Abstract
Intramuscular injection of plasmid DNA (pDNA) to express a therapeutic protein is a promising method for the treatment of many diseases. However, the therapeutic applications are usually hindered by gene delivery efficiency and expression level. In this study, critical factors in a pDNA-based gene therapy system, such as gene delivery materials, a therapeutic gene, and its regulatory elements, were optimized to establish an integrated system for the treatment of mouse hindlimb ischemia. The results showed that Pluronic® L64 (L64) was an efficient and safe material for gene delivery into mouse skeletal muscle. It also showed intrinsic ability to promote in vivo angiogenesis in a concentration-dependent manner, which might be through the activation of nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB)-regulated angiogenic factors. The combination of 0.1% L64 with a hybrid gene promoter (pSC) increased the gene expression level, elongated the gene expression duration, and enhanced the number of transfected muscle fibers. In mice ischemic limbs, a gene medicine (pSC-HIF1αtri/L64) composed of L64 and pSC-based expression plasmid encoding hypoxia-inducible factor 1-alpha triple mutant (HIF-1αtri), improved the expression of stable HIF-1α, and in turn, the expression of multiple angiogenic factors. As a result, the ischemic limbs showed accelerated function recovery, reduced foot necrosis, faster blood reperfusion, and higher capillary density. These results indicated that the pSC-HIF1αtri/L64 combination presented a potential and convenient venue for the treatment of peripheral vascular diseases, especially critical limb ischemia.
Keywords: gene therapy, Pluronic L64, angiogenesis, SV40 enhancer, HIF-1α
Introduction
Skeletal muscle is an attractive tissue for gene therapy because myocytes can act as the “factory” to produce and secrete therapeutic proteins.1 Viral vectors have been widely used due to their potent transgene expression in skeletal muscle,2,3 but they have potential risks to human beings, such as insertional or reverse mutation, immunogenicity and toxicity. By contrast, intramuscular injection of naked plasmid DNA (pDNA) is a safer, cheaper, and easier approach. However, pDNA-based therapeutic trials usually failed to meet expectations due to the low gene delivery efficiency and low expression level. The successful cases were mainly focused on reporter gene expression and DNA vaccination.4 To set up an effective pDNA-based gene therapy system, the key determinants, such as the gene delivery materials, therapeutic gene, and its regulatory elements, should be comprehensively designed and optimized so that they can function efficiently and synergistically.
The bottleneck in muscle-based gene therapy is the pDNA transfer efficiency governed by gene delivery materials. Cationic lipids and polymers can promote pDNA delivery into many cell lines in vitro and several tissues in vivo.5–7 However, they usually offer negative results on skeletal muscle gene delivery, as their gene expression levels are much lower than that of naked pDNA. Pluronics, also known as poloxamers, are a group of non-ionic amphiphilic block copolymers with two chains of hydrophilic polyethylene oxide (PEO) group flanking a hydrophobic polypropylene oxide (PPO) group. With a long record of safety, Pluronics have been widely used as pharmaceutical adjuvants, and some of them were approved by the US Food and Drug Administration (FDA).8–10 In addition, several studies showed that Pluronics such as L64, P85, and F127 increased the reporter gene expression after co-injection of pDNA/Pluronics mixture into rodent skeletal muscle, indicating their potential for in vivo muscle-based gene therapy.11,12 However, very few successful cases have been obtained to treat animal disease models using therapeutic genes.
Although Pluronics facilitate gene transfer into myocytes, the amounts of expressed molecules are usually not high enough for therapeutic purpose. Nuclear import of pDNA is still the crucial barrier for transgene expression. In a dividing cell, pDNA can be encompassed into the nucleus during the nuclear membrane disintegration and the subsequent nucleus reconstruction. In a non-dividing muscular cell, special measures should be taken to help pDNA enter the nucleus. DNA nuclear targeting sequences (DTSs) are special DNA sequences capable of facilitating nuclear entrance of pDNA.13 Therefore, DTSs can be integrated in the pDNA sequence to help nuclear import of the pDNA. The simian virus 40 enhancer (SV40E) possesses several sequences to bind transcriptional factors such as NF-κB, the activating protein AP-2, octamer transcription factor Oct-1, and transcriptional enhancer factor TEF-1.14,15 Upon combination, the transcriptional factors will introduce the DNA fragment into the nucleus. For this reason, SV40E can serve as a typical DTS. Here, we incorporated SV40E close to the upstream of cytomegalovirus (CMV) promoter to construct a plasmid with hybrid promoter, pSV40E/CMV (pSC). As Pluronics increased the gene transfer in a promoter-selective manner,10 we aimed in this study to evaluate the synergistic effects of the Pluronics/pSC combination on gene expression in skeletal muscle.
To treat ischemia, angiogenesis cytokines, such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), platelet derived growth factor (PDGF), Angiopoietin-1 (Ang-1) and stromal cell-derived factor (SDF-1), were commonly used.16,17 As a complex biological process, angiogenesis requires cooperative effects of the cytokines. Over-expression of a single cytokine may be insufficient to induce functional blood vessel formation, whereas inconsonant co-expression of several cytokines may lead to aberrant vascular proliferation, or bring the risks of hemangioma and tumor growth.18,19 The transcription factor hypoxia-inducible factor 1 (HIF-1) has been considered to be a more rational choice, because it functions as an upstream “switch” regulating the transcription of multiple angiogenesis cytokines in a synergistic manner.2,3,20 HIF-1 is a heterodimeric protein that consists of an O2-regulated α-subunit and a stable β-subunit. In hypoxic condition, HIF-1α translocates to the nucleus, dimerizes with HIF-1β, binds the hypoxia response elements (HREs) on the target gene’s promoter, and subsequently regulates the transcription.21,22 Under normoxic conditions, HIF-1α will be hydroxylated at two proline residues (Pro402 and Pro564) by prolyl-4-hydroxylase (PHD) and then recognized by von Hippel-Lindau (VHL) protein, the recognition component of an E3 ubiquitin ligase complex, leading to ubiquitination and rapid proteosomal degradation of HIF-1α. Meanwhile, HIF-1α can also be hydroxylated at an asparagine residue (Asn803) under normoxic conditions by an asparaginyl hydroxylase FIH-1 (factor inhibiting HIF-1), resulting in the interaction disrupture between HIF-1α and the transcriptional co-activators p300 and CBP (CREB binding protein), thereby inhibiting the transcription activity of HIF-1.23,24 Therefore, site-directed mutation of the codons of Pro402, Pro564, and Asn80 can abrogate the hydroxylation sites in HIF-1α and generate a constitutively stable and active form of HIF-1α protein. Over-expression of such a HIF-1α has shown a good angiogenic potency as well as safety profile in several viral vector-mediated gene therapy studies.25,26
In this study, the gene delivery materials, therapeutic gene, and its regulatory element were optimized and integrated to establish an angiogenic gene therapy system. We hope that the system will have therapeutic effects on the mouse model of ischemic hindlimb, and thereafter present promising potential for the treatment of peripheral vascular diseases, such as diabetic foot, thromboangiitis obliterans, and chronic venous insufficiency.
Materials and methods
Reagents
Pluronic L64, polyvinylpyrrolidone (PVP, 50 kD) and polyethyleneimine (PEI, branched 25 kD) were purchased from Sigma-Aldrich (St Louis, MO, USA). Pluronic P85 was a kind gift from Chengzhong (Michael) Yu (University of Queensland, QLD, Australia). Restriction enzymes were purchased from Fermentas (St Leon-Rot, Germany). The arginine-functionalized polypeptide dendrimer G5 and cationic liposome were synthesized, respectively, as described in previous research.5,6
Cell culture and transfection
Mouse myoblast cell line C2C12 (ATCC Number CRL-1772) was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS; Hyclone 30084.03, Invitrogen) and 1% penicillin-streptomycin in a CO2 (5%) incubator at 37°C under saturated humidity. For transfection, cells were seeded in 6-well plates and transfected one day later with 4 μg/well plasmid using Lipofectamine 2000 (Invitrogen) when the cell confluence was about 80%–90%.
Construction of plasmids
Structures of the plasmids used in this study are shown in Figure 1. They were constructed based on pcDNA3.1(+) (Invitrogen; referred to below as pCMV) which contained the CMV promoter. The plasmid pSC containing the SV40E/CMV promoter was constructed in three steps. Firstly, the SV40E fragment was amplified from pGL3-control (Promega, Madison, WI, USA) using primers SV40E-F and SV40E-R (Table 1), and cloned into pMD19-T (TaKaRa, Dalian, People’s Republic of China) in the forward orientation to get pMD19T-SV. Secondly, the CMV promoter was cut from pCMV using Bgl II and Kpn I, and inserted into pMD19T-SV between BamH I and Kpn I, generating pMD19T-SC. Finally, to construct pSC, the SV40E/CMV hybrid promoter separated from pMD19T-SC was used to replace the CMV promoter in pCMV between Bgl II and Kpn I sites.
Figure 1.
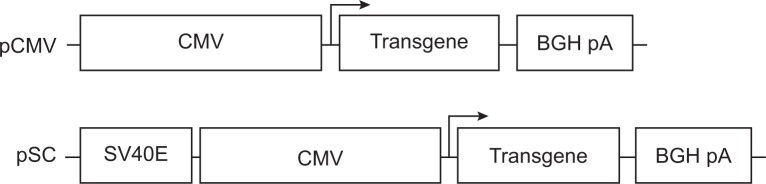
Structure of the constructed plasmids.
Abbreviations: CMV, cytomegalovirus; SV40E, SV40 enhancer; SC, CMV promoter/SV40 enhancer; BGH pA, bovine growth hormone polyadenylation signal sequence.
Table 1.
Sequences of the primers
| Primers | Sequences |
|---|---|
| SV40E-F | 5′-GAAGATCTTGGTAAAATCGATAAGGATCTGAACGATG-3′ |
| SV40E-R | 5′-CGGGATCCGCTGTGGAATGTGTGTCAGTTAGG-3′ |
| Luc-F | 5′-ATAAGAATGCGGCCGCACCATGGAAGACGCCAAAAACATAAAG-3′ |
| Luc-R | 5′-GCTCTAGATTACACGGCGATCTTTCCGCC-3′ |
| LacZ-F | 5′-ATAAGAATGCGGCCGCATAATGGTAGATCCCGTCGTTTTACAACG-3′ |
| LacZ-R | 5′-ATAAGAATGGGCCCTTATTTTTGACACCAGACCAACTGGTAATG-3′ |
| HIF1α-F3 | 5′-CAAACTTAAGAAGGAACCTGATGCTTTAACTTTGCTGGCCGCAGCCGCTGGAG-3′ |
| HIF1α-R3 | 5′-CTACTTCGAAGTGGCTTTGGCGTTTCAGCGGTG-3′ |
| HIF1α-F | 5′-ATAAGAATGCGGCCGCAATGGAGGGCGCCGGCGG-3′ |
| HIF1α-R | 5′-GCTCTAGATCAGTTAACTTGATCCAAAGCTCTGAG-3′ |
| HIF1α-qF | 5′-CCACAGGACAGTACAGGATG-3′ |
| HIF1α-qR | 5′-TCAAGTCGTGCTGAATAATACCACT-3′ |
| VEGF-qF | 5′-TCACTGTGAGCCTTGTTCAGAGCG-3′ |
| VEGF-qR | 5′-CACCGCCTTGGCTTGTCACATCT-3′ |
| PDGF-qF | 5′-CTTCCCCTACGTTCACTTCCG-3′ |
| PDGF-qR | 5′-CATTACAACCTTGCTCACCCTGC-3′ |
| SDF1-qF | 5′-ACCAGTCAGCCTGAGCTACC-3′ |
| SDF1-qR | 5′-CGGGTCAATGCACACTTGTCTGT-3′ |
| β-actin-qF | 5′-CTCCTCCCTGGAGAAGAGCTA-3′ |
| β-actin-qR | 5′-CCTTCTGCATCCTGTCGGCAA-3′ |
| IL-6-qF | 5′-CTCTGGGAAATCGTGGAAATGAG-3′ |
| IL-6-qR | 5′-CTGTATCTCTCTGAAGGACTCTG-3′ |
| MMP-2-qF | 5′-CTTTTGTATGCCCTTCGCTCG-3′ |
| MMP-2-qR | 5′-CAAGAAGGCTGAGCAGGAAG-3′ |
| MMP-9-qF | 5′-AACCCACAGAATGCTTACTGTGTG-3′ |
| MMP-9-qR | 5′-GGTTTCAGCAGATTTACAGGACAC-3′ |
Abbreviations: SV40E, SV40 enhancer; Luc, luciferase; LacZ, β-galactosidase; HIF1α, hypoxia-inducible factor 1-alpha; VEGF, vascular endothelial growth factor; PDGF, platelet-derived growth factor; SDF1, stromal cell-derived factor 1; IL-6, interleukin 6; MMP-2, matrix metallopeptidase 2; MMP-9, matrix metallopeptidase 9; F, forward primer; R, reverse primer; q, quantitative primer.
To construct the plasmids pCMV-Luc and pSC-Luc, the firefly luciferase (Luc) complementary DNA (cDNA) was amplified from pGL3-control using primers Luc-F and Luc-R (Table 1), and cloned into pCMV and pSC between Not I and Xba I sites, respectively. To construct the plasmids pCMV-LacZ and pSC-LacZ, the β-galactosidase (LacZ) cDNA was amplified from pUB6/V5-His/lacZ (Invitrogen) using primers LacZ-F and LacZ-R (Table 1), and cloned into pCMV and pSC between Not I and Apa I, respectively.
Plasmids expressing wild-type HIF-1α (HIF-1αwt) and P564A/N803A double mutant HIF-1α were gifts from Dr Daniel J Peet (University of Adelaide). To generate the P402A mutation in HIF-1α gene, a fragment was amplified from the HIF-1αwt plasmid using primers HIF1α-F3 and HIF1α-R3 (Table 1), and inserted into the double mutant HIF-1α plasmid between Afl II and Bsp119 I. The P402A/P564A/N803A triple mutant HIF-1α (HIF1αtri) fragment was amplified using primers HIF1α-F and HIF1α-R (Table 1), and respectively inserted into pCMV and pSC between Not I and Xba I sites, generating pCMV-HIF1αtri and pSC-HIF1αtri. The inserted fragments in the plasmids were confirmed by DNA sequencing.
Preparation of pDNA/material mixture
Mixtures of dendrimer G5/pDNA, liposome/pDNA and PEI/pDNA were prepared as described.5,6 Stock solutions (10×, w/v [weight/volume]) of Pluronic L64, P85 and PVP were prepared in pure water and stored at 4°C, respectively. Before intramuscular injection, L64, P85 and PVP were diluted to 2× solutions with saline, gently and thoroughly mixed with equal volume of pDNA, and incubated for 10 minutes at room temperature before injection.
In vivo assay of reporter gene expression
All animals were handled in compliance with the national and local animal welfare rules, and experiments were approved by the local ethics committee (Animal Care and Use Committee of Sichuan University). BALB/c mice (6-week-old males; West China Animal Culture Center of Sichuan University) were used to evaluate the reporter gene expression. Ten micrograms of pDNA encoding luciferase or β-galactosidase in 50 μL of saline with or without material was injected into mouse tibialis anterior (TA) muscles at both sides.
For luciferase activity assay, cells or tissues were homogenized in 1 mL lysis buffer (Promega). The homogenized lysates were extensively vortexed and centrifuged at 12,000× g for 5 minutes at 4°C. The supernatants were used for luciferase activity analysis using a reporter assay kit (Promega, E1501) according to the manufacturer’s protocol. Relative light unit (RLU) was normalized to the total protein concentration in the lysate supernatant, measured using a bicinchoninic acid (BCA) Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA).
For β-galactosidase activity assay, TA muscles were isolated and immersed in a freshly prepared fixative (2% paraformaldehyde and 0.2% glutaraldehyde in phosphate-buffered saline [PBS]) for 1 hour on ice. Fixed samples were washed three times in PBS for 5 minutes each at room temperature and then incubated in X-gal staining solution at 37°C for 6 hours. After staining, samples were further fixed in 4% paraformaldehyde and photographed with a digital camera.
Serum biochemical test
Mice were intramuscularly injected with 50 μL solution of pDNA and pDNA formulated with 0.1% L64 (pDNA/L64), respectively. Three days later, their bloods were collected and sera were separated by centrifugation at 3,000 rpm for 10 minutes at 4°C. Sera biochemical tests were analyzed using the Analyzer Medical System AUTOLAB (AMS, Italy).
Mouse hindlimb ischemia surgery
The animal model of hindlimb ischemia was established using male BALB/c mice aged 10–12 weeks. Based on previous description, the surgery protocol gave a modification on the ligation site, namely to ligate the iliac artery before it branches.27 In brief, animals were anesthetized by intraperitoneal administration of sodium pentobarbital (45 mg/kg), and both the hindlimbs were shaved, depilated, and wiped with iodophor. Through a longitudinal incision in the left thigh, the external iliac artery was carefully separated from the vein and nerve fiber, and tightly ligated at the site just before it bifurcates to the profunda femoris, epigastrica, and femoral arteries.
After the operation, mice were randomized into five groups: saline, L64, pSC-HIF1α, pSC-HIF1α/L64, and pCMV-HIF1α/L64. The final concentration of injected L64 was 0.1%. For each mouse, 100 μL of solution containing 20 μg of plasmid was intramuscularly injected at five sites on the ischemic limb to guarantee a homogenous distribution.
Damage assessment of ischemic limb
At 0, 14, and 28 days post surgery, limb morphology was visually scored to evaluate the degree of ischemia-induced damage. Three grades were used to measure the degree of limb necrosis: I, necrosis in the nail; II, necrosis in the toe; and III, necrosis in the distal half of the foot. Evaluations were performed by an observer blinded to treatment groups.
Blood flow measurement
Blood flow in anesthetized mouse hindlimb was evaluated by laser Doppler flowmetry (LDF) (Periflux System 5,000, Perimed, Stockholm, Sweden) as described.28 For each mouse, six sites on the gastrocnemius muscles of ischemic and normal limbs were detected using a deep measurement probe at different time points within 4 weeks after surgery. The reperfusion rate of ischemic hindlimb was defined as the ratio of ischemic to normal hindlimb perfusion value in the same mouse, so as to minimize the variability due to individual basal perfusion.
Immunohistochemistry and quantification of capillary density
Specimens were fixed in 10% formalin, embedded in paraffin, and sectioned with 5 μm thickness. After antigen retrieval, the sections were incubated with anti-CD31 polyclonal antibody (sc-1506; Santa Cruz Biotechnology, Santa Cruz, TX, USA) and a horseradish peroxidase (HRP)-conjugated secondary antibody (Zhongshan Golden Bridge Biotechnology, Beijing, People’s Republic of China). Antibody binding was visualized with freshly prepared 3,3′-diaminobenzidine tetrahydrochloride solution (Zhongshan Golden Bridge Biotechnology) and then counterstained with hematoxylin. CD31+ cells were counted in six random fields per slide captured with a 5× objective lens (Leica DMI 4000B, Wetzlar, Germany) and the mean number of positive cells per square millimeter was used as an index of capillary density.
Western blot
Samples were homogenized in ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer (Thermo Fisher) containing protease inhibitors (Hoffman-La Roche Ltd, Basel, Switzerland). The lystes were separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electronically transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore) at 100 V for 1 hour. After blocking with 5% (w/v) nonfat dry milk in PBST, the membrane was incubated with primary antibody (H-206 for anti-HIF-1α, R-22 for anti-β-actin; Santa Cruz) and HRP-conjugated secondary antibodies. Immunoreactive bands were visualized using SuperSignal™ West Pico Chemiluminescent Substrate (Thermo Fisher) and photographed with the ChemiDoc™ XRS+ System with Image Lab™ Software (Bio-Rad, Hercules, CA, USA).
Quantitative polymerase chain reaction
Total RNAs were extracted from gastrocnemius muscles using TRIzol® reagent (Invitrogen) according to the manufacturer’s protocol. RNA samples were treated with RNase-free DNase I (Fermentas) prior to cDNA synthesis to eliminate any residual plasmid or genomic DNA. First strand cDNAs were reversely transcribed as previously described.29 Quantitative polymerase chain reaction (qPCR) was designed according to the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines and performed on the CFX96 Touch™ real-time PCR detection system (BioRad).30 Data were detected and analyzed using CFX Manager™ software (Bio-Rad). All reactions were done in triplicate using SYBR Premix Ex Taq™ II (TaKaRa) in a 20 μL volume. The expression of target messenger RNA (mRNA) was normalized to the β-actin mRNA reference and calculated using the Pfaffl method.30,31 Data on relative gene expression were presented as the fold change compared with the saline-treated control. Primers of qPCR are shown in Table 1.
Statistical analysis
Quantitative data are presented as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to evaluate the significance of differences between groups. When a significant difference was detected, multiple-comparison analysis was performed using the Student-Newman-Keuls test. In some cases, evidence of interaction was tested for using two-way ANOVA. A P-value of <0.05 was considered statistically significant.
Results
Identification of L64 as an effective and safe vehicle for muscular gene delivery
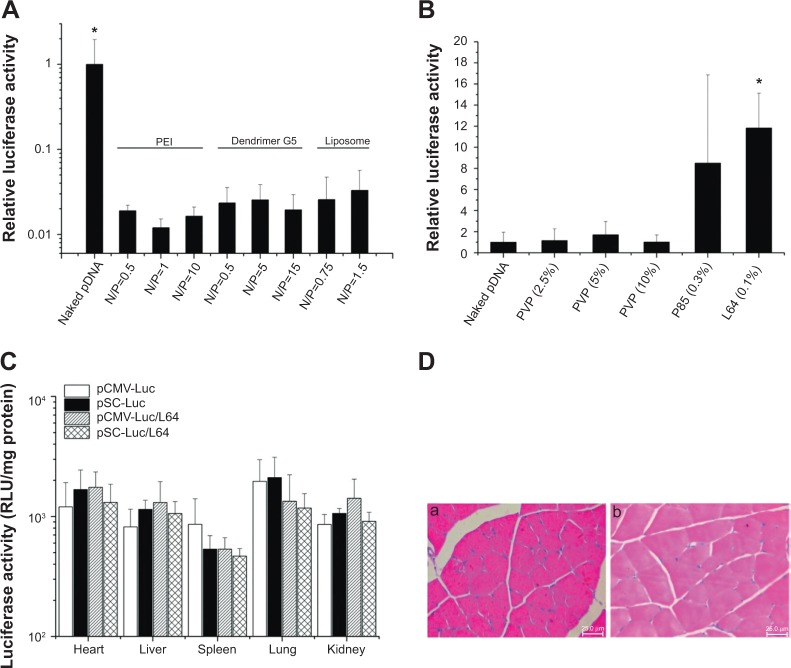
Cationic gene delivery materials, such as PEI, polypeptide dendrimer G5, and liposome, are widely used materials for in vitro gene transfection.5–7 However, they showed much lower levels of transgene expression in skeletal muscle than naked pDNA (Figure 2A). In contrast, some kinds of nonionic materials had similar or much better performance than naked pDNA, while L64 showed the best and most stable results (Figure 2B).
Figure 2.
Screening of materials for local gene delivery into skeletal muscle and the in vivo biocompatibility evaluation of pDNA/L64 combination.
Notes: (A and B) Screening of materials for local gene delivery into skeletal muscle. The bilateral tibialis anterior (TA) muscles were injected with 10 μg of naked pCMV-Luc or pCMV-Luc/material mixtures. Samples were obtained 7 days after injection. The luciferase activities in each group were measured and presented as the relative ratios compared with the naked pDNA group. (A) Cationic gene delivery materials (n=10). *P<0.05 versus all other groups. (B) Nonionic gene delivery materials (n=10). *P<0.05 versus naked DNA and the three PVP groups. (C and D) In vivo biocompatibility evaluation of pDNA/L64 combination. Ten micrograms of pCMV-Luc were injected into the left TA muscles of each mouse with or without 0.1% L64. The organs and muscles were detached 3 and 7 days after injection for detection. (C) Organ distribution of gene expression 3 days after intramuscular injection (n=6). (D) Representative tissue sections in the injected muscles 3 days after treatment: (a) pDNA, and (b) pDNA/L64 (n=6). Pluronic® L64 (Sigma-Aldrich, St Louis, MO, USA).
Abbreviations: pDNA, plasmid DNA; RLU, relative light units; CMV, cytomegalovirus; SV40E, SV40 enhancer; SC, CMV promoter/SV40 enhancer; Luc, luciferase; PVP, polyvinylpyrrolidone; PEI, polyethyleneimine; L64, Pluronic® L64; N/P, charge ratio between amino groups of materials and phosphate groups of DNA.
The ectopic transgene expression is an important safety concern because unrestricted gene expression in other organs or tissues may produce unexpected effects other than therapeutic purpose. To investigate the in vivo distribution of L64-mediated transgene expression, major organs of mice were examined for luciferase expression after intramuscular injection. In the examined organs, no significant difference in luciferase expression levels was observed among the four treatments at day 3 (Figure 2C). Similar results were obtained at day 7 (data not shown). To further determine the safety profile of L64 as a gene carrier, the local and systemic toxicity was evaluated after intramuscular injection of naked pDNA and pDNA/L64 into normal BALB/c mice. Tissue sections from injected TA muscles did not show any pathological changes in both the pDNA and pDNA/L64 treatments at day 3 (Figure 2D). There was also no significant difference in serum biochemical indexes, such as liver function, renal function, and blood glucose, between the two groups (Table 2). Similar results were observed at day 7 (data not shown).
Table 2.
Serum biochemical test of mice
| Items | pDNA | pDNA/L64 | |
|---|---|---|---|
| Liver function | ALT (U/L) | 35.7±3.55 | 33.62±1.93 |
| AST (U/L) | 103±13.22 | 111±14.4 | |
| ALP (U/L) | 183.7±17.43 | 191.26±15.83 | |
| GGT (U/L) | 2.64±0.38 | 2.51±0.51 | |
| TP (g/L) | 36.17±3.33 | 40.42±4.15 | |
| ALB (g/L) | 24.2±1.15 | 23.9±1.81 | |
| TBIL (μmol/L) | 25.6±1.79 | 30.2±1.85 | |
| DBIL (μmol/L) | 2.64±0.86 | 2.29±0.92 | |
| Renal function | BUN (mmol/L) | 7.85±0.68 | 7.27±0.91 |
| CRE (μmol/L) | 51.66±5.56 | 55.13±6.51 | |
| Blood glucose | GLU (mmol/L) | 5.18±0.83 | 5.76±0.66 |
Note: Pluronic® L64 (Sigma-Aldrich, St Louis, MO, USA).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, γ-glutamyl transpeptidase; TP, total protein; ALB, albumin; TBIL, total bilirubin; DBIL, direct bilirubin; BUN, blood urea nitrogen; CRE, creatinine; GLU, glucose; pDNA, plasmid DNA; L64, Pluronic® L64.
Effects of L64 and SV40E on transgene expression in mouse skeletal muscle
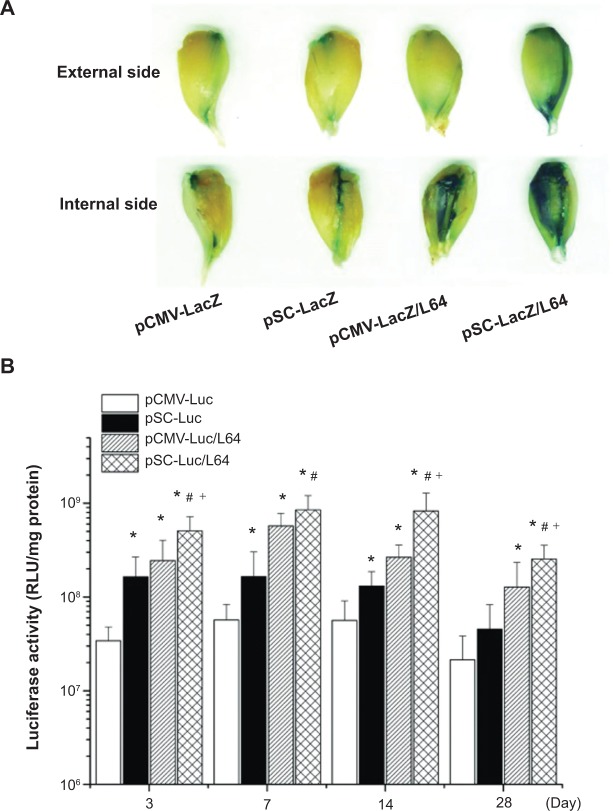
To visually evaluate the level and spatial distribution of transgene expression in mouse TA muscle, histological analysis of the β-galactosidase (LacZ) expression was performed at day 7 after injection. The pSC-LacZ/L64 treatment showed the highest level expression in the four groups, evident from the degree and distribution of stained muscles on the sample surfaces (Figure 3A). The inside muscle fibers offered information consistent with their external and internal sides, and only the fibers close to the needle tract were transfected in pCMV-LacZ and pSC-LacZ groups (data not shown). It is worth noting that the difference induced by the addition of L64 was more obvious than that by SV40E, indicating that the former was the major contributing factor to the superior performance observed for pSC-LacZ/L64.
Figure 3.
Qualitative and quantitative assays of reporter gene expression in the tibialis anterior (TA) muscles.
Notes: (A) Representative figures of LacZ expression in mice TA muscles. Samples were obtained 7 days after injection (n=6). (B) Quantitative assays of the Luc gene expression. The TA muscles of each mouse were injected with 10 μg pDNA and the muscles were detached for detection at different time points (n=10). *P<0.05 versus pCMV-Luc; #P<0.05 versus pSC-Luc; +P<0.05 versus pCMV-Luc/L64. Pluronic® L64 (Sigma-Aldrich, St Louis, MO, USA).
Abbreviations: pDNA, plasmid DNA; CMV, cytomegalovirus; SV40E, SV40 enhancer; SC, CMV promoter/SV40 enhancer; LacZ, β-galactosidase; Luc, luciferase; RLU, relative light units; L64, Pluronic® L64.
Results from luciferase activity assays revealed some characteristics of the four treatments in mouse skeletal muscles (Figure 3B): i) L64 significantly increased the activity of both CMV and pSC promoters on gene expression during 4 weeks. When L64 was added, the peak expression level of transgene at day 7 increased 10-fold for CMV promoter (pCMV-Luc/L64 versus pCMV-Luc; P<0.05), and 5-fold for SC promoter (pSC-Luc/L64 versus pSC-Luc; P<0.05). ii) SV40E significantly increased the activity of CMV promoter on gene expression during the entire 28-day period, whether L64 was incorporated or not (pSC-Luc versus pCMV-Luc, pSC-Luc/L64 versus pCMV-Luc/L64; P<0.05). iii) In combination with L64, SV40E elongated the duration of gene expression (pSC-Luc/L64 versus pCMV-Luc/L64). The gene expression of pCMV-Luc/L64 peaked at day 7 and decreased thereafter, while pSC-Luc/L64 kept the highest level for at least 14 days. A two-way ANOVA analysis on the data of day 14 also showed a statistically significant synergism between L64 and SV40E (P<0.05 for L64; P<0.05 for SV40E, P=0.02 for interaction). iv) Transgene expression in the target TA muscles was generally four to five orders of magnitude higher than that in remote organs, irrespective of the type of formulation (Figure 3B versus Figure 2C).
The qualitative and quantitative data indicated that the combination of Pluronic L64 with SV40E resulted in significant increases in reporter gene expression level, distribution, and duration.
The stability of wild and mutant HIF-1α mRNA and protein
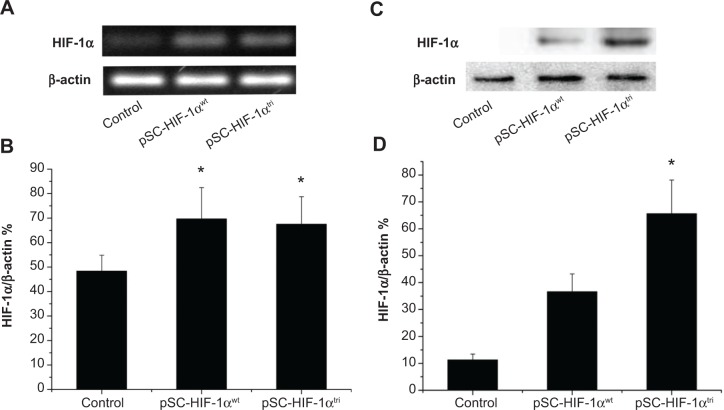
To investigate if the triple mutant HIF-1α protein is more stable than its wild type counterpart under normoxic condition, the pSC-HIF1αtri and pSC-HIF1αwt plasmids were respectively transfected into normally cultured mouse myoblast C2C12. Expressions of HIF-1α mRNA and protein in HIF1αtri- and HIF1αwt-transfected cells were significantly higher than those in untransfected cells. Although HIF1αtri- and HIF1αwt-transfected cells had identical HIF-1α mRNA expression (Figure 4A and B; P>0.05), their HIF-1α protein expressions were significantly different (Figure 4C and D; P<0.05). It was mainly due to the rapid degradation of HIF-1αwt protein in normoxic condition, whereas the HIF-1αtri protein was still stable. The efficiency and stability of protein expression of transfected genes showed the potential application of HIF1αtri for in vivo gene therapy.
Figure 4.
Stability of wild type and mutant HIF-1α mRNA and protein in C2C12 cells.
Notes: Cells were transfected with 0.9% saline (Control), pSC-HIF1αwt and pSC-HIF1αtri respectively, and harvested 3 days later for semi-quantitative RT-PCR and Western blot analysis. (A) Representative image of RT-PCR products showing HIF-1α mRNA levels. (B) Band intensity of the RT-PCR products was quantified using the Image Lab™ Software (Bio-Rad, Hercules, CA, USA). HIF-1α mRNA levels were presented as the relative values compared with β-actin expression in the same sample (n=3). *P<0.05 versus the control. (C) Representative image of Western blot showing HIF-1α protein levels. (D) Band intensity in the Western blot assay was quantified using the Image Lab™ Software (Bio-Rad). HIF-1α protein levels were presented as the relative values compared with β-actin expression in the same sample (n=3). *P<0.05 versus all other groups.
Abbreviations: HIF-1αwt, wild-type hypoxia-inducible factor 1-alpha; HIF-1αtri, hypoxia-inducible factor 1-alpha triple mutant; mRNA, messenger RNA; RT-PCR, reverse transcription polymerase chain reaction; SC, cytomegalovirus promoter/SV40 enhancer; SV40E, SV40 enhancer.
Treatment of mouse ischemic hindlimb via L64-mediated stable HIF-1α expression
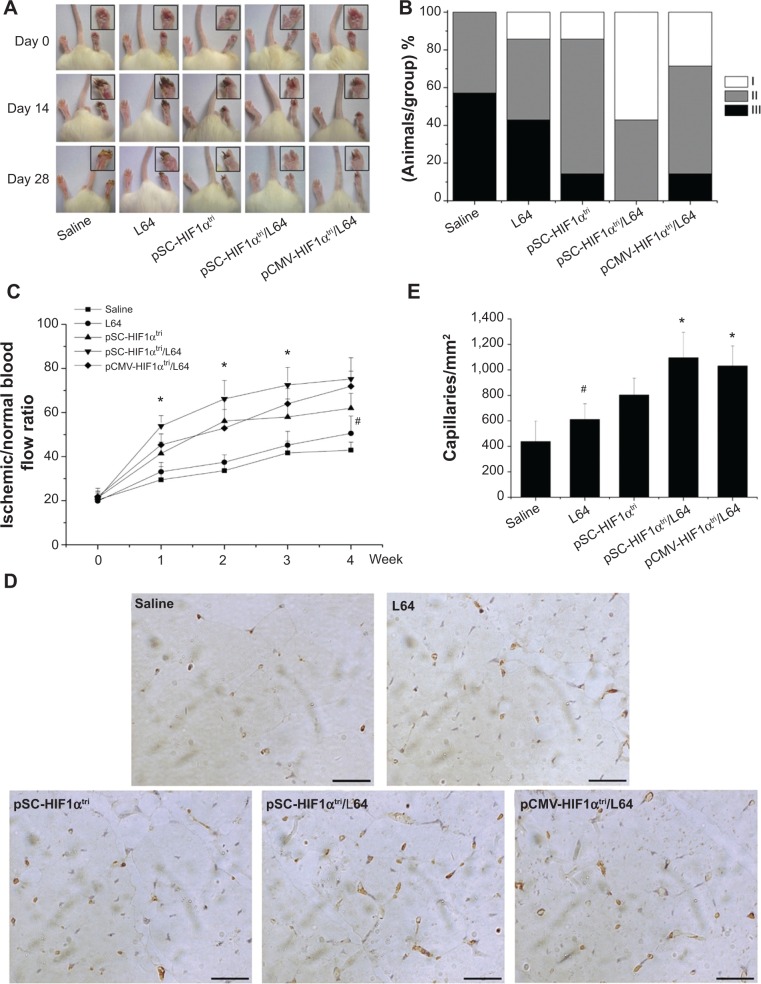
The onset of ischemia was confirmed by the LDF measure after the surgery (day 0). Photographs of the mice hindlimbs were recorded at every time point using a digital camera (Figure 5A). The ischemic status of mice hindlimbs in each group was assessed at day 28 using the 3-grade method (Figure 5B). In the saline group, 57% of the animals had necrosis in the distal half of the foot (grade III), whereas the ratios in pSC-HIF1αtri and pCMV-HIF1αtri/L64 treatments reduced to 14%. The pSC-HIF1αtri/L64 treatment got the best therapeutic results: no animal had necrosis in grade III; 43% of them had necrosis limited to the toe (grade II) and 57% to the nail (grade I).
Figure 5.
Therapeutic effects on mouse ischemic hindlimbs.
Notes: (A) Representative photographs of hindlimbs on days 0, 14, and 28 after treatments. (B) Grading the hindlimb necrosis on day 28 after treatment. Three grades were used to measure the degree of limb necrosis: I, necrosis in the nail; II, necrosis in the toe; and III, necrosis in the distal half of the foot. (C) The blood reperfusion ratio in ischemic hindlimbs. *P<0.05 versus all other groups; #P<0.05 versus the saline group. (D) Representative immunohistochemistry photographs of sections in ischemic hindlimbs. Capillaries were marked in brown. Scale bar =25 μm. (E) Quantification of capillary density. *P<0.05 versus the saline, L64, and pSC-HIF1α groups; #P<0.05 versus the saline group (n=7). Pluronic® L64 (Sigma-Aldrich, St Louis, MO, USA).
Abbreviations: CMV, cytomegalovirus; L64, Pluronic® L64; SC, CMV promoter/SV40 enhancer; HIF-1αtri, hypoxia-inducible factor 1-alpha triple mutant; SV40E, SV40 enhancer.
Blood flow is a precise index to evaluate the reperfusion extent in ischemic limbs. The reperfusion extent was presented as the blood flow ratio between the ischemic and normal limbs in a mouse (Figure 5C). In line with the reduced necrosis, the pSC-HIF1αtri/L64 treatment exhibited a significantly higher reperfusion extent than other groups from day 7 to day 21 after treatment (P<0.05). The other two HIF1αtri-treated groups were also able to improve perfusion recovery from day 7, albeit to a lesser extent. At day 28, the mice receiving pSC-HIF1αtri/L64 treatment yielded the highest reperfusion level (75.2%±9.7%). A similar result was also observed in the pCMV-HIF1αtri/L64 group (71.9%±6.9%). Interestingly, injection of L64 in the absence of the therapeutic pDNA also exhibited a beneficial effect on perfusion recovery, as reflected by the reperfusion values between the L64 (50.5%±7.9%) and saline (42.9%±3.7%) groups at day 28 (P<0.05).
The accelerated blood perfusion recovery rate was possibly due to an improved angiogenesis progress. This was revealed by quantification of capillary density in ischemic limb at day 28. Cluster of differentiation 31 (CD31), also known as platelet endothelial cell adhesion molecule (PECAM-1), is normally found on the surface of endothelial cells, platelets, macrophages and Kupffer cells, etc.32 In immunohistochemistry, CD31 is primarily used to demonstrate the presence of endothelial cells in tissue sections, which can help to evaluate the degree and speed of angiogenesis.33 As shown in Figure 5D, the brownish yellow spots indicated the stained endothelial cells in capillaries. Injection of pSC-HIF1αtri/L64 resulted in a significantly higher muscle capillary density than all other treatments (P<0.05), except the pCMV-HIF1αtri/L64 (Figure 5E). Meanwhile, the L64 group also showed an enhancement in capillary formation when compared with the saline group (P<0.05), consistent with the enhanced reperfusion level shown in Figure 5C.
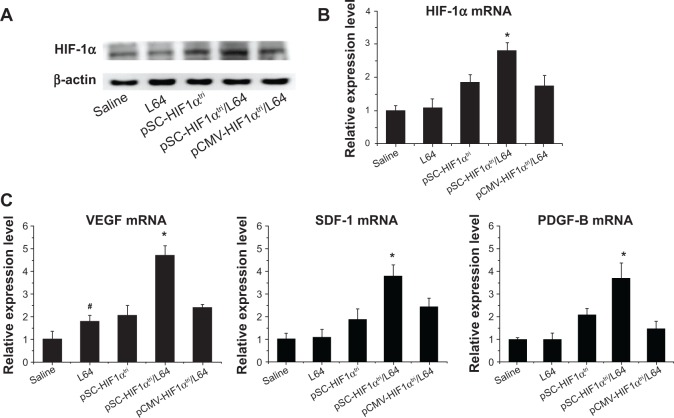
HIF-1α and its target gene expression in mouse ischemic limb
We further investigated the expression of HIF-1α and several cytokines in mouse ischemic limb to get insights into the molecular mechanism by which pSC-HIF1αtri/L64 stimulated blood vessel growth. Western blot analysis showed that pSC-HIF1αtri/L64 treatment conferred higher HIF-1α protein level than others (Figure 6A). Quantitative PCR results further confirmed that the mRNA levels of HIF-1α among groups were consistent with the protein levels (Figure 6B; P<0.05).
Figure 6.
Detection of HIF-1α and induced gene expression.
Notes: Seven days after treatment, the gastrocnemius muscles in mice ischemic hindlimbs were harvested for Western blot and qPCR analysis. (A) Representative Western blot image for HIF-1α protein detection. (B) qPCR analysis of HIF-1α gene expression. *P<0.05 versus all other groups; #P<0.05 versus the saline group. (C) qPCR analysis of HIF-1α induced gene expression. *P<0.05 versus all other groups; #P<0.05 versus the saline group (n=7). Pluronic® L64 (Sigma-Aldrich, St Louis, MO, USA).
Abbreviations: L64, Pluronic® L64; qPCR, quantitative polymerase chain reaction; mRNA, messenger RNA; CMV, cytomegalovirus; SC, CMV promoter/SV40 enhancer; HIF-1αtri, hypoxia-inducible factor 1-alpha triple mutant; VEGF, vascular endothelial growth factor; SDF-1, stromal cell-derived factor; PDGF-B, platelet derived growth factor; SV40E, SV40 enhancer.
The transcriptions of several important angiogenic factors (also known as the targets of HIF-1α) were quantitatively analyzed in the same muscle samples. The mRNA levels of VEGF, SDF-1, and PDGF-B in the pSC-HIF1αtri/L64 group increased 4.6, 3.8, and 3.7 fold, respectively, as compared with mRNA levels in the saline group. They were also significantly higher than those in the treatments without L64 or SV40E (Figure 6C; P<0.05). These data demonstrated the superiority of an integrated pSC-HIF1αtri/L64 system in promoting the target gene expression in muscle, and also provided molecular evidence confirming the blood vessel regeneration.
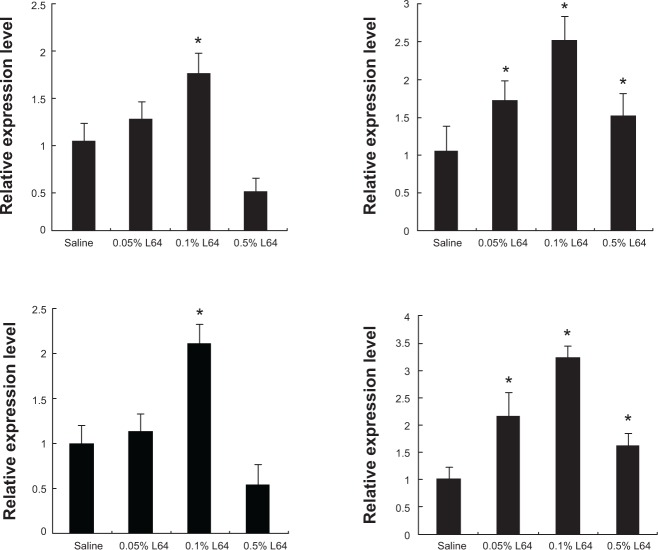
Influence of Pluronic L64 on expression of NF-κB-related angiogenic factors
A notable phenomenon is that the injection of L64 can up-regulate the transcription of VEGF (Figure 6C; P<0.05) but not that of SDF-1α and PDGF-B (P>0.05). Considering that VEGF is one of the important factors in the NF-κB signal pathway and that Pluronics SP107 and P85 activated expression of the luciferase gene driven by promoters containing NF-κB-response elements,10 we were interested in knowing whether L64 can induce the transcriptions of NF-κB-related angiogenic genes. To address this issue, we quantitatively analyzed the transcriptions of many NF-κB-regulated angiogenic genes, including VEGF, IL-6, MMP-2, and MMP-9 in L64-treated mouse TA muscles. The data showed that 0.1% L64 significantly increased the transcription of these four genes compared to 0.9% saline (Figure 7). However, such stimulatory effect of L64 on gene transcription diminished at a higher concentration of 0.5%.
Figure 7.
Expression of NF-κB related angiogenic factors induced by L64.
Notes: The left tibialis anterior (TA) muscles were injected with saline or L64 and separated 3 days later. Expressions of the angiogenic factors were tested using qPCR and presented as the relative values compared with those in the saline groups. (A) mRNA levels of VEGF. (B) mRNA levels of IL-6. (C) mRNA levels of MMP-2. (D) mRNA levels of MMP-9. *P<0.05 versus the saline group. n=6. Pluronic® L64 (Sigma-Aldrich, St Louis, MO, USA).
Abbreviations: NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; L64, Pluronic® L64; VEGF, vascular endothelial growth factor; IL-6, interleukin 6; MMP-2, matrix metallopeptidase 2; MMP-9, matrix metallopeptidase 9; qPCR, quantitative polymerase chain reaction.
Discussion
Intramuscular injection of pDNA to express therapeutic molecules is a promising method for the treatment of many diseases. The most difficult goal in pDNA-based gene therapy is to obtain optimal gene transfer efficiency mediated by non-viral gene delivery materials. Here, we showed that Pluronic L64 is one of the most effective non-viral gene delivery materials for in vivo gene transfer and expression in skeletal muscles (Figure 2A and B). One of the major safety concerns for the in vivo application of gene therapy is the expression of transgene in non-target tissues. In this study, ectopic transgene expression in distal organs was not stimulated by the pDNA/L64 expression system (Figure 2D) and was negligible when compared with that in the target muscle (Figure 2D versus 3B). Moreover, no in situ toxicity (Figure 1D) or general systemic toxicity (Table 2) was observed. It suggested that the pDNA/L64 expression system has a good safety profile and is promising for in vivo application.
It is very interesting to find that 0.1% L64 has an intrinsic ability to increase the blood reperfusion rate (Figure 5C) and capillary density (Figure 5E), as well as the transcription of VEGF in mice ischemic hindlimbs (Figure 6C). These results suggest that L64 can function as more than an inert gene carrier. Although the mechanism by which L64 induces angiogenic response is still unknown, the in vivo data demonstrated that transcriptions of many NF-κB-regulated angiogenic genes, including VEGF, IL-6, MMP-2 and MMP-9, were significantly up-regulated by 0.1% L64 (Figure 7). The diminished effect observed for the higher L64 concentration of 0.5% is possibly due to the activation of a negative feedback loop. Overall, these results provided evidence that activation of the NF-κB signal pathway may be a possible explanation regarding the biological mechanism of L64 on inducing angiogenesis after local muscle injection. NF-κB is a rapid response transcription factor that bears nuclear localization signals (NLSs). Its activation by L64 may, in turn, act at two steps. First, NF-κB can bind pDNA in cytoplasm and bring it into the nucleus through its NLSs. Then, it may further improve transgene expression by stimulating transcription.34
In a previous work on Pluronics P85, reporter gene expression was found to be greatly enhanced in mouse spleen, lymph nodes and, to a lesser extent, liver. This was associated with the activation of certain antigen-presenting cells (APCs) by P85.35 It was suggested that the activated APCs infiltrate into the injection site and carry the transgene to immune organs. This will bring potential risks for gene therapy, especially for angiogenesis cases. The activation of APCs may also reduce the duration of transgene expression.36 For these reasons, Pluronics with strong immune stimulatory effects are less suitable for use as carriers in gene therapy, except in some cases for specific immune-activating purposes. In this study, the data demonstrated that intramuscular injection of L64/pDNA mixture did not increase transgene expression in remote organs. The divergent results obtained from L64 and P85 may be attributed to the different immuno-adjuvant potential of Pluronics, which has been shown to depend on polymer size and molecular arrangement.
It was reported that Pluronics SP107 and P85 had selectivity for promoters containing NF-κB-response elements, such as CMV promoter. The Pluronics had little effect on gene expression driven by promoters without NF-κB-response element, including SV40 promoter, CRE and AP-1 response elements, and the muscle creatine kinase (MCK) promoter.8,10 Our research indicated that L64 worked harmoniously with SV40E/CMV promoter, and the combination of L64 with the hybrid promoter presented three advantages: increasing the gene expression level, elongating the expression duration, and enhancing the number of transfected muscle fibers (Figure 3). It offered a powerful tool for gene delivery and expression in muscle. When the triple mutant HIF-1α gene regulated by SV40E/CMV promoter was delivered into ischemic hindlimbs by L64, HIF-1α mRNA and protein were highly expressed and, accordingly, the expression of HIF1α-induced angiogenic cytokines were also increased (Figure 6). The effects of pSC-HIF1αtri/L64 treatment on mice ischemic hindlimbs were also displayed from different aspects, including functional recovery, visual observation, blood reperfusion, and capillary density (Figure 5). With one exception, however, this treatment did not significantly exhibit better physiological and histological parameters when compared with the pCMV-HIF1αtri/L64 group at day 28 (Figure 5C and E). The diminished superiority may be attributed to a ceiling rate of angiogenesis in ischemic limbs. As is known, angiogenesis depends on the balance between the stimulators and their inhibitors.37 It is conceivable that the continuously regenerated blood vessels may lead to aberrant vascular proliferation.38 Certain inhibitors may offer a counterbalance when the proangiogenic signals reached a concentration threshold and time limitation.39 This phenomenon also supports the notion that HIF-1 regulates the angiogenic progress in a natural and synergistic manner.3
In conclusion, our data demonstrated that pSC-HIF1αtri/L64 is a promising gene medicine for therapeutic angiogenesis of hindlimb ischemia. The significant therapeutic effects of this system rested on the combination of three optimized components: i) Pluronic L64, an effective and safe gene carrier that allowed high levels of therapeutic gene expression in skeletal muscle. ii) Plasmid vector modified with SV40E that increased both the level and duration of transgene expression. iii) Gene encoding stabilized HIF-1α, a master switch capable of activating natural angiogenic responses. Our current work is focused on further optimizing both the gene delivery vehicle and the plasmid construction to permit this gene therapy system to be used in larger animals and humans.
Acknowledgments
This work was supported by the National Basic Research Program of China (Program 973, Number 2011CB606206), National Natural Science Foundation of China (Numbers 31370972, 51133004, and 81361140343), and National Support Program of Science and Technology (Number 2012BAI17B06). We thank Dr Daniel J Peet (University of Adelaide) for his kind gift of plasmids expressing wild type and P564A/N803A double mutant HIF-1α.
Footnotes
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Lu QL, Bou-Gharios G, Partridge TA. Non-viral gene delivery in skeletal muscle: a protein factory. Gene Ther. 2003;10(2):131–142. doi: 10.1038/sj.gt.3301874. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar K, Talbot KF, Steenbergen C, Marce MB, Semenza GL. Adenoviral transfer of HIF-1 enhances vascular responses to critical limb ischemia in diabetic mice. Proc Natl Acad Sci U S A. 2009;106(44):18769–18774. doi: 10.1073/pnas.0910561106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rey S, Lee K, Wang CJ, et al. Synergistic effect of HIF-1alpha gene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia. Proc Natl Acad Sci U S A. 2009;106(48):20399–20404. doi: 10.1073/pnas.0911921106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen DN, Green JJ, Chan JM, Langer R, Anderson DG. Polymeric materials for gene delivery and DNA vaccination. Adv Mater. 2009;21(8):847–867. doi: 10.1002/adma.200801478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song H, Wang G, He B, et al. Cationic lipid-coated PEI/DNA polyplexes with improved efficiency and reduced cytotoxicity for gene delivery into mesenchymal stem cells. Int J Nanomed. 2012;7:4637–4648. doi: 10.2147/IJN.S33923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo K, Li CX, Li L, She WC, Wang G, Gu ZW. Arginine functionalized peptide dendrimers as potential gene delivery vehicles. Biomaterials. 2012;33(19):4917–4927. doi: 10.1016/j.biomaterials.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Dean DA. Nonviral gene transfer to skeletal, smooth, and cardiac muscle in living animals. Am J Physiol Cell Physiol. 2005;289(2):C233–C245. doi: 10.1152/ajpcell.00613.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sriadibhatla S, Yang Z, Gebhart C, Alakhov VY, Kabanov A. Transcriptional activation of gene expression by pluronic block copolymers in stably and transiently transfected cells. Mol Ther. 2006;13(4):804–813. doi: 10.1016/j.ymthe.2005.07.701. [DOI] [PubMed] [Google Scholar]
- 9.Lavigne MD, Pohlschmidt M, Novo JF, et al. Promoter dependence of plasmid-pluronics targeted alpha galactosidase A expression in skeletal muscle of Fabry mice. Mol Ther. 2005;12(5):985–990. doi: 10.1016/j.ymthe.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Zhu J, Sriadibhatla S, Gebhart C, Alakhov V, Kabanov A. Promoter- and strain-selective enhancement of gene expression in a mouse skeletal muscle by a polymer excipient Pluronic P85. J Control Release. 2005;108(2–3):496–512. doi: 10.1016/j.jconrel.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Kabanov AV, Lemieux P, Vinogradov S, Alakhov V. Pluronic block copolymers: novel functional molecules for gene therapy. Adv Drug Deliver Rev. 2002;54(2):223–233. doi: 10.1016/s0169-409x(02)00018-2. [DOI] [PubMed] [Google Scholar]
- 12.Kabanov A, Zhu J, Alakhov V. Pluronic block copolymers for gene delivery. Adv genet. 2005;53:231–261. [PubMed] [Google Scholar]
- 13.Dean DA. Import of plasmid DNA into the nucleus is sequence specific. Exp Cell Res. 1997;230(2):293–302. doi: 10.1006/excr.1996.3427. [DOI] [PubMed] [Google Scholar]
- 14.Zenke M, Grundström T, Matthes H, et al. Multiple sequence motifs are involved in SV40 enhancer function. EMBO J. 1986;5(2):387–397. doi: 10.1002/j.1460-2075.1986.tb04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean DA, Dean BS, Muller S, Smith LC. Sequence requirements for plasmid nuclear import. Exp Cell Res. 1999;253(2):713–722. doi: 10.1006/excr.1999.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atluri P, Woo YJ. Pro-angiogenic cytokines as cardiovascular therapeutics: assessing the potential. Bio Drugs. 2008;22(4):209–222. doi: 10.2165/00063030-200822040-00001. [DOI] [PubMed] [Google Scholar]
- 17.Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103(9):1231–1236. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 19.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shohet RV, Garcia JA. Keeping the engine primed: HIF factors as key regulators of cardiac metabolism and angiogenesis during ischemia. J Mol Med. 2007;85(12):1309–1315. doi: 10.1007/s00109-007-0279-x. [DOI] [PubMed] [Google Scholar]
- 21.Hon WC, Wilson MI, Harlos K, et al. Structural basis for the recognition of hydroxyproline in HIF-1α by pVHL. Nature. 2002;417(6892):975–978. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 22.Min JH, Yang H, Ivan M, Gertler F, Kaelin WG, Jr, Pavletich NP. Structure of an HIF-1α-pVHL complex hydroxyproline recognition in signaling. Science. 2002;296(5574):1886–1889. doi: 10.1126/science.1073440. [DOI] [PubMed] [Google Scholar]
- 23.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40(2):294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15(4):621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 25.Tal R, Shaish A, Rofe K, et al. Endothelial-targeted gene transfer of hypoxia-inducible factor-1alpha augments ischemic neovascularization following systemic administration. Mol Ther. 2008;16(12):1927–1936. doi: 10.1038/mt.2008.191. [DOI] [PubMed] [Google Scholar]
- 26.Rajagopalan S, Olin J, Deitcher S, et al. Use of a constitutively active hypoxia-inducible factor-1alpha transgene as a therapeutic strategy in no-option critical limb ischemia patients: phase I dose-escalation experience. Circulation. 2007;115(10):1234–1243. doi: 10.1161/CIRCULATIONAHA.106.607994. [DOI] [PubMed] [Google Scholar]
- 27.Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind limb ischemia. Nat Protocol. 2009;4(12):1737–1748. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- 28.Luo D, Luo Y, He Y, et al. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. Am J Pathol. 2006;169(5):1886–1898. doi: 10.2353/ajpath.2006.060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G, Cui Y, Zhang G, Garen A, Song X. Regulation of proto-oncogene transcription, cell proliferation, and tumorigenesis in mice by PSF protein and a VL30 noncoding RNA. Proc Natl Acad Sci U S A. 2009;106(39):16794–16798. doi: 10.1073/pnas.0909022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman PJ, Berndt MC, Gorski J, et al. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247(4947):1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 33.Ilan N, Madri JA. PECAM-1: old friend, new partners. Curr Opin Cell Biol. 2004;15(5):515–524. doi: 10.1016/s0955-0674(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 34.Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6(10):1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 35.Gaymalov ZZ, Yang Z, Pisarev VM, Alakhov VY, Kabanov AV. The effect of the nonionic block copolymer pluronic P85 on gene expression in mouse muscle and antigen-presenting cells. Biomaterials. 2009;30(6):1232–1245. doi: 10.1016/j.biomaterials.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato Y, Roman M, Tighe H, et al. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273(5273):352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 37.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 38.Simons M. Angiogenesis: where do we stand now? Circulation. 2005;111(12):1556–1566. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- 39.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]