Abstract
At rest, brain activity can be characterized not by an absence of organized activity but instead by spatially and temporally correlated patterns of activity. In this experiment, we investigated whether and to what extent resting state functional connectivity is modulated by sex hormones in women, both across the menstrual cycle and when altered by oral contraceptive pills. Sex hormones have been shown to have important effects on task-related activity, but few studies have investigated the extent to which they can influence the behavior of functional networks at rest. These hormones are dramatically altered by the use of hormonal contraception, which is used by approximately 100 million women worldwide. However, potential cognitive side effects of hormonal contraception have been given little attention. Here, we collected resting state data for naturally-cycling women (n=45) and women using combined oral contraceptive pills (n=46) and evaluated the differences in resting state activity between these two groups using Independent Components Analysis. We found that in the default mode network and in a network associated with executive control, resting state dynamics were altered both by the menstrual cycle and by oral contraceptive use. Specifically, the connectivity of the left angular gyrus, the left middle frontal gyrus, and the anterior cingulate cortex were different between groups. Because the anterior cingulate cortex and left middle frontal gyrus are important for higher-order cognitive and emotional processing, including conflict monitoring, changes in the relationship of these structures to the functional networks with which they interact may have important consequences for attention, affect, and/or emotion regulation.
Keywords: functional connectivity, resting state, independent components analysis, oral contraceptives, menstrual cycle, neuroendocrinology
1.1 INTRODUCTION
Sex hormones are neuroactive steroids that influence cognitive function in a variety of domains. These hormones change significantly over the course of the menstrual cycle, and are strongly suppressed by synthetic hormones in women who use hormonal contraceptives. Potential cognitive side effects of hormonal contraceptives have been only minimally explored, despite their widespread use. Effects of hormonal contraceptives on mate selection have been documented, both as they affect women’s preferences for men (Wedekind et al., 1995) and men’s preferences for women (Kuukasjärvi et al., 2004). Differences in long-term relationship outcomes have also been observed in hormonal contraceptive users (Roberts et al., 2012).
Although the intended purpose of hormonal contraceptives is primarily reproductive, and it might be assumed that behavioral effects of these medications are limited to the reproductive domain, recent studies have suggested more widespread cognitive changes may be associated with hormonal contraceptive use. Performance on verbal memory (Mordecai et al., 2008), verbal fluency, and mental rotation tasks differ in women using oral contraceptives (OCs; Griksiene and Ruksenas, 2011), as does the pattern of memory retention for recall of an emotional story (Nielsen et al., 2011).
Hormonal contraceptive use has been shown to significantly increase gray matter volume in prefrontal and temporal regions of the brain (Pletzer et al., 2010). Differences have also been observed in the white matter tracts of hormonal contraceptive users, specifically in the fornix (De Bondt et al., 2013). These differences in brain structure suggest that hormonal contraceptives may have functional effects on the brain as well, and indeed recent evidence has emerged suggesting that this is the case. Women using oral contraceptive pills have a larger blood-oxygen-level dependent (BOLD) response in the fusiform face area to images of faces compared to naturally-cycling women (Marečková et al., 2012), but a reduced BOLD response in the precentral gyrus to some categories of erotic stimuli relative to women in the follicular phase of the menstrual cycle (Abler et al., 2013). On a verb generation task, women using OCs showed different patterns of activation than naturally-cycling women, and the localization of the differences depended on the cycle phase of the naturally-cycling women (Rumberg et al., 2010).
Hormonal contraceptives have been shown to alter the endocrine response to stressors (Kirschbaum et al., 1999; Maes et al., 1992), and more recently this has been linked to differences in the neural activity associated with fear learning. Cortisol administration reduces hippocampal response to a learned fear stimulus in naturally-cycling women, but increases the hippocampal BOLD signal in women using OCs (Merz et al., 2012). Finally, OC use has also been associated with differences in the BOLD response that underlies emotional reactivity, with OC users showing less activity in several prefrontal regions and failing to show the attenuation of activity in the amygdala observed in naturally-cycling women (Gingnell et al., 2012).
With the emergence of these studies indicating that OCs may affect brain activity in response to the demands of specific tasks, we hypothesized that they may have important effects on the resting state of the brain. The brain at rest, rather than entering a quiescent state, has a characteristic pattern of synchronous low-frequency oscillations (Biswal et al., 1995, 1997). A number of functional networks have been observed that show correlated patterns of activity at rest, most notably the default mode network (DMN; Greicius et al., 2003), but also a number of other networks and subnetworks that have since been correlated with specific behavioral domains (Laird et al., 2011; Smith et al., 2009). Previous studies have shown sex influences on resting state functional connectivity (Biswal et al., 2010; Tian et al., 2011), but to our knowledge neither menstrual cycle nor OC use have been explored as potential modulators of resting state dynamics.
Because the tasks previously shown to be affected by OCs were cognitive and affective in nature, and as a result of preliminary data analysis, we hypothesized that the resting state networks most sensitive to OCs would be those associated with cognitive and affective domains. Thus, in this experiment we examined the dynamics of two resting state networks, both of which have been implicated in cognitive and affective tasks: the anterior portion of the default mode network (aDMN), and a network previously described as the executive control network (ECN; Smith et al., 2009).
Differences in the connectivity of these networks were examined across four hormonally distinct groups: (1.) early follicular naturally-cycling women, (2.) luteal naturally-cycling women, (3.) OC users during the inactive week of pill use, and (4.) OC users during the active phase of pill use. These four groups represent, respectively: women with low endogenous sex hormones; women with high endogenous sex hormones; women with low endogenous hormones and low synthetic hormones; and finally, women with high synthetic hormones and low endogenous hormones. The two groups of naturally-cycling women were selected to maximize the contrast between endogenous hormones, as the early follicular phase is characterized by low levels of sex hormones, and the luteal phase is characterized by relatively elevated levels of both estrogen and progesterone. Further, by including both naturally-cycling women and OC users, we were able to examine the extent to which the synthetic hormones in OCs mimicked the effects of endogenous hormones, and by including OC users during the inactive pill week, we were able to examine whether the effects of synthetic hormones (if any) were acute or chronic.
METHODS AND MATERIALS
2.1 Participants
Participants were recruited from the University of California, Irvine student population and the surrounding community. Signed, informed consent was obtained before beginning the experiment. Participants were screened by phone and excluded for age under 18 or over 40; a reported history of drug or alcohol abuse; a previous diagnosis of psychiatric, endocrine, or neurological disorders; epilepsy; strokes; brain tumors; current pregnancy or breastfeeding; irregular periods; left-handedness; or non-removable metal implants. Participants did not differ on basic demographic characteristics (see Table S1 in supplementary materials).
Naturally-cycling women were excluded if they had used any form of hormonal contraception in the previous 3 months. Women assigned to the early follicular group were scanned during cycle days 2 to 6, and women assigned to the luteal group were scanned during cycle days 18 to 24. These days were selected in accordance with those in previous literature (Andreano and Cahill, 2008; Andreano and Cahill, 2010; Maki, Rich and Rosenbaum, 2002). We elected to narrow the follicular window very slightly in light of unpublished data from our laboratory showing that salivary hormone levels are sometimes higher on cycle day 1 compared to cycle day 2, and our goal was to minimize hormone levels in the early follicular group. We also sought to avoid the possibility that women in this group were near enough to ovulation to be experiencing the pre-ovulatory estrogen surge, hence the cutoff of cycle day 6.
Women in the birth control group were excluded if they had used OCs for fewer than 3 consecutive months prior to scanning, if their OCs were progestin-only, or if they were not using a 28-day cycle. Inactive pill users were scanned during days 2–6 of inactive pill use, and active pill users were scanned during the 11th to 17th days of active pill use. We treated the beginning of the inactive week from the preceding month’s pack as cycle day 1, rather than treating the beginning of the active pill week as cycle day 1. This way, in both the naturally-cycling and the OC group, cycle day 1 corresponded approximately with the onset of menses, and thus days 11 to 17 of pill use corresponded to cycle days 18 to 24 in the naturally-cycling group.
One participant was consented but withdrew before scanning due to feelings of claustrophobia, another was consented but could not be scanned due to technical problems at the imaging center, and three participants in the naturally-cycling group were scanned but later excluded for menstrual irregularities leading to abnormal cycles within the study cycle; their data were not analyzed. After data pre-processing, an additional 2 subjects were excluded for excessive head motion (>4mm or 4 degrees). Data from 91 subjects was available for analysis: 20 follicular, 25 luteal, 22 inactive pill users, and 24 active pill users.
2.2 Saliva collection and assay
Saliva was collected immediately before and after scanning via direct expectoration into 15mL Falcon tubes. Each sample was approximately 2mL. Samples were frozen at −20°C until the day of assaying, when they were defrosted and centrifuged for 15 minutes at 3000rpm. The supernatant was decanted into a clean Falcon tube and centrifuged again for 10 minutes at 3000rpm before assaying.
Salivary progesterone and 17β-estradiol assays were performed using commercially available immunoassay kits (Salimetrics, State College, PA, USA). The detection sensitivity levels reported for these kits are 5 and 0.1 pg/mL, respectively.
Each of the two samples from each participant was assayed in duplicate. The two samples were averaged together to provide the average hormone level at the time of the resting scan. The average inter-assay coefficient of variance for the progesterone assays was 9.86%, and for the estradiol assays, 7.18%. The intra-assay coefficient of variance was 9.32% and 3.71% for the progesterone and estradiol assays, respectively.
2.3 MRI data collection and preprocessing
Data was collected on a Philips Achieva 3T MR scanner (Eindhoven, The Netherlands) equipped with an 8-channel SENSE head coil. Functional echoplanar imaging data was collected in 30 slices with an 80×79 acquisition matrix size, a 70° flip angle, 2 s repetition time, 30 ms echo time, and 3.0×1.5×3mm3 voxel size. Two hundred and fourteen volumes were collected. Structural T1-weighted data was collected in 160 slices with 1.0×1.0×0.67mm3 voxel size.
Once positioned in the scanner, participants were instructed, “Relax, and try to stay as still as you can until the scan is over. The scan will take about 8 minutes.” After the resting data was collected, participants remained in the scanner to participate in a separate experiment.
Imaging data was preprocessed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK) modules via the toolbox Data Processing Assistant for Resting-State fMRI (DPARSF; http://www.restfmri.net; Yan and Zang, 2010) implemented in MATLAB R2012b. The first volume of each run was dropped to allow for magnetic field stabilization. Subsequent volumes were slice timing corrected to the middle volume, realigned to the first volume, normalized to MNI space using a standard echoplanar imaging template, and smoothed with a 4×4×4mm full-width half-maximum Gaussian kernel.
Six head motion parameters were extracted by DPARSF and compared between groups by one-way ANOVA. No significant differences in head motion were found between groups in any direction, all ps > 0.1.
2.4 Data analysis
Preprocessed data was analyzed using the Group ICA Toolbox v. 2.0e (GIFT; http://mialab.mrn.org/software/gift). Group-level independent components were estimated using the Infomax algorithm (Bell and Sejnowski, 1995) and 20 iterations were performed using ICASSO (Himberg et al., 2004) with a minimum cluster size of 16 and maximum cluster size of 20 to increase the stability of the components. Individual subject ICA maps were back-reconstructed using GICA (Erhardt et al., 2011).
Of the 20 components ICA produced, 11 were discarded as likely artifactual findings and 9 were retained. These were identified as the medial visual, somatosensory, left frontoparietal, right frontoparietal, poster default mode, anterior default mode, lateral visual, auditory, and executive control networks. The anterior default mode network (aDMN) and executive control network (ECN) were selected as the networks of interest. These networks were initially identified by visual inspection, then compared to each of the 20 intrinsic connectivity networks (ICNs) identified by Laird et al., 2011 (http://www.brainmap.org/icns/). The component visually identified as the aDMN (Figure 1, Table S3) correlated most strongly with the Laird default mode ICN (r=0.44), and the component visually identified as the ECN (Figure 2, Table S4) correlated most strongly with Laird ICN 4 (r=0.44), described as a network that connects cognition with emotion/interoception. Clusters with an extent of at least 15 voxels and family-wise error (FEW) corrected p < 0.05 are reported.
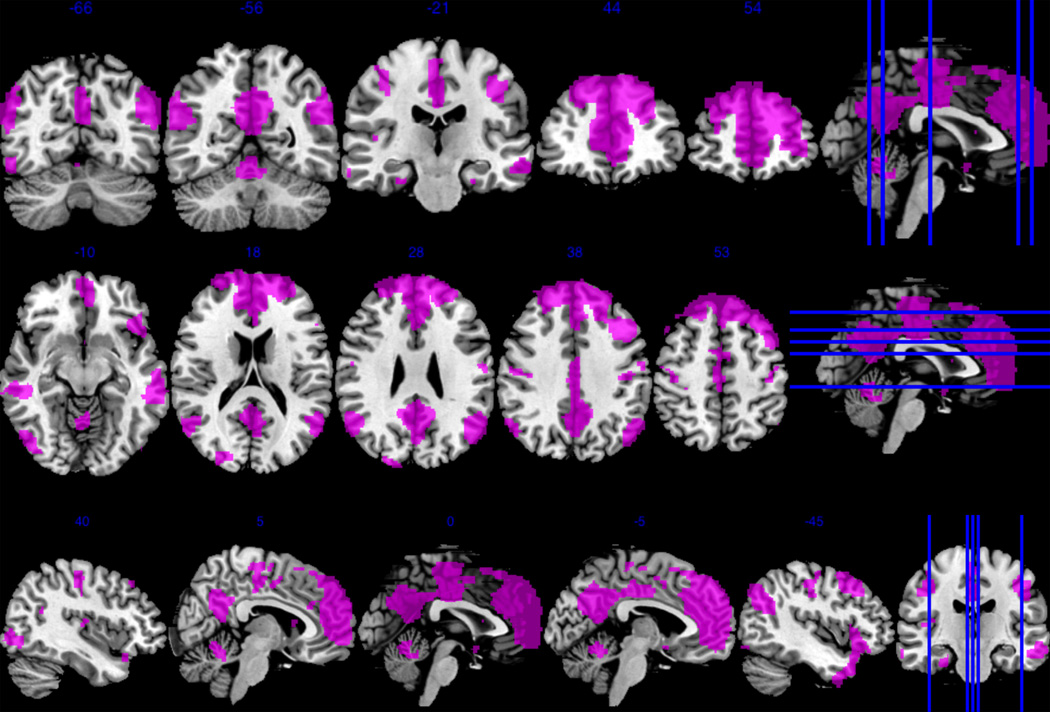
Figure 1.
The anterior default mode network included the bilateral superior medial gyri, bilateral cingulate cortex, bilateral angular gyri, bilateral inferior frontal gyri, bilateral temporal poles, cerebellar vermis, right parahippocampal gyrus, right insula lobe, and right caudate nucleus.
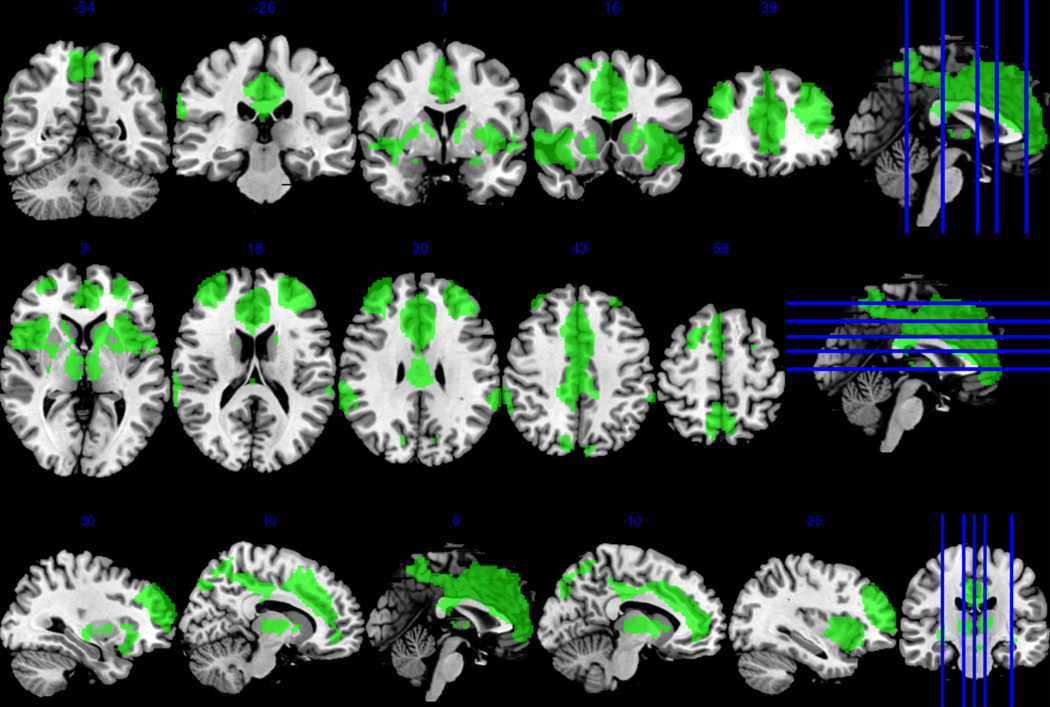
Figure 2.
The executive control network included bilateral cingulate cortex, bilateral bilateral supramarginal gyri, left insula lobe, bilateral middle frontal gyri, and right cuneus.
Each of these two components was transformed into a NifTI image file using the MARSeille Boîte À Région d’Intérêt toolbox (MarsBaR; http://marsbar.sourceforge.net; Brett et al., 2002). The image file was used as an explicit mask in SPM to limit the voxel extent to the network of interest (NifTI files downloadable at http://cahill.bio.uci.edu/ComponentMasks.zip). Finally, the component images for each subject in all four groups were entered into one-way ANOVAs in SPM and contrast files were generated. The contrast files showed the difference in connectivity between each group and the three others.
Correlations were also performed in SPM. The time course of each component (aDMN and ECN) for each participant was regressed against baseline salivary progesterone and estrogen levels. The hormone levels used were an average of the two samples taken over the course of the experimental session.
RESULTS
3.1 Salivary hormone levels
Differences in hormone levels between the four groups (follicular, luteal, inactive pill, and active pill) were analyzed by a one-way ANOVA. The groups differed significantly in mean salivary estradiol levels, F(3,87) = 3.12, p = 0.03. Post-hoc t-tests revealed that the luteal group differed significantly from the active pill users, p=0.01, and from the inactive pill users, p = 0.03. The follicular group’s baseline estrogen levels were marginally different from the active pill users, p = 0.05. However, the other groups did not differ significantly from one another (Figure 3).
Figure 3.
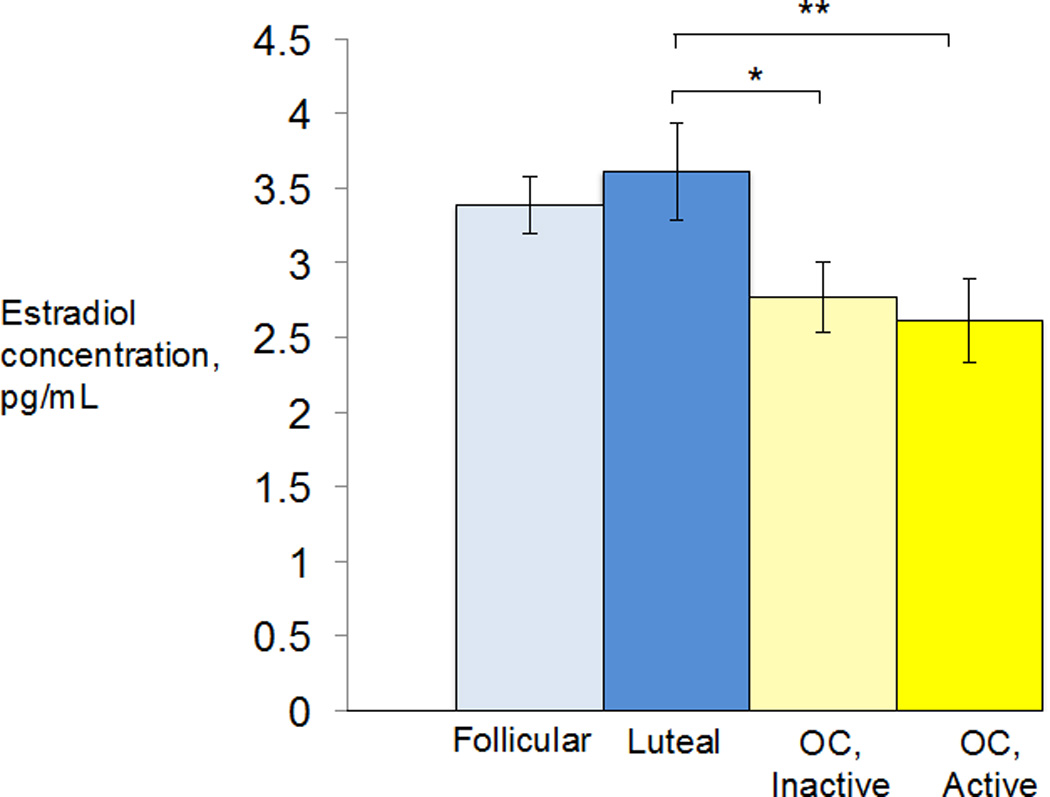
The luteal group’s baseline estradiol levels differed significantly from both OC groups. *p< 0.05, **p<0.01.
The groups also differed in baseline salivary progesterone levels, F(3,87) = 9.00, p < 0.0011. Post-hoc t-tests found that the luteal group differed significantly from the inactive pill group, p < 0.0011, from the active pill group, p < 0.0011, and from the follicular group, p < 0.05. The follicular group differed significantly from the inactive pill group, p < 0.05. The difference between follicular women’s and active pill users’ progesterone levels was marginally significant, p = 0.06. The active and inactive pill users’ progesterone levels did not differ from one another, p > 0.5 (Figure 4).
Figure 4.
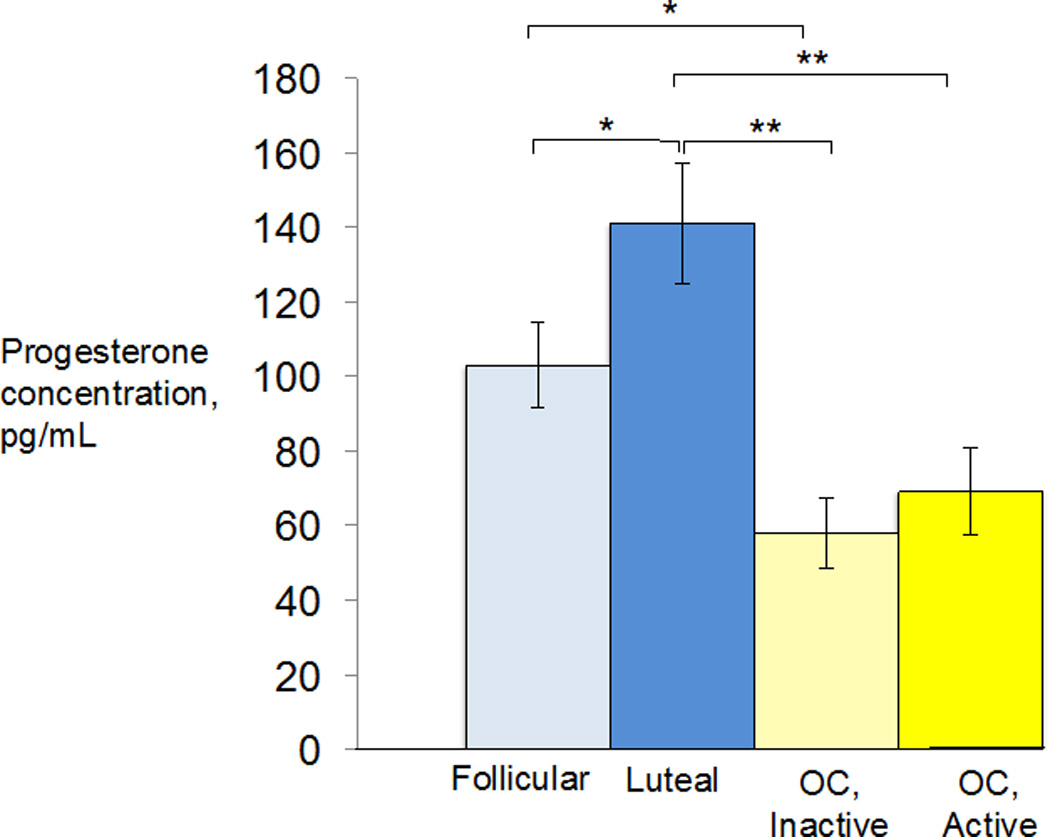
The luteal group had significantly higher baseline progesterone levels compared to each other group. The follicular group also had significantly higher baseline progesterone than the inactive pill users. *p<0.05, **p<0.0011.
Mean salivary hormone levels for both estrogen and progesterone are included in supplementary materials (Table S2).
3.2 Group differences in anterior Default Mode Network connectivity
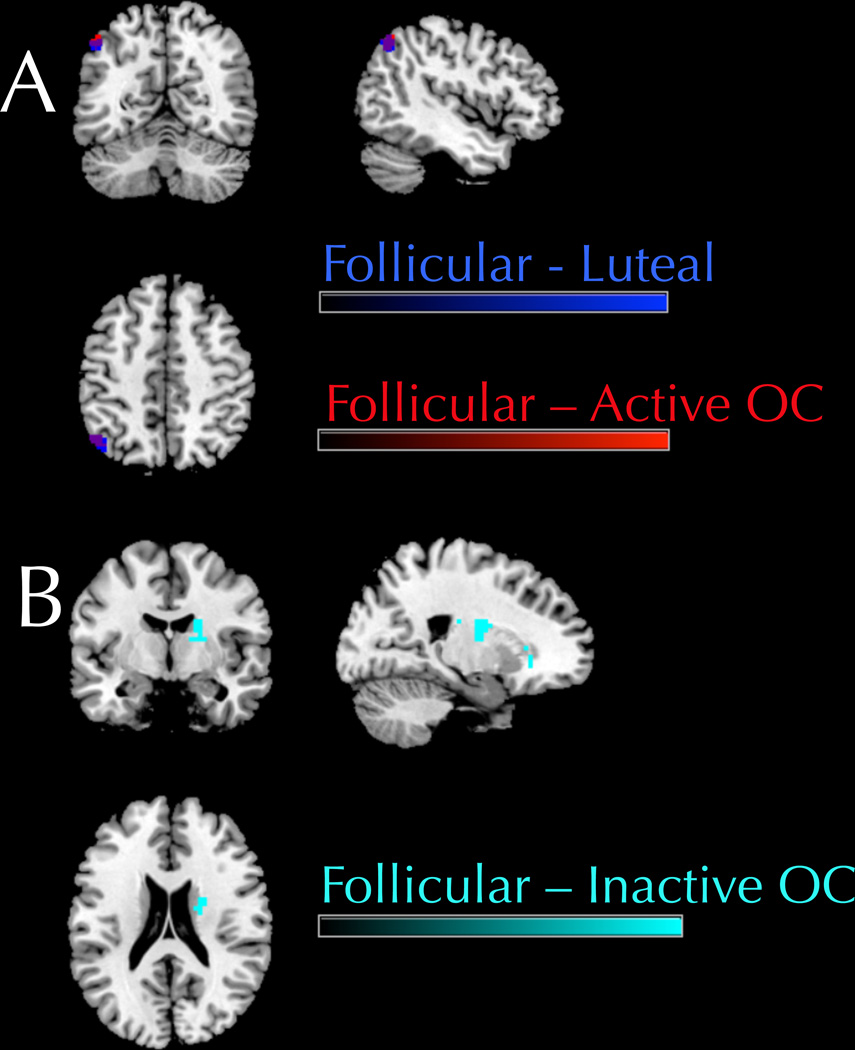
A one-way ANOVA showed that the connectivity of the aDMN differed significantly between the follicular and luteal groups. The follicular group showed increased connectivity with the aDMN in the left angular gyrus, t(1,87) = 5.42, cluster-level pFWE = 0.005, 34 voxel extent relative to the luteal group. Follicular women also showed increased connectivity with the aDMN in the left angular gyrus compared to active OC users, t(1,87) = 5.30, cluster-level pFWE = 0.017, 27 voxel extent (Figure 5a; bar graph available in supplementary materials Figure S1).
Figure 5.
(a): Follicular women show greater connectivity between the aDMN and the left angular gyrus compared to both luteal women (peak difference at −45, −66, 48) and active pill users (peak difference at −48, −63, 48). The follicular versus luteal comparison is shown in blue, and follicular versus active OC comparison in red. Purple indicates areas of overlap, i.e., voxels in which altered functional connectivity was found in both comparisons. 5(b): Follicular women show greater connectivity between the aDMN and the right caudate nucleus compared to inactive pill users (peak difference at 21, −6, 21).
A one-way ANOVA showed that the connectivity of the default mode network did not differ between OC users on the active pill week compared to the inactive pill week. However, follicular women differed significantly from the inactive pill users. Compared to inactive pill users, follicular women showed increased connectivity with the aDMN in the right caudate nucleus, t(1,87) = 5.69, cluster-level pFWE = 0.044, 22 voxel extent, (Figure 5b; bar graph available in supplementary materials Figure S2).
3.3 Group differences in Executive Control Network connectivity
Menstrual cycle phase was associated with significant differences in the connectivity of the ECN. Here, follicular women showed increased connectivity with the ECN relative to the luteal group in the right anterior cingulate cortex, t(1,87) = 5.42, cluster-level pFWE < 0.001, 52 voxel extent.
Active pill and inactive pill users also showed differences in ECN connectivity. A one-way ANOVA showed increased connectivity with the ECN in the left middle frontal gyrus, approximately BA10, in the inactive pill group relative to the active pill users, t(1,87)=4.59, cluster-level pFWE = 0.019, 19 voxel extent.
OC users also showed differences compared to naturally-cycling women in the ECN. Follicular women showed greater connectivity with the ECN than active pill users in both the left anterior cingulate cortex, t(1,87) = 4.82, cluster-level pFWE < 0.001, 61 voxels, and in the left middle frontal gyrus near BA10, t(1,87) = 4.57, cluster-level pFWE = 0.001, 37 voxels (Figure 6; bar graph available in supplementary material Figure S3 and S4).
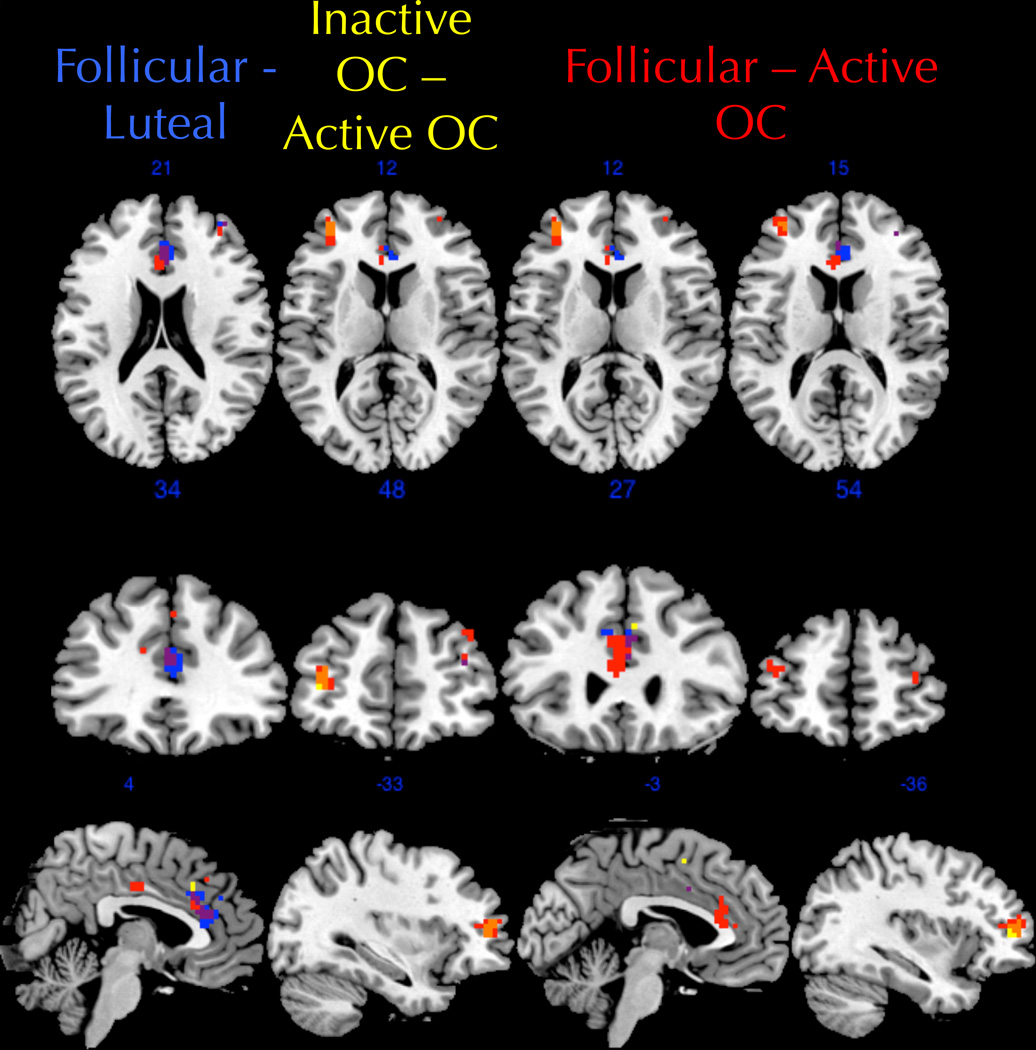
Figure 6.
Follicular women showed greater connectivity than luteal women between the right anterior cingulate cortex (peak difference at 3, 33, 21) and the ECN, shown in blue (column 1 slices selected to optimally show this contrast). Active pill users had reduced connectivity between the left middle frontal gyrus (peak difference at −33, 48, 12) and the ECN compared to inactive pill users, shown in yellow (column 2 slices selected to optimally show this contrast). OC users taking active pills also had reduced connectivity compared to follicular women in the left anterior cingulate cortex (peak difference at −3, 27, 12), shown in red (column 3 slices selected to optimally show this contrast), and in the left middle frontal gyrus (peak difference at −36, 54, 15), also shown in red (column 4 slices selected to optimally show this contrast). Purple indicates areas of overlap between the follicular/luteal contrast and follicular/active OC contrast. Orange indicates areas of overlap between the follicular/active OC contrast and the inactive OC/active OC contrast.
As depicted in Figure 6, connectivity of the left middle frontal gyrus was altered in the active OC users relative to both the inactive OC users and naturally-cycling follicular women. Connectivity was also reduced relative to naturally-cycling luteal women, but this difference was only significant at a much higher threshold (cluster-level p < 0.05, uncorrected). Normalized average connectivity values for the left middle frontal gyrus cluster in the ECN component are shown in Figure 7.
Figure 7.
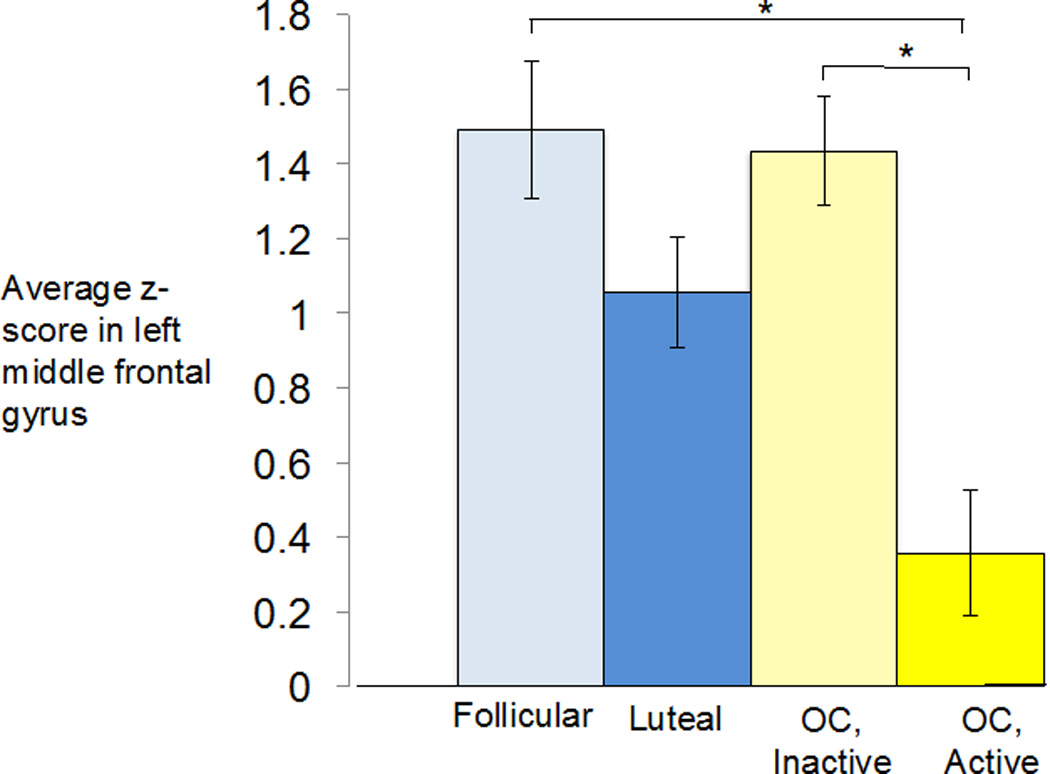
The average z-score of the left middle frontal gyrus cluster in the ECN component was significantly lower in the active OC users compared to either the inactive OC users or follicular women, *p<0.05, cluster-level FWE-corrected.
Luteal women did not show any regions of increased connectivity relative to OC users in the ECN. In neither network did the luteal group show any areas of increased connectivity relative to the follicular group.
3.4 Hormone correlations
Linear regression analyses were performed to determine if a linear relationship could be observed between salivary estrogen or progesterone levels and the time course of each component for each participant. No significant correlations were found, either positive or negative, for estrogen or progesterone.
4.1 DISCUSSION
In this investigation of the effects of hormones on resting state functional connectivity, we found that both endogenous hormone cycling due to the menstrual cycle and exogenous hormone administration through the use of oral contraceptive pills are associated with altered resting state dynamics in two separate functional networks, the aDMN and ECN. In the aDMN, luteal women showed reduced coherence between the left angular gyrus and the rest of the network when compared to follicular women. OC users in the active phase of their pill cycle also showed reduced coherence between the left angular gyrus and the rest of the network when compared to follicular women. In the ECN, luteal women showed reduced coherence between right anterior cingulate (ACC) and the rest of the network when compared to follicular women. Compared to naturally-cycling women in the follicular phase, OC users in the active pill phase showed reduced coherence between the left ACC and the rest of the network, and also showed reduced coherence between the left middle frontal gyrus and the ECN. This region also showed differences in resting state connectivity when active pill users were compared to those on the inactive week of their pill cycle; the inactive pill users had greater coherence between the left MFG and the ECN than did active pill users.
Activity in the intrinsic connectivity network defined as the default mode network has previously been shown to decrease during cognitive tasks (Greicius et al., 2003) and to be negatively correlated with task performance across a number of behavioral domains (Smith et al., 2009). In healthy individuals, evidence suggests the DMN is active during stimulus-free rest periods (Greicius et al., 2003; Gusnard and Raichle, 2001; Raichle et al., 2001) as well as during tasks that solicit an abstract, internal, self-representational state (D’Argembeau et al., 2005; Gusnard et al., 2001). For instance, DMN structures are activated by autobiographical remembering, prospection, and theory-of-mind imagining, tasks which place the self in the past, the future, and in the mind of another person, respectively (Spreng and Grady, 2009).
Altered functional connectivity in the DMN has previously been associated with young (Supekar et al., 2010) and old age (Sambataro et al., 2010), as well as a number of mental disorders (Broyd et al., 2008) including Alzheimer’s disease (Greicius et al., 2004) and major depressive disorder (Grimm et al., 2009). At least two studies have failed to find significant effects of sex on the DMN (Bluhm et al., 2008; Weissman-Fogel et al., 2010), initially suggesting that sex does not impact resting state dynamics. However, both studies failed to control for menstrual cycle position or oral contraceptive use, both of which, we have demonstrated here, can affect DMN connectivity. These data suggest that hormone status should be taken under consideration when investigating resting state functional connectivity. Additionally, because the DMN is involved in a variety of domains, both in healthy individuals as well as disease states, we can speculate that altered DMN connectivity related to altered hormone levels has the potential to affect abstract, self-referential reasoning, or perhaps in extreme cases may be related, positively or negatively, to the development of disease states that show altered DMN connectivity.
In the ECN, active pill users showed decreased connectivity in the left middle frontal gyrus compared to both inactive pill users and follicular women. This suggests that the hormones in OCs acutely disrupt the connectivity of this region to the rest of the ECN. Furthermore, this connectivity can be at least partly restored during the brief period of inactive pill use, evidenced by the difference in connectivity patterns between the active and inactive pill groups. The left prefrontal cortex has been generally associated with language processing (Binder et al., 1997; Crozier et al., 1999; Gabrieli et al., 1998; Poldrack et al., 1999; Vigneau et al., 2006), and the middle frontal gyrus in particular has been associated with a number of cognitive tasks, including but not limited to: recognition memory for verbal stimuli (Donaldson et al., 2001), explicit contingency learning (Carter et al., 2006), and error processing tasks (Kiehl et al., 2000) such as the Stroop task (Adleman et al., 2002). Because we observed a change in the coherence of the left middle frontal gyrus with the rest of the ECN, it may be the case that performance on any of these tasks that involve the left middle frontal gyrus may be altered by OC use.
Active pill OC users also showed decreased connectivity in the left anterior cingulate compared to naturally-cycling women, but only in comparison to those in the follicular phase. Interestingly, luteal women also showed decreased connectivity in the anterior cingulate, but in the opposite hemisphere. Previous literature has shown that the connectivity of the ACC is sensitive to hormone changes, and administration of micronized progesterone significantly increased the connectivity of the dorsal ACC to the amygdala (Van Wingen et al., 2008). Our data suggest that increases in hormone levels alter the connectivity of the anterior cingulate to the rest of the ECN, and the lateralization of the effect suggests that endogenous and synthetic hormones may influence functional connectivity in a similar, but not identical manner.
The ACC has previously been established as an important brain region for cognitive and affective control (Bush et al., 2000), activated by conflict monitoring tasks (Botvinick et al., 2004; Carter et al., 1998, 2007) such as the Stroop task (MacDonald et al., 2000; Pardo et al., 1990). Patients with attentional disorders such as ADHD show abnormalities in the ACC and often struggle with these conflict monitoring tasks (Bush et al., 1999; Seidman et al., 2006). The ACC has also been implicated in affective processing, both related to conflict monitoring (Etkin et al., 2006) and emotional processing independent of any conflict (e.g., Lane et al., 1998; Maddock et al., 2003). The ACC is consistently activated in emotional reappraisal tasks (Ochsner and Gross, 2005) and appears to be structurally (Bremmer et al., 2002) and functionally (Gotlib et al., 2005; Pezawas et al., 2005; Pizzagalli et al., 2001) abnormal in patients with major depression. Abnormal activity has also been shown in patients with PTSD (Etkin and Wager, 2007; Shin et al., 2001) as well as healthy people experiencing transient anxiety (Kimbrell et al., 1999).
Increases in ACC connectivity with other brain regions have been reported in ADHD patients (Tian et al., 2006) and in severely depressed patients (Horn et al., 2010); by contrast, reduced ACC connectivity has been shown in autism patients (Cherkassy et al., 2006). In healthy participants, ACC connectivity with orbitofrontal and prefrontal regions increases during high-risk decision-making (Cohen et al., 2005), and increased ACC-PFC connectivity has also been shown during conflict-monitoring (Fan et al., 2008; Mohanty et al., 2007). Further, different spatio-temporal patterns of ACC connectivity may influence different aspects of task performance: Lenartowicz and Macintosh (2005) reported one network associated with an ACC seed that predicted lower reaction times at the cost of reduced accuracy, and a separate network associated with the same ACC seed that positively predicted accuracy.
Thus, the changes we observed in the connectivity of the ACC to the rest of the executive control network, which is comprised predominantly of PFC regions, could have effects on tasks that integrate cognition and emotion. Future investigations may find differences in performance on these ACC-dependent tasks either across the menstrual cycle or in women using OCs. Of note, both the ACC and left middle frontal gyrus have been associated with conflict monitoring/error processing, and both regions showed altered resting state dynamics across the different hormonal groups. These tasks may be of particular interest in future investigations of menstrual cycle and OC effects on the brain.
In no comparison did luteal women show increased connectivity relative to follicular women, and in no comparison did OC users show increased connectivity relative to NC women. Thus, our data suggest that increases in hormone levels, whether due to endogenous hormone cycling or exogenous synthetic hormone administration, may decrease functional connectivity in these networks. Luteal women typically have higher estrogen and progesterone levels than follicular women; in our data we observed a differenced only in salivary progesterone levels. We also observed significant differences in basal levels of the hormones we measured between the naturally-cycling and OC women. To the extent that a mechanistic argument can be made, it appears that progesterone is a more likely candidate for modulating functional connectivity than 17β-estradiol, since we observed differences in the connectivity of the two naturally-cycling groups in the absence of a significant difference in estradiol levels. Further, we observed differences in connectivity between the two OC groups in the absence of differences in basal hormone levels. Therefore, it appears that endogenous hormone levels are associated with changes in resting state functional connectivity across the menstrual cycle, possibly due to changes in progesterone. Further, it appears that the synthetic hormones in OCs produce changes in resting state functional connectivity that imperfectly mimic the resting state dynamics seen in the normal menstrual cycle, producing a unique pattern of resting state functional connectivity.
However, an important limitation of this hypothesis is that no linear relationship could be found between salivary hormone levels and the time course of either component. This suggests that functional connectivity changes between these groups cannot be fully explained by hormones alone, nor by a straightforward linear relationship between hormone levels and resting state activity. Estrogen and progesterone have important roles as neuromodulators (of norepinephrine, see Etgen, Ungar and Petitti, 1992; of BDNF, see Sohrabji and Lewis, 2006; of oxytocin, see de Kloet, et al., 1986; of GABA and glutamate, see Smith et al., 1987). Further, both hormones have neuroactive metabolites, and allopregnanolone, a metabolite of progesterone, has been shown to alter activity in brain regions associated with emotion regulation (Sripada et al., 2013). However, interactions between sex hormones and other neurotransmitters are beyond the scope of this investigation, and would be compelling targets of future investigations. It may be the case that, related to interactions with these other neurotransmitters and neuroactive metabolites, any relationship between ovarian hormones and resting state characteristics is nonlinear in nature.
This study has several other important limitations that constrain the interpretation of these findings. First, although the evidence suggests that OCs and the menstrual cycle may affect cognition, we do not provide evidence of this here. This investigation is limited to blood flow changes at rest rather than demonstrating any behavioral changes. While we believe these findings provide a compelling basis for investigating cognitive changes across the menstrual cycle, and cognitive changes due to OC use, the data here should not be taken to represent cognitive or behavioral consequences of these hormonal changes. Second, all scans were between rather than within subjects. A replication of these effects in a single group of women, especially with placebo controls for OC use, would provide much stronger evidence that these effects are observable in the general population. Third, participants were not given explicit instructions regarding whether they were to keep their eyes open or closed, a condition that has been shown to alter resting state dynamics (Yan et al., 2009).
This investigation also did not specifically investigate or account for potential differences in brain structure between groups. Previous investigations have shown that the use of hormonal contraception may be associated with changes in gray matter volume in frontal and temporal regions (Pletzer et al., 2010), and that morphology of the hippocampus and basal ganglia may change over the course of the menstrual cycle (Protopopescu et al., 2008). Thus, it is possible that the changes in resting state functional connectivity detected in this investigation may in fact reflect changes in the underlying brain structure in the studied groups. This possibility represents an important target for future investigations.
Whether the observed changes in functional connectivity translate into disruptions, or perhaps even enhancements, in cognitive or affective function is an open question. Because this was an investigation of the brain at rest rather than a comparison of behaviors between groups, we must remain agnostic as to whether these differences in functional connectivity may have any effect on behavior. However, we suggest that changes in the baseline state of the brain may cause differences in behavior or subjective experience. Previous investigations have shown that regional task-related BOLD signals can be negatively predicted by that region’s connectivity with the DMN (Mennes et al., 2010), DMN activity negatively predicts task performance (Weissman et al., 2006; Laird et al., 2009), and the relationship between DMN activity and task demands is altered in people suffering from major depression (Grimm et al., 2009; Sheline et al., 2009). Thus, we hope these data will provide useful guidance to future investigations of the effects of the menstrual cycle and oral contraceptive pills on cognition, affect, and the relationship between the two.
5.1 CONCLUSIONS
We found significant differences in the resting state networks associated with cognition and affect in hormonally distinct groups. Both oral contraceptive pills and menstrual cycle phase are associated with significantly altered resting state dynamics in the anterior portion of the default mode network and the executive control network. The general pattern appears to be that both synthetic hormones in oral contraceptive pills and endogenous hormones that fluctuate across the menstrual cycle are associated with decreased functional connectivity in the networks we investigated. These findings provide evidence that both endogenous and synthetic hormones influence the baseline state of the brain, which may in turn influence cognitive performance and affective experience.
Supplementary Material
ACKNOWLEDGMENTS
We thank NIH grant R01MH057508 for financial support. We also thank Annie Hu and the staff at the Research Imaging Center for providing technical support.
Footnotes
FINANCIAL DISCLOSURES: We report no direct or indirect conflicts of interest, financial or otherwise.
REFERENCES
- 1.Abler B, Kumpfmüller D, Grön G, Walter M, Stingl J, Seeringer A. Neural correlates of erotic stimulation under different levels of female sexual hormones. PLOS ONE. 2013;8(2):e54447. doi: 10.1371/journal.pone.0054447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. NeuroImage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage. 2010;53(4):1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 2008;33(6):874–882. doi: 10.1016/j.psyneuen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural comput. 1995;7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 4.Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17(1):353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswal BB, Mennes M, Zuo X, et al. Toward discovery science of human brain function. PNAS. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR in Biomedicine. 1997;10:165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Biswal B, Yetkin FZ, Haughton VC, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 8.Bluhm RL, Osuch EA, Lanius RA, Boksman K, Neufeld RWJ, Théberge J, Williamson P. Default mode network connectivity: effects of age, sex, and analytic approach. NeuroReport. 2008;19(8):887–891. doi: 10.1097/WNR.0b013e328300ebbf. [DOI] [PubMed] [Google Scholar]
- 9.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Bremmer JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51(4):273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 11.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract]. Available on CD-ROM in NeuroImage; Presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2–6, 2002; Sendai, Japan. [Google Scholar]
- 12.Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci Biobehav Rev. 2008;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J. Anterior cingulate cortex dysfunction in Attention- Deficit/Hyperactive Disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry. 1999;45(12):1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 14.Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 15.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 16.Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- 17.Carter RM, O’Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. NeuroImage. 2006;29(3):1007–1012. doi: 10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Cherkassy VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. NeuroReport. 2006;17(16):1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- 19.Cohen MX, Heller AS, Ranganath C. Functional connectivity with anterior cingulate and orbitofrontal cortices during decision-making. Cognitive Brain Research. 2005;23(1):61–70. doi: 10.1016/j.cogbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Crozier S, Sirigu A, Lehéricy S, van de Moortele P, Pillon B, Grafman J, Agid Y, Dubois B, LeBihan D. Distinct prefrontal activations in processing sequence at the sentence and script level: An fMRI study. Neuropsychologia. 1999;37(13):1469–1476. doi: 10.1016/s0028-3932(99)00054-8. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Luxen A, Salmon E. Self-referential reflective activity and its relationship with rest: A PET study. NeuroImage. 2005;25(2):616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 21.De Bondt T, Van Hecke W, Veraart J, Leemans A, Sijbers S, Jacquemyn Y, Parizel PM. Does the use of hormonal contraceptives cause microstructural changes in cerebral white matter? Preliminary results of a DTI and tractography study. Eur Radiol. 2013;1(23):57–64. doi: 10.1007/s00330-012-2572-5. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Voorhuis DAM, Boschma Y, Elands J. Estradiol modulates density of putative oxytocin receptors in discrete rat brain regions. Neuroendocrinology. 1986;44(4):415–421. doi: 10.1159/000124680. [DOI] [PubMed] [Google Scholar]
- 22.Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. NeuroImage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- 23.Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Human Brain Mapping. 2011;32(12):2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen AE, Ungar S, Petitti N. Estradiol and progesterone modulation of norepinephrine neurotransmission: Implications for the regulation of female reproductive behavior. Journal of Neuroendocrinology. 1992;4(3):255–271. doi: 10.1111/j.1365-2826.1992.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 24.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 25.Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan J, Hof PR, Guise KG, Fossella JA, Posner MI. The functional integration of the anterior cingulate cortex during conflict processing. Cereb Cortex. 2008;18(4):796–805. doi: 10.1093/cercor/bhm125. [DOI] [PubMed] [Google Scholar]
- 27.Gabrieli JDE, Poldrack R, Desmond JE. The role of left prefrontal cortex in language and memory. PNAS. 1998;95(3):906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gingnell M, Engman J, Frick A, Moby L, Wikström J, Fredrikson M, Sundström- Poromaa I. Oral contraceptive use changes brain activity and mood in women with previous negative affect on the pill—A double-blinded, inactive-controlled randomized trial of a levonorgestrel-containing combined oral contraceptive. Psychoneuroendocrinology. 2012;38(7):1133–44. doi: 10.1016/j.psyneuen.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Gotlib IH, Sivers H, Gabrieli JDE, Whitfield-Gabrieli S, Goldin P, Minor KL, Canli T. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. NeuroReport. 2005;16(16):1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- 30.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. PNAS. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. PNAS. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griksiene R, Ruksenas O. Effects of hormonal contraceptives on mental rotation and verbal fluency. Psychoneuroendocrinology. 2011;36(8):1239–1248. doi: 10.1016/j.psyneuen.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, Ernst J, Hell D, Boeker H, Northoff G. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34:932–943. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. PNAS. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 34.Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage. 2004;22(3):1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 35.Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, Eckert U, Zierhut KC, Schiltz K, He H, Biswal B, Bogerts B, Walter M. Glutamatergic and resting- state functional connectivity correlates of severity in major depression – the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci. 2010;4(33):1–10. doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: An event-related fMRI study. Psychophysiology. 2000;37(2):216–223. [PubMed] [Google Scholar]
- 37.Kimbrell TA, George MS, Parekh PI, Ketter TA, Podell DM, Danielson Al, Repella JD, Benson BE, Willis MW, Herscovitch P, Post RM. Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biol Psychiatry. 1999;46(4):454–465. doi: 10.1016/s0006-3223(99)00103-1. [DOI] [PubMed] [Google Scholar]
- 38.Kirschbaum C, Kudielka BM, Gaab J, Schommer N, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Kuukasjärvi S, Eriksson CJP, Koskela E, Mappes T, Nissinen K, Rantala MJ. Attractiveness of women’s body odors over the menstrual cycle: the role of oral contraceptives and receiver sex. Behavioral Ecology. 2004;15(4):579–584. [Google Scholar]
- 40.Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29(46):14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT. Behavioral interpretations of intrinsic connectivity networks. Journal of Cogn Neurosci. 2011;23(12):4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of emotional awareness: Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 1998;10(4):525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- 43.Lenartowicz A, McIntosh AR. The role of anterior cingulate cortex in working memory is shaped by functional connectivity. Journal of Cognitive Neuroscience. 2005;17(7):1026–1042. doi: 10.1162/0898929054475127. [DOI] [PubMed] [Google Scholar]
- 44.MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 45.Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping. 2003;18(1):30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maes M, Claes M, Schotte C, Delbeke L, Jacquemyn Y, Verkerk R, De Meester I, Scharpé S. Disturbances in dexamethasone suppression test and lower availability of L-tryptophan and tyrosine in early puerperium and in women under contraceptive therapy. J Psychosom Res. 1992;36(2):191–197. doi: 10.1016/0022-3999(92)90028-z. [DOI] [PubMed] [Google Scholar]
- Maki PM, Rich JB, Rosenbaum RS. Implicit memory varies across the menstrual cycle: Estrogen effects in young women. Neuropsychologia. 2002;40(5):518–529. doi: 10.1016/s0028-3932(01)00126-9. [DOI] [PubMed] [Google Scholar]
- 47.Marečková K, Perrin JS, Khan IN, Lawrence C, Dickie E, McQuiggan D, Paus T. Hormonal contraceptives, menstrual cycle and brain response to faces. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal BB, Castellanos FX, Milham MP. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. NeuroImage. 2010;50(4):1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Horm Behav. 2012;62(4):531–538. doi: 10.1016/j.yhbeh.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Mohanty A, Engels AS, Herrington JD, Heller W, Ho MHR, Banich MT, Webb AG, Warren SL, Miller GA. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44(3):343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 51.Mordecai KL, Rubin LH, Maki PM. Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Horm Behav. 2008;54(2):286–293. doi: 10.1016/j.yhbeh.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Nielsen SE, Ertman N, Lakhani YS, Cahill L. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiol Learn Mem. 2011;96(2):378–384. doi: 10.1016/j.nlm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 54.Pardo JV, Pardo PJ, Janer KH, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. PNAS. 1990;87(1):256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 56.Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladumer G, Kerschbaum HH. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Res. 2010;1348:55–62. doi: 10.1016/j.brainres.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 57.Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ. Anterior cingulate activity as a predictor of degree of treatment response in major depression: Evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158(3):405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 58.Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- 59.Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, McEwen B, Silbersweig D, Stern E. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18(10):985–988. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- 60.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. PNAS. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roberts SC, Klapilová K, Little AC, Burriss RP, Jones BC, DeBruine LM, Petrie M, Havlíček J. Relationship satisfaction and outcome in women who meet their partner while using oral contraception. Proc R Soc B. 2012;279(1732):1430–1436. doi: 10.1098/rspb.2011.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rumberg B, Baars A, Fiebach J, Ladd ME, Forsting M, Senf W, Gizewski ER. Cycle and gender-specific cerebral activation during a verb generation task using fMRI: Comparison of women in different cycle phases, under oral contraception, and men. Neurosci Res. 2010;66(4):366–371. doi: 10.1016/j.neures.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 63.Sambataro F, Murty VP, Callicott JH, Tan H, Das S, Weinberger DR, Mattay VS. Age-related alterations in default mode network: impact on working memory performance. Neurobiol Aging. 2010;31(5):839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J. Dorsolateral prefrontal and anterior cingulate cortex volumetrix abnormalities in adults with Attention-Deficit/Hyperactivity Disorder identified by magnetic resonance imagining. Biol Psychiatry. 2006;60(10):1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 65.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. PNAS. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 67.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. PNAS. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith SS, Waterhouse BD, Chapin JK, Woodward DJ. Progesterone alters GABA and glutamate responsiveness: a possible mechanism for its anxiolytic action. Brain Research. 1987;2(6):353–359. doi: 10.1016/0006-8993(87)90634-2. [DOI] [PubMed] [Google Scholar]
- 69.Sohrabji F, Lewis DK. Estrogen-BDNF interactions: Implications for neurodegenerative diseases. Frontiers in Neuroendocrinology. 2006;27(4):404–414. doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci. 2009;22(6):1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- 71.Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I. Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits. Biological Psychiatry. 2013;73(11):1045–11053. doi: 10.1016/j.biopsych.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. NeuroImage. 2010;52(1):290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, Sui M, Cao Q, Hu S, Peng M, Zhuo Y. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett. 2006;400(1–2):39–43. doi: 10.1016/j.neulet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 74.Tian L, Wang J, Yan C, He Y. Hemisphere- and gender-related differences in small-world brain networks: A resting-state functional MRI study. NeuroImage. 2011;54:191–202. doi: 10.1016/j.neuroimage.2010.07.066. [DOI] [PubMed] [Google Scholar]
- 75.Van Wingen GA, van Broekhoven F, Verkes RJ, Petersson KM, Bäckström T, Buitelaar JK, Fernádez G. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. 2008;13:325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- 76.Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Wedekind C, Seebeck T, Bettens F, Paepke AJ. MHC-dependent mate preferences in humans. Proc R Soc Lond B. 1995;260(1359):245–249. doi: 10.1098/rspb.1995.0087. [DOI] [PubMed] [Google Scholar]
- 78.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 79.Weissman-Fogel I, Moayedi M, Taylor KS, Pope G, Davis KD. Cognitive and default-mode resting state networks: Do male and female brains -rest differently? Human Brain Mapping. 2010;31(11):1713–1726. doi: 10.1002/hbm.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan C, Liu D, He Y, Zou Q, Zhu C, Zuo X, Long X, Zang Y. Spontaneous brain activity in the default mode network is sensitive to different resting-state conditions with limited cognitive load. PLoS ONE. 2009;4(5):e5743. doi: 10.1371/journal.pone.0005743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yan C, Zang Y. DPARSF: a MATLAB toolbox for -pipeline data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.