Abstract
Hormone systems evolved over 500 million years of animal evolution to motivate feeding behavior and convert excess calories to fat. These systems produced vertebrates, including humans, who are famine-resistant but sensitive to obesity in environments of persistent overnutrition. We looked for cell-intrinsic metabolic features, which might have been subject to an evolutionary drive favoring lipogenesis. Mitochondrial protein acetylation appears to be such a system. Because mitochondrial acetyl-coA is the central mediator of fuel oxidation and is saturable, this metabolite is postulated to be the fundamental indicator of energy excess, which imprints a memory of nutritional imbalances by covalent modification. Fungal and invertebrate mitochondria have highly acetylated mitochondrial proteomes without an apparent mitochondrially-targeted protein lysine acetyltransferase. Thus, mitochondrial acetylation is hypothesized to have evolved as a nonenzymatic phenomenon. Because the pKa of a nonperturbed Lys is 10.4 and linkage of a carbonyl carbon to an ε amino group cannot be formed with a protonated Lys, we hypothesize that acetylation occurs on residues with depressed pKa values, accounting for the propensity of acetylation to hit active sites and suggesting that regulatory Lys residues may have been under selective pressure to avoid or attract acetylation throughout animal evolution. In addition, a shortage of mitochondrial oxaloacetate under ketotic conditions can explain why macronutrient insufficiency also produces mitochondrial hyperacetylation. Reduced mitochondrial activity during times of overnutrition and undernutrition would improve fitness by virtue of resource conservation. Micronutrient insufficiency is predicted to exacerbate mitochondrial hyperacetylation. Nicotinamide riboside and Sirt3 activity are predicted to relieve mitochondrial inhibition.
Keywords: overnutrition, calorie restriction, lipogenesis, Ac-coA, oxaloacetate, pKa, Sirt3, NAD+
Animal evolution
Animals, by definition, are heterotrophic organisms that acquire macronutrients from other organisms. Because animals cannot be at the bottom of the food chain, competition creates a reward system for acquiring, conserving and retaining resources. Animals evolved in parallel in many different environments, such that modern animals are remarkably numerous and diverse. Though no single beetle, insect, mollusk or vertebrate ended up with hegemonic control of planetary macromolecules, many of the systems for resource acquisition, conservation and retention are conserved.
The ability to survive, migrate and take advantage of newly discovered riches is fundamental to animal success. Indeed, the ability to acquire and digest complex macromolecular structures made particular organisms such as termites remarkably successful. Because resource availability is never guaranteed into the future and can be expected to be limited in lengthy competitions, the ability to survive famine is among the most highly selected traits over 500 million years of animal evolution.
Animal metabolic pathways have remarkable efficiencies: cellular and extracellular material from other organisms is broken down into glycolytic and lipolytic inputs. Protein is digested to amino acids and the corresponding keto acids. That which is needed to make sugars, building blocks and macromolecules is directed into anapleurotic and biosynthetic processes. That which can be oxidized in the citric acid cycle is consumed to generate ATP. In animals, all three classes of macronutrients have a storage form that protects against future needs. Carbohydrates are stored as glycogen, which can be phosphorylyzed to supply glucose-1-phosphate on demand. Protein anabolism in muscle can be reversed by proteolysis and conversion to other metabolites, such as in the glucose-alanine cycle. In addition, dietary fat can be stored and excess fuel is converted to fat. Of the three storage macromolecules, fat is the highest in energy content and the only one that does not require hydration to store.
Regulatory mechanisms of metabolism have been gleaned from many systems including bacteria, yeast and vertebrates. However, hormonal regulation of metabolism and energy balance is best understood in vertebrates. Complex hormonal regulatory systems control multiple levels of energy conservation from the signaling pathways that regulate anabolism and catabolism within tissues to organismal feeding behaviors and satiety. For example, the mobilization of glucose-1-phosphate from glycogen is stimulated by the hormone, glucagon, which is released from pancreatic α-cells in response to decreasing blood glucose. Glucagon binds to G-protein coupled receptors in liver and other tissues, producing successive activation of cAMP-dependent protein kinase, phosphorylase kinase and glycogen phosphorylase to release glucose-1-phosphate from glycogen (Jiang and Zhang, 2003). The glucose-alanine cycle and the processes of lipogenesis and lipolysis are also controlled by ancient, hormonal systems that regulate whole body energy balance (Felig, 1973, Scherer et al., 2011). In addition, in recent years, hormones produced by fat, the gut and the brain, and which act in multiple tissues, have been found to control hunger, feeding behavior, the set point for fat storage, and other mechanisms that control the disposition of fat (Meier and Gressner, 2004).
In addition to hormonal systems for carbon storage and energy metabolism, we considered whether a more ancient, cell-intrinsic mechanism might underlie lipogenesis. Here we hypothesize that mitochondrial protein acetylation and acylation is an ancient, conserved system that leads to inhibition of fuel utilization when energy is in excess, producing a cell-intrinsic switch to storage of fat.
Our model proposes that mitochondrial protein acetylation creates an erasable, covalent memory of an overfed state, whose initial metabolic signature consists of high acetyl-coA (Ac-coA), high NADH, and high ATP. This model does not require a highly evolved mitochondrial protein Lys acetyltransferase to modify fuel-utilizing enzymes. Instead, we propose that organisms evolved in a manner that put critical Lys residues of mitochondrial enzymes “in harm’s way” by depressing the pKa values of acetylation target sites, thereby rendering them susceptible to chemical modification—typically inhibition—during times of overnutrition. This would reduce the ability of mitochondria to restore rapid fuel-utilization after periods of plenty, and tend to promote the storage of fat.
This model simultaneously explains the ability of animals to survive scarcity, the tendency of modern humans to gain weight and experience mitochondrial decline in environments of continual energy excess, and—because of the micronutrient requirements for protein acetylation and deacetylation—explains why macronutrient overnutrition with a scarcity of micronutrients promotes lipogenesis.
Fuel utilization in mitochondria
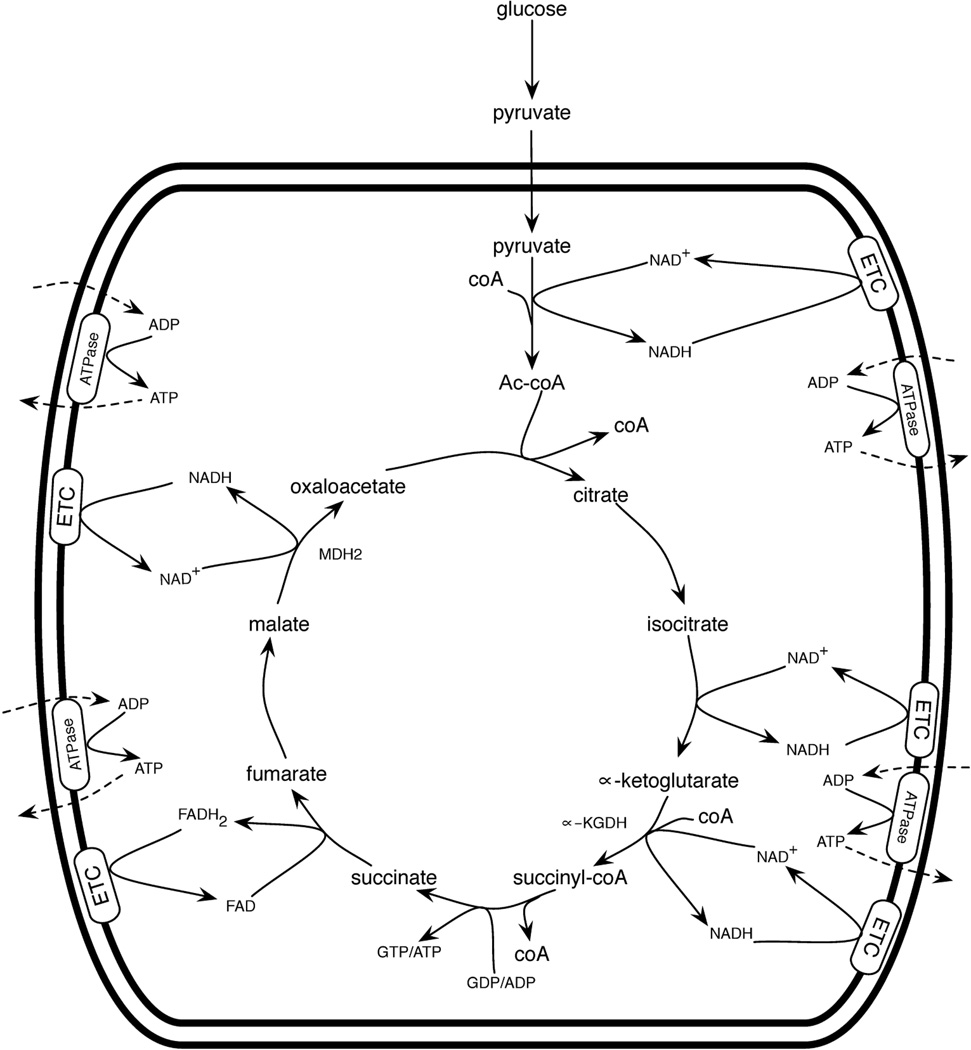
For the citric acid cycle to oxidize fuel, there must be fuel entering mitochondria as pyruvate, fatty acids, and/or amino acids. In addition, mitochondria possess a saturable pool of coenzyme A (coA) and a pool of hydride transfer cofactors that must be in the oxidized, i.e., NAD+ and FAD, states for the citric acid cycle to run. After formation of Ac-coA or other anapleurotic inputs, the citric acid cycle requires free coA for the α-ketoglutarate dehydrogenase (α-KGDH) complex to form succinyl-coA. In addition, there must be GDP or ADP for the succinyl-coA synthetase reaction to form succinate, and there must be a supply of each citric acid cycle intermediate to keep the cycle running. Fuel oxidation is coupled to function of the electron transport chain (ETC) in which O2 must be present as the ultimate electron acceptor— this allows reoxidation of NADH and FADH2 back to NAD+ and FAD (Figure 1).
Figure 1.
Fuel oxidation by mitochondria. Complete oxidation of pyruvate is depicted. Progression through the citric acid cycle depends on reoxidation of NADH and FADH2 by the electron transport chain (ETC). The net result is fuel utilization, CO2 production (not shown), and ATP export.
The oxidizing state of hydride transfer coenzymes is critical for the direction of multiple steps in the citric acid cycle, especially formation of oxaloacetate from malate by malate dehydrogenase 2 (MDH2). Under standard reaction conditions, i.e. 1M NAD+, malate, NADH and oxaloacetate, the fuel oxidizing direction of MDH2, i.e., malate + NAD+ → oxaloacetate + NADH, is thermodynamically unfavorable by ~ 30 kJ/mol. The thermodynamic favorability of citrate synthase, i.e., the “first” step in the citric acid cycle, drives the citric acid forward so long as there is NAD+ to produce malate and fuel to produce Ac-coA. Reoxidation of NADH to NAD+ and continuous production of malate allows oxaloacetate, the limiting substrate, to be formed. Though reversal of the MDH2 reaction is highly favorable, the collision of oxaloacetate with citrate synthase and Ac-coA allows oxaloacetate to be converted to citrate.
These characteristics create several speed limits that collectively govern the citric acid cycle. First, if excessive fuel enters mitochondria, the mitochondrial coA pool could be largely acetylated/acylated and have little free coA available for α-KGDH to function. Second, when the ETC is running at capacity, the hydride transfer coenzymes will begin to accumulate in reduced (NADH and FADH2) forms, which limits formation of the oxaloacetate that is necessary for Ac-coA to enter the cycle at citrate. By definition, if the ETC is running at capacity and protons are being pumped into the mitochondrial intermembrane space for maximal ATP generation, the intermembrane space is maximally acidified. This also means that the pH in the mitochondrial matrix will be alkalinized, potentially to a value as high as 8.2 (Santo-Domingo and Demaurex, 2012).
Consider the consequences of high mitochondrial Ac-coA, ATP and NADH, which may occur in a state of overnutrition in the liver. In addition to MDH2 running backwards at high NADH, pyruvate dehydrogenase (PDH) and citrate synthase are negatively regulated by NADH, and multiple enzymes are inhibited by ATP. In the early steps of the citric acid cycle, retardation or reversal (Des Rosiers et al., 1994) of isocitrate dehydrogenase (IDH) and inhibition of α-KGDH result in isocitrate flowing back to citrate. In the latter steps of the cycle, malate accumulates, largely due to the fact that the equilibrium for the MDH2 reaction (NAD+ + malate ←→ NADH + oxaloacetate) is strongly in favor of malate. Citrate and malate are the predominant organic acids exported by mitochondria into cytosol during energy excess. These signals of mitochondrial oversufficiency are important because malate export from liver mitochondria supplies gluconeogenesis, which is diabetogenic and indirectly contributes to lipogenesis. Cytosolic citrate is the direct precursor of cytosolic Ac-coA, which is used for production of malonyl-coA and lipogenesis.
Thus, mitochondrial metabolism possesses intrinsic satiety features that allow fuel utilization to be increased up to a point. Beyond that point, carbon inputs are converted to citrate and malate, which are exported to the cytosol for production of fat, sugar and other molecules.
Not all fuels are the same
Because the PDH complex requires both NAD+ and FAD in oxidized forms, it is difficult for pyruvate, the carbohydrate-derived mitochondrial fuel, to saturate mitochondrial oxidation capacity on its own. For example, if NADH and FADH2 produced by PDH and by oxidation of PDH-derived Ac-coA were at such great concentrations that they could not be reoxidized by the ETC, then PDH would be retarded by the tendency of NADH to bind to the NAD+ site and the consequent persistent occupancy of FADH2 at the FAD site. This would slow formation of Ac-coA, such that the ETC would be able to reoxidize NADH to NAD+, thus restoring the oxidizing environment essential for further pyruvate utilization.
In a similar manner, there are systems that limit the ability of dietary fatty acids to saturate mitochondrial oxidative capacity. Dietary fatty acids are long chain fatty acids (LCFA), which require the carnitine shuttle system for mitochondrial import. When lipogenesis is occurring, the cytosol contains malonyl-coA, which inhibits carnitine palmitoyltransferase 1 and LCFA import (McGarry et al., 1978). Thus, mitochondria that have already achieved energy satiety, i.e., which are exporting citrate for production of cytosolic Ac-coA and malonyl-coA, do not import LCFA.
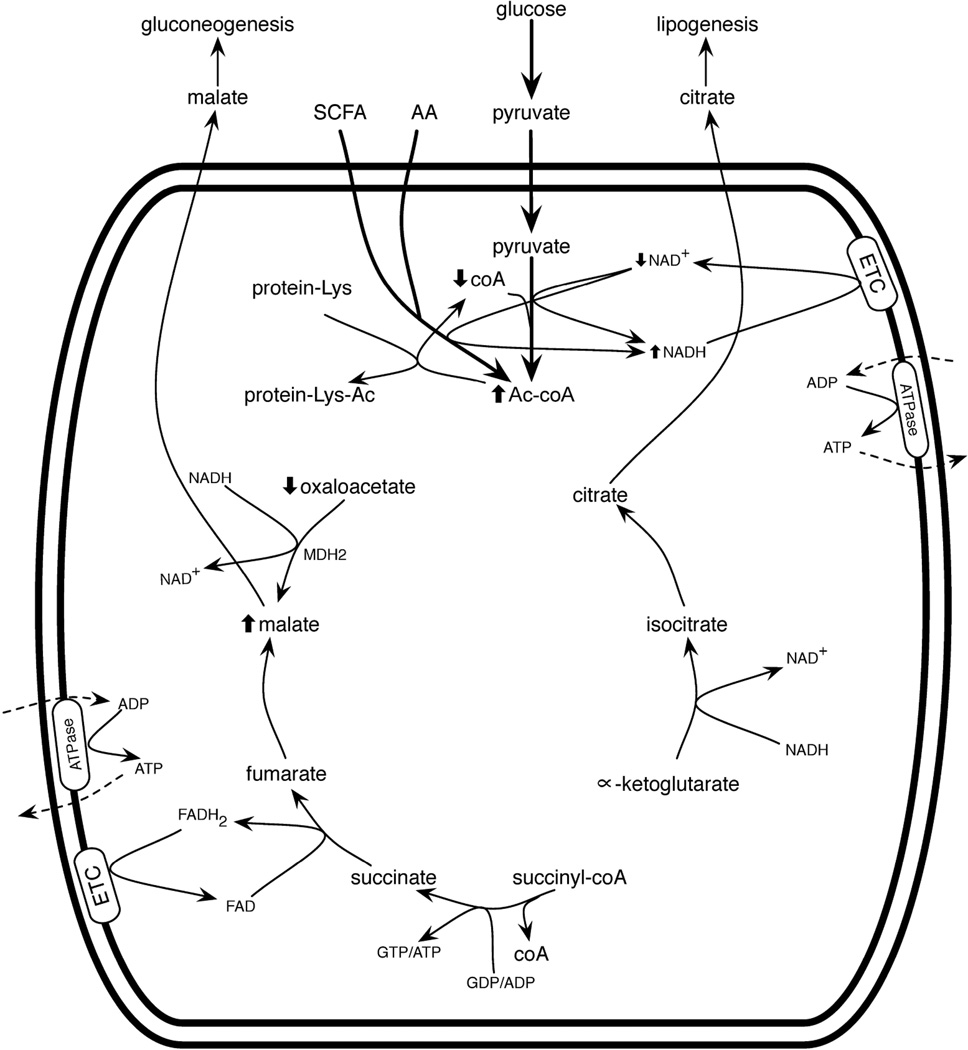
Short and medium chain fatty acids (SCFA and MCFA) and amino acids are not subject to these systems. Such molecules cross the mitochondrial inner membrane and gain access to mitochondrial enzymes, thereby producing Ac-coA and acetoacetyl-coA at the expense of free coA. Thus, SCFA, which are produced by the intestinal microbiome, particularly in diet-induced obesity (Turnbaugh et al., 2006), and protein-rich meals may have the tendency to saturate the mitochondrial coA pool. The combination of high carbohydrate, high protein and SCFA would tend to convert free mitochondrial coA to Ac-coA very effectively, thereby inhibiting citric acid cycle function at the α-KGDH complex (Figure 2).
Figure 2.
Mitochondrial function during macronutrient overnutrition. Here, mitochondria are importing pyruvate, short chain fatty acids (SCFA) and amino acids (AA) at rates that exceed the capacity of the ETC to reoxidize NADH. Under these conditions, mitochondrial coA accumulates in the Ac-coA form, in part, due to the inability of MDH2 to form oxaloacetate in the presence of high NADH. Without free coA for α-ketogluarate dehydrogenase (α-KGDH) to run, metabolites early in the citric acid cycle leave mitochondria as citrate, precursor for cytoplasmic Ac-coA and lipogenesis. Metabolites late in the citric acid cycle leave as malate, precursor for cytoplasmic oxaloacetate and gluconeogenesis. At high Ac-coA, protein acetylation occurs.
Whether the fats are SCFA or LCFA, it is a “red letter day” when an animal encounters supersaturating amounts of carbohydrate, protein and fat. We term this state “macronutrient overnutrition.” Carbon inputs to mitochondria initially cause fuel to be oxidized. At respiratory capacity, Ac-coA and NADH are expected to rise, thereby inhibiting mitochondrial fuel oxidation, and leading to export of malate and citrate. LCFA then tend to be deflected directly into lipogenesis, while SCFA, pyruvate and amino acids would tend to continue to enter mitochondria, producing a “mitochondria are full” metabolic signature of high Ac-coA, high NADH, high ATP and potentially high matrix pH. This metabolic signature does not require any hormonal system to produce—it is predicted to occur simply because of the finite capacity of respiratory metabolism. When mitochondria are full, citrate flows out for production of cytosolic Ac-coA and subsequent lipogenesis. The red letter day is marked by production of valuable fat stores that protect against famine.
Consequences of high mitochondrial Ac-coA include protein acetylation
Thus far, textbooks depict two fates for mitochondrial Ac-coA: oxidation by the citric acid cycle and ketone body production. Oxidation requires NAD+, free coA, nucleoside diphosphates and oxaloacetate. Ketone body production in liver mitochondria occurs during carbohydrate shortages, largely because oxidation of fats causes a buildup of Ac-coA and a shortage of oxaloacetate, with malate being exported to the cytosol for gluconeogenesis (McGarry and Foster, 1980). Fuel oxidation and ketogenesis both relieve high Ac-coA and release free coA. However, whereas citric acid cycle oxidation occurs when there is NAD+, free coA and oxaloacetate, ketogenesis can occur without free coA or NAD+ in the oxidized form. Is there another fate for mitochondrial Ac-coA, which would occur specifically under the conditions in which mitochondria are overloaded with fuel?
In vitro (Zhao et al., 2010) and in vivo (Hirschey et al., 2011) experiments have recently established that when fuel is increased, mitochondrial protein lysine acetylation is increased. Because the source of protein acetylation is Ac-coA (Bondy et al., 1970), mitochondrial Ac-coA must be considered to have a third major fate. As fuel is increased and mitochondrial Ac-coA increases, more protein Lys acetylation occurs. Such protein modifications would tend to relieve mitochondrial Ac-coA and could have important regulatory roles.
One of the first mitochondrial acetylomic analyses (Zhao et al., 2010) showed that metabolic enzymes are highly modified by acetylation and that acetylation is increased when fuel is increased. However, it was claimed that the hyperacetylated form of MDH2 promotes improved fuel oxidation. What the study actually shows is that MDH2 becomes acetylated at Lys185, Lys301, Lys307 and Lys314 and that enzyme activity is increased for hyperacetylated MDH2 but not for MDH2 in which the four Lys residues were replaced with Arg (Zhao et al., 2010). However, the fact that hyperacetylated MDH2 is more active does not support the idea that fuel oxidation is improved during such conditions. Because of the thermodynamic favorability of oxaloacetate reduction, MDH2 is assayed in the direction contrary to fuel oxidation, i.e., NADH + oxaloacetate → NAD+ + malate. During conditions of high fuel, i.e., high NADH, this is not only the convenient manner to assay MDH2, it is the biologically relevant direction. This means that hyperacetylated MDH2 in the presence of high NADH would more rapidly reduce oxaloacetate to malate (and keep malate from forming net oxaloacetate), thereby opposing fuel oxidation and promoting malate export. In fact, this biochemical analysis would be consistent with a diabetogenic effect of increased fuel. Increased fuel would not promote improved fuel oxidation because of MDH2 protein acetylation: increased fuel and consequent hyperacetylated MDH2 would promote malate export and potentially an increase in gluconeogenesis and hyperglycemia.
Mitochondrial acetylation is almost always inhibitory
Though the notion that mitochondrial protein acetylation increases fuel oxidation does not have much currency, the observation that mitochondrial protein acetylation is regulatory has been proven again and again. MDH2 is an unusual enzyme that is activated by hyperacetylation. Among the mitochondrial enzymes that are inactivated by Lys acetylation are Ac-coA synthetase 2 (Hallows et al., 2006, Schwer et al., 2006), glutamate dehydrogenase (Schlicker et al., 2008), complex I of the ETC (Ahn et al., 2008) IDH2 (Schlicker et al., 2008, Yu et al., 2012), long chain acyl-coA dehydrogenase (Hirschey et al., 2010), 3-hydroxy-3- methylglutaryl coA synthetase 2 (Shimazu et al., 2010), superoxide dismutase (Qiu et al., 2010, Tao et al., 2010), and ornithine transcarbamylase (Hallows et al., 2011). This represents a wide range of key mitochondrial functions including fuel oxidation, energy generation, waste disposal, and detoxification of reactive oxygen species.
Evidence that these enzymes are inactivated by Lys acetylation is derived by knocking out Sirtuin 3 (Sirt3), a nuclear-encoded enzyme, which accounts for mitochondrial protein Lys deacetylase activity (Lombard et al., 2007). Multiple studies have established that mitochondrial proteins accumulate in acetylated or hyperacetylated forms in sirt3−/− animals (Lombard et al., 2007, Ahn et al., 2008, Hirschey et al., 2010, Shimazu et al., 2010, Qiu et al., 2010, Tao et al., 2010, Hallows et al., 2011, Hebert et al., 2013, Yu et al., 2012). With the exception of MDH2, which is apparently activated by hyperacetylation (Zhao et al., 2010), all of the characterized mitochondrial acetylation targets have lower activity in the acetyl-modified forms. Indeed, it has recently been contended that deacetylation by Sirt3 is always activating (Chhoy et al., 2013).
The literature tells us that Sirt3, expression of which is increased during calorie restriction (Shi et al., 2005, Palacios et al., 2009, Hirschey et al., 2010), activates the functions of a wide swath of mitochondrial enzymes. This is particularly interesting because Sirt3, like all sirtuins, is an NAD+-dependent protein lysine deacetylase (Sauve et al., 2006, Belenky et al., 2007a, Feldman et al., 2012). However, we would suggest that because Sirt3 targets are synthesized and are presumably imported into mitochondria without Lys modifications, they are really inactivated by acetylation and have their activities restored by NAD+-dependent protein lysine deacetylation.
Protein acetylation as a memory of a metabolic signature
Metabolites themselves, in particular the ratios of key metabolites such as NAD+ to NADH and ATP to ADP, can direct metabolic flux. However, metabolite-driven changes in metabolic flux are so instantly homeostatic that they are often reinforced by covalent modifications that create a memory of metabolic conditions. Consider a cell that is experiencing a state of anoxia, which drives down ATP formation and can be considered a state of cellular hunger for energy inputs. The initial response to severe declines in the ATP:ADP ratio is mediated by formation of AMP. The glycolytic increase, producing new ATP, that results from appearance of AMP is so instantaneous that it erases the AMP signal. Were it not for AMP-dependent kinase-mediated protein phosphorylation, there would be no lasting memory of a spike in AMP. We will draw an analogy between AMP signaling and Ac-coA signaling.
When cellular ATP is low, ADP is high. Under these conditions, adenylate kinase converts two ADP to ATP plus AMP. AMP functions as an activator of catabolism by displacing an inhibitory ATP bound to an allosteric site on phosphofructokinase 1 (PFK1) (Wegener and Krause, 2002). So long as there is AMP bound to PFK1, the enzyme is activated and flux is increased through glycolysis. However, because PFK1 activation leads to rapid conversion of glucose to pyruvate with production of net ATP, AMP formation is rapidly followed by ATP production. With rising ATP, adenylate kinase reverses its net direction by using ATP to phosphorylate AMP, thereby erasing the initiating signal of low energy.
The instantly homeostatic nature of AMP as an allosteric regulator (i.e., AMP leads to production of ATP, which erases AMP) leaves a need for a covalent modification system that can sustain an increase in glycolysis and other responses to cellular hunger. Because AMP-activated protein kinase phosphorylates GLUT4 and phosphofructokinase 2 (PFK2), anoxic cells can ramp up glucose transport and glycolysis and sustain this flux using the fructose-2,6-bisphosphate activator of PFK1 beyond the time at which AMP is converted to ADP (Hardie, 2011). Indeed, because AMP-activated protein kinase creates covalent marks on multiple targets, a subsequent decline in glycolytic flux must await protein dephosphorylation or protein turnover. This example of the AMP metabolite directing metabolic flux through an instantly homeostatic mechanism linked to a longer lasting covalent modification system has parallels in mitochondrial Ac-coA metabolic signaling.
If AMP represents a cellular state of hunger, the metabolic signature of high Ac-coA, high NADH and high ATP represent mitochondrial overnutrition. These conditions inhibit fuel oxidation because the activities of pyruvate dehydrogenase and other NAD+-dependent oxidation reactions are disfavored at high NADH. Citrate synthetase slows down because of the lack of oxaloacetate, and IDH runs in reverse (Des Rosiers et al., 1994), which leads to citrate export and lipogenesis. Under these conditions, pyruvate carboxylase is allosterically activated by Ac-coA and generates oxaloacetate from pyruvate and ATP (Jitrapakdee et al., 2008). However, at high NADH, the oxaloacetate is reduced to malate by MDH2, such that pyruvate carboxylase does not provide the limiting substrate for citrate synthetase. At high Ac-coA, high NADH and high ATP in the overfed liver, pyruvate carboxylase initiates gluconeogenesis.
Just as glycolysis is activated by low ATP and the presence of AMP, mitochondrial metabolism is initially self-correcting. When IDH and MDH2 run in reverse, NADH is reoxidized to NAD+ and, as pyruvate carboxylase continues to run, high ATP can be worked off. Suddenly, pyruvate can be used by pyruvate carboxylase and PDH to make both citrate synthetase substrates and the citric acid cycle can be restarted. The ability of systems such as these to work off the “mitochondria are full” metabolic signature makes mitochondria resilient to transient overnutrition and is routine in the post-prandial state. However, while restoring the ability to oxidize fuel sounds appealing, it is not necessarily of the greatest evolutionary benefit to an organism.
If the “mitochondria are full” metabolic signature could be imprinted on mitochondrial proteins in a manner that were to retard oxidative functions more than transiently, then an organism that encounters conditions of macronutrient surplus might more effectively retard its metabolism and be able to store valuable fat. Specifically, if rising Ac-coA is converted to covalent modification of mitochondrial proteins, then protein acetylation could be an imprint of nutritional conditions that lasts longer than the metabolic signature itself.
For the vast majority of metabolic enzymes, the functional consequences of whose acetylation have been determined, acetylation is inhibitory (Hallows et al., 2006, Schwer et al., 2006, Schlicker et al., 2008, Ahn et al., 2008, Hirschey et al., 2010, Shimazu et al., 2010, Qiu et al., 2010, Tao et al., 2010, Hallows et al., 2011, Hebert et al., 2013, Yu et al., 2012). Indeed, if one looks at the citric acid cycle, the perfect storm to retard fuel oxidation by regulatory acetylation would be to retard nearly all enzymes and accelerate MDH2 at high NADH (Zhao et al., 2010). This would tend to prevent oxaloacetate conversion to citrate, despite high Ac-coA, such that malate will accumulate and be transported into the cytosol. Because α-KGDH requires free coA, citric acid cycle intermediates will flow backwards from α-ketoglutarate so that citrate can leave mitochondria for lipogenesis (Figure 2).
Thus, it is reasonable to suggest that mitochondrial protein acetylation evolved as a system to imprint a memory of macronutrient overnutrition on mitochondrial enzymes—so long as the acetyl marks remain on these enzymes, fuel oxidation would be impaired and fat storage would be improved. This is the phenotype of sirt3−/− mice: they are hypersensitive to weight gain, insulin resistance, hyperlipidemia, and fatty liver compared to wild-type animals on chronic high fat diet (Hirschey et al., 2011).
And the mitochondrial protein acetyltransferase is?
Mitochondrial enzymes have been found to be highly acetylated as far back as the yeast S. cerevisiae (Henriksen et al., 2012). In a dataset of acetylated proteins from the fruitfly D. melanogaster, Lys residues that are acetylated were found to be highly conserved between flies, worms, zebrafish and humans, and mitochondrial proteins were found to be highly enriched in acetylation (Weinert et al., 2011). These data suggest an ancient co-evolved system reminiscent of a protein kinase and protein kinase substrates.
Despite significant efforts to identify a sequence encoding a mitochondrial protein lysine acetyltransferase in invertebrates, none has been found. At least nine families of protein lysine acetyltransferases have been identified: HAT1, Gcn5/PCAF, MYST, p300/CBP, Rtt109, ACTR/AIB1, TAF250, ATF-2 and CLOCK (Yuan and Marmorstein, 2013). The diversity of protein lysine acetyltransferase sequences makes sequence identification of a mitochondrial protein lysine acetyltransferase challenging.
What are the possibilities to explain the origin and conservation of mitochondrial protein lysine acetylation?
Mitochondrial proteins are acetylated in the cytoplasm by nucleocytoplasmic acetyltransferases and by nucleocytoplasmic Ac-coA.
Mitochondrial proteins are acetylated by a known protein lysine acetyltransferase, a fraction of which is imported into mitochondria.
Mitochondrial proteins are acetylated by a novel mitochondrial enzyme, not recognizable as a protein lysine acetyltransferase.
Mitochondrial proteins are chemically acetylated.
The first possibility would fail to explain how mitochondrial proteins are dynamically acetylated (Zhao et al., 2010). However, the second and third possibilities cannot currently be excluded. In support of the second possibility, there is recent evidence for mitochondrial forms of calmodulin-dependent kinase II (Joiner et al., 2012) and protein kinase A (Sastri et al., 2013), which were long considered to be cytosolic or nucleocytosolic enzymes, are not in Mitocarta (Pagliarini et al., 2008), and do not contain mitochondrial targeting sequences. The major problem with the fourth possibility, which has recently been suggested (Newman et al., 2012), is how to explain the remarkable propensity of mitochondrial acetylation to hit active site residues and otherwise regulate enzyme function. If mitochondrial protein acetylation is driven by simple chemistry, how can the resulting protein modification be so site-specific?
Role of Lys pKa in nonenzymatic acetylation
Reactions that are driven by “simple” chemistry are controlled by concentration of reactions and also by regiospecific reactivity—the critical statistic is the concentration of the reactive species. No matter which enzyme catalyzes acetyl group transfer reaction to a Lys sidechain, the ε amino group must be deprotonated to be attacked by the partially positive carbonyl carbon of Ac-coA. Without an enzyme to recognize the polypeptide and present a base to abstract a proton from the substrate Lys, most Lys ε amino groups are poorly reactive. This is because the pKa of a nonperturbed Lys amino group is 10.4. Accordingly, near neutral pH, a typical, surface-exposed Lys spends almost 100% of the time in the protonated state and would be quite resistant to acetylation at any concentration of Ac-coA.
However, Lys residues in special locations can have pKa values depressed into the neutral range or lower. Indeed, Lys residues within enzyme active sites and other unique locations exhibit depressed pKa values by virtue of nearby positive charges or by desolvation. In both cases, the local environment destabilizes the protonated NH3+ group with respect to the neutral, lone electron pair-bearing NH2 group.
This model has the potential to explain the site-specificity of mitochondrial protein acetylation. Though not all Lys residues at enzyme active sites have depressed pKa values, the requirement of a depressed pKa value for nonenzymatic acetylation would tend to enrich for active site residues. Moreover, this system would have been the subject of 500 million years of target site evolution. If increasing Ac-coA tends to lead to mitochondrial protein modification, which inhibits mitochondrial function and promotes lipogenesis during times of energy excess, then organisms with susceptible Lys residues in key mitochondrial enzymes would tend to store more fat and have improved survival and fecundity, particularly during eras of uncertain food.
Other mitochondrial Lys modifications
Though Sirt3 is responsible for mitochondrial protein deacetylation (Lombard et al., 2007), the mitochondrial Sirt5 isozyme reverses succinyl and malonyl modifications of Lys (Du et al., 2011, Peng et al., 2011) and the mitochondrial Sirt4 isozyme may have an enzymatic activity on a distinct modification. Though it will be hard to eliminate the possibility that specific succinyl-coA and malonyl-coA transferases exist, we suggest that mitochondrial sirtuin substrates are formed by chemical means, i.e., as a function of the concentrations of the corresponding coA thioesters and a function of the concentration and reactivity of substrate Lys residues. This would also explain why multiple modifications tend to be found on the same substrate Lys residues.
Evolutionary mutability of Lys pKa and the role of elevated matrix pH
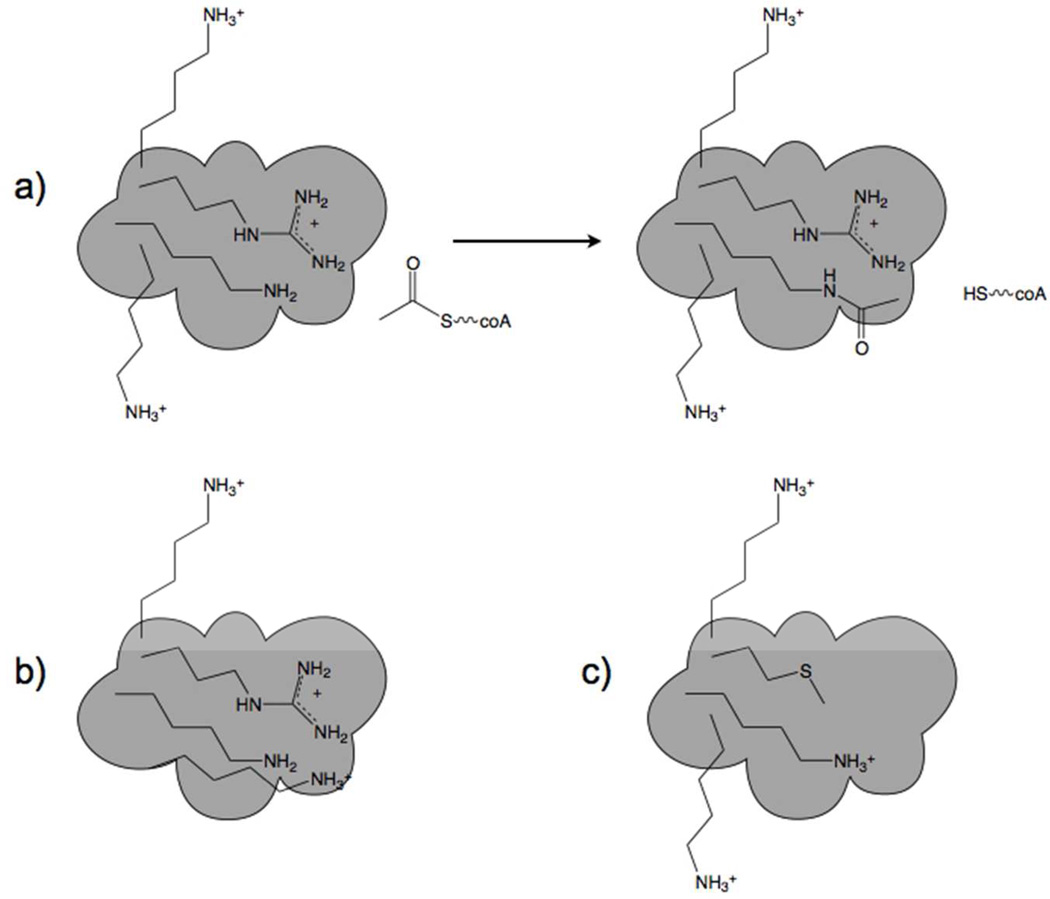
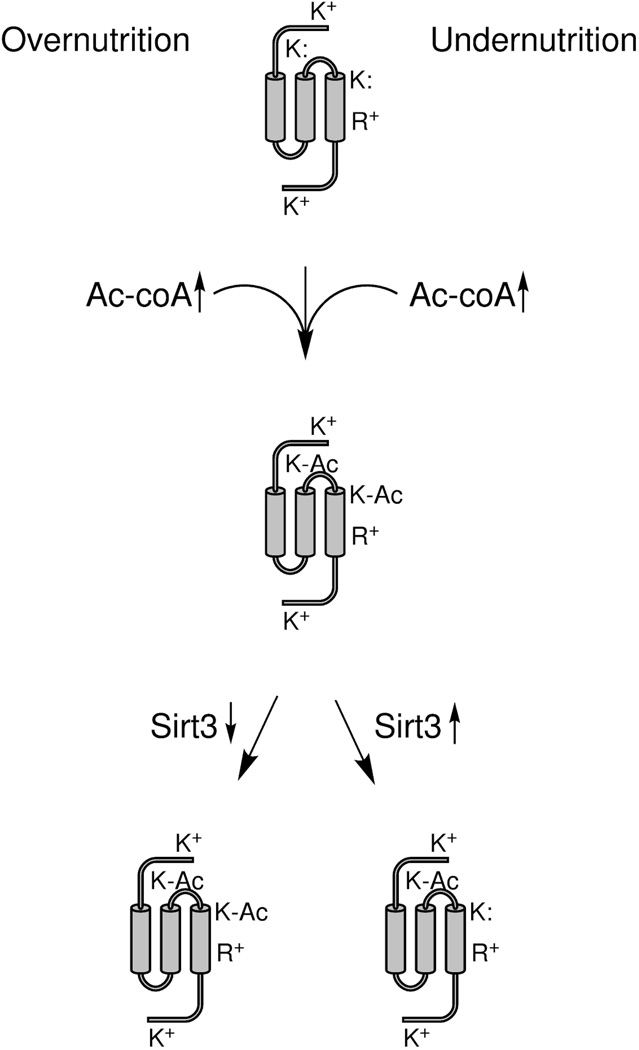
We propose that Lys acetylation in mitochondria arose simply as a function of elevated Ac-coA and the susceptibility of Lys residues in specific locations. If a particular Lys residue on an enzyme that promotes fuel utilization had a depressed pKa value, say to 8.4, it would tend to become acetylated during conditions of rising Ac-coA, creating a memory or imprint of high Ac-coA and retarding fuel oxidation, thereby promoting carbon export from mitochondria and the production of fat. If this were to create an evolutionary advantage, one would expect that additional enzymes would be selected to possess similar properties and that the local environment of target Lys residues could be driven to still lower pKa values, either by nearby appearance of Lys and Arg residues or by partial desolvation. In contrast, if an enzyme essential for survival were to be susceptible to inactivation by virtue of acetylation, then evolutionary forces would tend to remove positive charges nearby, thereby elevating Lys pKa and allowing resistance to rising Ac-coA (Figure 3).
Figure 3.
Chemistry and mutability of acetylation. a) Near neutral pH, Lys residues with nonperturbed pKa values are resistant to acetylation. One Lys near an Arg has a depressed pKa value and is modified by Ac-coA. b) A susceptible Lys residue has its pKa value further depressed by virtue of accumulation of an additional nearby positive charge. This is the postulated mechanism by which regulatory Lys residues frequently acquired susceptibility to chemical acetylation. Largely, mitochondrial enzyme inactivation is proposed to be under positive selective pressure by favoring lipogenesis. c) Here a formerly accessible Lys residue acquires greater resistance to acetylation by virtue of loss of a nearby positive charge. This adaptive mechanism would protect against loss of functions that are essential, particularly during times of nutritional oversufficiency or undersufficiency.
The metabolic signature of fuel-saturated mitochondria is predicted to consist not only of high Ac-coA, high NADH and high ATP, but also elevated matrix pH. This would be the case because, at respiratory capacity, protons have been maximally pumped into the intermembrane space, thereby alkalinizing the matrix (Santo-Domingo and Demaurex, 2012). As the mitochondrial matrix pH rises, protons are abstracted from more and more Lys residues, which would tend to drive more productive reactions of Ac-coA with protein Lys residues. Thus, with persistent overnutrition, the “mitochondria are full” metabolic signature would be increasingly converted to high occupancy acetylation of Lys residues with the lowest pKa values and imprinting of additional low pKa Lys residues on abundant proteins.
Whereas protein acetylation will tend to relieve high Ac-coA and thereby allow coA-dependent enzymes such as PDH and α-KGDH to run, if acetylated proteins are largely inhibited, then a switch from carbon export/lipogenesis back to fuel oxidation has to await reversal of these modifications by the NAD+-dependent deacetylase, Sirt3. In this manner, the high Ac-coA metabolic signature is converted to a longer lasting, covalent metabolic regulatory mechanism.
The relationship between macronutrients and micronutrients
When an animal encounters food in the wild, the energy inputs are typically living or formerly living biomass. Such “whole foods” can be expected to contain micronutrients in proportion to macronutrients. Because all cellular biomasses contain coA metabolites, pantothenate equivalents can be salvaged along with carbohydrate, protein and fat. Dietary pantothenate is used to generate coA, which is reportedly transported into the mitochondrial matrix by the Graves’ disease protein (Prohl et al., 2001). Pantothenate deficiency could make saturation of mitochondrial coA occur at a lower level of fuel. How mitochondria regulate their total levels of coA synthesis as a function of dietary pantothenate is not known (Leonardi et al., 2005).
The most abundant NAD+ metabolite is NAD+ itself (Evans et al., 2010, Trammell and Brenner, 2013). During digestion, NAD+ metabolites are broken down to the salvageable precursor vitamins, nicotinate, nicotinamide, and nicotinamide riboside (NR) (Bieganowski and Brenner, 2004, Belenky et al., 2007b, Bogan and Brenner, 2008). Trp is also used to produce NAD+ via the de novo pathway in the tissues in which such enzymes are expressed. Whereas any of the four NAD+ precursors can produce nucleocytoplasmic NAD+, Trp and nicotinic acid produce NAD+ through the nicotinate mononucleotide (NaMN) intermediate, whereas nicotinamide and NR produce NAD+ through the nicotinamide mononucleotide (NMN) intermediate (Trammell and Brenner, 2013). Because mitochondrial NAD+ depends on import of NMN (Nikiforov et al., 2011), nicotinamide and NR are predicted to be superior to nicotinate and Trp as mitochondrial NAD+ precursors (Trammell and Brenner, 2013). Because nicotinamide inhibits sirtuins at high dose (Anderson et al., 2003), NR may be the most effective NAD+ precursor to elevate mitochondrial sirtuin activities (Trammell and Brenner, 2013).
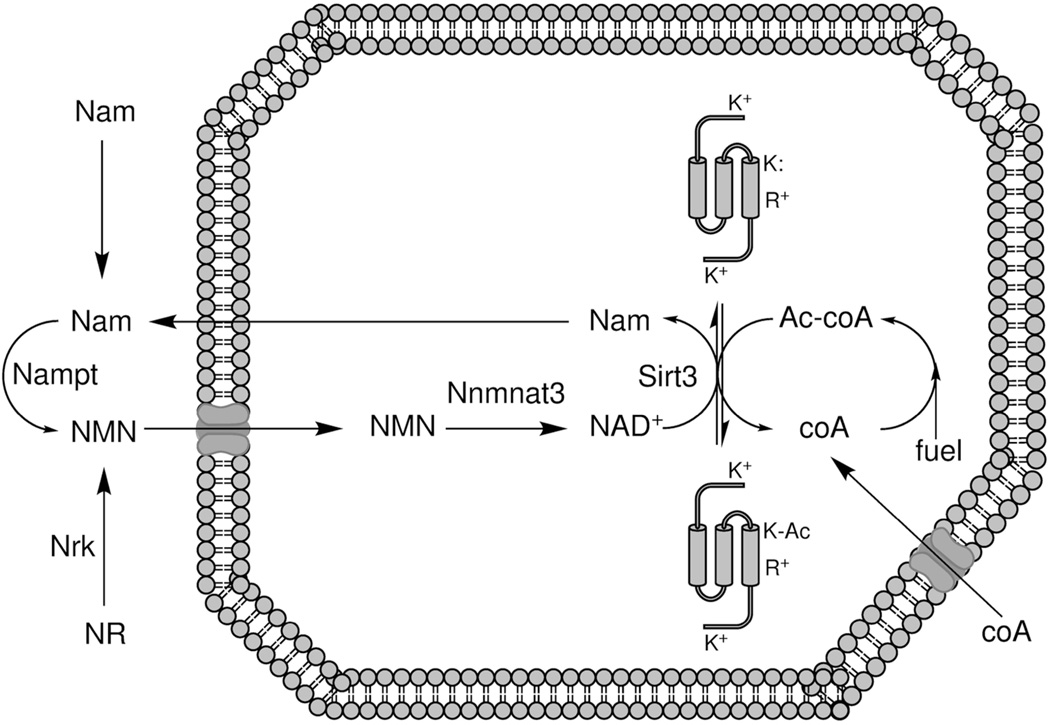
We have argued that mitochondrial acetylation forms a covalent memory of elevated mitochondrial Ac-coA and that such modifications are largely inhibitory for mitochondrial function. Because mitochondrial acetylation is reversed by Sirt3 in a manner that requires consumption of one equivalent of NAD+, and NADH is not a sirtuin substrate, reoxidation of NADH and/or continual resynthesis of mitochondrial NAD+ is required for Sirt3 activity. Thus, while mitochondrial protein acetylation is an erasable memory of overnutrition, as long as the mitochondrial NAD+ pool is largely in the NADH form, these modifications would be difficult to erase. In addition, because Sirt3 activity cleaves NAD+ into a nicotinamide moiety and an Ac-ADPribosyl product (Sauve et al., 2006, Belenky et al., 2007a, Feldman et al., 2012), Sirt3 activity without mitochondrial NAD+ resynthesis may not effectively restore the ability to oxidize fuel. Mitochondrial NAD+ resynthesis requires cytosolic nicotinamide phosporibosyltransferase (Revollo et al., 2004) and/or NR kinase (Bieganowski and Brenner, 2004), the corresponding NAD+ precursor, and the mitochondrial NMN adenylyltransferase, Nmnat3 (Nikiforov et al., 2011) (Figure 4).
Figure 4.
Relationship between co-enzymes and reversible acetylation. coA, which is synthesized from pantothenate in cytoplasm and mitochondria (not shown) is ultimately imported into mitochondria, where it becomes acetylated by pyruvate and fatty acid oxidation and amino acid catabolism. Protein acetylation releases free coA. Mitochondrial protein deacetylation depends on NAD+ and Sirt3, producing nicotinamide (Nam) and Ac- ADPribose (not shown) in addition to nonmodified protein. Salvage to nicotinamide mononucleotide (NMN) occurs in the cytoplasm as a function of Nam and Nam phosphoribosyltransferase (Nampt) or nicotinamide riboside (NR) and nicotinamide riboside kinase (Nrk). NMN is imported to mitochondria, where it is a substrate of NMN adenylytransferase 3 (Nmnat3).
Sirt3 protein expression is under the control of the nutritional status of an organism. In mice, high fat diet leads to a decline in Sirt3 protein accumulation (Palacios et al., 2009, Hirschey et al., 2011), whereas calorie restriction leads to an increase in Sirt3 expression (Shi et al., 2005, Palacios et al., 2009, Hirschey et al., 2010). Thus, chronic overnutrition is expected to lead to an increased rate and persistence of mitochondrial protein acetylation. Declining Sirt3 would tend to fix these inhibitory marks and lead to progressive mitochondrial dysfunction, which is frequently seen in obesity and its complications (Mantena et al., 2008). While the intrinsic programming that leads to reduced Sirt3 expression in overfed animals sounds like a bad thing, this is precisely the programming that would preserve fat stores and be selected by evolution, so long as it does not interfere with survival, mating or child-rearing.
The epidemic of human obesity is not associated with hyperphagy of minimally processed foods. Though our energy intake exceeds our energy expenditure, the foods we increasingly eat are those, which our food industry aims to sell (Chandon and Wansink, 2012). These are typically refined and concentrated in macronutrients. Ironically, the epidemic of pellagra of 100 years ago was traced to the diet of poor people in the American South, who ate corn rations and lard. The American snack food industry is built around similar ingredients. Though pellagra was cured with fresh foods and has been largely prevented with nicotinate and/or nicotinamide enrichment of cereals, the ~15 mg per day recommended daily allowance of this vitamin might be suboptimum today. This could be because there are lower niacin equivalents in current processed food diets; because current food consumption is greater and produces a greater protein acetylation phenotype, necessitating more NAD+ salvage; or because people today are bigger and have a different body composition. Indeed, mice on high fat diet who were supplemented with NR were able to resist some increase in adiposity (Canto et al., 2012). Though many other mechanisms may be at work, this may be evidence that increased mitochondrial NAD+ biosynthesis would tend to reduce mitochondrial protein acetylation, improve fuel utilization, and resist lipogenesis. Experiments to test this hypothesis are in progress and have the potential to improve human nutrition and health in the face of over-macronutrient nutrition.
According to this theory, NAD+ precursors will only aid in deacetylating and reactivating mitochondrial function if Sirt3 is still expressed. It is therefore of interest to note that muscle Sirt3 expression has been reported to decline in older sedentary people but that Sirt3 expression remains high in physically active people independent of their age (Lanza et al., 2008).
Gcn5l1, a potential component of vertebrate mitochondrial protein acetylation
Gcn5 is a 439 amino acid histone acetyltransferase from S. cerevisiae that acetylates histone H3 on Lys14. In the context of larger enzyme complexes and nucleosomes, Gcn5 acetylates an expanded repertoire of Lys residues (Grant et al., 1999). Recent work identified a vertebrate-specific Gcn5-related protein, termed Gcn5l1, which consists of a 31 amino acid mitochondrial targeting sequence followed by 94 amino acids of Gcn5-like catalytic domain. Knockdown of Gcn5l1 resulted in a hypoacetylation phenotype, suggesting that it may mediate transfer of the acetyl group from Ac-coA to mitochondrial protein targets (Scott et al., 2012).
Because this enzyme is absent from yeast and more ancient metazoans, Gcn5l1 cannot be responsible for the ancient evolutionary history of mitochondrial protein acetylation. Moreover, the lack of protein domains for protein substrate recognition would argue against a role for Gcn5l1 in targeting specific Lys residues for modification. Rather, we suggest that vertebrates may have recruited a minimalist enzyme to catalyze acetyl group transfer and that the specificity for Lys residue modification remains a function of substrate pKa values.
The appearance of Gcn5l1 as a candidate vertebrate mitochondrial protein acetyltransferase may constitute evidence that inhibitory hyperacetylation of mitochondrial proteins is not a flaw in vertebrate mitochondrial protein function, but rather a feature of our biology only recently exposed to an environment of persistent energy excess. Indeed, the evolutionary benefit of retarding fuel utilization and promoting fat storage when energy is in excess may have favored ancestors in which key enzyme mutations put Lys residues in harm’s way by reducing pKa and increasing susceptibility to acetylation at high Ac-coA. This process may have predated the appearance of vertebrate Gcn5l1 by hundreds of millions of years.
If mitochondrial hyperacetylation occurs during overnutrition, how can mitochondrial hyperacetylation also occur during undernutrition?
One of the paradoxes of mitochondrial protein acetylation is that not only does a high fat diet produce this phenotype (Hirschey et al., 2011), but calorie restriction also induces mitochondrial protein acetylation (Schwer et al., 2009, Hebert et al., 2013). Though targets are not necessarily the same in different tissues, liver—the best studied organ for analysis of mitochondrial protein acetylation—is subject to hyperacetylation in overnutrition and in calorie restriction, which we term macronutrient undernutrition. Deletion of Sirt3 and overnutrition both produce a fatty liver phenotype (Hirschey et al., 2011), suggesting that Sirt3 is responsible for removing acetylation marks that occur as a result of overfeeding and the “mitochondria are full” metabolic signature. Interestingly, the hyperacetylation signature from calorie restriction is not the same as that of Sirt3 deletion and it was confirmed that Sirt3 expression is increased upon calorie restriction (Hebert et al., 2013).
One way to explain this would be to invoke an overnutrition-induced mitochondrial acetyltransferase and a distinct undernutrition-induced mitochondrial acetyltransferase. This would depend on discovery of such enzymes or, potentially, different specificity factors that work with Gcn5l1. However, the chemical modification theory can also account for hyperacetylation during undernutrition and can account for the qualitative differences between accumulated acetylation marks in the two conditions.
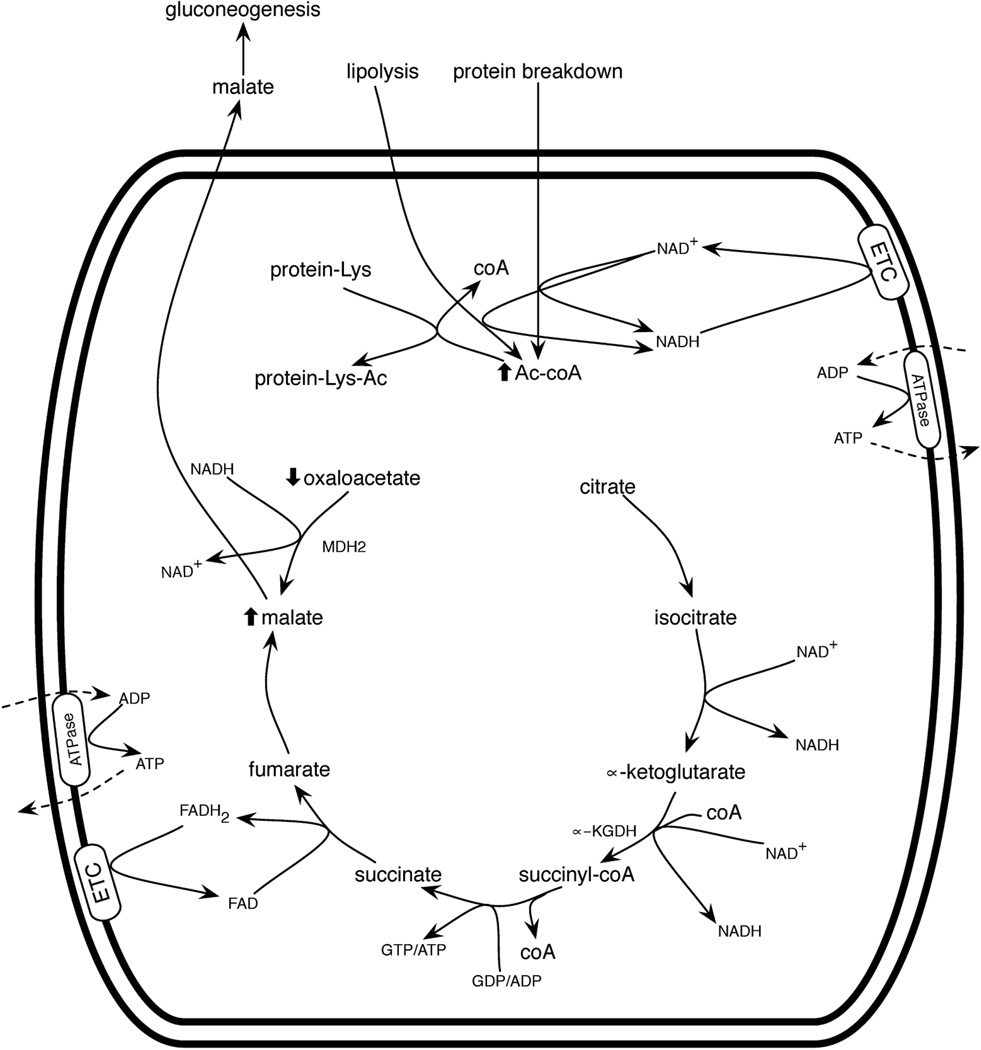
During macronutrient undernutrition and ketogenic diets, the liver enters a ketotic state in which fatty acids and amino acids are oxidized. However, fat-derived Ac-coA cannot run a complete cycle when the malate produced is drawn off for gluconeogenesis. Under these conditions, Ac-coA from fat and muscle breakdown accumulates, but there is virtually no mitochondrial oxaloacetate. Just as carbohydrate limitation results in liver production of ketone bodies from high mitochondrial Ac-coA (McGarry and Foster, 1980), calorie restriction would elevate mitochondrialCharles Brenner Page 22 8/16/13 Ac-coA. Unlike the “mitochondria are full” metabolic signature of high Ac-coA, high NADH and high ATP, the “mitochondria are ketotic” signature would retain levels of NAD+ and ADP to run the cycle but would be low in oxaloacetate (Figure 5). If mitochondrial protein acetylation is largely driven by high Ac-coA, then underfed and overfed mitochondria might exhibit similar rates of acetylation. However, because Sirt3 expression increases in response to underfeeding (Shi et al., 2005, Palacios et al., 2009, Hirschey et al., 2010) and decreases in response to overfeeding (Palacios et al., 2009, Hirschey et al., 2011), then acetylation during calorie restriction would be expected to be more dynamic: the net result of a high on-rate and a high off-rate. In contrast, acetylation in high fat diet-fed animals would tend not to be reversed as rapidly.
Figure 5.
Mitochondrial function during macronutrient undernutrition. When carbohydrates become low, mitochondria oxidize fats and protein from bodily stores. Lipolysis and amino acid catabolism produce mitochondrial Ac-coA, which enters the citric acid cycle. However, under conditions of low blood glucose, mitochondria in gluconeogenic tissues export malate such that oxaloacetate is depleted, leading to Ac-coA accumulation. In liver, ketone bodies are produced (not shown). Elevated Ac-coA leads to protein acetylation.
What are the specific hyperacetylation signatures of Sirt3 deletion and calorie restriction?
A recent study used quantitative acetylomic methods to characterize the liver mitochondrial proteome as a function of calorie restriction and Sirt3 deletion. Though calorie restriction increases acetylation-site occupancy of 135 acetyl sites by >2-fold when compared to the control diet, another 100 peptides exhibited decreased acetylation under calorie restriction (Hebert et al., 2013). This is consistent with the idea that acetylation and deacetylation are simultaneously increased in calorie restriction—the net increase in acetylation may represent the class of peptides that are relatively resistant to Sirt3 activity. The majority of sites with decreased acetylation in calorie restriction show dramatically increased acetylation (8- to 100-fold) in Sirt3 knockout animals (Hebert et al., 2013). These sites are apparently the ones that are readily deacetylated by Sirt3—they may also represent the sites that would accumulate in overfed animals as the “mitochondria are full” signature takes hold and Sirt3 expression level declines.
It is important to remember that a protein Lys deacetylase does not control the substrates it sees. Unlike a protein kinase that is faced with a variety of protein Ser, Thr and Tyr residues, and whose specificity is determined by its kcat/Km for each of those sites, a deacetylase encounters a protein decorated with acetylated Lys residues at particular locations. Thus, Sirt3 can erase (or not) the marks that are made but it doesn’t have the opportunity to make any specific marks on its own. We contend that the specificity of protein acetylation—as the marks go on—is driven by chemistry and protein evolution, and that the difference in accumulated acetylation marks is a function of which sites are relatively resistant to Sirt3. Sirt3-resistant sites would accumulate during calorie restriction when Sirt3 is up-regulated. Moreover, we would expect that if acetylation is driven by substrate pKa values and nonperturbed Lys residues have pKa values of 10.4, then undernutrition-induced acetylation sites will have evolved in the same way as overnutrition-induced sites: by providing a fitness benefit to the organism to become modified in conditions of elevated Ac-coA.
Despite the tendency of mitochondrial protein acetylation to hit active site residues and impair enzyme function, we contend that this provides a fitness benefit to overfed organisms because covalent modification of mitochondrial function would last longer than the transient increase in mitochondrial Ac-coA. In the case of overnutrition, this would tend to shunt citrate to the cytoplasm to promote lipogenesis. In the case of undernutrition, mitochondrial Ac-coA will rise due to a shortage of oxaloacetate, but fasting conditions would promote lipolysis and muscle wasting to provide malate and glucogenic amino acids for blood glucose. As soon as carbohydrates become available, one would expect that citric acid cycle intermediates would be replenished and complete Ac-coA oxidation would be restarted. However, if mitochondrial Ac-coA acetylates the mitochondrial proteome, then respiration in the tissues of a calorie restricted individual would tend to stay slow over the period of time required to remove the acetyl marks. Thus, just as AMP-dependent protein phosphorylation creates a covalent memory of cellular hunger, which lasts longer than the initiating AMP signal, mitochondrial protein acetylation is postulated to create a memory of overnutritional and undernutritional imbalances that are initially signaled by high Ac-coA and low oxaloacetate.
The evolutionary logic of retarding mitochondrial function in the overfed individual is to maintain lipogenesis while the surfeit of food winds down. The evolutionary logic of retarding mitochondrial function in the underfed individual is to slow metabolism even when food reappears. In both cases, though acetyl groups appear to be wasted by virtue of attaching them to protein sidechains, the anticipated result is overall resource conservation that can maintain viability. Accordingly, this model views undernutrition-associated and overnutrition-associated acetylation sites as largely inhibitory modifications that promote survival of an organism even though mitochondrial function is attenuated.
There are two ways to depress the pKa of a Lys. Positive charges can be located proximal to the Lys or the Lys amino group can be desolvated.
Remarkably, by quantitative analysis, Sirt3-responsive sites, which one would predict to accumulate during overnutrition, are overrepresented with Arg, Lys and Lys in positions 1, 2 and 3 amino acids carboxyl to the acetyl modification, respectively. Moreover, these sites are overrepresented on α–helices (Hebert et al., 2013). Placement of a positive charge one helical turn away from Lys is an excellent way to depress the Lys pKa. Because of their depressed pKa values, such sites would be expected to be modified during elevated mitochondrial Ac-coA and further accumulate when Sirt3 is genetically deleted or declines due to overfeeding.
Just as strikingly, the calorie restriction-induced sites were found to be located in hydrophobic sequences predicted to be inaccessible to Sirt3 (Hebert et al., 2013). These Lys residues potentially have their pKa values depressed due to partial desolvation (Isom et al., 2011). We predict that such lone pair-bearing Lys residues are reactive with small molecules such as Ac-coA and acyl-coAs but, because of their partially buried locations, do not permit enzymes such as Sirt3 to relieve acetylation/acylation (Figure 6).
Figure 6.
Net acetylation during macronutrient overnutrition and macronutrient undernutrition. As depicted in Figures 2 and 5, overnutrition and undernutrition can produce high mitochondrial Ac-coA and protein acetylation of susceptible Lys residues. In this schematic, two of four Lys residues have depressed pKa values, depicted as K: to represent the lone electron pair of a susceptible amino group. Two Lys residues without perturbed pKa values and an Arg are depicted as positively charged. One susceptible Lys is on an α-helix, one turn away from Arg. The other susceptible Lys is buried and partially desolvated. Under conditions of overnutrition, Sirt3 expression declines, such that both classes of acetylation are sustained. Under conditions of undernutrition, Sirt3 expression increases, leading to deacetylation of the exposed but not the buried Lys residue.
Precedents for high occupancy chemical modification
Biochemists have been trained to think that all important reactions in biology are enzyme catalyzed. However, the nonenzymatic glycosylation, termed glycation, of hemoglobin is site-specific and occurs at an occupancy of 5 to 15%, depending on blood glucose concentration, in human beings. More than 20 years ago, the site specificity of hemoglobin glycation was shown to vary with the pKa of the reactive amino groups (Acharya et al., 1991). More recently, 1,3-bisphosphoglycerate has been shown to chemically modify lysine residues in glycolytic enzymes in a manner that depends on target site reactivity and that retards glycolytic flux (Moellering and Cravatt, 2013).
Conclusions
Macronutrient undernutrition and overnutrition can both produce mitochondrial metabolic profiles that elevate Ac-coA. Whereas Ac-coA and oxaloacetate initiate the citric acid cycle, nutritional imbalances tend to deplete oxaloacetate either because it is needed for gluconeogenesis or because the overfed mitochondria are too reducing. Because the mitochondrial coA pool is saturable, undernutrition conditions will tend to drive Ac-coA formation from fat and protein stores, whereas overnutrition conditions will tend to drive Ac-coA formation from ingested calories. Though the citric acid cycle cannot run without oxaloacetate or free coA, mitochondrial metabolism would bear no memory of recent nutritional imbalances without the formation of covalent impressions of these metabolic states. Discovery of mitochondrial protein acetylation that is caused by episodes of undernutrition and overnutrition prompted us to consider how such phenomena evolved, particularly without any reported evidence of mitochondrially localized protein Lys acetyltransferases in yeast or invertebrates. Here we have proposed that modern organisms have mitochondrial enzymes with a number of Lys residues that were subject to selective pressure to depress pKa in order to render such residues sensitive to elevated Ac-coA. This mechanism would tend to slow the recovery of mitochondrial function to returning nutrients for organisms experiencing undernutrition and maintain a state of lipogenesis in organisms that have experienced overnutrition. In both cases, efficient mitochondrial reactivation is expected to depend on the activity of Sirt3 and mitochondrial NAD+ synthesis from a precursor such as NR that resupplies the mitochondrial NAD+ precursor, NMN, and which does not inhibit sirtuins.
Significantly, diets that are rich in processed foods may be relatively deficient in NAD+ precursors. Individuals who practice a ketogenic diet and individuals whose energy intake chronically exceeds their energy expenditure might be impairing mitochondrial function if their mitochondrial proteomes are heavily acetylated and acylated by related modifications. Such people would be expected to benefit from supplementation with NAD+ precursors to promote the deacetylation and activation of mitochondrial functions. In the case of individuals who are in chronic over-macronutrient nutritional states, an increase in the mitochondrial pools of NAD+ and Ac-coA might also contribute to increased fuel oxidative capacity independent of sirtuin functions, simply by elevating levels of coenzymes to activate fuel inputs and transfer reducing equivalents through the ETC.
This thesis—that animals have evolved mechanisms that impair mitochondrial function in order to preserve body mass—has struck some as contradictory. How can an evolved system impair something as central as mitochondrial function, including fuel oxidation and the ability to detoxify reactive oxygen species? Indeed, it is easy to cite data, which suggest that the epidemic of obesity will lead to a decline in life expectancy (Olshansky et al., 2005), which is clearly a measure of fitness, though not reproductive fitness. However, the thesis is based on the long evolutionary history of animal evolution, principally in environments of scarcity. The current environment of nutritional excess is not a driver of the evolution of lysine pKa values in mitochondrial proteins. The current environment is not one to which people or our companion animals are well adapted.
Acknowledgments
The authors thank members of the Brenner laboratory, reviewers, and multiple conferees at NAD Metabolism & Signaling and Evolution in Core Processes in Gene Regulation for encouragement and helpful discussions.
SG was supported by a Center for Biocatalysis and Bioprocessing training grant. Work on reversible acetylation in the CB laboratory was supported by the National Science Foundation and the Roy J. Carver Trust.
Footnotes
Declaration of Interest statement
The authors report no conflicts of interest.
References
- Acharya AS, Roy RP, Dorai B. Aldimine to ketoamine isomerization (Amadori rearrangement) potential at the individual nonenzymic glycation sites of hemoglobin A: preferential inhibition of glycation by nucleophiles at sites of low isomerization potential. J Protein Chem. 1991;10:345–358. doi: 10.1007/BF01025633. [DOI] [PubMed] [Google Scholar]
- Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky P, Bogan KL, Brenner C. NAD(+) metabolism in health and disease. Trends Biochem Sci. 2007a;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Belenky P, Racette FG, Bogan KL, Mcclure JM, Smith JS, Brenner C. Nicotinamide Riboside Promotes Sir2 Silencing and Extends Lifespan via Nrk and Urh1/Pnp1/Meu1 Pathways to NAD(+) Cell. 2007b;129:473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/s0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- Bogan KL, Brenner C. Nicotinic Acid, Nicotinamide and Nicotinamide Riboside: A Molecular Evaluation of NAD+ Precursor Vitamins in Human Nutrition. Ann Review Nutrition. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- Bondy SC, Roberts S, Morelos BS. Histone-acetylating enzyme of brain. Biochem J. 1970;119:665–672. doi: 10.1042/bj1190665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The NAD(+) Precursor Nicotinamide Riboside Enhances Oxidative Metabolism and Protects against High-Fat Diet-Induced Obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandon P, Wansink B. Does food marketing need to make us fat? A review and solutions. Nutr Rev. 2012;70:571–593. doi: 10.1111/j.1753-4887.2012.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhoy P, Anderson KA, Herschberg KA, Huhnh FK, Martin AS, Mcdonnell E, Peterson BS, Startzenski LA, Backos DS, Fritz KS, Hirschey MD. Deacetylation by SIRT3 relieves inhibition of mitochondrial function. Springer; 2013. [Google Scholar]
- Des Rosiers C, Fernandez CA, David F, Brunengraber H. Reversibility of the mitochondrial isocitrate dehydrogenase reaction in the perfused rat liver. Evidence from isotopomer analysis of citric acid cycle intermediates. J Biol Chem. 1994;269:27179–27182. [PubMed] [Google Scholar]
- Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, Lin H. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C, Bogan KL, Song P, Burant CF, Kennedy RT, Brenner C. NAD+ metabolite levels as a function of vitamins and calorie restriction: evidence for different mechanisms of longevity. BMC Chem Biol. 2010;10:2. doi: 10.1186/1472-6769-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Dittenhafer-Reed KE, Denu JM. Sirtuin catalysis and regulation. J Biol Chem. 2012;287:42419–42427. doi: 10.1074/jbc.R112.378877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P. The glucose-alanine cycle. Metabolism. 1973;22:179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Grant PA, Eberharter A, John S, Cook RG, Turner BM, Workman JL. Expanded lysine acetylation specificity of Gcn5 in native complexes. J Biol Chem. 1999;274:5895–5900. doi: 10.1074/jbc.274.9.5895. [DOI] [PubMed] [Google Scholar]
- Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, Shortreed MR, Prolla T, Markley JL, Smith LM, Zhao S, Guan KL, Denu JM. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell. 2011;41:139–149. doi: 10.1016/j.molcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. Adenosine monophosphate-activated protein kinase: a central regulator of metabolism with roles in diabetes, cancer, and viral infection. Cold Spring Harb Symp Quant Biol. 2011;76:155–164. doi: 10.1101/sqb.2011.76.010819. [DOI] [PubMed] [Google Scholar]
- Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, Westphall MS, Pagliarini DJ, Prolla TA, Assadi-Porter F, Roy S, Denu JM, Coon JJ. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell. 2013;49:186–199. doi: 10.1016/j.molcel.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen P, Wagner SA, Weinert BT, Sharma S, Bacinskaja G, Rehman M, Juffer AH, Walther TC, Lisby M, Choudhary C. Proteome-wide analysis of lysine acetylation suggests its broad regulatory scope in Saccharomyces cerevisiae. Mol Cell Proteomics. 2012;11:1510–1522. doi: 10.1074/mcp.M112.017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RV, Jr, Kahn CR, Verdin E. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44:177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom DG, Castaneda CA, Cannon BR, Garcia-Moreno B. Large shifts in pKa values of lysine residues buried inside a protein. Proc Natl Acad Sci U S A. 2011;108:5260–5265. doi: 10.1073/pnas.1010750108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284:E671–E678. doi: 10.1152/ajpendo.00492.2002. [DOI] [PubMed] [Google Scholar]
- Jitrapakdee S, St Maurice M, Rayment I, Cleland WW, Wallace JC, Attwood PV. Structure, mechanism and regulation of pyruvate carboxylase. Biochem J. 2008;413:369–387. doi: 10.1042/BJ20080709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, Yang J, Moore SA, Scholz TD, Strack S, Mohler PJ, Sivitz WI, Song LS, Anderson ME. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491:269–273. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, Mcconnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi R, Zhang YM, Rock CO, Jackowski S. Coenzyme A: back in action. Prog Lipid Res. 2005;44:125–153. doi: 10.1016/j.plipres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr, Weissman S, Verdin E, Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantena SK, King AL, Andringa KK, Eccleston HB, Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol Med. 2008;44:1259–1272. doi: 10.1016/j.freeradbiomed.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Mcgarry JD, Leatherman GF, Foster DW. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978;253:4128–4136. [PubMed] [Google Scholar]
- Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- Moellering RE, Cravatt BF. Functional lysine modification by an intrinsically reactive primary glycolytic metabolite. Science. 2013;341:549–553. doi: 10.1126/science.1238327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, He W, Verdin E. Mitochondrial Protein Acylation and Intermediary Metabolism: Regulation by Sirtuins and Implications for Metabolic Disease. J Biol Chem. 2012 doi: 10.1074/jbc.R112.404863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforov A, Dolle C, Niere M, Ziegler M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem. 2011;286:21767–21778. doi: 10.1074/jbc.M110.213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, Zwaans BM, Tishkoff D, Ho L, Lombard D, He TC, Dai J, Verdin E, Ye Y, Zhao Y. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10:M111 012658. doi: 10.1074/mcp.M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohl C, Pelzer W, Diekert K, Kmita H, Bedekovics T, Kispal G, Lill R. The yeast mitochondrial carrier Leu5p and its human homologue Graves' disease protein are required for accumulation of coenzyme A in the matrix. Mol Cell Biol. 2001;21:1089–1097. doi: 10.1128/MCB.21.4.1089-1097.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- Santo-Domingo J, Demaurex N. Perspectives on: SGP symposium on mitochondrial physiology and medicine: the renaissance of mitochondrial pH. J Gen Physiol. 2012;139:415–423. doi: 10.1085/jgp.201110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastri M, Haushalter KJ, Panneerselvam M, Chang P, Fridolfsson H, Finley JC, Ng D, Schilling JM, Miyanohara A, Day ME, Hakozaki H, Petrosyan S, Koller A, King CC, Darshi M, Blumenthal DK, Ali SS, Roth DM, Patel HH, Taylor SS. A kinase interacting protein (AKIP1) is a key regulator of cardiac stress. Proc Natl Acad Sci U S A. 2013;110:E387–E396. doi: 10.1073/pnas.1221670110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve AA, Wolberger C, Schramm VL, Boeke JD. The Biochemistry Of Sirtuins. Annu Rev Biochem. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- Scherer T, O'hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, Zielinski E, Vempati P, Su K, Dighe S, Milsom T, Puchowicz M, Scheja L, Zechner R, Fisher SJ, Previs SF, Buettner C. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13:183–194. doi: 10.1016/j.cmet.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382:790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc Natl Acad Sci U S A. 2006;103:10224–10229. doi: 10.1073/pnas.0603968103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B, Eckersdorff M, Li Y, Silva JC, Fermin D, Kurtev MV, Giallourakis C, Comb MJ, Alt FW, Lombard DB. Calorie restriction alters mitochondrial protein acetylation. Aging Cell. 2009;8:604–606. doi: 10.1111/j.1474-9726.2009.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott I, Webster BR, Li JH, Sack MN. Identification of a molecular component of the mitochondrial acetyltransferase programme: a novel role for GCN5L1. Biochem J. 2012;443:655–661. doi: 10.1042/BJ20120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280:13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- Shimazu T, Hirschey MD, Hua L, Dittenhafer-Reed KE, Schwer B, Lombard DB, Li Y, Bunkenborg J, Alt FW, Denu JM, Jacobson MP, Verdin E. SIRT3 deacetylates mitochondrial 3-hydroxyl-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 2010;12:654–661. doi: 10.1016/j.cmet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes Mcdonald W, Olivier AK, Spitz DR, Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trammell SA, Brenner C. Targeted, LCMS-based Metabolomics for Quantitative Measurement of NAD+ Metabolites. Computational and Structural Biotechnology Journal. 2013;4:e201301012. doi: 10.5936/csbj.201301012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Wegener G, Krause U. Different modes of activating phosphofructokinase, a key regulatory enzyme of glycolysis, in working vertebrate muscle. Biochem Soc Trans. 2002;30:264–270. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Weinert BT, Wagner SA, Horn H, Henriksen P, Liu WR, Olsen JV, Jensen LJ, Choudhary C. Proteome-wide mapping of the Drosophila acetylome demonstrates a high degree of conservation of lysine acetylation. Sci Signal. 2011;4:ra48. doi: 10.1126/scisignal.2001902. [DOI] [PubMed] [Google Scholar]
- Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem. 2012;287:14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Marmorstein R. Histone acetyltransferases: Rising ancient counterparts to protein kinases. Biopolymers. 2013;99:98–111. doi: 10.1002/bip.22128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]