Abstract
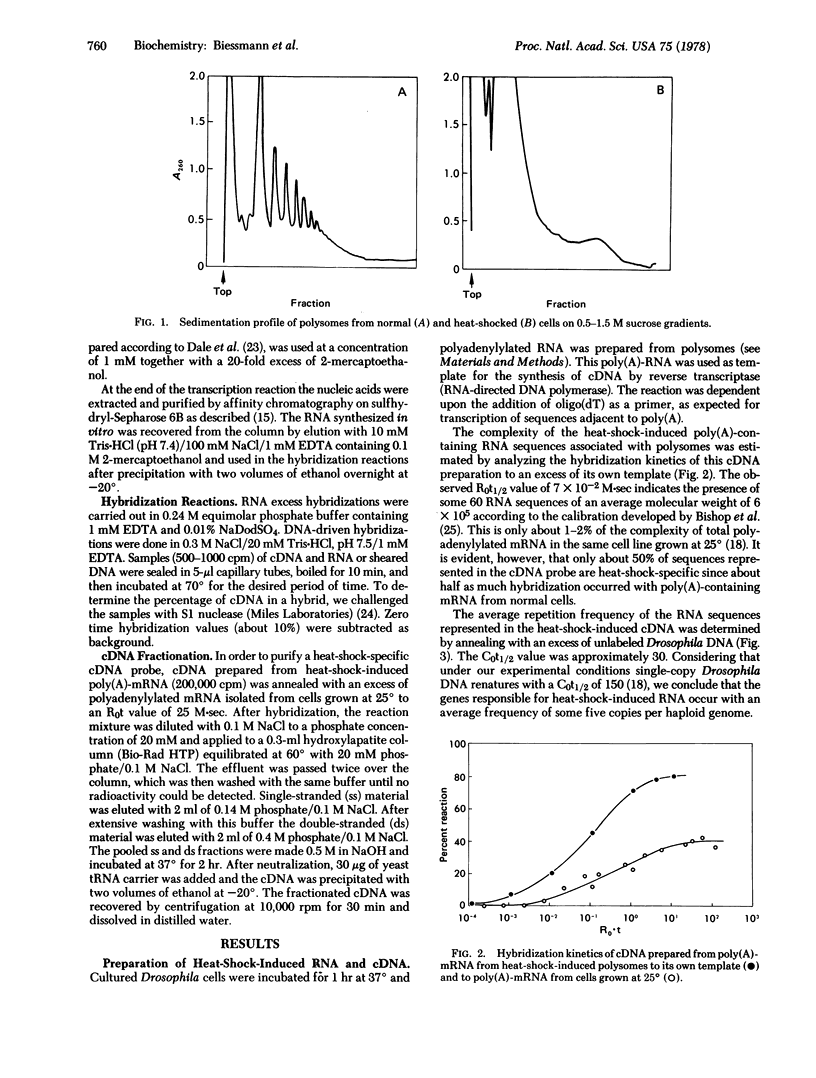
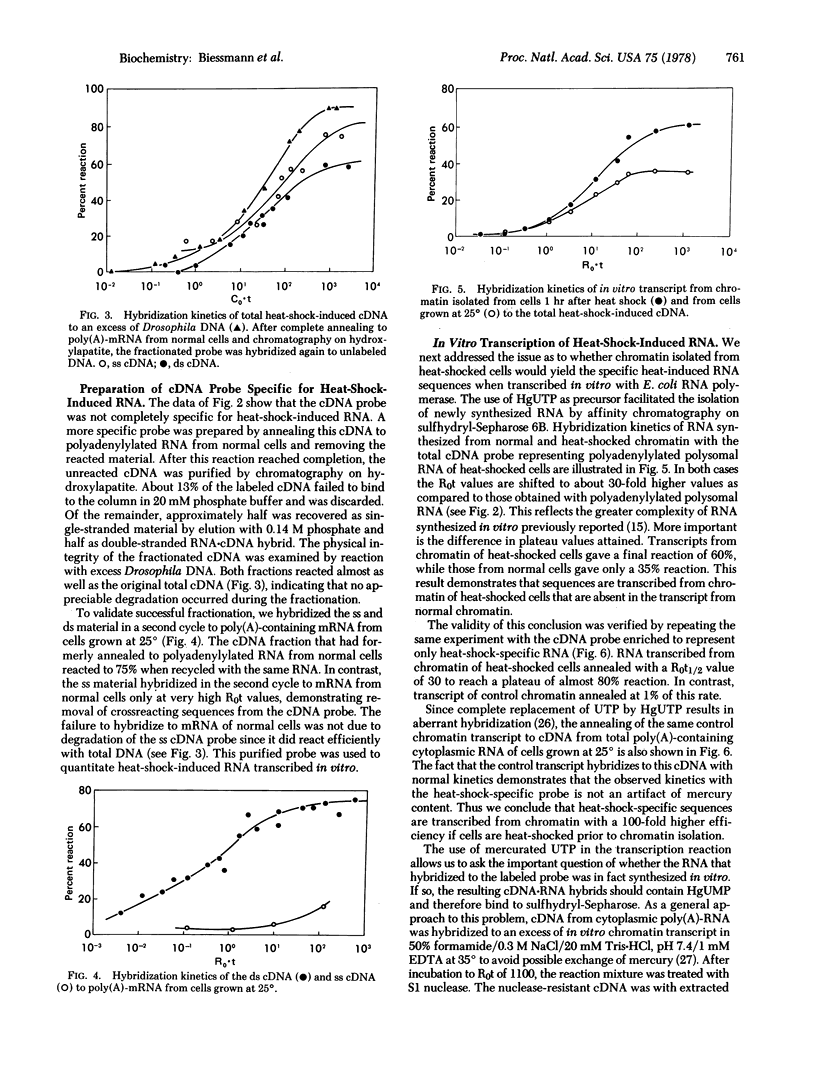
Polyadenylylated RNA synthesized after heat shock was isolated from polysomes of cultured cells of Drosophila melanogaster and used as template to prepare cDNA. An excess of poly(A)-RNA from heat-shocked cells hybridized to 80% of the cDNA, whereas cytoplasmic poly(A)-RNA from cells grown at 25 degrees could drive only half of the cDNA probe into hybrid. These sequences were removed from the cDNA population by annealing to poly(A)-RNA from cells grown at 25 degrees. The unreacted material represented only heat-shock-induced mRNA sequences, as shown by a second cycle of hybridization. Isolated chromatin was transcribed in vitro at 25 degrees with Escherichia coli RNA polymerase, with mercurated UTP as precursor. RNA transcribed from chromatin that was prepared from cells 1 hr after the temperature was shifted to 37 degrees hybridized with 100-fold faster kinetics to the heat-shock-specific cDNA probe than did RNA transcribed from chromatin of cells grown at 25 degrees. Therefore, heat shock results in a change in chromatin structure recognizable by E. coli RNA polymerase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M. Patterns of puffing activity in the salivary gland chromosomes of Drosophila. V. Responses to environmental treatments. Chromosoma. 1970;31(3):356–376. doi: 10.1007/BF00321231. [DOI] [PubMed] [Google Scholar]
- Astrin S. M. Mapping of the SV40 specific sequences transcribed in vitro from chromatin of SV40 transformed cells. Biochemistry. 1975 Jun 17;14(12):2700–2704. doi: 10.1021/bi00683a022. [DOI] [PubMed] [Google Scholar]
- Axel R., Cedar H., Felsenfeld G. Synthesis of globin ribonucleic acid from duck-reticulocyte chromatin in vitro. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2029–2032. doi: 10.1073/pnas.70.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatriz L. W., McCarthy B. J. Messenger RNA complexity in Drosophila melanogaster. Biochemistry. 1975 Jun 3;14(11):2440–2446. doi: 10.1021/bi00682a026. [DOI] [PubMed] [Google Scholar]
- Beebee T. J., Butterworth P. H. The use of mercurated nucleoside triphosphate as a probe in transcription studies in vitro. Eur J Biochem. 1976 Jul 15;66(3):543–550. doi: 10.1111/j.1432-1033.1976.tb10580.x. [DOI] [PubMed] [Google Scholar]
- Berendes H. D., Boyd J. B. Structural and functional properties of polytene nuclei isolated from salivary glands of Drosophila hydei. J Cell Biol. 1969 May;41(2):591–599. doi: 10.1083/jcb.41.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendes H. D. Synthetic activity of polytene chromosomes. Int Rev Cytol. 1973;35:61–116. doi: 10.1016/s0074-7696(08)60352-6. [DOI] [PubMed] [Google Scholar]
- Biessmann H., Gjerset R. A., Levy B., McCarthy B. J. Fidelity of chromatin transcription in vitro. Biochemistry. 1976 Oct 5;15(20):4356–4363. doi: 10.1021/bi00665a002. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Morton J. G., Rosbash M., Richardson M. Three abundance classes in HeLa cell messenger RNA. Nature. 1974 Jul 19;250(463):199–204. doi: 10.1038/250199a0. [DOI] [PubMed] [Google Scholar]
- Bonner J. J., Pardue M. L. The effect of heat shock on RNA synthesis in Drosophila tissues. Cell. 1976 May;8(1):43–50. doi: 10.1016/0092-8674(76)90183-5. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Martin E., Livingston D. C., Ward D. C. Direct covalent mercuration of nucleotides and polynucleotides. Biochemistry. 1975 Jun 3;14(11):2447–2457. doi: 10.1021/bi00682a027. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C. Mercurated polynucleotides: new probes for hybridization and selective polymer fractionation. Biochemistry. 1975 Jun 3;14(11):2458–2469. doi: 10.1021/bi00682a028. [DOI] [PubMed] [Google Scholar]
- FOX C. F., ROBINSON W. S., HASELKORN R., WEISS S. B. ENZYMATIC SYNTHESIS OF RIBONUCLEIC ACID. III. THE RIBONUCLEIC ACID-PRIMED SYNTHESIS OF RIBONUCLEIC ACID WITH MICROCOCCUS LYSODEIKTICUS RIBONUCLEIC ACID POLYMERASE. J Biol Chem. 1964 Jan;239:186–195. [PubMed] [Google Scholar]
- Gilmour R. S., Harrison P. R., Windass J. D., Affara N. A., Paul J. Globin messenger RNA synthesis and processing during haemoglobin induction in Friend cells. I. Evidence for transcriptional control in clone M2. Cell Differ. 1974 Jun;3(1):9–22. doi: 10.1016/0045-6039(74)90036-0. [DOI] [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. Tissue-specific transcription of the globin gene in isolated chromatin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3440–3442. doi: 10.1073/pnas.70.12.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerset R. A., McCarthy B. J. Limited accessibility of chromatin satellite DNA to RNA polymerase from Escherichia coli. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4337–4340. doi: 10.1073/pnas.74.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M., Garrard W. T., Bagi G., Wilson R. F., Bonner J. Partial purification of the template-active fraction of chromatin: a preliminary report. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2193–2197. doi: 10.1073/pnas.71.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouse L., Chilton M. D., McCarthy B. J. Hybridization of ribonucleic acid with unique sequences of mouse deoxyribonucleic acid. Biochemistry. 1972 Feb 29;11(5):798–805. doi: 10.1021/bi00755a019. [DOI] [PubMed] [Google Scholar]
- Harris S. E., Schwartz R. J., Tsai M. J., O'Malley B. W., Roy A. K. Effect of estrogen on gene expression in the chick oviduct. In vitro transcription of the ovalbumin gene in chromatin. J Biol Chem. 1976 Jan 25;251(2):524–529. [PubMed] [Google Scholar]
- Honjo T., Reeder R. H. Transcription of Xenopus chromatin by homologous ribonucleic acid polymerase: aberrant synthesis of ribosomal and 5S ribonucleic acid. Biochemistry. 1974 Apr 23;13(9):1896–1899. doi: 10.1021/bi00706a018. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M., Helmsing P. J., Ashburner M. Parallel changes in puffing activity and patterns of protein synthesis in salivary glands of Drosophila. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3604–3608. doi: 10.1073/pnas.72.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S. L., Henikoff S., Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1117–1121. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder R. H. Transcription of chromatin by bacterial RNA polymerase. J Mol Biol. 1973 Oct 25;80(2):229–241. doi: 10.1016/0022-2836(73)90169-1. [DOI] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972 Apr;27(2):353–365. [PubMed] [Google Scholar]
- Spradling A., Penman S., Pardue M. L. Analysis of drosophila mRNA by in situ hybridization: sequences transcribed in normal and heat shocked cultured cells. Cell. 1975 Apr;4(4):395–404. doi: 10.1016/0092-8674(75)90160-9. [DOI] [PubMed] [Google Scholar]
- Tissières A., Mitchell H. K., Tracy U. M. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974 Apr 15;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wilson G. N., Steggles A. W., Nienhuis A. W. Strand-selective transcription of globin genes in rabbit erythroid cells and chromatin. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4835–4839. doi: 10.1073/pnas.72.12.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M., Felsenfeld G. Use of mercury-substituted ribonucleoside triphosphates can lead to artefacts in the analysis of in vitro chromatin transcrits. Biochem Biophys Res Commun. 1977 Apr 11;75(3):598–603. doi: 10.1016/0006-291x(77)91514-5. [DOI] [PubMed] [Google Scholar]



