Abstract
There is growing evidence that cytokine expression is linked to hepatitis C virus (HCV) pathogenesis and treatment response rates among HCV-monoinfected persons. However, because of the profound effects of human immunodeficiency virus (HIV) coinfection on HCV, it is not clear if these observations are also true for HCV/HIV-coinfected individuals. Serum expression of the proinflammatory cytokines interleukin-8 (IL-8) and tumor necrosis factor-α (TNF-α) and the fibrogenic cytokine transforming growth factor-β1 (TGF-β1) were measured in HCV/HIV-coinfected persons at baseline and at week 24 of HCV therapy. Higher levels of IL-8 and TGF-β were demonstrated among nonwhite subjects at baseline. Increases in TNF-α and IL-8 expression were found at week 24 of HCV therapy, suggesting that enhanced proinflammatory cytokine production may occur during HCV treatment. However, cytokine levels were not predictive of HCV virologic, biochemical, or histologic response. Although previous studies conducted among HCV-monoinfected individuals have suggested that cytokine levels could predict the virologic response to therapy, no such associations were observed among HCV/HIV-coinfected persons, suggesting that they may respond differently to treatment than do their HCV-monoinfected counterparts.
INTRODUCTION
In the United States, an estimated 150,000–300,000 persons are infected with both hepatitis C virus (HCV) and human immunodeficiency virus (HIV). The critical roles that cytokines play in immune responses, inflammation, and fibro-genesis suggest that this complex network of molecules may contribute to viral persistence in chronic hepatitis C infection as well as regulate the response to therapeutic interventions. Mounting evidence suggests that the cytokine environment is altered during HCV monoinfection.1,2 For instance, interleukin-2 (IL-2) and interferon-γ (IFN-γ), are upregulated in chronic HCV patients compared with uninfected controls, and levels of these cytokines are correlated with histologic fibrosis,3 whereas IL-2, IFN-γ, and IL-8 are associated with inflammatory activity.4 There is growing evidence that cytokine expression is also linked to HCV treatment response, with lower baseline levels generally correlated with virologic response.4–13 Thus, certain cytokines may be useful as easily measured, predictive markers of treatment response.
We, therefore, quantified serum expression of IL-8, tumor necrosis factor-α (TNF-α), and transforming growth factor-β1 (TGF-β1) among HCV/HIV-coinfected persons enrolled in an HCV treatment trial to explore (1) the demographic and clinical variables associated with baseline levels and changes in cytokine expression after HCV treatment initiation and (2) the effects of HCV therapy on cytokine expression.
MATERIALS AND METHODS
Study design
Study subjects were 52 individuals enrolled in the U.S. Adult AIDS Clinical Trial Group (ACTG) study A5071 between December 2000 and April 2001 who had baseline and week 24 sera available for cytokine quantification. A5071 was a prospective, randomized, phase II/III trial evaluating the safety and efficacy of pegylated IFN plus ribavirin (PegIFN-RBV) vs. IFN plus RBV (IFN-RBV) in HCV/HIV-coinfected individuals with stable HIV disease who had not previously been treated with IFN. The study was described in detail in the original publication.14 Serum IL-8, TNF-α and TGF-β levels were assessed prior to treatment (baseline) and after 24 weeks of PegIFN-RBV or IFN-RBV. As measures of HCV treatment response, HCV virologic response (undetectable HCV RNA) and histologic response (≥2 point drop in total hepatic activity index [HAI] from baseline) were assessed at week 24. Biochemical response was defined as normalization of serum alanine aminotransferase (ALT) levels in persons with ALT greater than the upper limit of normal (ULN) at entry. All study patients were treated for 24 weeks, but only virologic responders and histologic responders completed 48 weeks of treatment. Plasma HCV RNA levels were quantified using the Roche Amplicor Monitor kit (Roche Diagnostic Systems, Inc., Branchburg, NJ), with a lower limit of detection (LLD) of 60 IU/mL for the primary study end point at week 24. Plasma HIV viral loads were quantified using the Roche Amplicor Monitor kit, with an LLD of 50 copies/mL. HCV genotypes were determined using the LiPA assay (Innogenetics, Gent, Belgium).
Immunoassays
Whole blood was separated by centrifugation at 1500 rpm for 15 min, and serum aliquots were frozen at −80°C until use. Serum levels of IL-8, TNF-α, and TGF-β1 were quantified using standard enzyme-linked immunoassays (ELISAs) according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). The LLD for each assay was 3.5 pg/mL for IL-8, 1.6 pg/mL for TNF-α, and 7.0 pg/mL for TGF-β1. Sample measurements less than the LLD were assigned a value of 0.
Statistical analysis
Associations between dichotomous or categorical variables were assessed using Fisher’s exact tests, and McNemar’s exact tests were conducted on changes over time. Comparisons between two groups involving continuous data were evaluated using Wilcoxon rank-sum tests, whereas changes over time were assessed by signed rank tests. Correlations were evaluated using Spearman rank tests. For baseline IL-8 analysis, log-normal regression models that accounted for left-censored observations were used to evaluate joint effects in multivariate models, and likelihood ratio tests were used to assess the significance of covariate effects. All p values were two-sided, and only p values < 0.05 were considered statistically significant without adjustments for multiple testing.
RESULTS
Study population characteristics
Baseline characteristics of the 52 participants prior to initiation of HCV therapy are shown in Table 1. No statistically significant differences were found in baseline characteristics between the study subjects and the remaining 81 A5071 participants, except for the HIV RNA detection rate. Whereas 29% of the current study participants had detectable HIV RNA at baseline, 47% of the remaining A5071 participants had detectable HIV RNA (p = 0.05). Consistent with the parent A5071 study results, PegIFN-RBV was found to be superior to IFN-RBV in reducing HCV RNA in our study sample (p = 0.0006) but their effects were similar on HAI and ALT changes. Similarly, abnormal ALT levels were reduced after treatment (p = 0.0002), although there was no change in HAI.
Table 1.
Baseline Population Characteristics
| Characteristic | Total (n = 52) |
|---|---|
| Male gender | 42 (81)a |
| Race/ethnicity | |
| White | 23 (44) |
| Black | 20 (38) |
| Hispanic | 6 (12) |
| Other | 3 (6) |
| Age (years) | |
| Median (range) | 45 (27–60) |
| HCV genotype 1 | 41 (79) |
| HCV therapy | |
| IFN-RBV | 27 (52) |
| PegIFN-RBV | 25 (48) |
| HIV therapy | |
| Naive/not reported | 6 (12) |
| NRTIb | 8 (15) |
| NRTI + NNRTI (or PI) | 27 (42) |
| NRTI + NNRTI + PI | 11 (21) |
| CD4 count (cells/mL) | |
| Median (range) | 445 (131–1240) |
| HCV RNA (log10 IU/mL) | |
| Median (range) | 6.31 (4.35–6.63) |
| HIV RNA (log10 copies/mL) | |
| Detectable | 15 (29) |
| Hepatic activity index | |
| Median (range) | 5 (2–10) |
| Fibrosis score | |
| Median (range) | 2 (0–6) |
| Abnormal ALT | 36 (69) |
Data are number of participants (%) unless otherwise noted.
NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Cytokine levels at baseline and associations with clinical variables
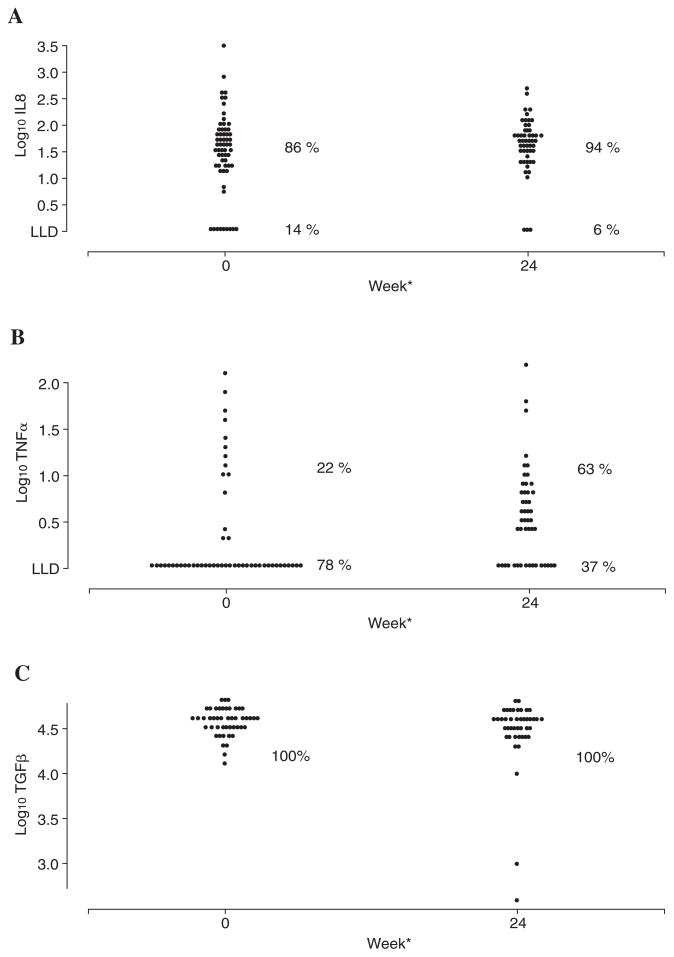
At baseline, 86% of the participants had detectable serum IL-8, and 22% had detectable TNF-α (Fig. 1). TGF-β1 was detectable for all participants. Baseline medians (ranges) were 1.52 (0–3.52), below the LLD (0–2.07), and 4.58 (4.10–4.81) log10 pg/mL for IL-8, TNF-α and TGF-β1, respectively. We next explored associations between baseline serum cytokine expression and baseline demographics and clinical variables of interest. HIV RNA detection, HCV genotype, HCV RNA, and HAI were not significantly associated with IL-8 levels. Non-white race/ethnicity (p = 0.026) and higher fibrosis score (p = 0.0044) were associated with increased IL-8, and decreased CD4 cell count (p = 0.061) and age (p = 0.070) were marginally associated with baseline IL-8 in univariate analyses. Their joint effects were assessed in a multivariate log-normal regression model. Race/ethnicity (p = 0.025) and baseline fibrosis score (p = 0.026) remained significant; however, CD4 count and age were no longer significant after adjusting for race/ethnicity and fibrosis score (Table 2). For TNF-α, there was a marginal negative correlation with HCV RNA (p = 0.09); however, no other associations were found to be statistically significant. For TGF-β1, there was an association between increased TGF-β1 and nonwhite race/ethnicity (p = 0.002), although no other significant associations were found.
FIG. 1.
(A) IL-8, (B) TNF-α, and (C) TGF-β1 levels at baseline and at 24 weeks of HCV treatment. Values are expressed as log10 transformed values (pg/mL). LLD on (A) and (B) denotes the lower level of detection for each assay. (A) *p = 0.082 comparing the median change between weeks 0 and 24. (B) *p = 0.007 comparing the median change between weeks 0 and 24. (C) *p = 0.19 comparing the median change between weeks 0 and 24.
Table 2.
Log-Normal Regression Analysis of Baseline IL-8
| Characteristic | Univariate p value | Multivariate p value |
|---|---|---|
| Race/ethnicity (nonwhite) | 0.026 | 0.025 |
| Baseline HIV RNA (detectable vs. undetectable) | 0.82 | a |
| HCV genotype (1 vs. non-1) | 0.32 | a |
| Baseline ALT (≤ ULN vs. > ULN) | 0.12 | a |
| Baseline CD4 | 0.061 | 0.360 |
| Baseline HCV RNA | 0.53 | a |
| Baseline hepatic activity index | 0.25 | a |
| Baseline fibrosis score | 0.0044 | 0.026 |
| Age | 0.070 | 0.460 |
Not entered in the multivariate model (p > 0.10).
Changes in cytokine levels after 24 weeks of HCV treatment
As no differences in cytokine levels were detected between PegIFN-RBV and IFN-RBV, they were grouped together for all subsequent analyses. After 24 weeks of HCV treatment, IL-8 detection increased from 86% at baseline to 94%, whereas TNF-α detection increased from 22% to 63%. Median IL-8 level increased marginally from 1.52 log10 pg/mL at baseline to 1.68 log10 pg/mL (range 0.00–2.68) at week 24 (p = 0.08). Median TNF-α level increased significantly from undetectable to 0.46 log10 pg/mL (range 0.00–2.16, p = 0.007). TGF-β1 was detectable in all samples at both times, and median TGF-β1 remained steady at 4.58 log10 pg/mL over 24 weeks of HCV treatment (p = 0.19).
Association analyses
No significant associations were found between week 24 changes from baseline (Δ0 → 24) in IL-8 or TGF-β1 levels and Δ0 → 24 in HIV RNA, CD4 cell count, HCV RNA, HAI, or fibrosis score. No significant associations were observed between IL-8, TNF-α, or TGF-β1 Δ0 → 24 and HCV treatment, race/ethnicity, HCV genotype, HCV virologic response, histologic response, or biochemical response. The associations between baseline IL-8, TNF-α, or TGF-β1 levels and week 24 HCV virologic, histologic, or biochemical response were also not significant. Furthermore, baseline levels of the three cytokines, as well as changes in their levels from baseline, were not significantly correlated with each other.
DISCUSSION
Several previous studies have examined cytokine production in response to therapy during HCV monoinfection.12,13,15,16 These data suggest that serum levels of cytokines, such as IL-8, TNF-α, and TGF-β1, might be important predictors of the virologic response to HCV therapy. In the present analysis, we measured cytokine levels to determine if cytokine expression was also associated with HCV virologic, histologic, or biochemical response among HCV/HIV-coinfected persons.
We found a statistically significant association between race/ethnicity and IL-8 expression, with higher IL-8 levels among nonwhite participants. Fibrosis score was also positively correlated with IL-8 at baseline. In multivariate analysis, race/ethnicity and fibrosis score remained significantly associated with IL-8 expression. Nonwhite race/ethnicity was also associated with significantly higher levels of TGF-α1 but not TNF-β, suggesting that differences in cytokine expression among diverse ethnic groups could have important implications for HCV.
A5071 subjects showed a significant reduction in HCV RNA after HCV treatment; thus, we evaluated if virologic response was mediated by proinflammatory or fibrogenic cytokines, such as IL-8, TNF-α and TGF-β1. Whereas detection rates for IL-8 and TNF-α increased after initiation of HCV therapy, median TGF-β1 levels were stable over the 24-week treatment period. Importantly, the associations between baseline (or changes at week 24 from baseline) IL-8, TNF-α, or TGF-β1 levels and week 24 HCV virologic, histologic, or biochemical response were not significant. The study sample size was sufficient to detect a correlation magnitude of ~0.4 between cytokine expression and HCV RNA changes after HCV treatment with 83% power. Therefore, either the statistical power of the measured cytokine levels to predict virologic response is lower than what is allowed with this sample size (16 responders and 36 nonresponder), or despite the increased proinflammatory cytokine expression demonstrated in the current study, these cytokines are not critical mediators of HCV treatment response in HCV/HIV-coinfected persons.
Several limitations to our study should be considered. First, coinfected persons were selected from a larger clinical trial based on sample availability; thus, negative findings may reflect limited power to detect small differences in cytokine expression. Although our study participants had lower HIV RNA detection compared with the remaining A5071 subjects, they were otherwise representative of the A5071 study population. Second, studies using paired serum and liver biopsies are lacking from the HCV literature, making it difficult to determine empirically whether peripheral cytokine levels accurately reflect intrahepatic cytokine expression. As liver biopsies were performed only on study participants who did not achieve HCV virologic response, biopsy material was not available for the majority of A5071 study participants. Therefore, we sought to evaluate serum cytokines as noninvasive markers of treatment response.3,5,17–19 Finally, it is likely that multiple cytokines, particularly endogenous IFNs, are also involved in mediating interactions between HIV and HCV but were not evaluated in the current study. Although a direct comparison between HCV-monoinfected and HCV/HIV-coinfected persons was not considered in the original A5071 study design, the current substudy demonstrates the inability of cytokine levels to predict virologic response to therapy in HCV/HIV-coinfected persons, as has been documented previously in HCV-monoinfected individuals.
Acknowledgments
We are indebted to the A5071 study clinicians, coordinators, and participants. This work was supported by the NIAID–AIDS Clinical Trials Group (AI38858 and AI38855) and the Partners/Fenway/Shattuck Center for AIDS Research (NIH P30-AI42851). We would also like to thank Ms. Norah Shire for her editing of the manuscript.
References
- 1.Heydtmann H, Shields P, McCaughan G, Adams D. Cytokines and chemokines in the immune response to hepatitis C infection. Curr Opin Infect Dis. 2001;14:279–287. doi: 10.1097/00001432-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson-Brown P, Neuman M. Immunopathogenesis of hepatitis C viral infection: Th1/Th2 responses and the role of cytokines. Clin Biochem. 2001;34:167–171. doi: 10.1016/s0009-9120(01)00210-7. [DOI] [PubMed] [Google Scholar]
- 3.Napoli J, Bishop A, McGuinness P, Painter D, McCaughan G. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology. 1996;24:759–765. doi: 10.1002/hep.510240402. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda R, Ishimura N, Ishihara S, Chowdhury A, Morlyama N, Nogami C, Miyake T, Niigaki M, Tokuda A, Satoh S, Sakai S, Akagi S, Watanabe M, Fukumoto S. Intrahepatic expression of proinflammatory cytokine mRNAs and interferon efficacy in chronic hepatitis C. Liver. 1996;16:390–399. doi: 10.1111/j.1600-0676.1996.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 5.Neuman M, Benhamou J, Bourliere M, Ibrahim A, Malkiewicz I, Asselah T, Martinot-Peignoux M, Shear NH, Katz GG, Akremi R, Benali S, Boyer N, Lecomte L, Le Breton V, Le Guludec G, Marcellin P. Serum tumour necrosis factor-α and transforming growth factor-b levels in chronic hepatitis C patients are immunomodulated by therapy. Cytokine. 2002;17:108–117. doi: 10.1006/cyto.2001.0997. [DOI] [PubMed] [Google Scholar]
- 6.Grungreiff K, Reinhold D, Ansorge S. Serum concentrations of sIL-2R, IL-6, TGF-β1, neopterin, and zinc in chronic hepatitis C patients treated with interferon-alpha. Cytokine. 1999;11:1076–1080. doi: 10.1006/cyto.1999.0504. [DOI] [PubMed] [Google Scholar]
- 7.Masaki N, Fukushima S, Hayashi S. Lower Th1/Th2 ratio before interferon therapy may favor long-term virological responses in patients with chronic hepatitis C. Dig Dis Sci. 2002;47:2163–2169. doi: 10.1023/a:1020114722763. [DOI] [PubMed] [Google Scholar]
- 8.Edwards-Smith CJ, Jonsson JR, Purdie DM, Bansal A, Shorthouse C, Powell EE. Interleukin-10 promoter polymorphism predicts initial response of chronic hepatitis C to interferon alfa. Hepatology. 1999;30:526–530. doi: 10.1002/hep.510300207. [DOI] [PubMed] [Google Scholar]
- 9.Kawamura C, Nakajima S, Kuroki T, Monna T. Two-dimensional analysis of production of IL-6 and TNF-alpha can predict the efficacy of IFN-alpha therapy. Hepatogastroenterology. 1999;46:2941–2945. [PubMed] [Google Scholar]
- 10.Cramp M, Rossel S, Chokshi S, Carucci P, Williams R, Naoumov N. Hepatitis C virus-specific T cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology. 2000;118:346–365. doi: 10.1016/s0016-5085(00)70217-4. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro S, Gershtein V, Elias N, Zuckerman E, Salman N, Lahat N. mRNA cytokine profile in peripheral blood cells from chronic hepatitis C virus (HCV)-infected patients: effects of interferon-alpha (IFN-alpha) treatment. Clin Exp Immunol. 1998;114:55–60. doi: 10.1046/j.1365-2249.1998.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polyak S, Khabar K, Rezeiq M, Gretch D. Elevated levels of interleukin-8 in serum are associated with hepatitis C infection and resistance to interferon therapy. J Virol. 2001;75:6209–6211. doi: 10.1128/JVI.75.13.6209-6211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuman M, Benhamou J, Malkiewicz I, Akremi R, Shear NH, Asselah T, Ibrahim A, Boyer N, Martinot-Peignoux M, Jacobson-Brown P, Katz GG, Le Breton V, Le Guludec G, Suneja A, Marcellin P. Cytokines as predictors for sustained response and as markers for immunomodulation in patients with chronic hepatitis C. Clin Biochem. 2001;34:173–182. doi: 10.1016/s0009-9120(01)00212-0. [DOI] [PubMed] [Google Scholar]
- 14.Chung R, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, Peters MG, Koziel MJ, Bhan AK, Alston B, Colquhoun D, Nevin T, Harb G, van der Horst C. Peginterferon alpha-2a plus ribavirin versus interferon alpha-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuman M, Blendis L, Shear N, Malkiewicz IM, Ibrahim A, Katz GG, Sapir D, Halpern Z, Brill S, Peretz H, Magazinik S, Konikoff FM. Cytokine network in nonresponding chronic hepatitis C patients with genotype 1: role of triple therapy with interferon alpha, ribavirin, and ursodeoxycholate. Clin Biochem. 2001;34:183–188. doi: 10.1016/s0009-9120(01)00213-2. [DOI] [PubMed] [Google Scholar]
- 16.Neuman M, Benhamou J, Martinot M, Boyer N, Shear NH, Malkiewicz I, Katz GG, Suneja A, Singh S, Marcellin P. Predictors of sustained response to alpha interferon therapy in chronic hepatitis C. Clin Biochem. 1999;32:537–545. doi: 10.1016/s0009-9120(99)00053-3. [DOI] [PubMed] [Google Scholar]
- 17.Neuman M, Benhamou J, Malkiewicz I, Malkiewicz IM, Ibrahim A, Valla DC, Martinot-Peignoux M, Asselah T, Bourliere M, Katz GG, Shear NH, Marcellin P. Kinetics of serum cytokines reflect changes in the severity of chronic hepatitis C presenting minimal fibrosis. J Viral Hepatitis. 2002;9:134–140. doi: 10.1046/j.1365-2893.2002.00343.x. [DOI] [PubMed] [Google Scholar]
- 18.Nelson D, Lim H, Marousis C, Fang JW, Davis GL, Shen L, Urdea MS, Kolberg JA, Lau JY. Activation of tumor necrosis factor-alpha system in chronic hepatitis C virus infection. Dig Dis Sci. 1997;42:2487–2494. doi: 10.1023/a:1018804426724. [DOI] [PubMed] [Google Scholar]
- 19.Kaplanski G, Farnarier C, Payan M, Bongrand P, Durand J. Increased levels of soluble adhesion molecules in the serum of patients with hepatitis C: correlation with cytokine concentrations and liver inflammation and fibrosis. Dig Dis Sci. 1997;42:2277–2284. doi: 10.1023/a:1018818801824. [DOI] [PubMed] [Google Scholar]