Abstract
Polycyclic aromatic hydrocarbons (PAHs) substituted with a ketone or quinone functionality (OPAHs) may be important environmental contaminants. The OPAHs from environmental samples have demonstrated toxicity and may be more harmful than PAHs. Knowledge gaps concerning the occurrence of OPAHs in the total environment arise from analytical difficulties, as well as limited standards and methodologies. An optimized method was developed to quantify five ketone and four quinone OPAHs from matrices ranging from biological tissue to diesel particulates. Five National Institute of Standards and Technology Standard Reference Materials (SRMs) were analyzed. This is the first report of OPAH quantitation in SRM 2977 (mussel tissue), SRM 1944 (New York /New Jersey, USA waterway sediment), SRM 1975 (diesel extract) and SRM 1650b (diesel particulate matter) and among the few to report concentrations from SRM 1649 (urban dust). Furthermore, this is one of the first reports of OPAHs in biological tissue. Σ9OPAHs were 374 ± 59 μg/kg (mussel tissue), 5.4 ± 0.5 mg/kg (sediment), 16.9 ± 1.6 mg/kg (urban dust), 33.4 ± 0.4 mg/kg (diesel extract), and 150 ± 43 mg/kg (diesel particulate matter). In all SRMs, the levels of OPAHs were similar to or exceeded levels of PAHs. Of the OPAHs tested, the most frequently occurring in the environmental matrices were 9-fluorenone, 9,10-anthraquinone, benzofluorenone, and 7,12-benz[a]anthracenequinone.
Keywords: Oxygenated polycyclic aromatic hydrocarbons, Standard reference materials, Quinone, Environmental media, Ketone
INTRODUCTION
Reporting of oxygenated polycyclic aromatic hydrocarbons (PAHs) in environmental matrices, namely in soil and air, have been slowly increasing in recent years as analytical methods become available and researchers are realizing the prevalence of oxygenated PAHs. The oxygenated PAHs discussed in the present study have a ketone or quinone group attached to the PAH rings (OPAHs) providing a potentially more mobile, bioavailable and/or persistent compound than PAHs [1]. The OPAHs are thought to be stable [1] and early investigations attributed environmental prevalence to direct combustion emissions and degradation of PAHs in the atmosphere [2]. Subsequent research has shown that the OPAH quinones also result from further degradation of methylated, nitrated, and other oxygenated PAHs by light radiation [3, 4]. More recently, OPAHs have also been shown to form and accumulate after biological remediation of PAH contaminated soils [1]. Consequently, researchers have suggested calling OPAHs dead-end products. Given the many potential sources of OPAHs, they could be as ubiquitous as the much studied PAHs.
There is growing evidence that OPAHs have important toxicological significance. The formation of OPAHs during mammalian metabolism can lead to genotoxicity, promoting carcinogenesis [5]. The OPAH quinone has been suggested to be the toxic species, having the capability to exert effects through a number of pathways including direct DNA, lipid or protein binding and redox cycling [6]. Some OPAHs are considered extremely toxic compared to other oxygenated PAHs, killing the majority of cells in a human cell mutagenicity assay [7]. In addition, bioassays of environmental samples indicate that OPAHs significantly contribute to toxicity [8, 9]. The quinone-enriched fractions of diesel particulates and ultrafine particulate matter were more potent than the PAH fraction for a number of toxic cellular endpoints [10]. Partly due to data gaps concerning environmental distribution and fate of OPAHs, the influence of OPAH exposure on mixture toxicity remains unclear.
Determination of the sources and sinks, as well as transport of OPAHs in the environment is in its infancy. The OPAHs have been identified in soils as products of biological degradation according to Lundstedt et al. and references therein [11]. They have also been detected in soils before remediation began [12, 13] allowing the plausibility of soils to be both a source and sink of OPAHs. Atmospheric transformation by chemical oxidants can also degrade PAHs to OPAHs [14]. Indeed, several studies have measured OPAHs in atmospheres of urban [15-18] and rural [19] locations around the world. Identical OPAH species have also been reported as photoproducts in aqueous environments [4]. However, many atmospheric studies have suggested that the source of OPAHs in the atmosphere is a result of direct combustion [15, 20, 21].
Few combustion sources have been characterized or identified. Studies have reported identification of ninety seven oxygenated PAHs from a municipal waste incinerator [22] and over forty ketone OPAHs were tentatively identified from wood smoke, coal, and diesel combustion [23]. Diesel exhaust could be a significant source of OPAHs, but limited characterization in this media has been done [21, 24-27]. While the presence of OPAHs in soils and air has been demonstrated, other environmental media is largely underrepresented. Possible sinks of OPAHs are also yet to be determined. Emphasizing this point, to our knowledge, studies of OPAHs in food or organisms that may have the ability to uptake or bioaccumulate has not been done.
One likely contributing reason for the limited number of OPAH studies in non-air (or soil) matrices is the limited number of OPAH analytes for which authentic standards were available. Although OPAH extraction [11, 28] and instrumental methods have been slowly developed using gas chromatography (GC)-mass spectrometry (MS), [11, 29] GC- tandem mass spectrometry (MS/MS) [30] and liquid chromatography (LC)-MS and LC-MS/MS [20, 31], a complete method validation is missing due to a lack of certified concentrations of OPAHs in applicable matrices. Also, deuterated OPAH standards only recently became available and researchers were relegated to use un-substituted PAHs or nitro-PAHs for proxies of OPAH internal standards. Standard Reference Materials (SRMs) from the National Institute of Standards and Technology (NIST) are plentiful for PAHs, but none exist for OPAHs. To our knowledge, SRM 1649b (urban dust) is the only SRM to report values for OPAHs, and these are only provided as informational. Few researchers have reported concentrations of OPAHs in other SRMs matrices, making comparisons and validations challenging.
Given the unmet need for robust OPAH environmental distribution and fate information, we sought to develop an OPAH high through-put, selective and sensitive analytical method that combined a breadth of environmental matrices with the development of an expanded characterization of OPAHs. Using the analytical method, five widely and commercially available certified reference materials were characterized and OPAHs were identified. The complex profile of OPAHs is further demonstrated in a range of matrices using authentic standards and improved quantification by utilizing deuterated OPAH internal standards.
MATERIALS AND METHODS
Chemicals
The following OPAHs were purchased: acenapthenequinone (ACYQ), aceanthracenequinone (ACAQ), phenanthrene-1,4-quinone (1,4-PHEQ), benzo[c]phenanthrene-[1,4]quinone (B[c]PHE1,4Q), 1,4-anthraquinone (1,4-ANTQ) from Chiron AS (Norway), 9-fluorenone (9-FLUO), 4H-cyclopenta[def]phenanthren-4-one (CP[def]PHEO), 9,10-phenthrenequinone (9,10-PHEQ), 9,10-anthraquinone (9,10-ANTQ), benzofluorenone (BFLUO), benzanthrone (BEZO), 7,12-benzanthracenequinone (7,12-BaAQ), 5,12-napthacenequinone (5,12-NAPQ) and benzo[cd]pyrenone (B[cd]PYRO) from Sigma (St. Louis, MO, USA). The following PAHs, naphthalene (NAP), 2-methylnaphthalene (2-mNAP), 1-methylnaphthalene (1-mNAP), 1,6-dimethylnaphthalene (1,6-dmNAP), acenaphthylene (ACY), 1,2-dimethylnaphthalene (1,2-dmNAP), acenaphthene (ACE), fluorene (FLU), dibenzothiophene (DBT), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLA), pyrene (PYR), retene (RET), 1-methylpyrene (1-mPYR), benz[a]anthracene (BaA), chrysene (CHR), 6-methylchrysene (6-mCHR), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), indeno[1,2,3-cd]pyrene (IcdP), dibenz[a,h]anthracene (DahA) and benzo[ghi]perylene (BghiP) were purchased from AccuStandard (New Haven, CT, USA).
The following deuterated surrogates were used for quantitation and method recovery: 9,10-anthraquinone-D8 and 9-fluorenone-D8 (C/D/N Isotopes, PQ, Canada) for OPAHs and acenaphthylene-D8, benzo[a]pyrene-D12, benzo[ghi]perylene-D12, fluoranthene-D10, naphthalene-D8, phenanthrene-D10, pyrene-D10 (Cambridge Isotope Laboratories, Andover, MA, USA) for PAHs. Perlyene-D12 and chyrsene-D12 were used as internal standards for instrumental quantification (Cambridge). High purity optima grade or equivalent solvents (Fisher Scientific) were used in all studies. Anhydrous sodium sulfate (Fisher Chemical, Pittsburgh, PA, USA) was baked at 350 °C for 24 h prior to use.
Instrumentation
Pressurized solvent extractions were preformed using Accelerated Solvent Extraction (ASE® 300, Dionex, Sunnyvale, CA, USA). Samples were extracted with the following operating conditions: 100 °C, 10.3 MPa, 5 min static, 240 s purge, 100% flush volume with two cycles of dichloromethane. Matrix interferences from lipid-containing matrices (sediment and mussel tissue) were removed with size exclusion chromatography. The instrument was equipped with a 515 HPLC pump, two Environgel columns in sequence (100 Ǻ pore size, 15μm) and a 2487 dual wavelength detector (Waters, Milford, MA, USA). Operating conditions were as follows: 5 ml/min flow rate at 1000 psi, using dichloromethane. One fraction, containing all PAHs and OPAHs, was collected from 14 to 20 min.
All samples were analyzed using an Agilent 5975B GC-MS in electron impact mode (70 eV) utilizing selective ion monitoring (SIM) with a DB-5MS column (30 m length, 0.25 μm film thickness, 0.32 mm inner diameter, Aglient J&W, Santa Clara, CA USA). The GC-MS injection port was operated in split less mode fitted with a glass liner and wool with in an injection volume of 1 µl. Instrumental conditions for OPAHs were as follows: inlet was operated at 300 °C, initial oven temperature at 45 °C, 1 min hold, ramp to 180 °C at 25 °C/min, 2 min hold, ramp to 270 °C at 3.5 °C/min, 1 min hold, final ramp to 310 °C at 25 °C/min, 1 min hold for a total run time of 37.71 min and total column flow of 39 ml/min using helium as the carrier gas. The MS were 150, 230, and 280 °C for the quadrupole, source and transfer line, respectively. The SIM ions and an example chromatogram of a standard solution can be found in the Supplemental Data. Non-polar PAHs were run separately under the following instrumental parameters: inlet was operated at 300 °C, initial oven temperature at 70 °C, 1 min hold, ramp to 300 °C at 10 °C/min, 4 min hold, ramp to 320 °C at 10 °C/min with a 2 min hold for a total run time of 32 min and total column flow of 34 ml/min using helium as the carrier gas. The MS temperatures were 180, 280, and 280 °C for the quadrupole, source and transfer line, respectively.
Standard reference materials
Standard Reference Materials (SRMs) were purchased from the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA) and stored according to recommendations [32]. The SRMs included SRM 1649b (urban dust), SRM 1975 (diesel extract), SRM 1650b (diesel particulate matter), SRM 1944 (sediment), and SRM 2977 (mussel tissue). All SRM matrices were purchased as a solid with the exception of SRM 1975 that was a liquid.
Sample extraction
Individual samples were weighed using an analytical balance (Mettler SX64, Toledo, OH, USA). Samples were ground with anhydrous sodium sulfate at approximately 30 times the sample weight to ensure a homogenous sample and to minimize the dead volume in the extraction cell. Homogenates were transferred into stainless steel cells and spiked with deuterated surrogate OPAHs and PAHs. Cells were loaded onto the ASE® 300 and extracted with high pressure and temperature using dichloromethane. Since the diesel extract was purchased as a liquid, 100 μl aliquots of this SRM were added to the protocol during the cleanup step and deuterated surrogates were spiked at that time.
Sample clean-up
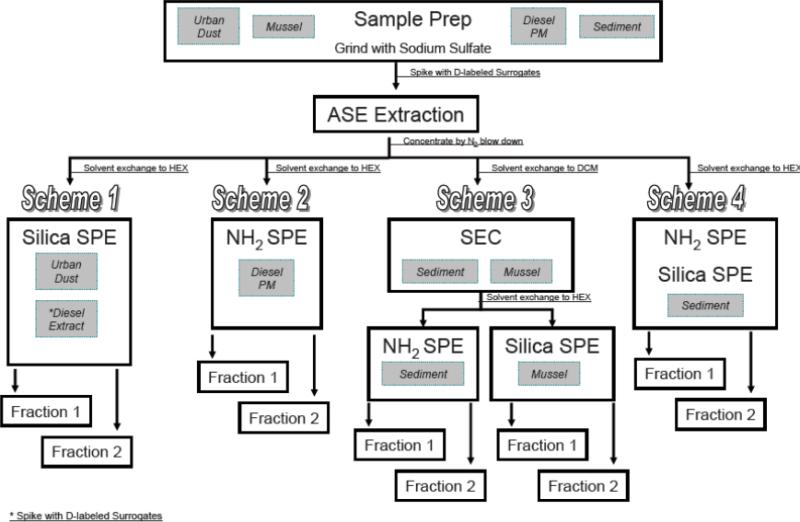
Size exclusion chromatography and solid phase chromatography were used for sample clean-up. Clean-up schemes were based on the level of complexity of each matrix and were modeled after established methods for organics per certificate of analysis from the SRMs [32]. For some matrices, a number of clean-up methods were acceptable. Different schemes were applied to some matrices to demonstrate the most efficient clean-up method specifically for OPAHs. The solid phase extraction tubes were matrix specific. 1000 mg Discovery® bonded silica and amino propyl solid phase extraction tubes (Supelco, Bellefonte, PA, USA) were fitted to a manifold and operated under vacuum at -10 mm Hg. Samples were selectively eluted into two fractions for discrimination of non-polar PAHs from more polar PAHs. The first elution was accomplished with 3 x 2 ml of 10% v/v dichloromethane: hexane. A second fraction with 3 x 2 ml of 20% v/v dichloromethane: hexane. A detailed schematic of the extraction and clean-up can be found in the Supplemental Data, Figure S2.
Scheme 1 was employed for urban dust and the diesel extract SRMs. Extracts from the ASE® were concentrated and solvent exchanged into hexane for a final volume of 1 ml using N2 at ambient temperatures. Concentrated extracts were cleaned-up using silica solid phase extraction tubes and two separate fractions were collected. Scheme 2 applied to diesel particulate matter only. The ASE extracts were also concentrated with N2 and solvent exchanged into hexane for a final volume of 1 ml. Samples were eluted through amino propyl solid phase extraction tubes and two fractions were collected. Scheme 3 was applied to sediment and mussel tissue SRMs. The ASE extracts were concentrated and solvent exchanged into dichloromethane using N2 at ambient temperatures. Concentrated extracts were subjected to size exclusion chromatography clean-up. The collected size exclusion chromatography fraction was solvent exchanged into hexane and re-concentrated to 1 ml. Mussel tissue samples were then taken through Scheme 1, while sediment was then taken through Scheme 2. Scheme 4 applied to sediment as an alternative clean-up scheme without using size exclusion chromatography. The ASE® extracts were concentrated using N2 at ambient temperatures and solvent exchanged into hexane to 1 ml. Samples were subjected to solid phase extraction using an amino propyl tube connected to a silica column in tandem. Two solid phase extraction fractions were collected. Fractions were recombined after PAH analysis.
Scheme 01-04.
OPAH and PAH quantification
After solid phase clean-up, extracts were concentrated and spiked with internal standards. All samples were then quantified for OPAHs and PAHs using GC-MS. Authentic standards, purchased and prepared individually, were used to accurately identify and quantitate the OPAHs. Since a small amount of OPAHs were present in the non-polar fraction, the polar and non-polar fractions were recombined subsequent to PAH analysis. Surrogates spiked before ASE extraction account for losses incurred during laboratory analysis. Analyte concentrations are determined from calibration curves of relative response ratios of analytes to spiked surrogates. Calibration curves consisted of five to ten standards each, with correlation coefficients > 0.98.
Quality assurance/control
Each analytical batch contained a minimum of 15% quality control samples including blanks, check standards and overspikes. The blank consisted of anhydrous sodium sulfate packed into the extraction ASE® cells and accounted for any procedural artifacts during analysis. Recoveries of OPAH overspikes from the extraction and clean-up (Scheme 1) were determined by spiking the target analytes into sodium sulfate packed ASE® cells with 0.3 μg of OPAHs. The OPAH overspikes were also taken through size exclusion chromatography clean-up (Scheme 3). Preliminary investigations suggested that losses of OPAHs occurring during solid phase extraction clean-up were independent of type (ie. silica vs. amino propyl). The polycyclic aromatic hydrocarbon (PAH) quality control samples consisted of overspikes of 0.3 μg PAHs taken through the entire extraction and clean-up (Scheme 1). Additionally, replicates of SRMs were analyzed in different analytical batches.
In order to ensure quality integrity of the GC-MS system, reagent blanks accompanied each analytical run. The GC-MS OPAH calibration standards were run every five to ten samples to ensure accuracy of quantification. The OPAH check standards were routinely analyzed and were typically within 15% of their spiked values. Recoveries of certified values of PAHs demonstrated, in part, the adequacy of the methods used. Method recoveries of PAHs from the SRMs were compared to those published by NIST and can be found in the Supplemental Data.
RESULTS
Detection limits and spike recoveries
The OPAH analyte information and instrument detection limits are listed in Table 1. Instrumental detection limits were calculated as a signal to noise ≥ 3 using the peak-to-peak ratio. Samples used in the instrumental detection limit determination were solutions of standards analyzed from several analytical batches over the course of weeks, adding robustness. The instrumental detection limits ranged from 0.5 to 50 pg for the analytes quantified. Quantitative analytes were defined as having calibration curves with correlation coefficients > 0.98 as well as reproducible and accurate quantitation (+/- 30% of their true value) from instrumental check standards from each analytical batch. Nine OPAHs met these criteria. Compounds with a semi-quantitative status did not meet one or both of the aforementioned criteria.
Table 1.
Ketone and quinone-sustituted polycyclic aromatic hydrocarbon (OPAH) properties and structures are provided for each analyte in the method. Quantition ion (Quant ion) for gas chromatography-mass spectrometry analysis is shown here. Qualifier ions can be found in the Supplemental Data. Analytes identified as Quantitative met quality control standards for accuracy and precision.
| OPAH | Abbreviation | CAS No. | MW | Formula | Quant Ion | IDL (pg) | Structure | Type |
|---|---|---|---|---|---|---|---|---|
| 9-Fluorenone | 9-FLUO | 486-25-9 | 180.2 | C13H8O | 180 | 0.5 | file | Quantitative |
| Acenapthenequinone | ACYQ | 82-86-0 | 182.2 | C12H6O2 | 126 | 50 | file | Semi-quantitative |
| 9-Fluorenone-D8 | na | 137219-34-2 | 188.3 | C13D8O | 188.1 | n/a | NA | Surrogate |
| 4H-Cyclopenta[def]phenanthren-4-one | CP[def]PHEO | 5737-13-3 | 204.2 | C15H8O | 204 | 1 | file | Quantitative |
| 9,10-Phenthrenequinone | 9,10-PHEQ | 84-11-7 | 208.2 | C14H8O2 | 180 | 500 | file | Semi-quantitative |
| 9,10-Anthraquinone | 9,10-ANTQ | 84-65-1 | 208.2 | C14H8O2 | 208 | 5 | file | Quantitative |
| 1,4-Anthraquinone | 1,4-ANTQ | 635-12-1 | 208.2 | C14H8O2 | 208 | 50 | file | Quantitative |
| Phenanthrene-1,4-dione | 1,4-PHEQ | 569-15-3 | 208.2 | C14H8O2 | 216 | 50 | file | Semi-quantitative |
| 9,10-Anthraquinone-D8 | NA | 10439-39-1 | 216.3 | C14D8O2 | 216.1 | n/a | NA | Surrogate |
| Benzanthrone | BEZO | 82-05-3 | 230.3 | C17H10O | 230 | 10 | file | Quantitative |
| Benzofluorenone | BFLUO | 479-79-8 | 230.3 | C17H10O | 230 | 0.5 | file | Quantitative |
| Aceanthracenequinone | ACAQ | 6373-11-1 | 232.2 | C16H8O2 | 204 | 50 | file | Semi-quantitative |
| Benzo[cd ]pyrenone | B[cd ]PYRO | 3074-00-8 | 254.3 | C19H10O | 254 | 10 | file | Quantitative |
| 7,12-Benz[a ]anthracenquinone | 7,12-Ba AQ | 2498-66-0 | 258.3 | C18H10O2 | 258 | 10 | file | Quantitative |
| 5,12-Naphthacenequinone | 5,12-NAPQ | 1090-13-7 | 258.3 | C18H10O2 | 258 | 10 | file | Quantitative |
| Benzo[c ]phenanthrene-[1,4]quinone | B[c]PHE1,4Q | 109699-80-1 | 258.3 | C18H10O2 | 258 | 100 | file | Semi-quantitative |
MW= molecular weight, Quant Ion = m/z ratio used for quantification, IDL= instrumental detection limit (peak to peak signal/noise >3), NA= not applicable
Non-corrected method recoveries of individual spiked OPAHs were on average > 82% with the exception of three-ring OPAHs, which ranged from 34 to 57%. Non-corrected recoveries were, in general, 10% lower with size exclusion chromatography clean-up than solid phase extraction clean-up schemes (see Supplemental Data). Using the internal standard and surrogate recovery method of analysis, reported values of analytes were recovery-corrected, meaning surrogates added before extraction accounted for losses incurred during sample extraction, solid phase extraction and size exclusion chromatography clean-up. Mean recoveries of the individual surrogate standards spiked before sample extraction (n=21) were 107.1 ± 26.2% (9,10-anthraquinone-D8) and 75.5 ± 14.2% (9-fluorenone-D8) and were consistent with that of the controls (n=12) with mean recoveries of 99.0 ± 18% (9,10-anthraquinone-D8) and 69.5 ± 13% (9-fluorenone-D8).
As evidenced from the PAH quality control (n=5), the extraction process was not a likely source of OPAHs. 9-FLUO and 9,10-ANTQ were the only OPAHs detected in the PAH spike quality control and were recovered at low concentrations (10-50 times instrumental detection limits). Recoveries of fluorene and anthracene spikes (n=5) were 107 ± 13% and 116 ±13%. All OPAH and PAH analytes were below detection limits in all blank quality control samples tested, except 9-FLUO which was detected (n=4) at low concentration, approximately ten times the instrumental detection limit. Sample concentrations were greater than 100 times the detection limit of 9-FLUO. A conceivable reason for the low concentrations of 9-FLUO and 9,10-ANTQ detected in the quality control samples could be from impurities of the spiked 9-fluorenone-D8 and 9,10-anthraquinone-D8 standards. These are manufactured at a purity of 98%; the undeuterated 9-FLUO and 9,10-ANTQ and low detection limits can explain the small quantities in the quality control samples.
Individual OPAHs
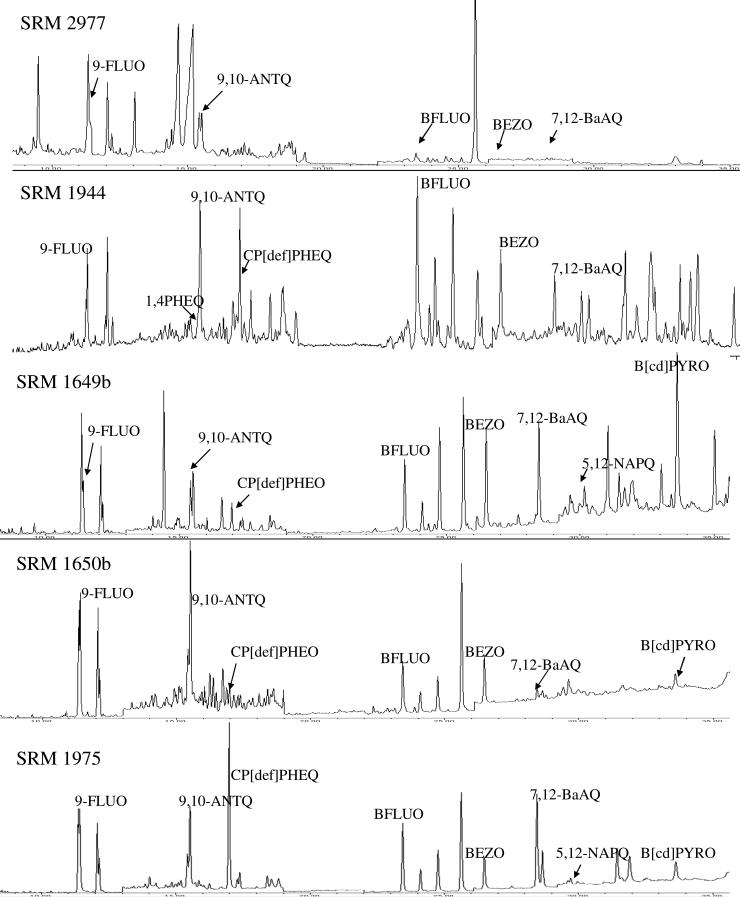
The OPAHs were present in all matrices included in the present study. The chromatograms in Figure 1 demonstrate that eight OPAHs were frequently detected out of nine that were considered quantitative. Clearly resolved and Gaussian shaped peaks allowed for easy identification. Five OPAHs, 9-FLUO, 9,10-ANTQ, BFLUO, BEZO, and 7,12-BaAQ were detected in all matrices (Fig. 1). The CP[def]PHEO was detected in all matrices except mussel tissue. The B[cd]PYRO was detected in all the airborne sources including diesel extract, diesel particulate matter and urban dust SRMs. The 5,12-NAPQ was detected in urban dust and the diesel extract, but not the diesel particulate matter. Out of all of the quantitative OPAHs, 1,4-ANTQ was the only analyte that was below analytical detection limits from each matrix.
Figure 1.
Example chromatograms of ketone or quinone-substituted polycyclic aromatic hydrocarbons identified in several standard reference material (SRM) matrices: SRM 2977 (mussel tissue), SRM 1944 (sediment), SRM 1649b (urban dust), SRM 1650b (diesel particulate matter) and SRM 1975 (diesel extract). Each chromatogram represents a selected ion monitoring (SIM) scan from gas chromatography-mass spectrometry with a non polar column. A more detailed chromatogram is provided in the Supplemental Data.
As shown in Table 2, the highest concentration of individual OPAHs in a matrix was measured from the diesel particulate matter and ranged from 7 to 48 mg/kg of OPAH. The lowest measured concentrations were from mussel tissue, where by individual OPAHs ranged from 20 to 200 μg/kg. The OPAHs from diesel extract ranged from < 1 to 8 mg/kg and were ten times less than diesel particulate matter, but similar to that of urban dust, which ranged from < 1 to 5 mg/kg. The individual OPAHs from sediment were measured at concentrations very similar to each other and ranged from less than 1 to approximately 2 mg/kg. While there was no statistical difference from the majority of OPAHs measured from sediment using clean-up Schemes 3 and 4, recoveries of CP[def]PHEO and BEZO were two fold higher with size exclusion chromatography (Table 2). We found that size exclusion chromatography clean-up (Scheme 3) greatly enhanced the chromatographic resolution of OPAHs from sediment. Therefore, Scheme 3 was the optimal clean-up procedure for sediment.
Table 2.
Recovered ketone and quionone-substituted polycyclic aromatic hydrocarbon (OPAH) concentrations from polycyclic aromatic hydrocarbon-contaminated Nationational Institute of Standards and Technology (NIST) standard reference materials (SRMs). Data represents mean values from at least two analytical batches.
| Mean OPAH Concentrations from NIST SRMs with Standard Deviation | ||||||
|---|---|---|---|---|---|---|
| SRM no. Matrix n Units | 2977 Mussel tissue 5 μg/kg (SD) | 1944 Sediment (Sch 3) 2 mg/kg (SD) | 1944 Sediment (Sch 4) 3 mg/kg (SD) | 1649b Urban dust 3 mg/kg (SD) | 1975 Diesel extract 3 mg/kg (SD) | 1650b Diesel particulate matter 5 mg/kg (SD) |
| 9-FLUO | 22.6 (4.9) | 0.653 (0.05) | 0.65 (0.03) | 0.78 (0.04) | 2.69 (0.08) | 24.9 (7.3) |
| CP[def ]PHEO | BDL | 0.366 (0.05) | 1.14 (0.03) | 0.62 (0.03) | 7.74 (0.29) | 6.9 (4.1) |
| 9,10-ANTQ | 180.8 (35.5) | 1.70 (0.43) | 1.53 (0.15) | 1.60 (0.11) | 5.23 (0.16) | 47.7 (19.9) |
| 1,4-ANTQ | BDL | BDL | BDL | BDL | BDL | BDL |
| BEZO | 21.8 (0.1) | 0.144 (0.25) | 1.28 (0.12) | 4.46 (0.50) | 4.39 (0.34) | 36.9 (8.6) |
| BFLOU | 46.5 (9.9) | 1.27 (0.01) | 1.17 (0.02) | 1.65 (0.09) | 3.43 (0.25) | 15.9 (4.8) |
| B[cd ]PYRO | BDL | BDL | BDL | 2.42 (0.71) | 1.87 (0.32) | 9.2 (2.5) |
| 7,12-Bn AQ | BDL | BDL | BDL | 2.20 (0.15) | 0.79 (0.03) | BDL |
| 5,12-NAPQ | 31.9 (7.6) | 0.613 (0.01) | 0.68 (0.01) | 3.16 (0.13) | 7.22 (0.35) | 9.0 (1.7) |
BDL = below detection limits, SD = standard deviation, Sch = scheme Values are reported on a dry weight basis and recovered from replicates of 1-2 g mussel tissue, 0.8 g sediment (comparison of clean up Schemes 3 and 4), 150 mg urban dust, 50 (il of diesel extract and 10 mg diesel particulate matter using pressurized solvent extraction, size exclusion chromotography and/or solid phase extraction clean-up with gas chromotography-electron impact-mass spectrometry.
Differences and similarities among the OPAH concentration profiles between matrices were also observed. The two diesel SRMs had very different concentration profiles. Both CP[def]PHEO and 7,12-BaAQ were abundant analytes in the diesel extract; however, these same compounds were minor in comparison to other analytes detected in diesel particulate matter. Three- and four-ring OPAHs present in both sediment and mussel tissue seemed to display a similar profile. Further, the lighter OPAHs with a molecular weight (MW) of less than 210 g/mol were more predominant in diesel particulate matter, sediment and mussel tissue. Urban dust stood out as the only SRM with greater abundance of the heavier MW OPAHs. It should be emphasized that 9,10-ANTQ was the most concentrated OPAH from three diverse matrices: mussel tissue, sediment and diesel particulate matter SRMs. Additionally, sediment and mussel tissue were the only SRMs lacking the five-ring OPAH, B[cd]PRYO.
OPAH vs PAH concentration
Concentrations of OPAHs from the environmental matrices were very similar to levels of PAHs even though more PAH analytes were monitored (~ three times as many). The sum of OPAHs (Σ9OPAH) and the sum of PAHs (Σ26PAHs) were on the same order of magnitude for the SRMs, with the exception of sediment (Table 3). No statistical difference (t test) was observed between Σ9OPAHs and Σ26PAHs in mussel tissue (p = 0.06) and the diesel particulate matter (p = 0.1). The Σ9OPAHs were slightly greater than the Σ26PAHs for the diesel extract. An additional calculation was also performed enabling direct comparison of OPAHs with the respective PAH analog. This comparison was possible for three OPAHs in the present method (9-FLUO, 9,10-ANTQ, and 7,12-BaAQ). These OPAHs were summed against the three PAHs: FLU, ANT and BaA. Apparent distinctions are realized using this calculation compared to the previous total summation. The total sum of PAHs was higher than the OPAHs for urban dust, yet comparing the equivalent numbers of OPAHs to PAHs, we find that the Σ3OPAHs were two times greater than Σ3PAHs. In fact, mussel tissue and both diesel SRMs showed that the Σ3OPAHs were an order of magnitude higher than the analog Σ3PAHs (Table 3). The OPAHs were more concentrated than the PAHs for most of the matrices analyzed here. The one exception is the sediment, but even in this case, the Σ3PAHs were only slightly higher than the Σ3OPAHs (Table 3).
Table 3.
Summation of measured ketone and quinone-substituted polycyclic aromatic hydrocarbons (OPAHs) and polycyclic aromatic hydrocarbons (PAHs) for each standard reference material (SRM). Direct analog summation refers to a comparision of the three OPAHs for which an unsubstitituted PAH analog was measured.
| Standard Reference Material: | Concentrations | ||||
|---|---|---|---|---|---|
| Total analytesa | Direct analogsb | ||||
| Mussel tissue SRM2977 | mass/kg | SD | mass/kg | SD | |
| ΣOPAHs (n =5, μg) | 282.1 | 68.8 | 222.5 | 57.6 | |
| ΣPAHs (n =5, μg) | 362.0 | 44.6 | 34.2 | 27.0 | |
| p -value | 0.061 | <0.001 | |||
| Sediment SRM1944 | |||||
| ΣOPAHs (n =5, mg) | 5.4 | 0.5 | 2.9 | 0.3 | |
| ΣPAHs (n =5, mg) | 53.0 | 6.1 | 4.6 | 0.8 | |
| p -value | <0.001 | 0.002 | |||
| Urban dust SRM1649b | |||||
| ΣOPAHs (n =3, mg) | 16.9 | 1.6 | 5.5 | 0.3 | |
| ΣPAHs (n =6, mg) | 39.7 | 1.4 | 2.6 | 0.3 | |
| p -value | <0.001 | <0.001 | |||
| Diesel extract SRM1975 | |||||
| ΣOPAHs (n =3, mg) | 33.4 | 0.4 | 15.1 | 0.3 | |
| ΣPAHs (n =5, mg) | 26.7 | 2.1 | 0.2 | 0.1 | |
| p -value | 0.029 | <0.001 | |||
| Diesel particulate matter SRM1650b | |||||
| ΣOPAHs (n =5, mg) | 150.4 | 42.7 | 81.6 | 28.4 | |
| ΣPAHs (n =5, mg) | 192.0 | 22.8 | 7.6 | 0.9 | |
| p -value | 0.095 | 0.008 | |||
Σ26 PAHs, Σ9 OPAHs
Σ3 PAHs (FLU, ANT and Ba A), Σ3 OPAHs (9-FLUO, 9,10-ANTQ & 7,12-Ba AQ) p -value is derived from the Student’s t-test of the ΣOPAHs and ΣPAHs SD = standard deviation
DISCUSSION
Method application
Instrumental detection limits for fourteen OPAHs (Table 1) including three, four, and five-ring OPAHs, ranged from 0.5 to 500 pg. Twelve of the OPAHs had instrumental detection limits less than or equal to 50 pg, which is comparable to previously reported GC and LC-based methods [33]. Detection limits of 0.1 to 6 pg using LC-MS/MS were reported, although only four OPAHs and their associated isomers were considered [33]. Excellent detection limits ranging from 0.01 to 2.6 pg for the two and three-ring OPAHs, have been reported using GC-(negative chemical ionization)-MS [29]. Other studies focusing on the analysis of one- to three-ring OPAHs by LC or GC-MS employed a laborious derivatization step, thereby producing a more stable and volatile quinone, but also higher instrumental detection limits of 11 to 650 pg [27] and 300 to 4800 pg [34], respectively. Additional rigor for the present study was achieved using deuterium-labeled OPAH standards as surrogates for recovery corrected quantitation. It is advantageous to have standards with identical physical and chemical properties to account for losses incurred during the extraction and analysis. The present study and Cho et al. are, to our knowledge, the only to report the use of deuterium-labeled OPAH standards [34]. Cho et al. custom synthesized four deuterium-labeled OPAHs for each OPAH in their study, thereby creating optimal quantitation conditions [34]. The use of deuterated OPAHs for quantitation was especially useful for the present study since some matrices had numerous processing steps differing across matrices.
The present method successfully recovered and quantified nine OPAHs from a wide diversity of environmental SRM matrices, many of which we report for the first time. The matrices included biological tissue, sediment, urban dust, and diesel particulate matter. Since multiple matrices with different physical and chemical properties were analyzed, we chose a comprehensive extraction process using pressured liquid extraction with 100% dichloromethane. Minimal losses and no evidence of OPAH formation from the extraction process was found. Several researchers have also reported good extraction efficiencies (> 80%) using pressurized liquid extraction from specific single matrices, such as soils [11] or air particulates [29]. Although dichloromethane with pressurized liquid extraction has been shown to efficiently extract OPAHs from soils, Lundstedt et al. developed a simultaneous extraction and fractionation method using 1:3 cyclohexane:dichloromethane as the optimal solvent [11]. Other groups have also reported ultra sonication with dichloromethane for diesel and ambient air particulates [34] and with ethyl acetate for air particulates [20]. Extraction efficiencies have yet to be determined from a biological tissue. Given that OPAHs were efficiently extracted using dichloromethane from air, soil and diesel in the literature and from the present experiments, this method is believed to be applicable to numerous environmental media, including biological tissues.
Overall, fourteen OPAHs, including two sets of structural isomers, ranging from three to five rings were included for a broad range of matrices, but the method was not without limitations. Although the isomers were easily resolved, the GC was not optimal for some OPAHs. Analytes with vicinal quinones (ACAQ, ACYQ, and 9,10-PHEQ) demonstrated detection limits five to 500 times that of other OPAHs. Poor chromatography was also observed from B[c]PHE1,4Q and 1,4-PHEQ. Although a clean injection port had a positive effect, the reproducibility was an issue for these compounds due any one or combination of the following: degradation, limited volatility and/or stationary phase non-compatibility. For these reasons, five OPAHs (ACYQ, 9,10-PHEQ, ACAQ, B[c]PHE1,4Q and 1,4-PHEQ) were considered semi-quantitative only. Furthermore, the non-detections of ACYQ, ACAQ, and 9,10-PHEQ and low detection frequency of 1,4-PHEQ may be explained by instrumental conditions rather than the absence in the SRMs. A more appropriate means of analysis for these difficult OPAHs would be LC-MS [20, 31].
OPAH method comparison for urban dust
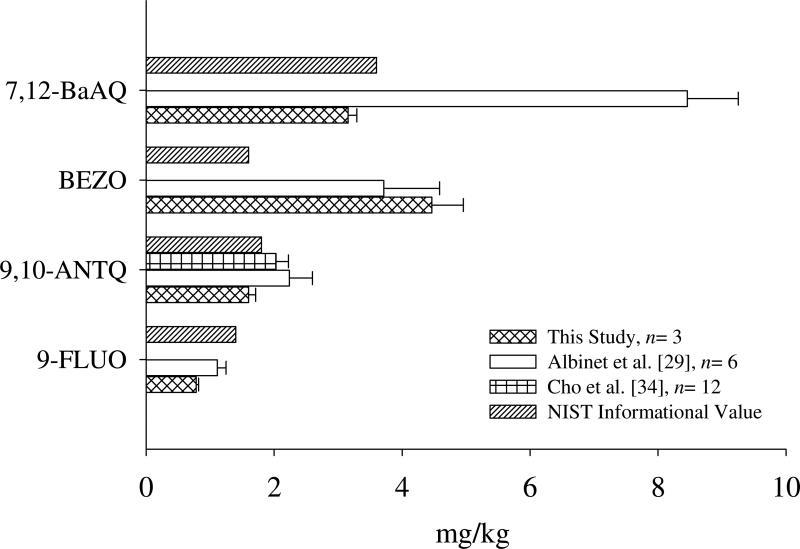
Quantities of OPAHs have been reported for urban dust. The NIST provides informational values for four OPAHs on the certificate of analysis [32] and a small number of researchers have reported levels of some OPAHs from a previously certified lot, SRM 1649a, according to Albinet et al. and studies therein [29]. In general, good agreement with NIST was achieved for 9,10-ANTQ at 1.6 mg/kg measured from the present study, compared to 1.4 mg/kg (NIST) and 7,12-BaAQ at 3.16 mg/kg (present study) and 3.6 mg/kg (NIST), Figure 2. Values are under and overestimated for 9-FLUO and BEZO (respectively) according to NIST, but similar to those values reported by Albinet at al. [29], Fig. 2. Comparable extraction (pressurized liquid extraction with dichloromethane), column (30 m DB-5), and instrumental (GC-MS) techniques were used in the studies. The fact that Albinet et al. used chemical ionization as opposed to election impact as the MS ionization source used by NIST and the present study, may explain the higher concentration of 7,12-BaAQ, but this did not seem likely considering the similarities from the other OPAH recoveries. Cho et al. [34] used a slightly different methodology involving a derivatization step to increase volatility and stability of certain OPAHs. Even so, very little difference is observed between all studies for the value reported by Cho et al. for 9,10-ANTQ. This derivatization step employed by Cho et al. also likely resulted in the detection of 9,10-PHEQ at 1.18 mg/kg in SRM 1649a [34] that was below detection limits from the present study due to limited volatility. In general, all listed comparison values were within the same range. Similar recovered concentrations for 9,10-ANTQ and 9-FLUO across research groups was encouraging (Fig. 2). One explanation for the differences observed for 7,12-BaAQ and BEZO could be that use of deuterated OPAH standards in the present study provided a more accurate estimation of OPAHs.
Figure 2.
Representative literature recoveries are plotted to demonstrate method comparison for the four ketone and quinone-substituted polycyclic aromatic hydrocarbons reported by the National Institute of Standards and Technology (NIST). Informational values are provided on the Certificate of Analysis for standard reference material (SRM) 1649b or urban dust [32]. Albinet et al. extracted the 50 or 100 mg samples using pressurized liquid extraction with dichloromethane, solid phase extraction clean-up and gas chromatography (GC)-negative chemical ionization-mass spectrometry (MS) [29]. Cho et al. extracted 1-2 mg samples using ultrasonic extraction with dichloromethane, derivatization and GC-electron impact-MS, missing values for Cho et al were not analyzed [34]. Albinet et al. and Cho et al. analyzed a previous lot, SRM 1649a.
Environmental occurrence
This method provides the previously unattainable opportunity to assess biological tissues, including foods, along with urban dust and sediment for OPAHs. This is the first to report concentrations of OPAHs in four out of the five SRMs tested. More importantly, this is the first report of quantifiable levels of OPAHs in mussel tissue and among the few to report concentrations of OPAHs in diesel particulate matter. Information collected here aids in the understanding of OPAH environmental distribution and provides insights on potential sources and sinks of these compounds.
Mussel tissue was collected from shucked mussels from Guanabara Bay, Brazil, a known PAH contaminated area [32]. The OPAHs detected in the mussel tissue were present in every other SRM analyzed here. The presence of OPAHs could indicate bioaccumulation, PAH metabolism or direct uptake from the surrounding sediment. Although bioaccumulation mechanisms have not yet been reported for OPAHs in organisms, one recent study found that mussels bio-transformed ANT to 9,10-ANTQ in a lab controlled study [35]. Only the absence of CP[def]PHEO in mussel tissue differentiated the OPAHs detected from that of the sediment which was also collected from a PAH contaminated site. The SRM sediment is a mixture of marine sediments from the New York and Newark Bays. Interestingly, even though the sediment and mussel tissue were taken from marine environments in different hemispheres, a similar OPAH profile was observed. Many of the OPAHs reported in the present study were also detected from sediment off the coast of Barcelona [36]. The presence of 9,10-ANTQ as well as 7,12-BaAQ were attributed to urban runoff since, at that time, these OPAHs had only been associated with diesel exhaust [36]. Biodegradation of contaminants in sediment [1], atmospheric fallout [37] and/or photo oxidation of shallow sediment [4] are all plausible reasons for these OPAHs to occur. The comparable OPAH profile from sediment and mussel tissue may also suggest that similar biotic mechanisms may produce these OPAHs or that mussels may be able to accumulate OPAHs already in the sediment.
Ketone and quinone-substituted PAHs have been identified in emissions from diesel and gas automobiles and they could be significant sources; but until recently, little research has been done to quantify OPAHs in auto exhaust media [27]. For the first time, quantification of OPAHs in diesel particulate matter and diesel extract is presented, while other researchers have only previously identified OPAHs in the diesel particulate matter [24, 30, 33]. Diesel particulate matter is considered representative of heavy-duty diesel engines, while the diesel extract is the extract of particulate matter collected from a filtering system designed for diesel-powered industrial forklifts. Individual OPAHs (BEZO, ANTQ, CP[def]PHEQ and fluorenones) have been qualitatively identified in previous studies from various diesel exhausts [2], but quantitative methods were not yet available. Concentrations of 9-FLUO and 9,10-ANTQ have been reported from lab-generated diesel particulates [25]. From the diesel SRMs, the quantitation of each of the above-mentioned OPAHs is reported, in addition to B[cd]PYRO, 7,12-BaAQ and 5,12-NAPQ (diesel extract only). The presence of OPAHs in the diesel SRMs, combined with previous reports, indicates that diesel combustion is a likely source of OPAHs.
The urban dust, collected in Washington D.C., represents urban atmospheric particulate matter. Individual OPAHs from the urban dust are frequently reported in airborne particulate matter. In particular, 9,10-ANTQ, BEZO and 9-FLUO were the predominate OPAHs found in urban air from Germany [17] and urban and rural locations in the French alpine valley [17, 19]. Although not as frequently monitored, Sklorz et al. also reported relatively high concentrations (~one third of 9,10-ANTQ concentrations) of the higher MW OPAHs (B[cd]PYRO and BFLUO) in urban air [17]. The OPAHs occurring in the urban dust SRM as well as airborne particulate matter occur by direct emission or photochemical oxidation of PAHs in the atmosphere [23, 26]. Typically, higher MW PAHs are more concentrated on particles than lighter (gas-phase) PAHs [18]. The evidence that higher MW OPAHs were more predominate than the lighter MW OPAHs from the urban dust, may suggest that OPAHs could be as plentiful and dynamic as PAHs since the lighter MW OPAHs would be expected to predominately occur in the gas phase.
Although occurring at different magnitudes, the detection of 9-FLUO, 9,10-ANTQ, BFLUO, and 7,12-BaAQ in all SRM matrices shows that these OPAHs are environmentally relevant and important to monitor. The CP[def]PHEO is infrequently reported in the literature, but high abundances in diesel extract and sediment could signify another environmentally relevant OPAH. In addition, B[cd]PYRO is not frequently reported, but detections in all of the airborne media, identifies an important atmospheric OPAH. The fact the 5,12-NAPQ was found in the diesel extract and not the diesel particulate matter, suggests that it could be a marker for certain types of combustion. These data imply that perhaps, in the same way as PAHs, chemical fingerprints or profiles of OPAH sources may become apparent as more investigations occur.
Environmental abundance
A method, spanning many environmental matrices, was developed demonstrating environmental occurrences of OPAHs in these diverse media. However, the abundances may be more useful to further understand the importance of OPAHs and the relative distribution in the environment. The Σ9OPAHs and Σ26PAHs were at similar levels in these SRM matrices. This is an important finding and one supported by previous investigations [1,36]. The aforementioned Barcelona sediment study was one of the first to report that the most concentrated OPAHs (9-FLUO, CP[def]PHEO and BEZO) were similar in concentration to the parent PAHs [36]. An early study from Paris, France measured four oxygenated PAHs (two quinones) and found them up to thirty times and ten times the concentration of PAHs in diesel exhaust and in ambient air, respectively [21]. Continued investigations, during air sampling campaigns in the French alpine valley in 2002 to 2003, reported concentrations of Σ6OPAHs and Σ10PAHs on the same order of magnitude [19]. From Sweden, six PAH-contaminated soils and one Certified Reference Material soil were analyzed and comparable levels of OPAHs and PAHs were found [1]. Further investigations from this study and references therein have found that biological and chemical remediation of PAHs can lead to OPAH accumulation in soils [1]. Relative concentrations of OPAHs compared to PAHs from food have not been reported in the literature, making this the first to report OPAHs and PAHs at the same levels in mussel tissue.
Comparing the total OPAH concentration to the U.S. Environmental Protection Agency (U.S. EPA) 16 priority pollutant PAHs (included in the present study) is another way to demonstrate the comparatively high concentrations of OPAHs in environmental media. Total OPAHs were 150% of the total U.S. EPA 16 PAHs for mussel tissue. The OPAHs from the airborne SRMs were 130, 97, and 45% of the U.S. EPA 16 PAHs for diesel extract, diesel particulate matter and urban dust, respectively. The OPAHs at 12% of the U.S. EPA 16 PAHs for sediment were the lowest measured values. This percentage was somewhat surprising considering sediment can be a sink or potential source due to biodegradation of PAHs. On the other hand, Lundstedt et al has reported that total OPAHs can be between 10 and 66% of the total PAHs depending on the type of PAH source contamination in the soils [1].
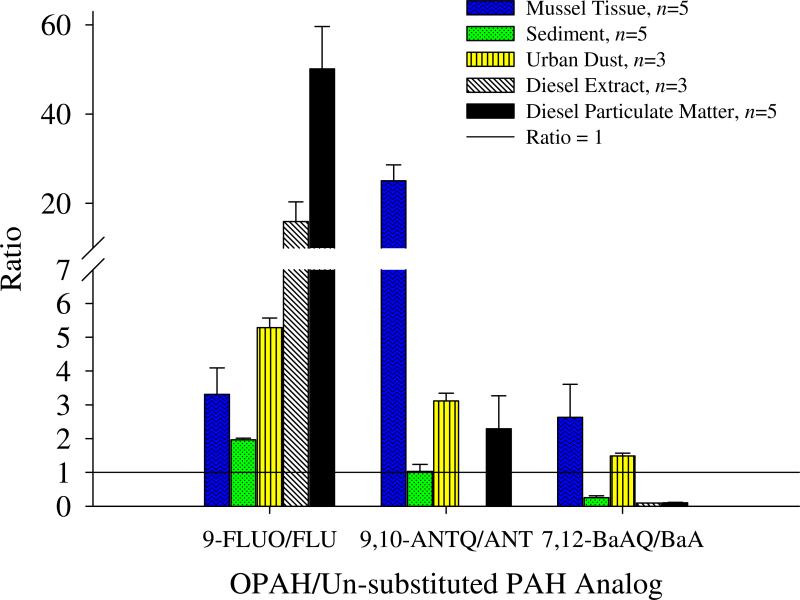
Important observations were also made by isolating the individual OPAHs. A unique finding was that 9,10-ANTQ was the most concentrated analyte in mussel tissue. The 9,10-ANTQ was measured at approximately three times that of the most concentrated PAH, pyrene at 77.4 ± 2.1 (certified value) and 51.3 ± 15 μg/kg (measured). This could have significant ramifications for exposure and toxicity studies; although, the relative toxicities of individual OPAHs vary and are not well established [1]. Considering the ratio of the OPAH to the respective (un-substituted) PAH analog (i.e., 9-FLUO/FLU), the oxygenated form was more abundant than the parent PAH in most cases (Fig. 3). In urban dust and mussel tissue, the individual OPAH (9-FLUO, 9,10-ANTQ, and 7,12-BaAQ) was more predominate than the parent PAH analog (FLU, ANT, and BaA) for all three pairs of ratios. When considering 9-FLUO, all SRMs ratios were greater than one. The largest disparity was observed for the diesel SRMs, whereby the 9-FLUO was 20 to 50 times greater than FLU. From mussel tissue, one to 25 times the amount of OPAH to the PAH analog was observed. Two ratios for sediment were less than one, indicating that the PAH analog was more concentrated than 9,10-ANTQ and 7,12-BaAQ.
Figure 3.
Ratios represent average concentration of ketone or quinone-substituted polycyclic aromatic hydrocarbon (OPAH) to the un-substituted polycyclic aromatic hydrocarbon (PAH) analog for each standard reference material (SRM) matrix. Values over one indicate that the OPAH is more concentrated than the un-substituted PAH in the respective media.
An interesting study from marine sediments in New England showed that contaminated sites had a 9,10-ANTQ/ANT ratio less than one, while remote sites, considered clean, had a ratio greater than two [37]. Based on this, McKinney et al. made the assumption that higher ratios can be attributed to atmospheric fallout of OPAHs [37]. Since urban dust and mussel tissue ratios from the known contaminated SRM media are well over one for the all three PAH ratios shown in Figure 3, a high ratio may not indicate a clean sample as McKinney et al suggested [37]. Additionally, 9-FLUO concentrations were found at higher levels than FLU from contaminated soil in Sweden [1]. Upon comparison of summation of total OPAHs and PAHs, OPAH to PAH analog, and OPAHs to the U.S. EPA 16 priority PAHs, OPAHs were measured at similar or higher levels than PAHs in the five SRM media. This evidence demonstrates the predominance of OPAHs in the environment.
CONCLUSIONS
The OPAHs are a challenging class of PAHs to analyze. A robust quantitative method for nine OPAHs and a semi-quantitative method for five additional OPAHs is demonstrated for a diverse set of matrices in the present study. Concentrations of OPAHs from the NIST Standard Reference Materials showed that OPAHs in air, soil and food were significant, even higher than PAH concentrations. The present study demonstrated that OPAHs are prevalent in environmental media and highlights the need for certified concentrations in established or new SRMs, enabling further method development and environmental monitoring. Continued method development for a high throughput method that includes OPAHs with two to three rings is also needed. High levels of OPAHs in mussel tissue imply that the investigation of uptake, metabolism and clearance of OPAHs from organisms may be justified. Information concerning half-lives, partitioning, and degradation would significantly aid understanding the fate and distribution of OPAHs. As well, the overall occurrence and comparatively high concentrations of OPAHs begs further research on source apportionment and individual OPAH toxicity.
Supplementary Material
Table S1 Selected ion monitoring Groups and Ions
Figure S1 Chromatogram of Standard Solution
Figure S2 Extraction and Clean-up Schematic PAH recoveries
Table S2 OPAH Method Recoveries
Acknowledgement-
The authors would like to thank Jeremy Riggle and Sarah Allan for their analytical expertise and laboratory assistance. The present study was developed under a STAR Research Assistance Fellowship, ID Number: F07D40790, awarded by the U.S. Environmental Protection Agency. It has not been formally reviewed by the U.S. EPA. The project described was supported in part by Award Number P42 ES016465, from the National Institute of Environmental Health Sciences. The views expressed in this presentation are solely those of the authors and do not necessarily represent the official views of U.S. EPA, NIEHS, or NIH and these organizations do not endorse any products or commercial services mentioned.
REFERERNCES
- 1.Lundstedt S, White PA, Lemieux CL, Lynes KD, Lambert LB, Oberg L, Haglund P, Tysklind M. Sources, fate, and toxic hazards of oxygenated polycyclic aromatic hydrocarbons (PAHs) at PAH-contaminated sites. Ambio. 2007;36:475–485. doi: 10.1579/0044-7447(2007)36[475:sfatho]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Spitzer T, Takeuchi T. Determination of benzanthrone in environmental samples. J Chromatogr A. 1995;710:109–116. [Google Scholar]
- 3.Yu HT. Environmental carcinogenic polycyclic aromatic hydrocarbons: photochemistry and phototoxicity. J Environ Sci Health, Part C Environ Carcinog Ecotoxicol Rev. 2002;20:149–183. doi: 10.1081/GNC-120016203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lampi MA, Gurska J, McDonald KIC, Xie FL, Huang XD, Dixon DG, Greenberg BM. Photoinduced toxicity of polycyclic aromatic hydrocarbons to Daphnia magna: Ultraviolet-mediated effects and the toxicity of polycyclic aromatic hydrocarbon photoproducts. Environ Toxicol Chem. 2006;25:1079–1087. doi: 10.1897/05-276r.1. [DOI] [PubMed] [Google Scholar]
- 5.Burdick A, Davis JW, Liu KJ, Hudson LG, Shi, Honglian, Monske ML, Burchiel SW. Benzo(a)pyrene quinones increase cell proliferation, generate reactive oxygen species, and transactivate the epidermal growth factor receptor in breast epithelial cells. Cancer Res. 2003;63:7825–7833. [PubMed] [Google Scholar]
- 6.Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 7.Durant JL, Busby WF, Jr, Lafleur AL, Penman BW. Human cell mutagenicity of oxygenated, nitrated, and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols. Mutat Res. 1996;371:123–157. doi: 10.1016/s0165-1218(96)90103-2. [DOI] [PubMed] [Google Scholar]
- 8.Lemieux CL, Lambert AB, Lundstedt S, Tysklind M, White PA. Mutagenic hazards of complex polycyclic aromatic hydrocarbon mixtures in contaminated soil. Environ Toxicol Chem. 2008;27:978–990. doi: 10.1897/07-157.1. [DOI] [PubMed] [Google Scholar]
- 9.De Martinis BS, Okamoto RA, Kado NY, Gundel LA, Carvalho LRF. Polycyclic aromatic hydrocarbons in a bioassay-fractionated extract of PM10 collected in Sao Paulo, Brazil. Atmos Environ. 2002;36:307–314. [Google Scholar]
- 10.Xia T, Korge P, Weiss JN, Li N, Venkatesen MI, Sioutas C, Nel A. Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: Implications for ultrafine particle toxicity. Environ Health Perspect. 2004;112:1347–1358. doi: 10.1289/ehp.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundstedt S, Haglund P, Oberg L. Simultaneous extraction and fractionation of polycyclic aromatic hydrocarbons and their oxygenated derivatives in soil using selective pressurized liquid extraction. Anal Chem. 2006;78:2993–3000. doi: 10.1021/ac052178f. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson M, Dalhammar G, Borg-Karlson AK. Biological degradation of selected hydrocarbons in an old PAH/creosote contaminated soil from a gas work site. Appl Microbiol Biotechnol. 2000;53:619–626. doi: 10.1007/s002530051667. [DOI] [PubMed] [Google Scholar]
- 13.Brooks LR, Hughes TJ, Claxton LD, Austern B, Brenner R, Kremer F. Bioassay-directed fractionation and chemical identification of mutagens in bioremediated soils. Environ Health Perspect. 1998;106(Suppl 6):1435–1440. doi: 10.1289/ehp.98106s61435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkinson R, Arey J. Atmospheric chemistry of gas-phase polycyclic aromatic hydrocarbons: formation of atmospheric mutagens. Environ Health Perspect. 1994;102(Suppl 4):117–126. doi: 10.1289/ehp.94102s4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sienra MD. Oxygenated polycyclic aromatic hydrocarbons in urban air particulate matter. Atmos Environ. 2006;40:2374–2384. [Google Scholar]
- 16.Schnelle-Kreis J, Gebefugi I, Welzl G, Jaensch T, Kettrup A. Occurrence of particle-associated polycyclic aromatic compounds in ambient air of the city of Munich. Atmos Environ. 2001;35:S71–S81. [Google Scholar]
- 17.Sklorz M, Briede JJ, Schnelle-Kreis J, Liu Y, Cyrys J, de Kok TM, Zimmermann R. Concentration of oxygenated polycyclic aromatic hydrocarbons and oxygen free radical formation from urban particulate matter. J Toxicol and Environ Health. Part A. 2007;70:1866–1869. doi: 10.1080/15287390701457654. [DOI] [PubMed] [Google Scholar]
- 18.Allen JO, Dookeran NM, Taghizadeh K, Lafleur AL, Smith KA, Sarofim AF. Measurement of oxygenated polycyclic aromatic hydrocarbons associated with a size-segregated urban aerosol. Environ Sci Technol. 1997;31:2064–2070. [Google Scholar]
- 19.Albinet A, Leoz-Garziandia E, Budzinski H, Villenave E, Jaffrezo JL. Nitrated and oxygenated derivatives of polycyclic aromatic hydrocarbons in the ambient air of two French alpine valleys - Part 1: Concentrations, sources and gas/particle partitioning. Atmos Environ. 2008;42:43–54. [Google Scholar]
- 20.Lintelmann J, Fischer K, Matuschek G. Determination of oxygenated polycyclic aromatic hydrocarbons in particulate matter using high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2006;1133:241–247. doi: 10.1016/j.chroma.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 21.Leotz-Gartziandia E, Tatry V, Carlier P. Sampling and analysis of organic compounds in diesel particulate matter. Environ Monit Assess. 2000;65:155–163. [Google Scholar]
- 22.Akimoto Y, Aoki T, Nito S, Inouye Y. Oxygenated polycyclic aromatic hydrocarbons from MSW incinerator fly ash. Chemosphere. 1997;34:263–273. [Google Scholar]
- 23.Ramdahl T. Polycyclic aromatic ketones in environmental samples. Environ Sci Technol. 1983;17:666–670. doi: 10.1021/es00117a008. [DOI] [PubMed] [Google Scholar]
- 24.Bayona JM, Markides KE, Lee ML. Characterization of polar polycyclic aromatic-compounds in a heavy-duty diesel exhaust particulate by capillary column gas-chromatography and high-resolution mass-spectrometry. Environ Sci Technol. 1988;22:1440–1447. doi: 10.1021/es00177a009. [DOI] [PubMed] [Google Scholar]
- 25.Sidhu S, Gullett B, Striebich R, Klosterman J, Contreras J, DeVito M. Endocrine disrupting chemical emissions from combustion sources: diesel particulate emissions and domestic waste open burn emissions. Atmos Environ. 2005;39:801–811. [Google Scholar]
- 26.Choudhury DR. Characterization of polycyclic ketones and quinones in diesel emission particulates by gas-chromatography mass-spectrometry. Environ Sci Technol. 1982;16:102–106. [Google Scholar]
- 27.Jakober CA, Riddle SG, Robert MA, Destaillats H, Charles MJ, Green PG, Kleeman MJ. Quinone emissions from gasoline and diesel motor vehicles. Environ Sci Technol. 2007;41:4548–4554. doi: 10.1021/es062967u. [DOI] [PubMed] [Google Scholar]
- 28.Zdrahal Z, Karasek P, Lojkova L, Buckova M, Vecera Z, Vejrosta J. Pressurised liquid extraction of ketones of polycyclic aromatic hydrocarbons from soil. J Chromatogr A. 2000;893:201–206. doi: 10.1016/s0021-9673(00)00748-2. [DOI] [PubMed] [Google Scholar]
- 29.Albinet A, Leoz-Garziandia E, Budzinski H, ViIlenave E. Simultaneous analysis of oxygenated and nitrated polycyclic aromatic hydrocarbons on standard reference material 1649a (urban dust) and on natural ambient air samples by gas chromatography-mass spectrometry with negative ion chemical ionisation. J Chromatogr A. 2006;1121:106–113. doi: 10.1016/j.chroma.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 30.Nicol S, Dugay J, Hennion MC. Determination of oxygenated polycyclic aromatic compounds in airborne particulate organic matter using gas chromatography tandem mass spectrometry. Chromatographia. 2001;53:S464–S469. [Google Scholar]
- 31.Grosse S, Letzel T. Liquid chromatography/atmospheric pressure ionization mass spectrometry with post-column liquid mixing for the efficient determination of partially oxidized polycyclic aromatic hydrocarbons. J Chromatogr A. 2007;1139:75–83. doi: 10.1016/j.chroma.2006.10.086. [DOI] [PubMed] [Google Scholar]
- 32.National Institute of Standards and Technology . SRMs 1649b, 1650b, 1944, 1975, 2977. Gaithersburg, MD, USA: 2009. Standard reference materials. - Certificate of analysis. [Google Scholar]
- 33.Lintelmann J, Fischer K, Karg E, Schröppel A. Determination of selected polycyclic aromatic hydrocarbons and oxygenated polycyclic aromatic hydrocarbons in aerosol samples by high-performance liquid chromatography and liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2005;381:508–519. doi: 10.1007/s00216-004-2883-8. [DOI] [PubMed] [Google Scholar]
- 34.Cho AK, Di Stefano E, You Y, Rodriguez CE, Schmitz DA, Kumagai Y, Miguel AH, Eiguren-Fernandez A, Kobayashi T, Avol E, Froines JR. Determination of four quinones in diesel exhaust particles, SRM 1649a, and atmospheric PM2.5. Aerosol Sci Technol. 2004;38:68–81. [Google Scholar]
- 35.Tomruk A, Guven KC. Biotransformation of 1-methylnaphthalene and anthracene in mussels (Mytilus galloprovincialis Lamarck, 1819). Fresenius Environ Bull. 2008;17:256–259. [Google Scholar]
- 36.Fernandez P, Grifoll M, Solanas AM, Bayona JM, Albaiges J. Bioassay-directed chemical-analysis of genotoxic components in coastal sediments. Environ Sci Technol. 1992;26:817–829. [Google Scholar]
- 37.McKinney RA, Pruell RJ, Burgess RM. Ratio of the concentration of anthraquinone to anthracene in coastal marine sediments. Chemosphere. 1999;38:2415–2430. doi: 10.1016/s0045-6535(98)00435-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Selected ion monitoring Groups and Ions
Figure S1 Chromatogram of Standard Solution
Figure S2 Extraction and Clean-up Schematic PAH recoveries
Table S2 OPAH Method Recoveries