 also exhibits much lower synteny with human AE3 than do the zebrafish ae1 and ae2 ohnologs with their corresponding human genes. This low synteny may reflect or result from evolutionary transposition of the ae3 gene into intron 1 of the hdac4 gene.
The zebrafish ae3 gene is embedded entirely within intron 1 of the zebrafish hdac4 gene on chromosome 9. Two 5′-variant alternate slc4a3 transcripts encode a single Slc4a3 polypeptide that lacks ~61–68 N-terminal amino acids corresponding to those encoded by proximal exon 3 and exon 2 of mammalian and other teleost Ae3 polypeptides (Fig. 2). This truncation likely reflects the insertion of 13 distinct non-transposon repeat elements into exon 2 and intron 2. Zebrafish slc4a3 mRNA is widely expressed at low levels as detected by RT-PCR, but was detected by in situ hybridization only in fin buds of 36–72 hpf embryos. HA-tagged Slc4a3 polypeptide was expressed at or near the surface of Xenopus oocytes, in which it mediated low rates of DIDS-sensitive Cl−/Cl− exchange sensitive to regulation by pHi and pHo, but not by hypertonicity or NH4+.
Introns and repeat elements of the ae3 gene of zebrafish
The zebrafish genome contains 3-to-7 times more intronic sequence than the genomes of other teleosts. Indeed, zebrafish intronic DNA exceeds that of stickleback by 471 Mb. However, the zebrafish genome has at the same time fewer introns per gene than other fish. Thus, the introns of the zebrafish ae3 gene are much larger than those of the corresponding human and rodent genes (Supplemental Table 2), consistent with the higher prevalence of large introns in zebrafish than in 4 other sequenced teleosts [22]. The large introns of the ae3 gene are also consistent with its low expression, as high gene expression correlates on average with smaller intron size [8].
As much as 47–52% of zebrafish intronic sequence is made of up of repetitive elements. This is the highest fraction known among vertebrate genomes, for which the average is about 30 %. [17] [22, 26]. The zebrafish ae3 gene is marked at its 5′-end by a remarkable collection of 13 repeat elements, each distinct in sequence and length. These repeat elements contribute to limited exonic sequence, as well as extensive intronic sequence upstream of ae3 exon 3 (Fig. 2). Imperfect copies of zebrafish ae3 repeats I–XIII were represented hundreds of times in the zebrafish genome, and included two LTR-type repeats, eight DNA-type repeats, and three repeats that did not match currently annotated categories [17]. DNA-type repeats XII and XIII and unclassifed repeat X have undergone exonization to contribute to the two confirmed alternate 5′-UTRs of zebrafish ae3 mRNA. Repeat IV encodes the predicted, but still unverified, far N-terminal coding region of a hypothetical longer Ae3 polypeptide variant. Curiously, the same repeat element has inserted into the zebrafish cav 1.3b gene cacna1db, where it has undergone exonization to encode the first 10 amino acid residues of the pore subunit of one of the L-type Ca2+ channels (Fig. 2c,d).
We speculate that the zebrafish ae3 gene repeat element insertions have reduced Ae3 expression levels through attenuation of promoter activity and mRNA levels. The low functional activity demonstrated by all recombinant Ae3 polypeptides studied to date [11, 14, 38], ~≤10 % of the activities of Ae2 and Ae1 polypeptides, has been attributed to properties of the Ae3 transmembrane domain [11]. Skate Ae3 activity in Xenopus oocytes was similarly undetectable [14].
Pseudonization of the zebrafish ae3 gene might well be without serious consequence in the setting of maintained expression of duplicated ae2 and ae1 genes through the course of evolution. However, the zebrafish Zv9 genome contains only 154 pseudogenes, in contrast to 13,000 human pseudogenes. A remarkable 75 % of zebrafish pseudogenes are unprocessed, compared to only 22 % in human, reflecting the larger role of retrotransposition evident in human genome evolution (the zebrafish genome harbors no identified active transposable elements.) [17]. In any case, the low expression levels of ae3 transcript encompassing a long open-reading frame that encodes functional activity of Ae3 polypeptide detectable under some circumstances, do not allow the conclusion that zebrafish ae3 is a pseudogene.
Functional activity of zebrafish Ae3
Zebrafish HA-Ae3 trafficked to or near the surface of the Xenopus oocyte, but, the Cl−/Cl− exchange function of the untagged protein was not consistently demonstrable. However, N-terminal addition of the Pro-rich, anionic HA-antigen epitope tag led to reproducible functional expression in Xenopus oocytes. Although the mechanism remains unclear, the presence of the HA-tag at the N-terminus of zebrafish Ae3 may prevent polypeptide degradation, as has been reported for heterologous mammalian NKCC2 expression in cultured cells [6]. Alternatively, the acidic HA-tag may promote a conformational change that increases intrinsic activity and/or surface expression in Xenopus oocytes. Such a conformational change might partially compensate for the absence in oocytes of an important regulatory cofactor or Ae3-binding protein that faciliates Ae3 function in the zebrafish.
The tagged HA-zAe3 was not active enough to allow detection of Cl−/HCO3 − exchange activity in the Xenopus oocyte expression system. (Cl−/HCO3 − exchange activity as measured by 36Cl− efflux is slower than Cl−/Cl− exchange activity for all Na+-independent SLC4 polypeptides (AE1, AE2, AE3) tested to date in Xenopus oocytes). HA-zAe3-mediated Cl−/Cl− exchange was sensitive to inhibition by acidic pHo and by acidic pHi, properties shared by zebrafish Ae2 polypeptides. However, the sensitivity to stimulation by hypertonic bath solution and by NH4Cl characteristic of zebrafish Ae2a and AE2b [28, 29] were lacking in HA-zAe3. Despite this lack of Ae3 stimulation by NH4 +, zebrafish Ae3 shared with Ae2a and Ae2b a lack of inhibition by the acidic pHi that consistently accompanies bath NH4 + exposure of Xenopus oocytes. In this respect, Ae3 shares with Ae2 a response to the pHi changes elicited in the presence of NH4 + that differs from the responses to changes in pHi in the absence of NH4 +. The increased anion exchange activity of zebrafish Ae3 evident at pHo 8.5 suggests that future studies, especially those focused on inhibitory regulatory stimuli, might best be conducted at this alkaline pH. Meanwhile, the physiological roles of Ae3-mediated anion exchange in development and in the adult fish remain to be elucidated.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 DK43495 (SLA) and P30 DK34854 (Harvard Digestive Diseases Center to SLA), and DK070838 and HL032262 (BHP). FRR was a fellow of the Robert Bosch Foundation. CC was supported by the Cooley’s Anemia Foundation.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00424-014-1494-2) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare they have no conflict of interest.
Contributor Information
Boris E. Shmukler, Renal Division and Molecular and Vascular Medicine Division, Beth Israel Deaconess Medical Center; Department of Medicine, Harvard Medical School, 99 Brookline Ave. RN-380 F, Boston, MA 02215, USA
Fabian R. Reimold, Renal Division and Molecular and Vascular Medicine Division, Beth Israel Deaconess Medical Center; Department of Medicine, Harvard Medical School, 99 Brookline Ave. RN-380 F, Boston, MA 02215, USA. Department of Medicine, Mount Auburn Hospital, Harvard Medical School, Cambridge, MA 02138, USA
John F. Heneghan, Renal Division and Molecular and Vascular Medicine Division, Beth Israel Deaconess Medical Center; Department of Medicine, Harvard Medical School, 99 Brookline Ave. RN-380 F, Boston, MA 02215, USA
Caiyong Chen, Department of Medicine, Division of Hematology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Tianxun Zhao, Department of Medicine, Division of Hematology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, USA.
Barry H. Paw, Department of Medicine, Division of Hematology-Oncology, Boston Children’s Hospital, Harvard Medical School, Boston, MA 02115, USA
Seth L. Alper, Email: salper@bidmc.harvard.edu, Renal Division and Molecular and Vascular Medicine Division, Beth Israel Deaconess Medical Center; Department of Medicine, Harvard Medical School, 99 Brookline Ave. RN-380 F, Boston, MA 02215, USA
References
- 1.Al Moamen NJ, Prasad V, Bodi I, Miller ML, Neiman ML, Lasko VM, Alper SL, Wieczorek DF, Lorenz JN, Shull GE. Loss of the AE3 anion exchanger in a hypertrophic cardiomyopathy model causes rapid decompensation and heart failure. J Mol Cell Cardiol. 2011;50:137–146. doi: 10.1016/j.yjmcc.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alper SL. Molecular physiology and genetics of Na+−independent SLC4 anion exchangers. J Exp Biol. 2009;212:1672–1683. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alper SL. Familial renal tubular acidosis. J Nephrol. 2010;23(Suppl 16):57–76. [PubMed] [Google Scholar]
- 4.Alvarez BV, Gilmour GS, Mema SC, Martin BT, Shull GE, Casey JR, Sauve Y. Blindness caused by deficiency in AE3 chloride/bicarbonate exchanger. PLoS One. 2007;2:e839. doi: 10.1371/journal.pone.0000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amigo JD, Ackermann GE, Cope JJ, Yu M, Cooney JD, Ma D, Langer NB, Shafizadeh E, Shaw GC, Horsely W, Trede NS, Davidson AJ, Barut BA, Zhou Y, Wojiski SA, Traver D, Moran TB, Kourkoulis G, Hsu K, Kanki JP, Shah DI, Lin HF, Handin RI, Cantor AB, Paw BH. The role and regulation of friend of GATA-1 (FOG-1) during blood development in the zebrafish. Blood. 2009;114:4654–4663. doi: 10.1182/blood-2008-12-189910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benziane B, Demaretz S, Defontaine N, Zaarour N, Cheval L, Bourgeois S, Klein C, Froissart M, Blanchard A, Paillard M, Gamba G, Houillier P, Laghmani K. NKCC2 surface expression in mammalian cells: down-regulation by novel interaction with aldolase B. J Biol Chem. 2007;282:33817–33830. doi: 10.1074/jbc.M700195200. [DOI] [PubMed] [Google Scholar]
- 7.Brunati AM, Bordin L, Clari G, James P, Quadroni M, Baritono E, Pinna LA, Donella-Deana A. Sequential phosphorylation of protein band 3 by Syk and Lyn tyrosine kinases in intact human erythrocytes: identification of primary and secondary phosphorylation sites. Blood. 2000;96:1550–1557. [PubMed] [Google Scholar]
- 8.Castillo-Davis CI, Mekhedov SL, Hartl DL, Koonin EV, Kondrashov FA. Selection for short introns in highly expressed genes. Nat Genet. 2002;31:415–418. doi: 10.1038/ng940. [DOI] [PubMed] [Google Scholar]
- 9.Cheung JC, Reithmeier RA. Palmitoylation is not required for trafficking of human anion exchanger 1 to the cell surface. Biochem J. 2004;378:1015–1021. doi: 10.1042/BJ20030847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coury F, Zenger S, Stewart AK, Stephens S, Neff L, Tsang K, Shull GE, Alper SL, Baron R, Aliprantis AO. SLC4A2-mediated Cl-/HCO3– exchange activity is essential for calpain-dependent regulation of the actin cytoskeleton in osteoclasts. Proc Natl Acad Sci U S A. 2013;110:2163–2168. doi: 10.1073/pnas.1206392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujinaga J, Loiselle FB, Casey JR. Transport activity of chimaeric AE2-AE3 chloride/bicarbonate anion exchange proteins. Biochem J. 2003;371:687–696. doi: 10.1042/BJ20030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Mice with a targeted disruption of the AE2 Cl-/HCO3– exchanger are achlorhydric. J Biol Chem. 2004;279:30531–30539. doi: 10.1074/jbc.M403779200. [DOI] [PubMed] [Google Scholar]
- 13.Gawenis LR, Bradford EM, Alper SL, Prasad V, Shull GE. AE2 Cl-/HCO3– exchanger is required for normal cAMP-stimulated anion secretion in murine proximal colon. Am J Physiol Gastrointest Liver Physiol. 2010;298:G493–G503. doi: 10.1152/ajpgi.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guizouarn H, Musch MW, Goldstein L. Evidence for the presence of three different anion exchangers in a red cell. Functional expression studies in Xenopus oocytes. J Membr Biol. 2003;193:109–120. doi: 10.1007/s00232-002-2012-6. [DOI] [PubMed] [Google Scholar]
- 15.Hailey DW, Roberts B, Owens KN, Stewart AK, Linbo T, Pujol R, Alper SL, Rubel EW, Raible DW. Loss of Slc4a1b chloride/bicarbonate exchanger function protects mechanosensory hair cells from aminoglycoside damage in the zebrafish mutant persephone. PLoS Genet. 2012;8:e1002971. doi: 10.1371/journal.pgen.1002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hentschke M, Wiemann M, Hentschke S, Kurth I, Hermans-Borgmeyer I, Seidenbecher T, Jentsch TJ, Gal A, Hubner CA. Mice with a targeted disruption of the Cl-/HCO3– exchanger AE3 display a reduced seizure threshold. Mol Cell Biol. 2006;26:182–191. doi: 10.1128/MCB.26.1.182-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assuncao JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Eliott D, Threadgold G, Harden G, Ware D, Mortimer B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Urun Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberlander M, Rudolph-Geiger S, Teucke M, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nusslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang D, Karbach D, Passow H. Anion transport function of mouse erythroid band 3 protein (AE1) does not require acylation of cysteine residue 861. Biochim Biophys Acta. 1994;1194:341–344. doi: 10.1016/0005-2736(94)90317-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee YC, Yan JJ, Cruz SA, Horng JL, Hwang PP. Anion exchanger 1b, but not sodium-bicarbonate cotransporter 1b, plays a role in transport functions of zebrafish H+−ATPase-rich cells. Am J Physiol Cell Physiol. 2011;300:C295–C307. doi: 10.1152/ajpcell.00263.2010. [DOI] [PubMed] [Google Scholar]
- 20.Ma M, Jiang YJ. Jagged2a-notch signaling mediates cell fate choice in the zebrafish pronephric duct. PLoS Genet. 2007;3:e18. doi: 10.1371/journal.pgen.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meier S, Hubner CA, Groeben H, Peters J, Bingmann D, Wiemann M. Expression of anion exchanger 3 influences respiratory rate in awake and isoflurane anesthetized mice. J Physiol Pharmacol. 2007;58(Suppl 5):371–378. [PubMed] [Google Scholar]
- 22.Moss SP, Joyce DA, Humphries S, Tindall KJ, Lunt DH. Comparative analysis of teleost genome sequences reveals an ancient intron size expansion in the zebrafish lineage. Genome Biol Evol. 2011;3:1187–1196. doi: 10.1093/gbe/evr090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mudumana SP, Hentschel D, Liu Y, Vasilyev A, Drummond IA. odd skipped related1 reveals a novel role for endoderm in regulating kidney versus vascular cell fate. Development. 2008;135:3355– 3367. doi: 10.1242/dev.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paw BH, Davidson AJ, Zhou Y, Li R, Pratt SJ, Lee C, Trede NS, Brownlie A, Donovan A, Liao EC, Ziai JM, Drejer AH, Guo W, Kim CH, Gwynn B, Peters LL, Chernova MN, Alper SL, Zapata A, Wickramasinghe SN, Lee MJ, Lux SE, Fritz A, Postlethwait JH, Zon LI. Cell-specific mitotic defect and dyserythropoiesis associated with erythroid band 3 deficiency. Nat Genet. 2003;34:59–64. doi: 10.1038/ng1137. [DOI] [PubMed] [Google Scholar]
- 25.Prasad V, Bodi I, Meyer JW, Wang Y, Ashraf M, Engle SJ, Doetschman T, Sisco K, Nieman ML, Miller ML, Lorenz JN, Shull GE. Impaired cardiac contractility in mice lacking both the AE3 Cl-/HCO3– exchanger and the NKCC1 Na+−K+−2Cl-cotransporter: effects on Ca2+ handling and protein phosphatases. J Biol Chem. 2008;283:31303–31314. doi: 10.1074/jbc.M803706200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sela N, Kim E, Ast G. The role of transposable elements in the evolution of non-mammalian vertebrates and invertebrates. Genome Biol. 2010;11:R59. doi: 10.1186/gb-2010-11-6-r59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semon M, Wolfe KH. Reciprocal gene loss between Tetraodon and zebrafish after whole genome duplication in their ancestor. Trends Genet. 2007;23:108–112. doi: 10.1016/j.tig.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Shmukler BE, Kurschat CE, Ackermann GE, Jiang L, Zhou Y, Barut B, Stuart-Tilley AK, Zhao J, Zon LI, Drummond IA, Vandorpe DH, Paw BH, Alper SL. Zebrafish slc4a2/ae2 anion exchanger: cDNA cloning, mapping, functional characterization, and localization. Am J Physiol Ren Physiol. 2005;289:F835–F849. doi: 10.1152/ajprenal.00122.2005. [DOI] [PubMed] [Google Scholar]
- 29.Shmukler BE, Clark JS, Hsu A, Vandorpe DH, Stewart AK, Kurschat CE, Choe SK, Zhou Y, Amigo J, Paw BH, Alper SL. Zebrafish ae2.2 encodes a second slc4a2 anion exchanger. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1081–R1091. doi: 10.1152/ajpregu.00690.2007. [DOI] [PubMed] [Google Scholar]
- 30.Sidi S, Busch-Nentwich E, Friedrich R, Schoenberger U, Nicolson T. gemini encodes a zebrafish L-type calcium channel that localizes at sensory hair cell ribbon synapses. J Neurosci. 2004;24:4213–4223. doi: 10.1523/JNEUROSCI.0223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart AK, Chernova MN, Kunes YZ, Alper SL. Regulation of AE2 anion exchanger by intracellular pH: critical regions of the NH(2)-terminal cytoplasmic domain. Am J Physiol Cell Physiol. 2001;281:C1344–C1354. doi: 10.1152/ajpcell.2001.281.4.C1344. [DOI] [PubMed] [Google Scholar]
- 32.Stewart AK, Kurschat CE, Alper SL. Role of nonconserved charged residues of the AE2 transmembrane domain in regulation of anion exchange by pH. Pflugers Arch. 2007;454:373–384. doi: 10.1007/s00424-007-0220-8. [DOI] [PubMed] [Google Scholar]
- 33.Stewart AK, Kurschat CE, Burns D, Banger N, Vaughan-Jones RD, Alper SL. Transmembrane domain histidines contribute to regulation of AE2-mediated anion exchange by pH. Am J Physiol Cell Physiol. 2007;292:C909–C918. doi: 10.1152/ajpcell.00265.2006. [DOI] [PubMed] [Google Scholar]
- 34.Stewart AK, Kurschat CE, Vaughan-Jones RD, Alper SL. Putative re-entrant loop 1 of AE2 transmembrane domain has amajor role in acute regulation of anion exchange by pH. J Biol Chem. 2009;284:6126–6139. doi: 10.1074/jbc.M802051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart AK, Shmukler BE, Vandorpe DH, Reimold F, Heneghan JF, Nakakuki M, Akhavein A, Ko S, Ishiguro H, Alper SL. SLC26 anion exchangers of guinea pig pancreatic duct: molecular cloning and functional characterization. Am J Physiol Cell Physiol. 2011;301:C289–C303. doi: 10.1152/ajpcell.00089.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart AK, Alper SL. The SLC4 Anion Exchanger Gene Family. In: Alpern RJ, Hebert SC, editors. The Kidney: Physiology and Pathophysiology. Elsevier; New York: 2012. pp. 1861–1915. [Google Scholar]
- 37.Vilas GL, Johnson DE, Freund P, Casey JR. Characterization of an epilepsy-associated variant of the human Cl-/HCO3(−) exchanger AE3. Am J Physiol Cell Physiol. 2009;297:C526–C536. doi: 10.1152/ajpcell.00572.2008. [DOI] [PubMed] [Google Scholar]
- 38.Yannoukakos D, Stuart-Tilley A, Fernandez HA, Fey P, Duyk G, Alper SL. Molecular cloning, expression, and chromosomal localization of two isoforms of the AE3 anion exchanger from human heart. Circ Res. 1994;75:603–614. doi: 10.1161/01.res.75.4.603. [DOI] [PubMed] [Google Scholar]
- 39.Zhang D, Kiyatkin A, Bolin JT, Low PS. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood. 2000;96:2925–2933. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.