Abstract
Purpose.
We have shown that Acanthamoeba interacts with a mannosylated protein on corneal epithelial cells and stimulates trophozoites to secrete a mannose-induced 133 kDa protease (MIP-133), which facilitates corneal invasion and induces apoptosis. The mechanism of MIP-133–induced apoptosis is unknown. The aim of this study was to determine if MIP-133 induces apoptosis and proinflammatory cytokines/chemokines in human corneal epithelial (HCE) cells via the cytosolic phospholipase A2α (cPLA2α) pathway.
Methods.
HCE cells were incubated with or without MIP-133 at doses of 7.5, 15, and 50 μg/mL for 6, 12, and 24 hours. The effects of cPLA2α inhibitors on cPLA2α, arachidonic acid (AA) release, and apoptosis were tested in vitro. Inhibition of cPLA2α involved preincubating HCE cells for 1 hour with cPLA2α inhibitors (10 μM methyl-arachidonyl fluorophosphonate [MAFP] or 20 μM arachidonyl trifluoromethyl ketone [AACOCF3]) with or without MIP-133 for 24 hours. Expression of cPLA2α mRNA and enzyme was examined by RT-PCR and cPLA2 activity assays, respectively. Apoptosis of corneal epithelial cells was determined by caspase-3 and DNA fragmentation assays. Expression of IL-8, IL-6, IL-1β, and IFN-γ was examined by RT-PCR and ELISA.
Results.
MIP-133 induced significant cPLA2α (approximately two to four times) and AA release (approximately six times) from corneal cells while cPLA2α inhibitors significantly reduced cPLA2α (approximately two to four times) and AA release (approximately three times) (P < 0.05). cPLA2α inhibitors significantly inhibited MIP-133–induced DNA fragmentation approximately 7 to 12 times in HCE cells (P < 0.05). MIP-133 specifically activates cPLA2α enzyme activity in HCE cells, which is blocked by preincubation with anti–MIP-133 antibody. In addition, MIP-133 induced significant IL-8, IL-6, IL-1β, and IFN-γ production, approximately two to three times (P < 0.05).
Conclusions.
MIP-133 interacts with phospholipids on plasma membrane of HCE cells and activates cPLA2α. cPLA2α is involved in apoptosis, AA release, and activation of proinflammatory cytokines/chemokines from HCE cells. cPLA2α inhibitors may be a therapeutic target in Acanthamoeba keratitis.
Results indicate that MIP-133 released by the interaction of mannose with the mannose receptors of A. castellanii cell membrane interacts with phospholipids on corneal epithelium and induces apoptosis and proinflammatory cytokines through cPLA2α signaling.
Introduction
Acanthamoeba keratitis (AK) is a sight-threatening chronic inflammatory disease of the cornea caused by several species of free-living pathogenic amoebae.1,2 Disease symptoms of AK include a ring-like corneal infiltrate, epithelial destruction, and disproportionately severe ocular pain. Topical or systemic treatment of AK with antibiotics, antifungals, and antivirals is often ineffective.3–5 It has been shown that Acanthamoeba binds to the corneal surface by mannose-binding protein (MBP), which induces a cytopathic effect.6,7 We have demonstrated that the binding of Acanthamoeba to corneal epithelial cells induces release of the mannose-induced 133 kDa protease (MIP-133). MIP-133 affects the subsequent steps in the pathogenic cascade of AK, including the cytopathic effects on the corneal epithelium and the stroma, penetration of the basement membrane, and the dissolution of the collagenous stroma.1,8–10 MIP-133 protein was found to be effective at activating a caspase-3-dependent apoptosis pathway in corneal epithelial cells as well as in keratocytes.1,8 We demonstrated that unlike “amoebapores,” the Entamoeba histolytica cytolytic peptides, MIP-133 does not perforate the lipid bilayers to cause cell death.1,11 How the MIP-133 protein interacts with the cell surface to cause apoptosis is still unknown. Recently, it has been demonstrated that Pseudomonas aeruginosa induces apoptosis in human lung fibroblasts and human conjunctiva epithelial cell lines through the activation of cytosolic phospholipase A2 (cPLA2) and arachidonic acid (AA) release via a contact-dependent mechanism.12 It is known that MIP-133 induces apoptosis upon contact with corneal cells1,8; however, the cytopathic signaling involved with this interaction is unknown. We hypothesized that cPLA2 is involved in apoptosis of corneal epithelial cells induced by MIP-133. PLA2 enzymes are divided into four major families: platelet-activating factor acetylhydrolases (PAF-AHs); secreted PLA2s (sPLA2s); intracellular Ca2+-independent PLA2s (iPLA2s); and cytosolic Ca2+-dependent PLA2s (cPLA2s). cPLA2s are classified into five subgroups, α through ζ.13–15 cPLA2α has been studied comprehensively because it is the only PLA2 that exhibits specificity for hydrolysis of sn-2 AA from phospholipids for eicosanoid biosynthesis in response to a wide variety of extracellular stimuli,16,17 and is regulated by phosphorylation and an increase in intracellular calcium.13 Phosphorylation of cPLA2α by mitogen-activated protein kinases (MAPKs) is required for cPLA2α-mediated release of AA in stimulated cells.16,17 Previous studies demonstrated the dual role of PLA2s in several eye diseases, which may be related to their enzymatic activities or to regulatory functions including signaling and protein–protein interactions.18 AA is one of the biologically important free fatty acids released by cPLA2α, which subsequently converts to prostanoids and leukotrienes stimulating apoptosis through activation of the mitochondrial pathway. The release of AA by the activation of cPLA2α in cells induced to undergo apoptosis is associated with loss of cell viability, caspase activation, and DNA fragmentation.14 The present study addressed the role of MIP-133 in the induction of apoptosis and proinflammatory cytokines due to AA accumulation by the cPLA2α pathway. Here, we demonstrate that MIP-133–induced apoptosis of human corneal epithelial (HCE) cells is associated with an increase in cPLA2α activity and increases the levels of cPLA2α, AA, and proinflammatory cytokines/chemokines.
Materials and Methods
Amoebae and Human Cell Lines
Acanthamoeba castellanii (ATCC 30,868), isolated from a human cornea, was obtained from the American Type Culture Collection (ATCC, Manassas, VA). Amoebae were grown as axenic cultures in peptone yeast extract glucose at 35°C with constant agitation on a shaker incubator at 125 rpm.19 Human telomerase-immortalized corneal epithelial (HCE) cells were a generous gift from James Jester (University of California-Irvine, Irvine, CA). HCE cells were cultured in keratinocyte medium (KGM-2 Bullet Kit; Lonza, Walkersville, MD) containing 10% fetal bovine serum (HyClone Laboratories, Inc., Logan, UT) at 37°C in a humidified 5% CO2 atmosphere.
Mouse Corneal Epithelial Cell Cultures
Fas-deficient (B6.MRL-Tnfrsf6lpr, H-2b) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were handled in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Cell cultures were established from freshly dissected corneal explants as described previously20 and cultured in minimum essential medium (MEM) containing 2 mM L-glutamine, 1 mM sodium pyruvate, 2 mM MEM vitamins, and 1% penicillin–streptomycin–fungizone solution (BioWhittaker; Lonza) and 10% heat-inactivated FBS (HyClone Laboratories, Inc.).
MIP-133
The MIP-133 protein was isolated and characterized as reported previously,21 and protein concentrations were determined using the bicinchoninic acid (BCA) protein assay.22
Caspase-3 Assay for Apoptosis
Corneal epithelial cells from Fas receptor–deficient mice (lpr/lpr) were cultured in 24-well plates at ∼90% confluence and incubated with and without MIP-133 for 24 hours at a dose of 15 μg/mL. Cells were collected by centrifugation at 2000g for 10 minutes at 4°C and used for apoptosis assay. Briefly, cells were washed three times in Hanks' balanced salt solution (HBSS) and resuspended in cytofix/cytoperm solution (permeabilization buffer; BD Biosciences Pharmingen, San Diego, CA), then washed in Perm-Wash buffer (BD Biosciences Pharmingen). Cells were stained with phycoerythrin (PE)-labeled rabbit anti-caspase-3 antibody (BD Biosciences Pharmingen) for 20 minutes in the dark. Active anti-caspase-3 antibody detects the active form of the enzyme that occurs in apoptotic cells. The samples were analyzed by FACS Scan flow cytometry (BD Biosciences, Franklin Lakes, NJ). As a positive control of apoptosis, the cells were treated with 3 μg/mL staurosporine (Sigma Chemical Co., St. Louis, MO), and apoptosis was determined by caspase-3 assay. For each sample, 5000 to 10,000 ungated events were acquired and the results were analyzed with CellQuest Software (BD Biosciences, San Jose, CA). Isotype control IgG was used as a control antibody. The results were expressed as the percentage of cells that stained positively with anti-caspase-3 antibody.
HCE Cell Cultures and Treatment Experiments
HCE cells were cultured in 24-well plates at ∼90% confluence in KGM-2 medium and incubated with or without MIP-133 at doses of 7.5, 15, and 50 μg/mL for 6, 12, and 24 hours. Inhibition of cPLA2α involved preincubating HCE cells for 1 hour with cPLA2α inhibitors (10 μM methyl-arachidonyl fluorophosphonate12 [MAFP; Cayman Chemical Company, Ann Arbor, MI] or 20 μM arachidonyl trifluoromethyl ketone12,23 [AACOCF3; Enzo Life Sciences, Inc., Farmingdale, NY]) or with the negative control compound arachidonyl methyl ketone (20 μM AACOCH3; BIOMOL Research Laboratories, Inc., Plymouth Meeting, PA), followed by incubation with or without 15 μg/mL MIP-133 for 24 hours. The inhibitors were dissolved in dimethyl sulfoxide (DMSO; Fisher BioReagents, Fair Lawn, NJ). HCE cells incubated with KGM-2 medium in each experiment without treatment with MIP-133 and cPLA2α inhibitors served as control untreated group. Cells and supernatants were collected by centrifugation at 2000g for 10 minutes at 4°C.
Isolation of RNA and RT-PCR
HCE cells were collected from 24-well plates at the indicated times after treatments. Total cellular RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The concentration of total RNA and RNA integrity were determined by measurement of the absorbance at 260 nm and 280 nm and by agarose gel electrophoresis, respectively. cDNA was synthesized from 2 μg total RNA by RT-PCR using random primers (High Capacity cDNA Reverse Transcription Kit; Applied Biosystems, Foster City, CA). PCR was performed using AmpliTaq Gold PCR Master Mix (Applied Biosystems). The amplification profile included one cycle of initial denaturation at 94°C for 5 minutes, 40 cycles of denaturation at 94°C for 1 minute, primer annealing at 60°C for 1 minute, and extension at 72°C for 1 minute, and subsequently, one cycle of final extension at 72°C for 10 minutes. The mRNA expression of each gene was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal control.
The oligonucleotide primers for cPLA2α, IL-8, IL-6, IL-1β, IFN-γ, and GAPDH were as follows:
cPLA2α (450 bp):
5′-GAGTTTTGGGCGTTTCTGGT-3′ (sense)
5′-ACGGCAGGTTAAATGTGAGC-3′ (anti-sense)
IL-8 (289 bp):
5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ (sense)
5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ (anti-sense)
IL-6 (200 bp):
5′-ATGAACTCCTTCTCCACAAGCGC-3′ (sense)
5′-GAAGAGCCCTCAGGCTGGACTG-3′ (anti-sense)
IL-β (204 bp):
5′-CCTGTGGCCTTGGGCCTCAA-3′ (sense)
5′-GGTGCTGATGTACCAGTTGGG-3′ (anti-sense)
IFN-γ (427 bp):
5′-GCATCGTTTTGGGTTCTCTTGGCTGTTACTGC-3′ (sense)
5′-CTCCTTTTTCGCTTCCCTGTTTTAGCTGCTGG-3′ (anti-sense)
GAPDH (450 bp):
5′-ACCACAGTCCATGCCATCAC-3′ (sense)
5′-TCCACCACCCTGTTGCTGTA-3′ (anti-sense)
All primers were verified by BLAST (Basic Local Alignment Search Tool; in the public domain, http://blast.ncbi.nlm.nih.gov/Blast.cgi) search of the National Center for Biotechnology Information database for specificity to the human genes of interest.
cPLA2α Activity in Cell Lysates
HCE cells were collected from 24-well plates at the indicated times after treatments and then centrifuged at 2000g for 10 minutes at 4°C. The cell pellets were washed with phosphate-buffered saline (PBS) by centrifugation at 2000 rpm for 5 minutes and then homogenized by Tissue-Tearor (Biospec Products, Inc., Bartlesville, OK) with its specific procedure in 1 mL cold buffer (i.e., 50 mM HEPES [Mediatech, Inc., Manassas, VA], pH 7.4, containing 1 mM EDTA [VWR International, LLC, West Chester, PA]). Cell lysates were collected by centrifugation at 10,000g for 15 minutes at 4°C. cPLA2α activity was measured using the PLA2 substrate arachidonoyl thio-phosphatidylcholine according to the protocol recommended by the manufacturer (Cayman Chemical Company). The absorbance was measured at 414 nm in a microplate reader (Gen51.10; BioTek Instruments, Inc., Winooski, VT). cPLA2α activity was expressed in nanomoles/milligram of protein/minute determined from the extinction coefficient of 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB) as instructed in the cPLA2 assay kit (Cayman Chemical Company), and the protein content in the supernatants was quantified using the BCA protein assay.22 Sensitivity of the activity assay was 3.5 to 42 nmol/min/mL without sample dilution.
Anti–MIP-133 Antibody Treatment
HCE cells were cultured in 24-well plates at ∼90% confluence in KGM-2 medium. Inhibition of experiments involved preincubating HCE cells for 1 hour with either 1:75 diluted chicken anti–MIP-133 antiserum (Aves Labs, Inc., Tigard, OR) or chicken preimmune serum control followed by incubation with or without 15 μg/mL MIP-133 for 24 hours. Additional control wells contained untreated confluent cells. Following incubation, all wells were washed three times with their respective growth medium. Cells were collected from 24-well plates and then centrifuged at 2000g for 10 minutes at 4°C. The cell pellets were washed with PBS, and cPLA2α activity was measured as described above.
AA Release Assay
HCE cells (1 × 105 cells/mL) were added to 24-well plates and then labeled for 18 hours with 0.05 μCi/mL [3H]arachidonic acid [5,6,8,9,11,12,14,15-3H(N)] (New England Nuclear, Boston, MA) as described previously.12 Prior to stimulation, cells were washed with KGM-2 medium without serum. Cells were preincubated for 1 hour with cPLA2α inhibitors (10 μM MAFP or 20 μM AACOCF3) and then incubated for 24 hours with or without 15 μg/mL MIP-133. To determine the release of extracellular AA, supernatants were collected after 24-hour incubation and centrifuged at 2000g for 5 minutes in a microcentrifuge to remove any cells that may have detached. The released radioactivity in the supernatants was evaluated by liquid scintillation counting. Maximum release values were obtained by quantitating the counts per minute (CPM) present in both the supernatants and the lysed cellular pellet remaining in the wells via addition of 0.5 mL 0.1% Triton X-100 in H2O. The spontaneous release of AA from radiolabeled cells was determined by incubating the radiolabeled cells in medium alone. The percent specific release of radiolabel of extracellular AA release was calculated as described previously.12
Apoptosis Assessment by DNA Fragmentation Assay
Apoptosis was determined by Cellular DNA Fragmentation ELISA (Cell Death Detection ELISAPlus kit; Roche Diagnostics GmbH, Mannheim, Germany) as described previously.24 In brief, HCE cells (1 × 105 cells in 200 μL supplemented KGM-2 medium) were preincubated for 1 hour with or without cPLA2α inhibitors (10 μM MAFP or 20 μM AACOCF3) or 20 μM AACOCH3 (a negative control compound) and then incubated for 24 hours with or without 15 μg/mL MIP-133. Cells were centrifuged for 10 minutes at 200g, and the cell pellets were incubated with lysis buffer supplied by the manufacturer for 30 minutes at room temperature. Twenty microliters of the supernatant (cytoplasmic fraction) was used in the Cellular DNA Fragmentation ELISA following the manufacturer's standard protocol. Absorbances were measured at 405 nm and 490 nm (reference wavelength) using a microplate reader (Gen51.10; BioTek Instruments, Inc.). Signals in the wells containing the substrate only were subtracted as background. The results were expressed in absorbance (A405nm − A490nm).
ELISA
Cytokines/chemokines (IL-8, IL-6, IL-1β, and IFN-γ) were quantified from cell supernatants using ELISA. Briefly, cell culture supernatants were collected at the indicated times after treatments and centrifuged to remove cell debris. Total protein concentrations of supernatants were determined by BCA protein assay.22 Levels of IL-8, IL-6, IL-1β, and IFN-γ were determined using specific ELISA test kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The absorbance was measured at 450 nm using a microplate reader (Gen51.10; BioTek Instruments, Inc.). The minimum detectable levels of IL-8, IL-6, IL-1β, and IFN-γ by ELISA were 3.5 pg/mL, >0.70 pg/mL, >1 pg/mL, and >8.0 pg/mL, respectively. The results were expressed in pg/mg protein of IL-8, IL-6, IL-1β, and IFN-γ.
Statistics
All experiments were performed in triplicate, and results are presented as mean ± SEM. Differences between two groups were determined by unpaired Student's t-test. Differences between multiple groups were determined by two-way analysis of variance (ANOVA). In all analyses, P < 0.05 was considered statistically significant.
Results
MIP-133 Induces Apoptosis in Corneal Epithelial Cells of Fas Receptor–Deficient Mice (lpr/lpr)
We have shown that MIP-133 induces apoptosis in human and Chinese hamster corneal epithelial cells.21 To determine the involvement of Fas/Fas ligand interactions in MIP-133–induced apoptosis in corneal cells, corneal epithelial cells of Fas receptor–deficient mice were treated with and without MIP-133, and apoptosis was detected by caspase-3 assay. MIP-133–treated corneal epithelial cells displayed a significant (P < 0.05) increase in apoptosis over the level in untreated cells. Staurosporine (positive control) induced a significant (P < 0.05) increase in apoptosis of the corneal epithelial cells (Table). These results indicate that MIP-133 induces apoptosis in corneal epithelial cells without involvement of Fas/Fas ligand interactions.
Table. .
Percent Apoptosis of Corneal Epithelial Cells of Fas Receptor–Deficient Mice
|
Groups |
Mean ± SEM |
P value* |
| Untreated corneal epithelial cells | 7 ± 3 | |
| MIP-133–treated corneal epithelial cells | 67 ± 8 | 0.011 |
| Staurosporine-treated corneal epithelial cells | 82 ± 11 | 0.04 |
Corneal epithelial cells from Fas receptor–deficient mice (lpr/lpr) were cultured in 24-well plates (1 × 106 cells/well) with and without MIP-133 for 24 hours, and apoptosis was determined by caspase-3 assay. As a positive control of apoptosis, the cells were treated with 3 μg/mL staurosporine. The data are mean ± SEM of three independent experiments.
P value < 0.05 by unpaired Student's t-test for treated corneal epithelial cells versus untreated corneal epithelial cells.
MIP-133 Upregulates cPLA2α mRNA Expression and cPLA2α Activity in HCE Cells
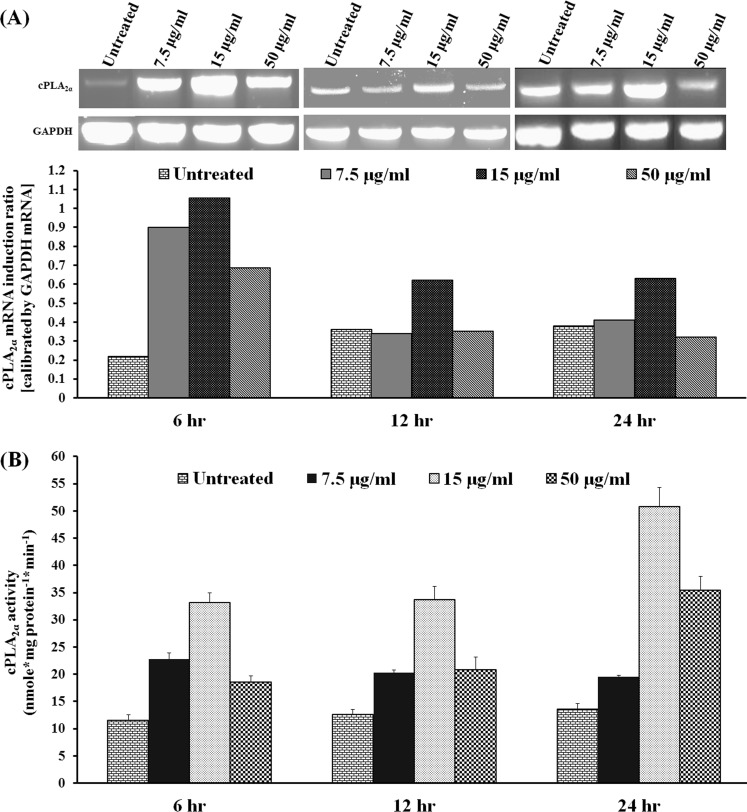
To determine if MIP-133 activates phospholipids on corneal epithelial cells, HCE cells were treated with different doses of MIP-133, and expression of cPLA2α was detected by RT-PCR. MIP-133 upregulated cPLA2α mRNA expression at doses of 7.5, 15, and 50 μg/mL after 6 hours of treatment. This upregulation was observed at the dose of 15 μg/mL at both 12 and 24 hours (Fig. 1A). cPLA2α enzyme activity was determined in cell lysates. All three doses of MIP-133 significantly (P < 0.05) increased cPLA2α activity at 6, 12, and 24 hours after incubation (Fig. 1B). These results indicate that MIP-133 induced upregulation of cPLA2α at mRNA and protein levels.
Figure 1. .
MIP-133 upregulates cPLA2α in HCE cells. HCE cells were exposed to MIP-133 at doses of 7.5, 15, and 50 μg/mL for 6, 12, and 24 hours, and then total RNA was isolated for RT-PCR. The amount of mRNA expression was quantified by densitometry of bands in comparison to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (A). HCE cells were homogenized, and then cell lysates were used to perform cPLA2α enzyme analysis (B). The data are mean ± SEM of three independent experiments. P values < 0.05 were obtained by ANOVA.
cPLA2α Upregulation by MIP-133 Is Diminished by cPLA2α Inhibitors in HCE Cells
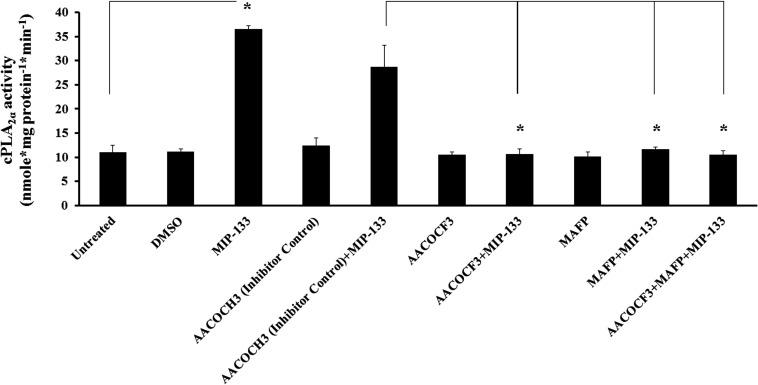
To determine if MIP-133 activates phospholipids in HCE cells via the cPLA2α pathway, the effect of various cPLA2α inhibitors on cPLA2α activity was examined by enzyme assays. cPLA2α activity increased significantly after MIP-133 treatment. Moreover, cPLA2α inhibitors AACOCF3 and MAFP alone or in combination significantly inhibited MIP-133–induced cPLA2α activity (P < 0.05). The inactive control compound AACOCH3 did not inhibit MIP-133–induced cPLA2α activity (Fig. 2). These results indicate that MIP-133 activates cPLA2α enzyme activity in HCE cells, which is blocked by preincubation with cPLA2α inhibitors.
Figure 2. .
Effect of cPLA2α inhibitors (AACOCF3 and MAFP) on MIP-133–induced cPLA2α enzyme activity in HCE cells. HCE cells were preincubated for 1 hour with cPLA2α inhibitors (10 μM MAFP or 20 μM AACOCF3) or an inactive negative control (20 μM AACOCH3), and then incubated with or without 15 μg/mL MIP-133 for 24 hours. Induction of cPLA2α enzyme activity was examined using a cPLA2 assay kit. The data are mean ± SEM of three independent experiments. Asterisk indicates P value < 0.05 by unpaired Student's t-test.
Anti–MIP-133 Antibody Attenuates cPLA2α Activity Induced by MIP-133 in HCE Cells
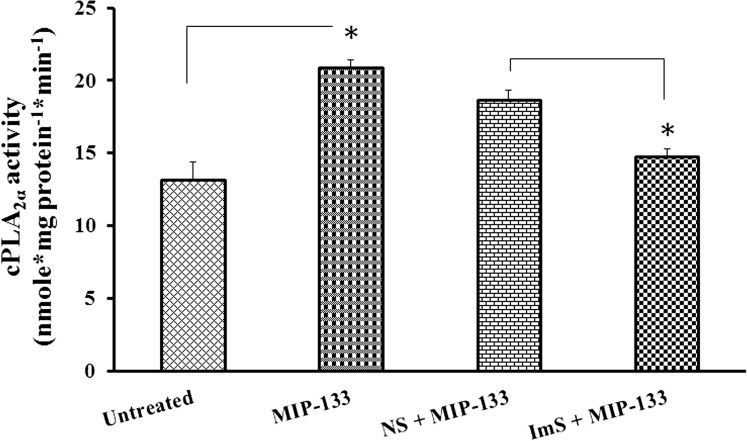
To determine if MIP-133 activates phospholipids in HCE cells via the specific cPLA2α pathway, the effect of chicken anti–MIP-133 antibody on cPLA2α activity was examined by enzyme assays. Previously, it has been demonstrated that anti–MIP-133 antiserum (Aves Labs, Inc.) inhibits cytopathic effects (CPEs) against HCE cells in vitro, and its specific binding to the MIP-133 protein was revealed through Western blot analysis and ELISA.1 We observed that cPLA2α activity increased significantly after MIP-133 treatment (P < 0.05). Moreover, chicken anti–MIP-133 antiserum significantly inhibited MIP-133–induced cPLA2α activity (P < 0.05). The chicken preimmune normal serum did not inhibit MIP-133–induced cPLA2α activity (Fig. 3). These results indicate that MIP-133 specifically activates cPLA2α enzyme activity in HCE cells, which is blocked by preincubation with chicken anti–MIP-133 antiserum.
Figure 3. .
Effect of anti–MIP-133 antiserum (ImS) on MIP-133–induced cPLA2α enzyme activity in HCE cells. HCE cells were preincubated for 1 hour with chicken anti–MIP-133 antiserum (ImS, 1:75 dilution) or chicken preimmune normal serum control (NS, 1:75 dilution), and then incubated with or without 15 μg/mL MIP-133 for 24 hours. Induction of cPLA2α enzyme activity was examined using a cPLA2 assay kit. The data are mean ± SEM of three independent experiments. Asterisk indicates P value < 0.05 by unpaired Student's t-test.
MIP-133–Induced AA Release Is Diminished by cPLA2α Inhibitors in HCE Cells
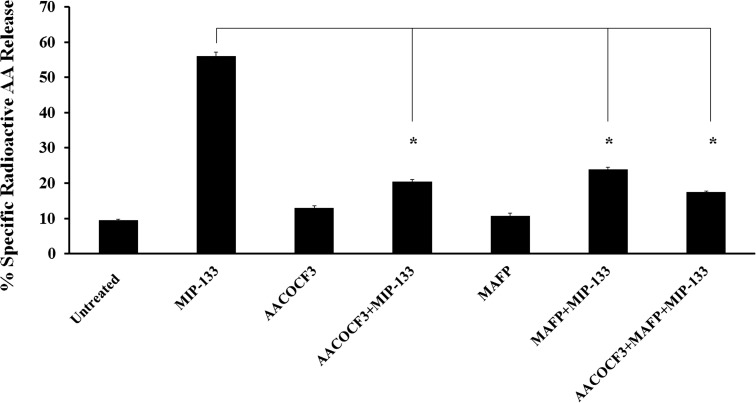
To determine whether cPLA2α is involved in MIP-133–induced extracellular AA release from HCE cells, we tested the effect of various inhibitors on AA release. HCE cells were incubated with 15 μg/mL MIP-133, and AA release was detected after 24 hours of stimulation. cPLA2α inhibitors AACOCF3 and MAFP significantly (P < 0.05) reduced AA release induced by MIP-133 from HCE cells (Fig. 4). The results suggest that cPLA2α pathway is involved in AA release.
Figure 4. .
Effect of cPLA2α inhibitors on MIP-133–induced AA release from HCE cells. The HCE cells were preincubated for 1 hour with cPLA2α inhibitors (10 μM MAFP or 20 μM AACOCF3) and then incubated with or without 15 μg/mL MIP-133 for 24 hours. AA release by MIP-133 treatment was determined by liquid scintillation counting. The data are mean ± SEM of three independent experiments. Asterisk indicates P value < 0.05 by unpaired Student's t-test.
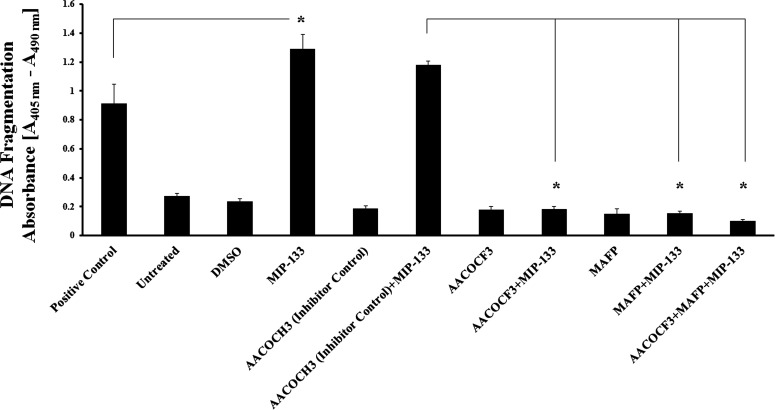
cPLA2α Inhibitors Reduce MIP-133–Induced Apoptosis in HCE Cells
We aimed to gain insight into the functional role of AA release from HCE cells after MIP-133–induced cPLA2α activity; therefore we studied apoptosis in HCE cells after stimulation with MIP-133. Treatment of HCE cells with MIP-133 induced significant (P < 0.05) apoptosis on HCE cells compared to untreated control cells (Fig. 5). cPLA2α inhibitors AACOCF3 and MAFP significantly (P < 0.05) inhibited this MIP-133–induced apoptosis. In contrast, the inactive control compound AACOCH3 did not block apoptosis induced by MIP-133. The results indicate that cPLA2α is involved in MIP-133–mediated apoptosis of HCE cells.
Figure 5. .
MIP-133–induced apoptosis is blocked by cPLA2α inhibitors in HCE cells. HCE cells were preincubated for 1 hour with cPLA2α inhibitors (10 μM MAFP or 20 μM AACOCF3) or an inactive negative control (20 μM AACOCH3), and then incubated with or without 15 μg/mL MIP-133 for 24 hours. Apoptosis was examined by Cell Death Detection ELISAPlus. MIP-133–induced apoptosis was confirmed by a positive control provided in the apoptosis kit. The data are mean ± SEM of three independent experiments. Asterisk indicates P value < 0.05 by unpaired Student's t-test.
MIP-133 Upregulates Proinflammatory Cytokines in HCE Cells
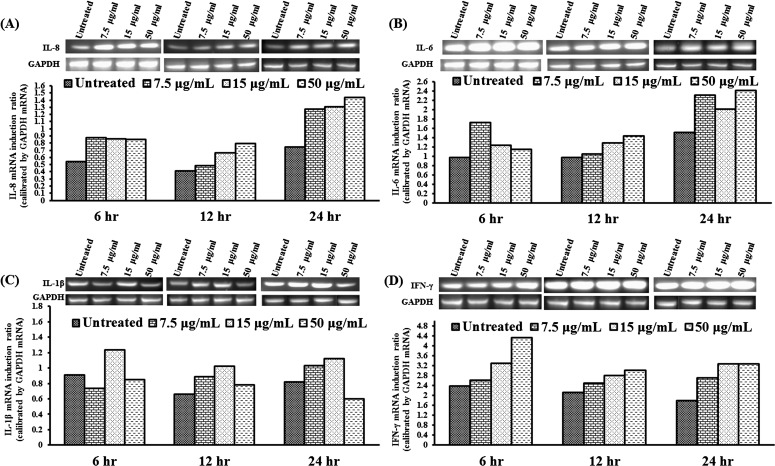
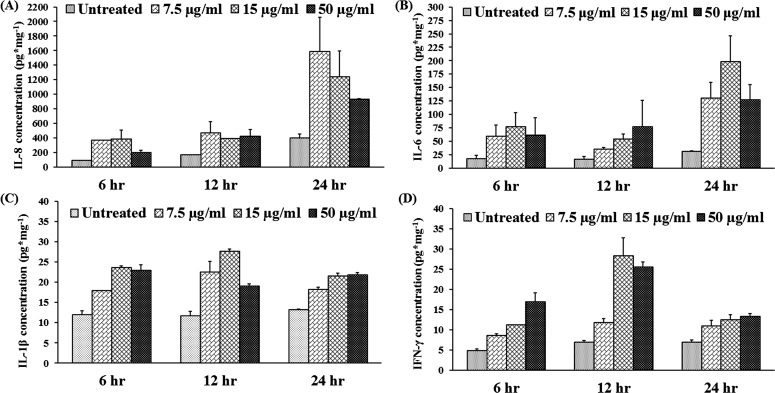
We have shown that MIP-133 plays an important role in the pathogenicity of AK by inducing cytolysis of the corneal cells and stimulating the accumulation of polymorphonuclear neutrophils (PMN) in the cornea (Alizadeh H, et al. IOVS 2011;52:ARVO E-Abstract 5795). Several studies demonstrated the expression of proinflammatory cytokines in HCE cells after inflammatory stimuli.25–35 We hypothesized that MIP-133 interacts with the corneal epithelial cells through activation of the cPLA2α pathway, leading to the production of cytokines and chemokines and recruitment of neutrophils into the cornea. To determine the expression and production of cytokines in cultured corneal cells, cells and supernatants were collected at indicated times after MIP-133 stimulation. The cells and supernatants were subjected to RT-PCR and ELISA for detection of IL-8, IL-6, IL-1β, and IFN-γ mRNA (Fig. 6) and protein (Fig. 7). Unstimulated HCE cells constitutively expressed low levels of IL-8, IL-6, IL-1β, and IFN-γ mRNA. MIP-133 (7.5–50 μg/mL) significantly increased mRNA and protein expression (P < 0.05) of these cytokines at all three doses and all three time points (6, 12, and 24 hours).
Figure 6. .
(A–D) MIP-133 upregulates IL-8 (A), IL-6 (B), IL-1β (C), and IFN-γ (D) mRNA in HCE cells. HCE cells were exposed to MIP-133 at doses of 7.5, 15, and 50 μg/mL for 6, 12, and 24 hours, and were then processed for total RNA isolation and RT-PCR analysis. The amount of mRNA expression was quantified by densitometry of bands in comparison to GAPDH. The experiment was repeated three times.
Figure 7. .
(A–D) MIP-133 induces IL-8 (A), IL-6 (B), IL-1β (C), and IFN-γ (D) protein expression in HCE cells. HCE cells were exposed to MIP-133 at doses of 7.5, 15, and 50 μg/mL for 6, 12, and 24 hours. Supernatants were collected from harvested cells and subjected to cytokine-specific ELISA. The data are mean ± SEM of three independent experiments. P values < 0.05 were obtained by two-way analysis of variance (ANOVA).
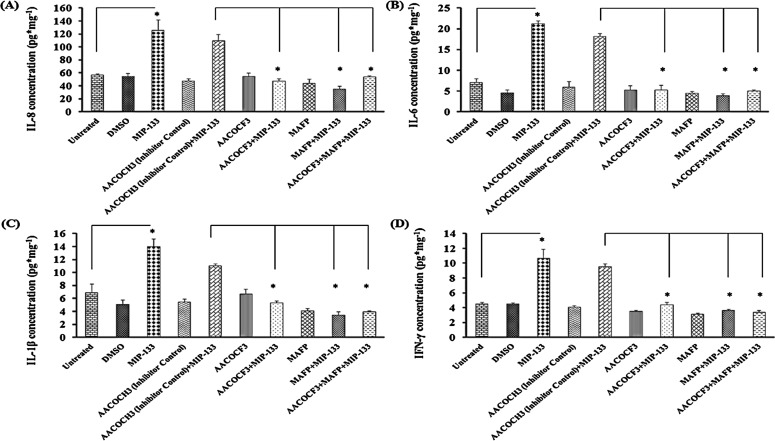
Proinflammatory Cytokine Induction by MIP-133 Is Diminished by cPLA2α Inhibitors
To determine whether cPLA2α is involved in MIP-133–induced proinflammatory cytokine release from HCE cells, the effect of various cPLA2α inhibitors on the production of IL-8, IL-6, IL-1β, and IFN-γ was tested in vitro. HCE cells were preincubated with the cPLA2α inhibitors MAFP or AACOCF3 and then incubated with 15 μg/mL MIP-133. IL-8, IL-6, IL-1β, and IFN-γ production was measured by ELISA 24 hours after MIP-133 stimulation. AACOCH3 was used as an inactive negative control. IL-8, IL-6, IL-1β, and IFN-γ production was increased significantly (P < 0.05) after MIP-133 stimulation (Fig. 8). MAFP and AACOCF3 significantly inhibited MIP-133–induced proinflammatory cytokine production (P < 0.05). However, the combination of MAFP and AACOCF3 did not have an additive effect, most likely because each inhibitor alone reduced cytokine expression to basal levels. These results indicate that cPLA2α is involved in MIP-133–induced proinflammatory cytokine production in HCE cells.
Figure 8. .
(A–D) MIP-133–induced upregulation of proinflammatory cytokines is blocked by cPLA2α inhibitors in HCE cells. Inhibition of cPLA2α involved preincubating HCE cells for 1 hour with cPLA2α inhibitors (10 μM MAFP or 20 μM AACOCF3) or an inactive control (20 μM AACOCH3), then incubating with or without 15 μg/mL MIP-133 for 24 hours. Cytokine production was examined by ELISA. The data are mean ± SEM of three independent experiments. Asterisk indicates P value < 0.05 by unpaired Student's t-test.
Discussion
In the present study, MIP-133 induced apoptosis in corneal epithelial cells of Fas receptor–deficient mice (lpr/lpr), which suggests that Fas receptors are not involved in MIP-133–induced apoptosis. How the MIP-133 protein interacts with the cell surface to cause apoptosis is still unknown. We investigated the role of cPLA2α in MIP-133–induced apoptosis of HCE cells. cPLA2α is a family of lipolytic enzymes that hydrolyze membrane phospholipids and cause release of fatty acids from glycophospholipids, particularly AA,13–15,17 a precursor of eicosanoids that are bioactive lipid molecules involved in numerous inflammatory processes.15,16 Our results indicate that MIP-133 interacts with phospholipids on HCE cells to activate cPLA2α, induce AA release, and employ this cPLA2α pathway to induce apoptosis of HCE cells. We have shown that specific inhibitors of cPLA2α enzymes (AACOCF3 and MAFP) and chicken anti–MIP-133 antiserum block MIP-133 induction of cPLA2α activity and inhibit MIP-133–mediated apoptosis of HCE cells. Although it is theoretically possible that induction of apoptosis and AA release is due to MIP-133 expression of cPLA2α activity, no cPLA2α activity was detected in our purified MIP-133 preparation (data not shown). Our previous findings of MIP-133 cytopathic activity suggested that MIP-133 is a serine protease.9,36 cPLA2α is activated by inflammation and oxidative stimuli by undergoing phosphorylation at amino acid residue serine-505 to become its soluble form, or by Ca2+ and MAPK phosphorylation to form p-cPLA2α, which translocates to the plasma membrane.37–39 However, during apoptosis, the cell membrane's phospholipid asymmetry changes, and phosphatidylserine is exposed on the outer membrane.40 Thus, our future targets on the basis of this study are to explore whether the cPLA2α upregulation mechanism by MIP-133 serine protease is due to phosphorylation of MAPKs to induce the translocation of cPLA2α in HCE cells, or due to the interaction of MIP-133 serine protease with phosphatidylserine on the outer membrane of HCE cells to induce cPLA2α.
Our current studies are largely in agreement with those of Kirschnek and Gulbins,12 who reported a role for PLA2 in Pseudomonas aeruginosa–induced apoptosis of conjunctiva epithelial cells and human lung fibroblast cell lines. In addition, P. aeruginosa produces several destructive proteins that associate with different ocular damage by the PLA2 pathway and cause cell death.41 In P. aeruginosa infection the induction of cPLA2 leading to AA release, cytokine production, and apoptosis could be due to the spatial reorganization of PLA2 and the metabolism of ceramide to ceramide-1-phosphate.12,41
We have shown that subconjunctival injection of the purified MIP-133 protein in Chinese hamsters induces epithelial ulceration, focal thickening, and PMN exocytosis. Stromal changes included lamellar connective tissue disruption, PMN infiltration, neovascularization, thickening, and edema (Alizadeh H, et al. IOVS 2011;52:ARVO E-Abstract 5795). These results indicate that MIP-133 plays an important role in the pathogenicity of AK by inducing cytolysis of the corneal cells and by stimulating the accumulation of PMN in the cornea. Our studies indicate that interaction of MIP-133 with the corneal epithelial cells induces a rapid immune response by the production of IL-8, IL-6, IFN-γ, and IL-1β, which can initiate an efficient host response to corneal infections. These results indicate a role of MIP-133 as a virulence protein, which may be responsible for PMN recruitment and inflammatory response to the corneal epithelium in vivo. The gene expression of proinflammatory cytokines and chemokines IL-6, IL-1β, TNF-α, and IL-8 also has been demonstrated in HCE cell lines challenged with P. aeruginosa.26 We showed that the pretreatment of HCE cells with AACOCF3 and MAFP blocks the protein production of IL-8, IL-6, IL-1β, and IFN-γ. Collectively, these results indicate that activation of cPLA2α induced by MIP-133 is responsible for the induction of proinflammatory signaling in cornea. We found that the corneal epithelial cells express IFN-γ and that the level of this cytokine increased 6 hours after infection; however, it is not clear whether IFN-γ was produced in response to MIP-133 or by induction of other cytokines or chemokines. The expression of IFN-γ by corneal epithelial cells is somewhat surprising, since it is known that IFNs are involved in cell signaling and are produced by varieties of immune cells in response to pathogenic microorganisms.41 Our results are in agreement with those of Ren et al.,42 who demonstrated that corneal epithelial cells are capable of producing IFN-β, and the level of this cytokine increased significantly following Acanthamoeba infection in vitro. Ueta et al.43 have shown that HCE produced IFN-β and that stimulation with poly(I:C) enhanced production of IFN-β in vitro. Rabbit corneal epithelial cells endogenously produce interferon, and its expression is increased in response to herpes simplex virus 1 (HSV-1) infection in vitro.44 HCE cells initiated a potent antiviral response resulting in an increase of IFN-β mRNA expression. Poly(I:C) stimulation also upregulated mRNA expression of the antiviral chemokine IFN-γ inducible protein 10 (IP10).45
The cornea is a nonvascular tissue that is significantly different from other tissues with regard to function and biological response to various stimuli.46 The activation of cPLA2α may have both beneficial and detrimental inflammatory effects on the cornea, depending on the effectiveness and duration of the host inflammatory response. There is a critical balance between generating a successful inflammatory response to eliminate the microorganism and an excessive inflammatory response that can result in corneal scarring and blindness.46 Understanding the molecular pathogenesis of MIP-133 that initiates proinflammatory cytokines and chemokines may permit the development of novel, specific therapies that can be delivered topically to prevent some of the destructive consequences of ocular infections.
In summary, MIP-133 is released from A. castellanii via interaction of corneal epithelial mannose with mannose receptors on the A. castellanii membrane. Our current results indicate that MIP-133 released by the interaction of mannose with the mannose receptors of A. castellanii cell membrane interacts with phospholipids on corneal epithelium and induces apoptosis and proinflammatory cytokines through cPLA2α signaling. Identification of MIP-133–induced apoptotic signaling may facilitate the development of more effective therapeutic strategies in Acanthamoeba keratitis.
Acknowledgments
We thank Abe Clark, Professor of Cell Biology and Anatomy and Director of the North Texas Eye Research Institute, University of North Texas Health Science Center, Fort Worth, for his critical review and comments on the manuscript.
Footnotes
Supported by Public Health Service Grant EY09756 from the National Institutes of Health.
Disclosure: T. Tripathi, None; A.D. Smith, None; M. Abdi, None; H. Alizadeh, None
References
- 1.Hurt M, Neelam S, Niederkorn J, Alizadeh H. Pathogenic Acanthamoeba spp secrete a mannose-induced cytolytic protein that correlates with the ability to cause disease. Infect Immun. 2003;71:6243–6255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke DW, Niederkorn JY. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol. 2006;22:175–180 [DOI] [PubMed] [Google Scholar]
- 3.Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148:487–499 [DOI] [PubMed] [Google Scholar]
- 4.Hay J, Kirkness CM, Seal DV, Wright P. Drug resistance and Acanthamoeba keratitis: the quest for alternative antiprotozoal chemotherapy. Eye (Lond). 1994;8:555–563 [DOI] [PubMed] [Google Scholar]
- 5.Ficker L, Seal D, Warhurst D, Wright P. Acanthamoeba keratitis--resistance to medical therapy. Eye (Lond). 1990;4:835–838 [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Cao Z, Panjwani N. Pathogenesis of Acanthamoeba keratitis: carbohydrate-mediated host-parasite interactions. Infect Immun. 1997;65:439–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garate M, Cao Z, Bateman E, Panjwani N. Cloning and characterization of a novel mannose-binding protein of Acanthamoeba. J Biol Chem. 2004;279:29849–29856 [DOI] [PubMed] [Google Scholar]
- 8.Leher H, Silvany R, Alizadeh H, et al. Mannose induces the release of cytopathic factors from Acanthamoeba castellanii. Infect Immun. 1998;66:5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alizadeh H, Neelam S, Hurt M, Niederkorn JY. Role of contact lens wear, bacterial flora, and mannose-induced pathogenic protease in the pathogenesis of amoebic keratitis. Infect Immun. 2005;73:1061–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niederkorn JY, Alizadeh H, Leher H, McCulley JP. The pathogenesis of Acanthamoeba keratitis. Microbes Infect. 1999;1:437–443 [DOI] [PubMed] [Google Scholar]
- 11.Leippe M, Ebel S, Schoenberger OL, et al. Pore-forming peptide of pathogenic Entamoeba histolytica. Proc Natl Acad Sci U S A. 1991;88:7659–7663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirschnek S, Gulbins E. Phospholipase A2 functions in Pseudomonas aeruginosa-induced apoptosis. Infect Immun. 2006;74:850–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke JE, Dennis ED. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50:S237–S242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taketo MM, Sonoshita M. Phospholipase A2 and apoptosis. Biochim Biophys Acta. 2002;1585:72–76 [DOI] [PubMed] [Google Scholar]
- 15.Hirabayashi T, Shimizu T. Localization and regulation of cytosolic phospholipase A(2). Biochim Biophys Acta. 2000;1488:124–138 [DOI] [PubMed] [Google Scholar]
- 16.Hefner Y, Borsch-Haubold AG, Murakami M, et al. Serine 727 phosphorylation and activation of cytosolic phospholipase A2 by MNK1-related protein kinases. J Biol Chem. 2000;275:37542–37551 [DOI] [PubMed] [Google Scholar]
- 17.Ghosh M, Loper R, Ghomashchi F, et al. Function, activity, and membrane targeting of cytosolic phospholipase A2ζ in mouse lung fibroblasts. J Biol Chem. 2007;282:11676–11686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Kolko M. Phospholipases A2 in ocular homeostasis and diseases. Biochimie. 2010;92:611–619 [DOI] [PubMed] [Google Scholar]
- 19.Visvesvara GS, Mirra SS, Brandt FH, et al. Isolation of 2 strains of Acanthamoeba castellanii from human tissue and their pathogenicity and isoenzyme profiles. J Clin Microbiol. 1983;6:1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y-G, McCulley JP. Growing human corneal epithelium on collagen shield and subsequent transfer to denuded cornea in vitro. Curr Eye Res. 1991;10:851–863 [DOI] [PubMed] [Google Scholar]
- 21.Hurt M, Niederkorn J, Alizadeh H. Effects of mannose on Acanthamoeba castellanii proliferation and cytolytic ability to corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:3424–3431 [DOI] [PubMed] [Google Scholar]
- 22.Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85 [DOI] [PubMed] [Google Scholar]
- 23.Panupinthu N, Zhao L, Possmayer F, et al. P2X7 nucleotide receptors mediate blebbing in osteoblasts through a pathway involving lysophosphatidic acid. J Biol Chem. 2007;282:3403–3412 [DOI] [PubMed] [Google Scholar]
- 24.Bonfoco E, Krainc D, Ankarcrona M, et al. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1995;92:7162–7166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hara Y, Shiraishi A, Ohashi Y. Hypoxia-altered signaling pathways of toll-like receptor 4 (TLR4) in human corneal epithelial cells. Mol Vis. 2009;15:2515–2520 [PMC free article] [PubMed] [Google Scholar]
- 26.Xue ML, Thakur A, Lutze-Mann L, Willcox MD. Pro-inflammatory cytokine/chemokine gene expression in human corneal epithelial cells colonized by Pseudomonas aeruginosa. Clin Experiment Ophthalmol. 2000;28:197–200 [DOI] [PubMed] [Google Scholar]
- 27.Selleri C, Maciejewski JP, Sato T, Young NS. Interferon-gamma constitutively expressed in the stromal microenvironment of human marrow cultures mediates potent hematopoietic inhibition. Blood. 1996;87:4149–4157 [PubMed] [Google Scholar]
- 28.Narayanan S, Glasser A, Hu YS, McDermott AM. The effect of interleukin-1 on cytokine gene expression by human corneal epithelial cells. Exp Eye Res. 2005;80:175–183 [DOI] [PubMed] [Google Scholar]
- 29.Burbach GJ, Naik SM, Harten JB, et al. Interleukin-18 expression and modulation in human corneal epithelial cells. Curr Eye Res. 2001;23:64–68 [DOI] [PubMed] [Google Scholar]
- 30.Heimer SR, Yamada A, Russell H, Gilmore M. Response of corneal epithelial cells to Staphylococcus aureus. Virulence. 2010;1:223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McInnis KA, Britain A, Lausch RN, Oakes JE. Human corneal epithelial cells synthesize ELR(-)alpha-chemokines in response to proinflammatory mediators. Ocul Immunol Inflamm. 2007;15:295–302 [DOI] [PubMed] [Google Scholar]
- 32.Xue ML, Zhu H, Thakur A, Willcox M. 1 alpha, 25-Dihydroxyvitamin D3 inhibits pro-inflammatory cytokine and chemokine expression in human corneal epithelial cells colonized with Pseudomonas aeruginosa. Immunol Cell Biol. 2002;80:340–345 [DOI] [PubMed] [Google Scholar]
- 33.Higuchi A, Kawakita T, Tsubota K. IL-6 induction in desiccated corneal epithelium in vitro and in vivo. Mol Vis. 2011;17:2400–2406 [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Zhang J, Kumar A, et al. Herpes simplex virus 1 infection induces the expression of proinflammatory cytokines, interferons and TLR7 in human corneal epithelial cells. Immunology. 2006;117:167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue ML, Zhu H, Willcox M, et al. The role of IL-1β in the regulation of IL-8 and IL-6 in human corneal epithelial cells during Pseudomonas aeruginosa colonization. Curr Eye Res. 2001;23:406–414 [DOI] [PubMed] [Google Scholar]
- 36.Hurt M, Niederkorn J, Alizadeh H. Effects of mannose on Acanthamoeba castellanii proliferation and cytolytic ability to corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:3424–3431 [DOI] [PubMed] [Google Scholar]
- 37.Sun GY, Horrocks LA, Farooqui AA. The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. J Neurochem. 2007;103:1–16 [DOI] [PubMed] [Google Scholar]
- 38.Balsinde J, Balboa MA, Insel PA, Dennis EA. Regulation and inhibition of phospholipase A2. Annu Rev Pharmacol Toxicol. 1999;39:175–189 [DOI] [PubMed] [Google Scholar]
- 39.Hurley JH, Tsujishita Y, Pearson MA. Floundering about at cell membranes: a structural view of phospholipid signaling. Curr Opin Struct Biol. 2000;10:737–743 [DOI] [PubMed] [Google Scholar]
- 40.van Engeland M, Ramaekers FC, Schutte B, Reutelingsperger CP. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry. 1996;24:131–139 [DOI] [PubMed] [Google Scholar]
- 41.Willcox MDP. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007;84:273–278 [DOI] [PubMed] [Google Scholar]
- 42.Ren M, Gao L, Wu X. TLR4: the receptor bridging Acanthamoeba challenge and intracellular inflammatory responses in human corneal cell lines. Immunol Cell Biol. 2010;88:529–536 [DOI] [PubMed] [Google Scholar]
- 43.Ueta M, Hamuro J, Kiyono H, Kinoshita S. Triggering of TLR3 by polyI:C in human corneal epithelial cells to induce inflammatory cytokines. Biochem Biophys Res Commun. 2005;331:285–294 [DOI] [PubMed] [Google Scholar]
- 44.Taylor JL, O'Brien WJ. Interferon production and sensitivity of rabbit corneal epithelial and stromal cells. Invest Ophthalmol Vis Sci. 1985;26:1502–1508 [PubMed] [Google Scholar]
- 45.Kumar A, Zhang J, Yu FS. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology. 2006;117:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song PI, Abraham TA, Park Y, et al. The expression of functional LPS receptor proteins CD14 and toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 2001;42:2867–2877 [PubMed] [Google Scholar]