Abstract
Background
Although several novel agents are currently in clinical trials for eosinophilic disorders, none has demonstrated efficacy in reducing blood and tissue eosinophilia in all subjects. Additional approaches are clearly needed.
Objective
To explore the potential of the human eosinophil surface receptor, EGF-like module containing mucin-like hormone receptor 1 (EMR1), as a therapeutic target for eosinophilic disorders.
Methods
EMR1 expression was assessed in blood and bone marrow specimens from eosinophilic and normal subjects, cell lines, CD34+ cells differentiated in vitro, and tissue biopsies using flow cytometry, quantitative PCR and immunostaining. Eosinophil targeting by a novel humanized afucosylated anti-EMR1 IgG1 was evaluated in vitro by NK killing assay and in vivo in cynomolgus monkeys.
Results
Analysis of blood and bone marrow cells from normal and eosinophilic donors and in vitro differentiated CD34+ cells confirmed restriction of human EMR1 surface and mRNA expression to mature eosinophils. Tissue eosinophils also expressed EMR1. Although EMR1 was highly expressed on eosinophils from all subjects, surface expression was negatively correlated with absolute eosinophil count (AEC) (r = -0.46, P <0.001) and soluble plasma levels correlated positively with AEC (r= 0.69, P<0.001), suggesting modulation of EMR1 in vivo. Nevertheless, afucosylated anti-EMR1 mAb dramatically enhanced NK killing of eosinophils from normal and eosinophilic donors and induced a rapid and sustained depletion of eosinophils in monkeys.
Conclusion
EMR1 expression is restricted to mature blood and tissue eosinophils. Targeting of eosinophils using afucosylated anti-EMR1 antibody shows promise as a treatment of eosinophilic disorders.
Keywords: EMR1, eosinophil, hypereosinophilic syndrome, monoclonal antibody therapy, afucosylated antibody, eosinophilia
Introduction
Eosinophils are multifunctional leukocytes implicated in the pathogenesis of a wide variety of disorders, including asthma, helminth infections, hematologic malignancies, and hypereosinophilic syndromes (HES)1. Although glucocorticoids are first-line therapy for many of these disorders, long-term toxicity is common, and resistance can occur. Whereas clinical trials of novel therapeutic agents targeting eosinophils, including monoclonal antibodies to IL-5 (mepolizumab and reslizumab) and IL-5 receptor α (IL-5Rα; benralizumab)2, 3 have demonstrated the safety of eosinophil-depletion in the treatment of various eosinophilic disorders, including eosinophilic asthma, eosinophilic esophagitis, HES and eosinophilic granulomatosis with polyangiitis, none has shown complete elimination of tissue eosinophils or clinical symptoms. Clearly, additional targeted therapies are needed.
Human epidermal growth factor (EGF)-like module containing mucin-like hormone receptor 1 (EMR1) is a surface receptor of unknown function that belongs to the EGF-seven-transmembrane (EGF-TM7) family of G-protein coupled receptors4. Other family members include EMR2 and CD97, molecules involved in modulation of neutrophil activation and leukocyte migration to sites of inflammation5-7. The human ortholog of F4/80, which is expressed on murine monocytes, macrophages, myeloid dendritic cells and eosinophils8, human EMR1 has been reported to be expressed exclusively on eosinophils9 and to be highly expressed in nasal polyps10. EMR1 is therefore, an ideal potential target for the treatment of eosinophilic disorders.
In the present study, we describe the expression profile of EMR1 in the blood, bone marrow and tissues of normal donors and subjects with a variety of eosinophilic disorders. Modulation of the receptor was also explored ex vivo and in vitro. Finally, the efficacy of an afucosylated chimeric antibody to EMR1 to target eosinophils for NK-mediated antibody-dependent cell lysis was assessed in vitro and in vivo in a primate model.
Methods
Anti-EMR1 monoclonal antibodies
Recombinant extracellular domains (ECD) from human and cynomolgus monkey EMR-1 were expressed as Fc-fusion proteins in CHO cells. After purification on protein-A columns, Fc tags were proteolytically removed using Factor Xa, and human EMR1 ECD was used for immunization in mice. The mouse hybridoma line, 1E7, which expresses high-affinity anti-EMR1 monoclonal antibody was grown in Hybridoma SFM media (Invitrogen). Afucosylated and fucosylated chimeric 1E7 antibodies with human IgG1 kappa constant regions were expressed in Potelligent CHOK1SV (Biowa/Lonza)11. Murine and chimeric 1E7 antibodies were purified by protein-A affinity chromatography.
Study subjects
Eosinophilic subjects (EOS, n=38) underwent detailed clinical and laboratory evaluation as part of an Institutional Review Board (IRB)–approved clinical protocol to study eosinophilia (NCT00001406) and included subjects with idiopathic HES (n=18), lymphocytic variant HES (n=7), helminth infection (n=4), hypereosinophilia of unknown significance (n=3), PDGFRA-associated myeloproliferative neoplasm (n=3) and familial eosinophilia (n=2). Six subjects were on therapy at the time of study, but had persistent eosinophilia (geometric mean (GM) absolute eosinophil count (AEC) 2167/μL, range 910-13000/μL). Healthy volunteers were recruited under an IRB-approved clinical protocol designed to obtain controls for in vitro research (NCT00090662). All participants gave written informed consent.
Cell purification
Granulocytes and peripheral blood mononuclear cells (PBMC) were separated by sedimentation over Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). Erythrocytes were lysed by hypotonic shock with ice-cold ddH2O (for granulocytes) or ACK lysing buffer (for PBMCs). Individual cell populations were purified using magnetic bead selection on an AutoMacs (Miltenyi Biotech, Cambridge, MA) according to the manufacturer's instructions. Eosinophils and neutrophils were purified from the granulocyte layer using the Eosinophil Isolation Kit. NK cells, CD14+ monocytes and CD34+ stem cells were purified from the PBMC layer using the NK Cell Isolation Kit, anti-CD14 beads and anti-CD34 beads, respectively (Miltenyi Biotech). Granulocyte purity was determined by counting a minimum of 300 cells on cytospin preparations stained with Diff-Quik (Siemens Healthcare Diagnostics). Purity of other cells was determined by flow cytometry. Purity was >98% for all cell populations studied. Cells for RNA expression analysis were counted and put directly in TriZol Reagent (Invitrogen, Carlsbad, CA) at a concentration of 10×106/ml.
Human cell lines and culture conditions
Purified peripheral blood eosinophils were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS, Biowhittaker), 25 mM HEPES, 2 mM L-glutamine, 10 mM sodium pyruvate, and 50 μg/mL of gentamycin (culture medium (CM)). The leukemic cell line EOL1 (DSMZ Institute, Braunschweig, Germany), the erythroleukemia cell line K562 (ATCC® CCL-243™, Manassas, VA), and the histiocytic lymphoma U937 (ATCC® CRL-1593.2™) were maintained in RPMI 1640 medium with 10% FCS at 37°C. AML14.3D10 (ATCC® CRL-12079™) was maintained in CM containing 50 μM β-mercaptoethanol. CHO cells transfected with EMR1 (CHOK1SV) were cultured in CD-CHO (Invitrogen) supplemented with 25 μM L-methionine sulfoximine.
Detection of surface EMR1 by flow cytometry
EMR1 expression in bone marrow aspirates and peripheral blood was assessed by multiparameter flow cytometry using directly-conjugated antibodies as previously described12 (see Online supplement for detailed methodology).
Real time quantitative PCR
Total RNA was extracted from purified cell populations and cell lines using TriZol Reagent (Invitrogen, Carlsbad, CA), and cDNA was synthesized from 1 μg total RNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) according to the manufacturer's protocol. cDNA from human CD34+ cells cultured under conditions to induce mast cell differentiation13 and from the mast cell lines, HMC-1.1 (lacking KIT D816V), HMC-1.2 (expressing KIT D816V), and LAD2 were provided by Dr. Todd Wilson, NIAID/NIH. Approximately 50 ng of RNA equivalent cDNA template was used per well and real-time amplification was performed in a 96- well plate using a GeneAmp 7900HT Sequence Detection System (Applied Biosystems). Primers used are provided in the Online supplement. Each sample was run in triplicate, and cycle threshold values were normalized using 18S cycle threshold values from corresponding samples.
CD34+cell differentiation in vitro
Human CD34+ cells isolated by positive selection from granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood of two healthy donors (provided by Dr. Harry Malech, NIAID/NIH) were cultured in IMDM (Invitrogen) supplemented with 10% FBS and GM-CSF (1 ng/ml), SCF, Flt3-L and TPO (each at 10 ng/ml), IL-5 (25 ng/ml), IL-3 (20 ng/ml) for 3 days, followed by IL-5 (25 ng/ml) only for another 21 days. Cells were collected on days 4, 6, 8, 10, 12, 15, 18, 21, and 24 of culture, and total RNA and cell lysates prepared at each time point. Eosinophil differentiation was assessed using Diff-Quik (Siemens Healthcare Diagnostics) and Fast Green/Neutral Red (Sigma-Aldrich) staining. At 21 days of culture, >60% of the cells were eosinophil-like by morphologic criteria and >20% were mature eosinophils based on Fast Green staining of cytoplasmic granules. Growth and viability were determined by light microscopic evaluation with trypan blue exclusion. Viability remained >95% throughout the 24-day experimental period.
Tissue immunostaining
A 5 mm skin punch biopsy obtained from a patient with HES was bisected. One half of the specimen was fixed in neutral buffered formalin, routinely processed, sectioned, and stained with Hematoxylin & Eosin (H&E) per standard procedures of the Laboratory of Pathology, NCI. Immunohistochemical staining for eosinophil peroxidase (EPX) was performed using a mouse anti-mouse monoclonal antibody (1:500, provided by Dr. J.J. Lee). The second portion was snap frozen for immunostaining with anti-EMR1 antibody (c1E7) using standard techniques (see Online supplement for detailed methodology).
Acetone-fixed nasal polyp frozen tissue section slides obtained from Capital Biosciences (female patient; CRSwNP and bronchial asthma; 39 years old) were stained with mouse 1E7-Alexa595 (0.6 μg/ml), mouse anti-EPX antibody (Abcam; diluted 1:200 with 2% BSA with PBS) and/or mouse anti-CD68 (Ventana; clone KP-1, diluted 1:2) using standard techniques for visualization by immunofluorescence microscopy (see Online supplement for detailed methodology).
Measurement of soluble receptors in plasma
Plasma levels of sEMR1 were measured by sandwich ELISA (MyBiosources). All assays were performed in singlicate, and values were calculated on the basis of a standard curve. The minimal detectable concentration of sEMR1 in plasma was 10 pg/mL.
In vitro killing assay
Purified eosinophils (8×104, target) were incubated with autologous NK cells (effector) at an effector/target ratio of 5:1 in 96-well U-bottom plates at 37°C in the presence or absence of 10 μg/ml afucosylated c1E7 or IgG1 isotype control antibodies. After 4 hours, the assay was stopped by putting the plates on ice and replacing the culture medium with binding buffer (100 μ1/well, BD Biosciences). Annexin V FITC (BD Biosciences) was added and after 15 min incubation at room temperature, samples were acquired and analyzed on a flow cytometer (LSRII, BD Biosciences). Eosinophils were identified based on their granularity (high side scatter). ADCC was determined by gating on Annexin V–positive target cells.
Administration of anti-EMR1 antibody in non-human primates
Male cynomolgus macaques received a single intravenous infusion of c1E7 at either 1 mg/kg (n=2) or 5 mg/kg (n=2) at Bio-Quant (San Diego) under a protocol approved by Bio-Quant's IACUC and the attending veterinarian. Whole blood was collected for PK analysis and eosinophil count at baseline, 5 minutes, 2,4, and 8, hours and 1,2,4,8,15,22,and 28 days after dosing. The plasma concentration of c1E7 was measured using a quantitative antigen-based ELISA (see Online Supplement for detailed methods). Complete blood counts and serum chemistries were performed pre-dose and on days 8, 28, 42 and 56.
Statistical analysis
Statistical analyses were performed using the non-parametric Mann-Whitney U and Wilcoxon tests for unpaired and paired comparisons of group means, respectively, and Spearman rank for correlation. P<0.05 was considered significant for all analyses. Pharmacokinetic analysis was performed using a non-compartmental model (see Online Supplement).
Results
Surface expression of EMR1 in humans is restricted to eosinophils
To confirm that EMR1 expression in human blood and bone marrow is restricted to eosinophils, flow cytometric analysis was performed using whole blood and bone marrow aspirates from normal donors and eosinophilic subjects using monoclonal antibody 1E7 generated against recombinant EMR1 extracellular domain. As reported previously, using a different anti-EMR1 monoclonal antibody9, EMR1 expression was detected exclusively on eosinophils (CD16-CD9+ granulocytes in blood and IL5Rα+CD16- siglec-8+ granulocytes in bone marrow aspirates) in all subjects tested (Fig 1, A-D). EMR1-positive cells were also positive for IL-5Rα, Siglec 8, CD9, CD15, CD45, and CD49d, and negative for CD3, CD14, CD16, CD19, CD34, CD64, and CD203 (data not shown). EMR1 was not detected on mast cells, basophils, monocytes, neutrophils, lymphocytes, the monocytic cell line U937, the erythroleukemic cell line K562, or either of two eosinophilic leukemia cell lines, Eol-1 and AMLD14.3D10 (Fig 1, E); whereas the positive control CHOK1SV cell line (transfected with full length EMR1 gene) showed uniformly high expression of EMR1 (Fig 1, E).
Figure 1. EMR1 is expressed exclusively on eosinophils.
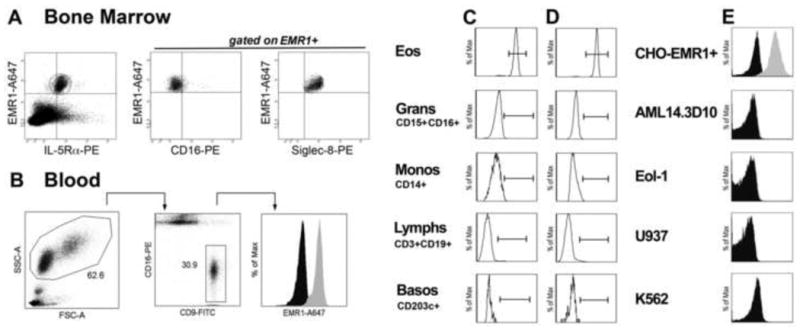
Representative staining of eosinophils using anti-EMR1 antibody in bone marrow (A) and blood (B) from a subject with HES. (C-E) Absence of EMR1 expression on cells other than eosinophils in bone marrow (C) and peripheral blood (D) and in cell lines (E). The CHOK1SV cell line (CHO cells transfected with EMR1) is shown as a positive control.
Quantitative reverse transcriptase PCR (qRT-PCR) confirmed the presence of EMR1 mRNA in eosinophils from eosinophilic subjects and normal controls (Table I). EMR1 mRNA expression was not detectable in purified PBMC, monocytes, or neutrophils, CD34+ cells isolated from peripheral blood, mast cells generated by in vitro differentiation of CD34+ cells (data not shown) or any of the human cell lines tested (Table I).
Table I.
EMR1 mRNA expression.
| Cell type | N | EMR1 mRNA* |
|---|---|---|
| EOS Eos | 28 | 19.7±4.6 |
| ND Eos | 11 | 8.9±2.5 |
| PBMC | 12 | 1.6±0.7 |
| Monocytes | 4 | 0.28±0.14 |
| Neutrophils | 4 | 0.06±0.02 |
| CD34+ | 4 | 0.06±0.03 |
| U937 | 1 | 0.045 |
| K562 | 1 | 0 |
| Eol-1 | 3 | 0.08±0.03 |
| AML14.3D10 | 3 | 0.20±0.10 |
| CHO-EMR1+ | 3 | 7.97±1.98 |
in arbitrary units (×10-5) related to 18S rRNA (mean ± std error)
EMR1 is expressed late in eosinophil development
EMR1 surface expression was examined on CD34+ cells in bone marrow aspirates from 9 eosinophilic and 2 normal donors (representative staining shown in Fig2, A) and on purified CD34+ cells isolated from peripheral blood of 4 normal donors (representative staining shown in Fig 2, B). Although a small population (1-4%) of IL-5Rα+CD34+ cells was identified, EMR1 expression was not detected on CD34+ cells in any of the samples studied (Fig 2, A-B). To assess the kinetics of EMR1 expression during eosinophilopoiesis, purified peripheral blood CD34+ cells from 2 donors were differentiated in vitro under conditions known to promote eosinophil development. By day 12 after the addition of IL-5, surface expression of CD34 was no longer detected by flow cytometry (Fig 2, C), and approximately 20% of the cells were mature eosinophils (Fast Green-positive) (Fig 2, D). As previously described14, 15, expression of mRNA for eosinophil granule proteins and IL-5Rα increased during the culture period, beginning approximately 3-4 days after addition of IL-5 and peaking at 10 days (Fig 2, F-G). Surface expression of EMR1 rose steadily beginning at approximately 8 days after addition of IL-5 (Fig 2, E and E1, B), and double-staining confirmed that EMR1-positive cells were also positive for IL-5Rα and siglec-8, consistent with an eosinophil phenotype (Figure E1, C). EMR1 mRNA expression was first detected at day 4 and slowly increased throughout the culture period (Fig 2, G).
Figure 2. EMR1 is expressed late in eosinophil development.
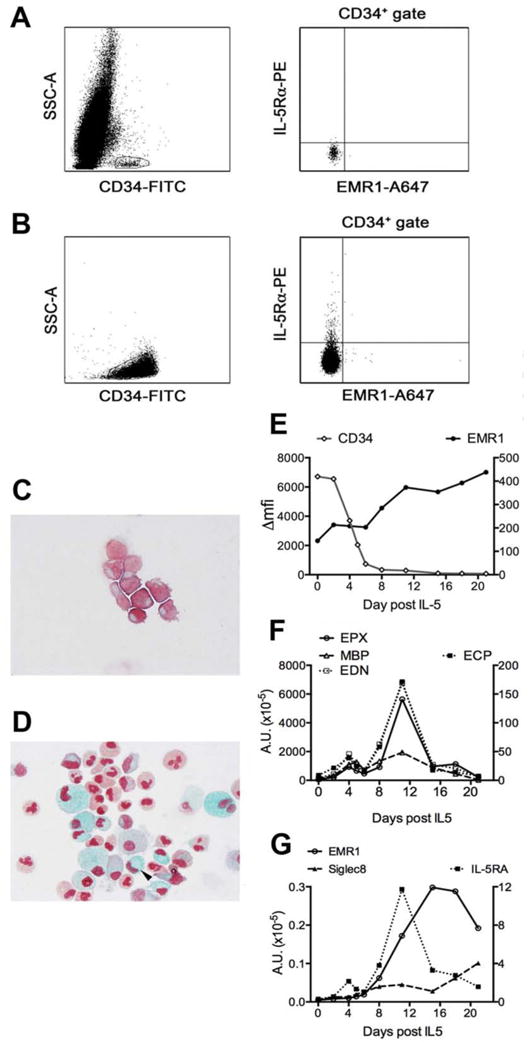
(A,B) Surface expression of IL-5Rα, but not EMR1, is detected on CD34+ cells in bone marrow from a patient with HES A) and on purified CD34+ cells isolated from peripheral blood (B). (C-G) In vitro differentiation of CD34+ cells. Fast-green/neutral red staining of cultured CD34+ cells 2 days prior to (C) and 21 days after (D) the addition of IL-5. The arrow indicates a mature eosinophil with bilobed red nucleus and green secondary granules. Surface expression of EMR1 and CD34 as a function of time (E). mRNA expression as a function of time for EPX, MBP, and EDN (F; left axis), ECP (F; right axis), EMR1 and Siglec-8 (G; left axis) and IL-5Rα (G; right axis).
EMR1 is expressed on tissue eosinophils
Since the pathogenesis of eosinophilic disorders is primarily due to tissue infiltration by eosinophils, EMR1 expression on eosinophils in skin and nasal polyp tissue was examined by immunostaining. A formalin-fixed skin biopsy from a subject with HES showed scattered eosinophils (Fig 3, A) with evidence of degranulation demonstrated on immunostaining with anti-EPX antibody (Fig 3, C). Anti-EMR1 antibody staining of an adjacent frozen section identified scattered cells in a pattern consistent with intact eosinophils (Fig 3, B). Immunofluorescence staining of nasal polyp tissue showed co-localization of cells staining positive with anti-EMR1 with EPX-positive eosinophils (Fig 3, D-F), but not CD68-positive macrophages (Fig 3, G-I).
Figure 3. EMR1 expression on tissue eosinophils.
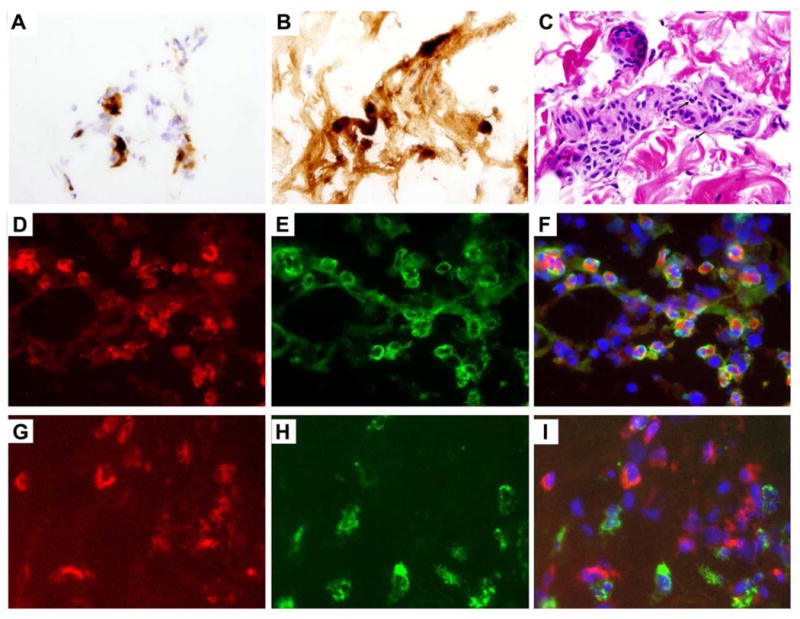
A-C, Skin biopsy from a subject with HES. Frozen section stained with antibody to EMR1 (A). Adjacent paraffin-embedded sections stained with antibody to EPX (B) and with H&E (C) showing eosinophilic infiltration and degranulation. The arrows indicate representative eosinophils (C). (B). Original magnification 600x (A, B,) and 100x (C). D-I, Nasal polyp biopsy. Immunofluorescence staining of EMR1 in red (D,G), EPX (E) or CD68 (H) in green, and overlay (F,I) showing EMR1 staining on eosinophils and not on macrophages. Original magnification 640x.
EMR1 expression is correlated with eosinophilia
Eosinophil surface expression of EMR1 was quantified in whole blood from normal donors (n=16), eosinophilic subjects on no treatment (n=14) and treated subjects with persistent eosinophilia (n=7). Although EMR1 was highly expressed on eosinophils from all subjects, the GM Δmfi [95% CI] was significantly increased in the normal donors (5645 [4006-7956]) compared to either the untreated (2832 [1786-4489]) or treated (2393 [1248-4587]) subjects with eosinophilia (p<0.05; Figure 4A). Furthermore, AEC was negatively correlated with surface expression of EMR1 (r = -0.46, P <0.001; Fig 4, B), suggesting that modulation of EMR1 expression occurs in vivo. To further explore this possibility, surface expression of EMR1 and CD69, an early activation marker, were assessed in vitro on purified eosinophils from 8 eosinophilic subjects and 6 normal donors following incubation for 2 hrs in the presence or absence of IL-5 (1 ng/ml). Whereas CD69 expression was upregulated in response to IL-5 in all subjects, expression of EMR1 decreased in 6/6 eosinophilic and 4/6 normal subjects, and GM Δmfi for EMR1 showed a significant, albeit small, decrease from 3845 to 3475 (P<0.05; Fig E2, A and B).
Figure 4. EMR1 expression in eosinophils is correlated with absolute eosinophil count.
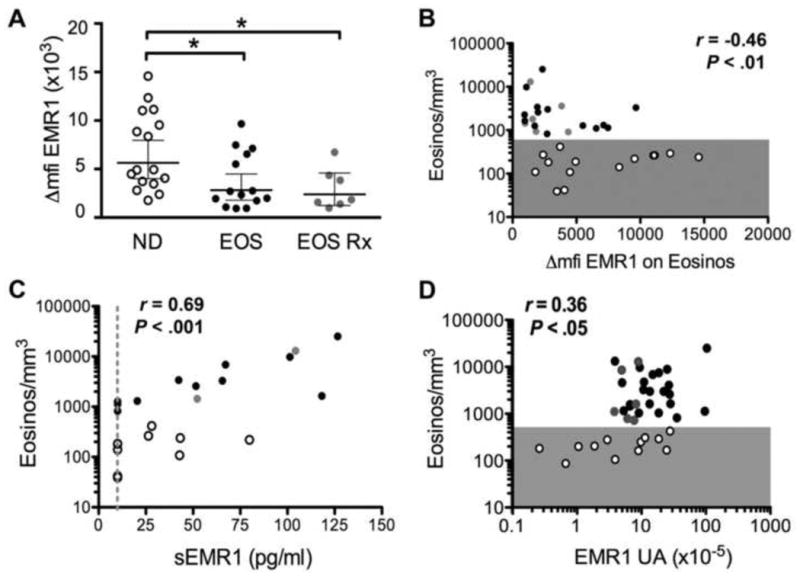
(A) Surface expression of EMR1 on eosinophils in whole blood expressed as the change in mean fluorescence intensity (Δmfi) in normal donors (ND), untreated (EOS) and treated (EOS Rx) subjects with eosinophilia. Geometric means with 95% CI intervals are indicated by horizontal lines. *P <0.05 versus ND. (B-D) Correlation of surface expression of EMR1 (Δmfi), plasma levels of soluble EMR1 (in pg/ml), and EMR1 mRNA levels (expressed as arbitrary units relative to 18S rRNA) with AEC. AEC < 500/mm3 is indicated by gray shading. Each symbol represents data for an individual donor. Normal donors are indicated by open circles, eosinophilic subjects on no treatment by black circles and eosinophilic subjects on therapy by gray circles.
Levels of sEMR1 were assessed by ELISA in undiluted plasma from 13 eosinophilic subjects and 9 normal controls. sEMR1 was detectable in 15 (68%) of the subjects tested, and levels were positively correlated with AEC (Fig 1 C; r=0.69, P<0.001). sEMR1 was not detected in culture supernatants of IL-5 activated eosinophils (n=5; data not shown).
EMR1 mRNA levels were positively correlated with AEC (r=0.36, n=39, P <0.05; Fig 4, D) and were significantly increased in eosinophilic donors as compared to normal donors (P<0.05, data not shown). In vitro stimulation of purified eosinophils with IL-5 at 1ng/ml, overnight or for 6hr, also induced upregulation of EMR1 transcription with GM mRNA levels increasing from 4.4 to 9.7 arbitrary units (×10-5) related to 18S rRNA (n=13, P <0.01, See Fig E2, C).
Eosinophil killing is enhanced in the presence of afucosylated anti-EMR1 antibody
Human IgG1 lacking the monosaccharide fucose in its carbohydrate moiety has been shown to have enhanced ADCC activity due to increased binding affinity for human FcyRIIIa receptor11. Consequently, an afucosylated chimeric anti-EMR1 monoclonal antibody (c1E7) was generated and tested in an in vitro NK cell-mediated killing assay using purified eosinophils as target cells from 4 normal and 2 eosinophilic human donors (Figure 5A and E3). The GM percentage of annexin V+ eosinophils increased from 15.1 to 62.3 in the presence of afucosylated c1E7 (P<0.05), but not in the presence of antibody isotype control (Figure 5A) or fucosylated c1E7 antibody at concentrations up to 10 μg/ml (data not shown). Afucosylated c1E7 had no effect on viability of eosinophils or NK cells when incubated with these cells separately (Fig 5B).
Figure 5.
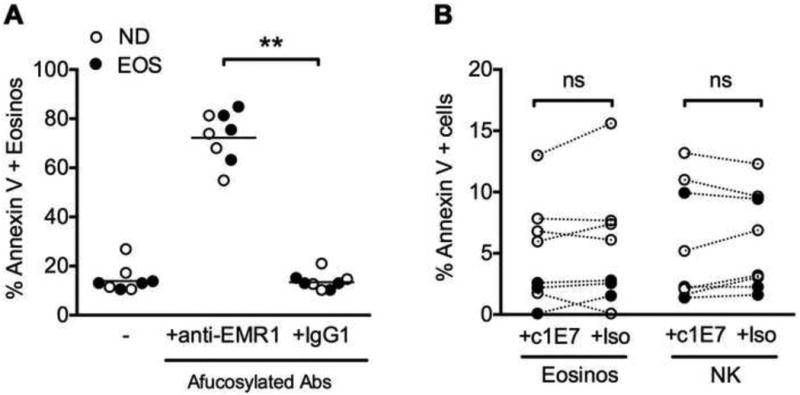
In vitro killing of peripheral blood eosinophils using afucosylated anti-EMR1 monoclonal antibody (c1E7). (A) Eosinophil (target) cell death after 4h incubation with c1E7 or afucosylated isotype control (Iso) and autologous NK cells (effector) (E:T ratio of 5:1). (B) Lack of eosinophil or NK cell death after 4h incubation with antibody alone. Each symbol represents data for an individual subject. Eosinophilic subjects (n=4) are indicated by black circles and normal donors (n=4) by open circles. CM=culture medium, ** P <0.01 (Wilcoxon t test).
Eosinophils are depleted in vivo following a single dose of afucosylated c1E7 antibody
Chimeric antibody, c1E7, binds with similar affinity to recombinant human or cynomolgus macaque EMR1 as determined by ELISA (data not shown). The antibody was administrered intravenously to cynomolgus monkeys at a dose of 1 mg/kg (group 1; n=2) or 5 mg/kg (group 2; n=2). Mean peak plasma concentrations (Cmax) of c1E7 were 41.1 μg/mL for group 1 and 146.7μg/mL for group 2. The plasma concentration of c1E7 showed a bi-exponential elimination profile and a terminal phase in all animals with a mean elimination-phase half-life (t1/2β) of 89 hours for group 1 and 210 hours for group 2. Mean Vc and Vss for each group approximated blood volume consistent with limited tissue distribution (Table E1).
Prior studies in cynomolgus monkeys have demonstrated little variability in the AEC over time in the absence of treatment16, 17. In contrast, peripheral blood eosinophils decreased post-infusion in all 4 animals, becoming undetectable at the first time point measured (8 hours) in group 1 and at 2 hours post-infusion in group 2 (Figure 6 and Table E2). Eosinophil counts returned to normal by day 28 post-infusion in the group 1 animals, but remained below baseline for 28-56 days in the 2 animals that received the 5mg/kg dose (Figure 6). A mild rebound eosinophilia was noted in 3 of the 4 animals at day 42, resolving by day 56 in the one animal (CYN 10-65c) for which data is available (Figure 6 and Table E2). The total leukocyte counts excluding eosinophils remained stable post-treatment, and no clinically significant adverse events were reported after antibody administration. Plasma levels of eosinophil granule proteins (major basic protein, eosinophil cationic protein, eosinophil-derived neurotoxin and eosinophil peroxidase) were measured at each of the time points using a suspension array assay in multiplex (see Supplemental Methods) and showed no consistent trend in the first 24 hours post-infusion, but decreased in all 4 animals over the 28 days post-infusion (Figure E4). Additional pharmacokinetic and safety data can be found in the Online supplement (Tables E1 and E3).
Figure 6.
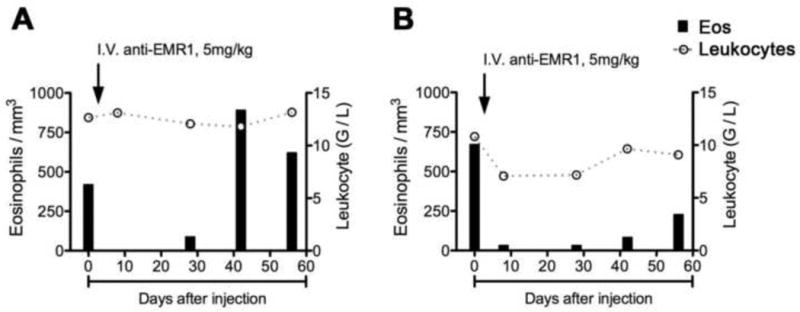
Eosinophil depletion over time following intravenous administration of a single 5mg/kg dose of afucosylated anti-EMR1 monoclonal antibody in two cynomolgus monkeys. Each panel shows the data for an individual monkey (CYNO 10-65c in panel A and CYNO 10-66c in panel B). The AEC (left axis) at each time point is represented by dark columns and the white blood count (right axis) by open circles connected by a dashed grey line.
Discussion
In their recent report, the NIH Taskforce on the Research Needs of Eosinophil-Associated Diseases (TREAD) listed the development of new eosinophil-targeted therapeutics as a research priority18. In this regard, the present study not only confirms previously published data demonstrating that EMR1 expression is restricted to eosinophils9, but expands this finding to eosinophils from patients with a wide variety of eosinophilic disorders and provides the first evidence that an afucosylated monoclonal antibody directed against EMR1 can selectively target and safely eliminate eosinophils in vivo in a primate model. Benralizumab (MedImmune), an afucosylated antibody against IL-5Rα, has already been used in a phase I trial in mild asthmatics, demonstrating that enhanced ADCC can be used to deplete eosinophils in humans17, 19. Although no severe adverse events were reported, none of the subjects in this study had marked eosinophilia (>1500/μL) at baseline.
A major limitation of targeted therapies currently in clinical trials for eosinophilic disorders has been incomplete depletion of tissue eosinophils. This is likely due, at least in part, to alterations in eosinophil surface receptor expression that occur during tissue migration and activation3. In the present study, EMR1 was detected on tissue eosinophils in skin from a patient with HES, nasal polyps and asthmatic lung (data not shown). Of note, EPX staining in the same tissues detected both intact eosinophils and free granules, consistent with eosinophil activation. Together these data suggest that EMR1 expression is preserved in activated tissue eosinophils. Nevertheless, the extent to which afucosylated antibody to EMR1 will deplete these tissue eosinophils awaits further study.
EMR1 offers several advantages as a potential therapeutic target. Unlike many eosinophil surface receptors, including IL-5Rα, EMR1 is not expressed on mast cells or basophils, precluding unanticipated adverse effects due to mediator release during cytolysis of these cells. Similar to siglec-820, EMR1 is expressed relatively late in eosinophil development and is unlikely to deplete CD34+ eosinophil precursors that play a potentially important role in plasma cell homeostasis21, 22. Finally, although the function of EMR1 is unknown, cynomolgus monkeys experienced no clinically significant adverse effects following administration of anti-EMR1 antibody, and mice deficient in F4/80 (the murine homolog of EMR1) are healthy and fertile23.
Modulation of cell surface receptors can occur by a number of different mechanisms, including receptor internalization, proteolytic cleavage, and decreased transcription of mRNA for the membrane form of the receptor. The observed correlation of the peripheral eosinophil count with decreased surface expression of EMR1 on eosinophils and increased plasma levels of sEMR1 supports the hypothesis that EMR1 is shed in vivo in response to signals that promote eosinophilia. The fact that soluble receptor was not detected in culture supernatants despite down-modulation of surface receptor by IL-5 is most likely due to technical constraints related to the concentration of eosinophils in culture, the small change in surface expression in response to IL-5 and the sensitivity of the EMR1 ELISA. Similar findings have been reported for the EGF-TM7 family member, CD97, where increased expression of CD97 in the synovium was accompanied by detectable soluble CD97 in the synovial fluid in subjects with rheumatoid arthritis, but not in normal serum or activated T cell supernatants24, 25. Although the mechanism of receptor shedding was not addressed in this study, autoproteolysis of CD97 and EMR2 has been demonstrated to occur via G-protein coupled receptor proteolytic sites (GPS) in their extracellular domains26. EMR1 also contains a GPS site; however, the sequence is imperfect and functionality of the EMR1 domain has not been demonstrated to date27.
Although decreased surface receptor and increased soluble receptor could both theoretically interfere with the efficacy of a therapeutic antibody to EMR1 in patients with marked eosinophilia, this is unlikely for the following reasons. First, despite the small, but significant, decrease with increasing AEC, eosinophil surface expression of EMR1 remained high in all subjects tested. Second, in vitro killing was equally efficient in subjects with and without eosinophilia. Third, plasma levels of soluble receptor (10-126pg/ml; equivalent to approximately 0.1 – 1.5pM) were several logs lower than the levels of chimeric afucosylated antibody (2.15μg/ml or 14.3nM after 15 days and 11.1 μg/ml or 74nM after 28 days for doses at 1 and 5mg/kg respectively) achieved in pharmacokinetic studies in non-human primates (Table E3).
In summary, EMR1 is expressed exclusively on mature eosinophils in the blood, bone marrow and tissues of patients with eosinophilia regardless of the underlying disorder. Although receptor expression is decreased on peripheral blood eosinophils in the setting of eosinophilia and/or IL-5 exposure, this downmodulation was not sufficient to impair in vitro killing by NK cells in the presence of afucosylated anti-EMR1 antibody. More importantly, administration of a single dose of afucosylated chimeric anti-EMR1 antibody induced rapid and sustained depletion of peripheral blood eosinophils without effect on other peripheral blood cells or evidence of clinical toxicity. Although the number of animals studied was small, these findings suggest that selectively targeting eosinophils using a humaneered® afucosylated anti-EMR1 antibody may provide a safe and effective strategy for the treatment of eosinophilic disorders.
Supplementary Material
Clinical Implications.
Afucosylated antibody to the human eosinophil-specific surface receptor, EMR1, shows promise as a new therapy for eosinophilic disorders.
Acknowledgments
The authors would like to acknowledge Nicole Holland-Thomas, Kathryn Spates and Amara Pabon for their assistance in procuring the subject samples for these studies. We thank Swathi Sujatha-Bhaskar for characterization of EMR1 antibodies, Jason Williams, Wendy Ching and Thomas Chang for EMR1 production and characterization, Kenneth Luehrsen and David Martinez for generating EMR1 DNA constructs.
Funding This research was supported by the Division of Intramural Research of the NIAID, NIH and by KaloBios Pharmaceuticals Inc. G.Y. and M.B. are employed by KaloBios Pharmaceuticals Inc, N.T, J.L, V.P. and C.B were previously employed by and own stock/stock options in KaloBios Pharmaceuticals, Inc.
Abbreviations
- ADCC
antibody-dependent cell mediated cytotoxicity
- AEC
absolute eosinophil count
- CHO
Chinese hamster ovary
- CM
culture medium
- CRSwNP
chronic rhinosinusitis with nasal polyps
- ECD
extracellular domain
- EGF
epidermal growth factor
- EGF-TM7
EGF-seven-transmembrane
- EMR
EGF-like module containing mucin-like hormone receptor
- EOS
eosinophilic donor
- EPX
eosinophil peroxidase
- FBS
fetal bovine serum
- Fc
constant fragment
- Flt3-L
fms-like tyrosine kinase-3 ligand
- G-CSF
granulocyte-colony stimulating factor
- GPS
G protein-coupled receptor proteolytic site
- GM
geometric mean
- HES
hypereosinophilic syndrome
- H&E
hematoxylin & eosin
- IL
interleukin
- IL-5R
interleukin-5 receptor
- IACUC
institutional animal care and utilization committee
- IRB
institutional review board
- MFI
mean fluorescence intensity
- ND
normal donor
- NK
natural killer
- PBMC
peripheral blood mononuclear cells
- PDGFRA
platelet derived growth factor receptor A
- PK
pharmacokinetic
- sEMR1
soluble EMR1
- SCF
stem cell factor
- TPO
thrombopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, Weller PF, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607–12 e9. doi: 10.1016/j.jaci.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wechsler ME, Fulkerson PC, Bochner BS, Gauvreau GM, Gleich GJ, Henkel T, et al. Novel targeted therapies for eosinophilic disorders. J Allergy Clin Immunol. 2012;130:563–71. doi: 10.1016/j.jaci.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bochner BS, Gleich GJ. What targeting eosinophils has taught us about their role in diseases. J Allergy Clin Immunol. 2010;126:16–25. doi: 10.1016/j.jaci.2010.02.026. quiz 6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwakkenbos MJ, Kop EN, Stacey M, Matmati M, Gordon S, Lin HH, et al. The EGF-TM7 family: a postgenomic view. Immunogenetics. 2004;55:655–66. doi: 10.1007/s00251-003-0625-2. [DOI] [PubMed] [Google Scholar]
- 5.Wandel E, Saalbach A, Sittig D, Gebhardt C, Aust G. Thy-1 (CD90) is an interacting partner for CD97 on activated endothelial cells. J Immunol. 2012;188:1442–50. doi: 10.4049/jimmunol.1003944. [DOI] [PubMed] [Google Scholar]
- 6.Yona S, Lin HH, Dri P, Davies JQ, Hayhoe RP, Lewis SM, et al. Ligation of the adhesion-GPCR EMR2 regulates human neutrophil function. FASEB J. 2008;22:741–51. doi: 10.1096/fj.07-9435com. [DOI] [PubMed] [Google Scholar]
- 7.McKnight AJ, Gordon S. The EGF-TM7 family: unusual structures at the leukocyte surface. J Leukoc Biol. 1998;63:271–80. doi: 10.1002/jlb.63.3.271. [DOI] [PubMed] [Google Scholar]
- 8.Gordon S, Hamann J, Lin HH, Stacey M. F4/80 and the related adhesion-GPCRs. Eur J Immunol. 2011;41:2472–6. doi: 10.1002/eji.201141715. [DOI] [PubMed] [Google Scholar]
- 9.Hamann J, Koning N, Pouwels W, Ulfman LH, van Eijk M, Stacey M, et al. EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur J Immunol. 2007;37:2797–802. doi: 10.1002/eji.200737553. [DOI] [PubMed] [Google Scholar]
- 10.Plager DA, Kahl JC, Asmann YW, Nilson AE, Pallanch JF, Friedman O, et al. Gene transcription changes in asthmatic chronic rhinosinusitis with nasal polyps and comparison to those in atopic dermatitis. PLoS One. 2010;5:e11450. doi: 10.1371/journal.pone.0011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87:614–22. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- 12.Maric I, Robyn J, Metcalfe DD, Fay MP, Carter M, Wilson T, et al. KIT D816V-associated systemic mastocytosis with eosinophilia and FIP1L1/PDGFRA-associated chronic eosinophilic leukemia are distinct entities. J Allergy Clin Immunol. 2007;120:680–7. doi: 10.1016/j.jaci.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–42. [PubMed] [Google Scholar]
- 14.Al-Rabia MW, Blaylock MG, Sexton DW, Thomson L, Walsh GM. Granule protein changes and membrane receptor phenotype in maturing human eosinophils cultured from CD34+ progenitors. Clin Exp Allergy. 2003;33:640–8. doi: 10.1046/j.1365-2222.2003.01639.x. [DOI] [PubMed] [Google Scholar]
- 15.Shalit M, Sekhsaria S, Mauhorter S, Mahanti S, Malech HL. Early commitment to the eosinophil lineage by cultured human peripheral blood CD34+ cells: messenger RNA analysis. J Allergy Clin Immunol. 1996;98:344–54. doi: 10.1016/s0091-6749(96)70159-8. [DOI] [PubMed] [Google Scholar]
- 16.Hart TK, Cook RM, Zia-Amirhosseini P, Minthorn E, Sellers TS, Maleeff BE, et al. Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys. J Allergy Clin Immunol. 2001;108:250–7. doi: 10.1067/mai.2001.116576. [DOI] [PubMed] [Google Scholar]
- 17.Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344–53 e2. doi: 10.1016/j.jaci.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Bochner BS, Book W, Busse WW, Butterfield J, Furuta GT, Gleich GJ, et al. Workshop report from the National Institutes of Health Taskforce on the Research Needs of Eosinophil-Associated Diseases (TREAD) J Allergy Clin Immunol. 2012;130:587–96. doi: 10.1016/j.jaci.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busse WW, Katial R, Gossage D, Sari S, Wang B, Kolbeck R, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol. 2010;125:1237–44 e2. doi: 10.1016/j.jaci.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Hudson SA, Herrmann H, Du J, Cox P, Haddad el B, Butler B, et al. Developmental, malignancy-related, and cross-species analysis of eosinophil, mast cell, and basophil siglec-8 expression. J Clin Immunol. 2011;31:1045–53. doi: 10.1007/s10875-011-9589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerutti A, Puga I, Cols M. New helping friends for B cells. Eur J Immunol. 2012;42:1956–68. doi: 10.1002/eji.201242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12:151–9. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 23.Schaller E, Macfarlane AJ, Rupec RA, Gordon S, McKnight AJ, Pfeffer K. Inactivation of the F4/80 glycoprotein in the mouse germ line. Mol Cell Biol. 2002;22:8035–43. doi: 10.1128/MCB.22.22.8035-8043.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray JX, Haino M, Roth MJ, Maguire JE, Jensen PN, Yarme A, et al. CD97 is a processed, seven-transmembrane, heterodimeric receptor associated with inflammation. J Immunol. 1996;157:5438–47. [PubMed] [Google Scholar]
- 25.Hamann J, Wishaupt JO, van Lier RA, Smeets TJ, Breedveld FC, Tak PP. Expression of the activation antigen CD97 and its ligand CD55 in rheumatoid synovial tissue. Arthritis Rheum. 1999;42:650–8. doi: 10.1002/1529-0131(199904)42:4<650::AID-ANR7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 26.Huang YS, Chiang NY, Hu CH, Hsiao CC, Cheng KF, Tsai WP, et al. Activation of myeloid cell-specific adhesion class G protein-coupled receptor EMR2 via ligation-induced translocation and interaction of receptor subunits in lipid raft microdomains. Mol Cell Biol. 2012;32:1408–20. doi: 10.1128/MCB.06557-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwakkenbos MJ, Matmati M, Madsen O, Pouwels W, Wang Y, Bontrop RE, et al. An unusual mode of concerted evolution of the EGF-TM7 receptor chimera EMR2. FASEB J. 2006;20:2582–4. doi: 10.1096/fj.06-6500fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


