Abstract
Background
Lipoprotein abnormalities are associated with a rapid decline in renal function in patients of chronic kidney disease. In addition, hyperlipidemia is associated with an increased risk of developing renal insufficiency. The underlying molecular mechanisms for these clinical findings are unclear. We have previously reported a role for inhibitor of differentiation 3 (ID3), a transcription factor, in regulating kidney disease in hyperlipidemia. Introducing a genetic deficiency of Id3 in spontaneously hyperlipidemic Apolipoprotein E knockout (Apoe-/-) mice led to accelerated mesangio-proliferative glomerulonephritis. The present study was carried out to further investigate the contribution of ID3 in hyperlipidemia associated kidney disease.
Methods
Female C57BL/6 mice that were ID3 sufficient wild type (WT) or ID3 deficient (Id3-/-) were fed a western diet and evaluated for proteinuria, glomerular pathology and immune infiltrating cells. Primary mesangial cell lines were generated from both mouse strains and stimulated with oxidized phospholipids. Cytokines and chemokines produced were measured by multiplex assays, ELISA, and QPCR. Glomerular isolates were studied for CXCL1 expression by QPCR.
Results
Id3-/- mice on a western diet developed accelerated proteinuria and mesangio-proliferative glomerulonephritis compared to WT controls. In vitro, Id3-/- glomerular mesangial cell lines produced higher levels of the monocyte chemoattractant, CXCL1 in response to oxidized phospholipids. This was consistent with the rapid increase in glomerular CXCL1 expression followed by macrophage infiltration in Id3-/- mice fed a western diet.
Conclusions
A functional ID3 influences susceptibility to kidney disease and prevents glomerular injury by regulating local chemokine production and inflammatory cell recruitment.
Introduction
The association between hyperlipidemia and progression of chronic kidney disease is well established [1]. Clinical studies show that individuals with elevated cholesterol levels are at a higher risk of developing kidney disease [2, 3]. These studies, and data from mouse models suggest that the development of kidney disease in the presence of hyerplipidemia may be influenced by additional factors [4]. We have shown that introducing a deficiency of inhibitor of differentiation 3 (ID3) in spontaneously hyperlipidemic Apolipoprotein E deficient (Apoe-/-) mice leads to accelerated atherosclerosis [5] and mesangio-proliferative glomerulonephritis (GN) [6]. In addition to causing hyperlipidemia, Apolipoprotein E deficiency also directly affects mesangial cell function [4, 18]. Therefore, to delineate the role of ID3 on hyperlipidemia associated kidney disease, without the effect of Apolipoprotein E deficiency, female WT and Id3-/- mice were fed an atherogenic western diet (TD.88137, 42% calories from fat) to cause diet induced hyperlipidemia. Our results suggest that the lack of ID3 facilitates the development of kidney disease, and a functional ID3 is reno-protective.
Inhibitor of differentiation 3 (ID3) is a broadly expressed transcription factor and belongs to the helix-loop-helix (HLH) family of proteins [7]. ID3 forms hetero-dimers with basic HLH transcription factors like E-proteins through the protein binding HLH domains. However, ID3 lacks the DNA binding domain and therefore, ID3-E-protein hetero-dimers fail to bind DNA. Thus, ID3 is a dominant negative regulator of transcription in multiple cell types including smooth muscle cells [7] immune cells [8], and adipocytes [9].
Oxidized phospholipids are increased in hyperlipidemic states and induce proinflammatory chemokines and cytokines in glomerular cells [10]. We hypothesized that production of inflammatory mediators is exacerbated in ID3 deficiency, resulting in recruitment of immune cells into the glomeruli. This hypothesis was tested using ID3 sufficient (WT) and ID3 deficient (Id3-/-) mice.
Methods
All methodologies are described in details in the supplementary materials.
Mice
All procedures followed NIH guidelines for humane use of animals and were approved by the Institutional Animal Care and Use Committee. C57BL/6 wild type (WT) and Id3-/- mice [5] were fed either western diet (TD.88137, Harlan-Teklad) or mouse chow (TD 7012) starting at 6-8wks of age. Urinary albumin and creatinine levels and lipid profile analyses were measured as previously described [6].
Evaluation of renal pathology
Mice were sacrificed after either 8wks or 15wks on diet and kidneys were studied for renal pathology, fibronectin deposition, and immune cell infiltration [6, 12-14].
Treatment of mesangial cells with oxidized phospholipids
Primary mesangial cell lines were generated from glomerular isolates [15] and used after 5th passage. Cells were stimulated with oxidized phospholipids, Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (oxPAPC), the active principle of minimally modified LDL [16]. Supernatants were collected and chemokines and cytokines were measured using the Mouse Grp1 23-plex and Grp2 9-plex Bio-Plex suspension array system (Bio-Rad). CXCL1 levels were determined by ELISA (R&D systems) for repeat experiments.
Gene expression analyses in mesangial cell lines and isolated glomeruli
CXCL1 expression in mesangial cells and isolated glomeruli were carried out as previously described [17, 18]. Data are represented as fold change over untreated B6 mesangial cell cDNA. GAPDH was used as a housekeeping gene control. For glomerular gene expression, results are presented as relative expression using GAPDH as a housekeeping control.
Statistical Analyses
Statistical analyses were carried out using one way ANOVA with Bonferroni correction for multiple comparisons, two way ANOVA, and Mann Whitney test using Graph Pad Prism 3.0 software.
Results
Diet induced hyperlipidemia leads to GN and proteinuria in Id3-/- mice
Both strains, WT and Id3-/- were fed a western diet for 8 wks and showed significant increases in weight, serum LDL, HDL, and total cholesterol compared to chow fed controls (Supplementary Table 1). Neither strain showed changes in blood glucose levels, in concordance with previous reports that female mice on high fat diets are resistant to metabolic syndrome-like disease [19]. Despite this, Id3-/- females developed significant proteinuria and GN after 8wks on the western diet (Figure. 1a-c). In contrast, WT mice on a western diet developed little proteinuria and showed mild glomerular pathology. Quantitative analyses showed a significant increase in glomerular area staining for fibronectin, an indicator of mesangial activation and mesangio-proliferative GN in Id3-/- mice (Figure 1d, and Supplementary Table 2). Representative images of glomerular pathology and fibronectin deposition are shown in Figure 2. In an additional cohort of mice sacrificed after 15wks of feeding, Id3-/- mice on the western diet showed further progression of mesangial expansion, inflammatory cell infiltration, and glomerulosclerosis (mean severity score 2.37±0.37; n=6) compared to WT mice (mean severity score 0.73±0.030; n=4; p=0.0079). WT and Id3-/- mice fed mouse chow did not develop kidney disease. Thus, diet induced hyperlipidemia resulted in mesangio-proliferative GN in Id3-/- mice.
Figure 1. Id3-/- female mice develop kidney disease after 8 wks on a western diet.
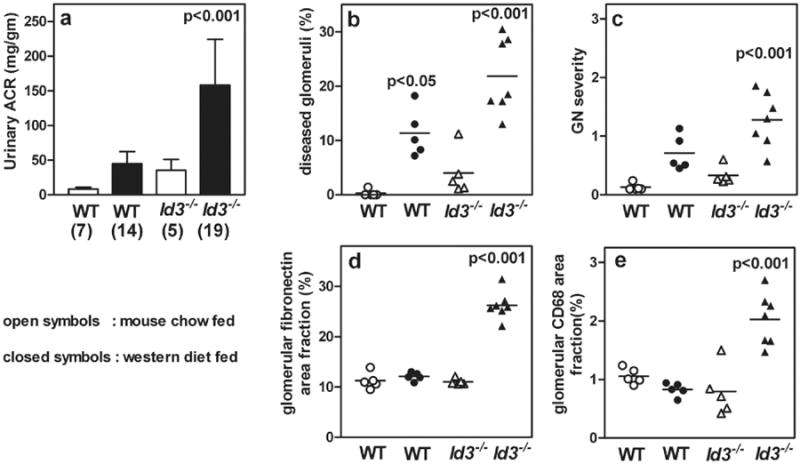
WT or Id3-/- female mice were fed regular mouse chow (open symbols) or western diet (closed symbols). Number of mice in each group is indicated in parenthesis. Urine samples (24 hr) were analyzed for urinary albumin to creatinine ratios (ACR) (a). PAS stained kidney sections were studied for frequency of glomeruli affected (b) and severity of GN was scored (c). Kidney sections were stained by immunofluorescence for fibronectin deposition and results show percent glomerular area positive for fibronectin (d). Inflammatory cell infiltration in the glomeruli was quantified by measuring glomerular area staining positive for CD68+ macrophages (e). Each data point represents one mouse. Statistical analyses by ANOVA with Bonferroni post test for multiple comparisons. p values indicate statistical significance compared with wild type controls. Detailed methodology and results of stereologic analyses for fibronectin and CD68 positive staining are presented in Supplementary Table 2.
Figure 2. Id3-/- females fed a western diet develop GN, glomerular fibronectin deposits and glomerular infiltration by activated macrophages.

Representative photomicrographs of paraffin embedded PAS stained kidney sections showing glomeruli from WT and Id3-/- female mice fed chow or western diets for 8 wks (a-d). Immunostaining for fibronectin deposits (e-h) showing increased glomerular fibronectin in western fed Id3-/- mice. Immunofluorescence microscopy shows macrophages CD68 (red) and MHCII (green) in periglomerular areas indicated by arrows and intra-glomerular shown by arrowheads (i-l). Nuclei (e-l) are stained blue with DAPI. Scale bar is 20 microns.
Glomerular pathology is associated with macrophage infiltration
To identify inflammatory cell infiltrates, kidney sections were stained with antibodies to T cells (CD3, CD4), B cells (B220), dendritic cells (CD11c) and macrophages (CD68 and F4/80). Macrophages were the predominant infiltrating cells seen in mice with GN. A quantitative analysis of CD68 positive cells within glomeruli showed a significant increase in Id3-/- mice on a western diet (Figure 1e, supplementary table 2). The CD68 cells were activated as indicated by co-expression of MHC class II (Figure 2). Glomerular infiltrates of CD68 cells were infrequent in the other mouse groups. After 15wks of western diet, increased renal pathology was associated with a further increase in glomerular CD68+ infiltration in Id3-/- mice (6.13+0.18; n=6) compared to WT mice (3.22+0.72; n=5; p<0.001). There was no increase in T, B, or dendritic cell infiltration in either group (data not shown). Neutrophils were not seen infiltrating the glomeruli in any of the groups. The pathology was restricted predominantly to the glomerular and peri-glomerular regions with little involvement of the tubulointerstitial regions.
Id3 regulates mesangial cell responses to oxidized phospholipids
To investigate the potential mechanism of mesangio-proliferative GN induced by ID3 deficiency, we established primary mesangial cell lines from WT and Id3-/- mice. The mesangial cell lines were checked for purity by staining cells with a panel of antibody reagents reacting with mesangial, epithelial, endothelial, podocyte, and fibroblast markers and were used after the 5th passage. All cell lines were >90% pure (Supplementary figure 1). Oxidized and minimally modified LDL are taken up by mesangial cells through the scavenger receptors and are known to modulate mesangial cell function [20]. The Id3+/+ and Id3-/- mesangial cells were stimulated with oxPAPC, an inflammatory oxidized phospholipid and an active principle of minimally modified LDL [16]. Supernatants collected 24 hrs later were analyzed for chemokines and cytokines using the Mouse Grp I 23-plex and Grp II 9-plex Bio-Plex suspension array system (BioRad). Of the 32 cytokines and chemokines studied, Id3-/- mesangial cells showed higher basal CXCL1 production than WT cells. Other chemokines detected include MCSF, PDGFbb and VEGFa, and they were comparable in both Id3-/- and WT cells (supplementary figure 2). Higher CXCL1 production by Id3-/- mesangial cells in response to oxPAPC was confirmed by ELISA using another set of independently generated WT and Id3-/- cell lines and the results are shown in Figure 3a. CXCL1 production in supernatant was associated with a corresponding increase in CXCL1 gene expression and mRNA levels (Figure 3b). CXCL1 is a neutrophil and monocyte chemoattractant and has been identified as the earliest chemokine critical for arrest of circulating monocytes at the site of endothelial injury [21, 22]. In the kidney, CXCL1 production in the glomeruli has been implicated in inflammatory cell recruitment in immune complex mediated glomerulonephritis [23]. This supports our hypothesis that ID3 regulates local responses to pro-inflammatory lipids and ID3 deficiency exacerbates recruitment of macrophages into the renal glomeruli.
Figure 3. oxPAPC induces CXCL1 in Id3-/- mesangial cells.
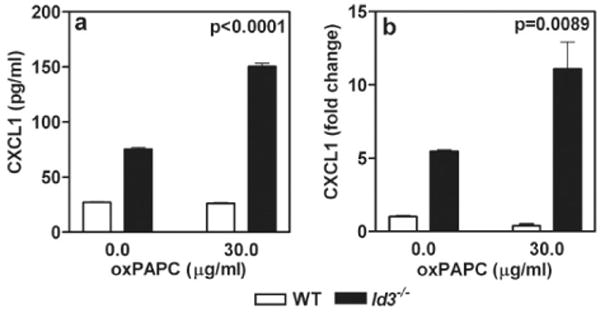
Primary mesangial cells (5×104cells/well) were incubated in RPMI medium with 0.5% low lipid fetal calf serum for 24 hrs, followed by incubation with oxPAPC (30μg/ml) for another 24 hrs. CXCL1 measured by ELISA in supernatants of cultured mesangial cells from Id3-/- and WT mice are shown (a). CXCL1 mRNA levels in the same cells were measured by QPCR (b). Similar results were obtained in additional experiments with two sets of independently generated primary mesangial cell lines from WT and Id3-/- mice. p values calculated using two way ANOVA using graph pad prism 3.0
A rapid upregulation of glomerular CXCL1in Id3-/- mice on a western diet
To investigate whether hyperlipidemia affected local CXCL1 production in vivo, WT and Id3-/- mice were fed mouse chow or a western diet and CXCL1 expression in isolated glomeruli was studied 2 wks later. A significant increase in glomerular CXCL1 expression was seen in Id3-/- mice on a western diet (0.1172+0.024; n=5) compared to chow fed Id3-/- mice (0.050+0.023; n=4;) (p=0.032 by Mann Whitney test). Glomerular CXCL1 expression was not different between WT mice fed regular chow (0.067+0.04; n=3) or western diet (0.060+0.04; n=3). These data are consistent with the mesangial cell results showing that ID3 deficiency is associated with CXCL1 upregulation in response to inflammatory lipids.
Discussion
In the present study, we have recapitulated the salient characteristics of kidney disease previously reported in Apoe-/-Id3-/- double knockout mice; and show that ID3 per se, in hyperlipidemic mice directly influences susceptibility to kidney disease. ID3 deficiency may exacerbate CXCL1 production by glomerular cells in response to inflammatory lipids and the resulting macrophage recruitment. Since Id3 is present in multiple cell types, it is also possible that other glomerular cells lacking Id3 may contribute to cytokine production in vivo. Thus, the reno-protective effect of ID3 may be through regulation of local chemokine production. ID3 is known to directly interact with more than 30 different transcription factors [24]. Clearly, a change in the ID3 function may impact a wide range of protein-protein interactions with potentially significant consequences.
In contrast to our findings in Apoe-/-Id3-/- mice with glomerulonephritis, the hyperlipidemic Id3-/- mice did not show significant increase in glomerular immune complex deposition compared to hyperlipidemic WT mice. Apoliprotein E deficiency is known to cause increased immune responsiveness [25] and these results add significance to the present study in dissecting the effects of ID3 alone on kidney disease. Clinical studies provide evidence for the relationship between lipids and chronic kidney disease. However, they fail to explain why certain individuals (in the absence of diabetes or metabolic syndrome) are likely to develop chronic kidney disease [3]. In humans, a single nucleotide polymorphism (SNP) in ID3 is associated with increased susceptibility to atherosclerosis [5]. Our preliminary findings in humans suggest a significant association between the same ID3 SNP and proteinuria, specifically influenced by small low density lipoproteins (p=0.0024) (Nackiewicz et al., Arthritis Rheum 2013; 65:S240 Abstract #552).
C57BL/6 male mice on high fat diets (60% calorie from fat) develop metabolic syndrome associated with obesity, elevated plasma glucose, proteinuria and GN and this may be due to decrease in renal AMP activated protein kinase, a cellular energy sensor [26]. However, C57BL/6 female mice on high fat diets develop GN and proteinuria only in the absence of ID3 suggesting distinct pathogenic mechanisms between females and males.
Investigating molecular mechanisms in mice has identified ID3 as a novel transcription factor that may contribute to kidney disease and provide mechanistic links between atherosclerosis, hyperlipidemia and kidney disease in humans.
Supplementary Material
Acknowledgments
The studies were supported by Grant-In-Aid from the National Kidney Foundation DC Metro Area (HB), Center for Immunity, Inflammation and Regenerative Medicine and Division of Nephrology Research Support (HB), R01 AI079621, and R21 DE022977 (USD). OxPAPC was a generous gift from Dr. Norbert Leitinger, Department of Pharmacology, University of Virginia.
Footnotes
Disclosures: The authors have not conflict of interest to disclose.
References
- 1.Samuelsson O, Mulec H, Knight-Gibson C, Attman PO, Kron B, Larsson R, Weiss L, Wedel H, Alaupovic P. Lipoprotein abnormalities are associated with increased rate of progression of human chronic renal insufficiency. Nephrol Dial Transplant. 1997;12:1908–1915. doi: 10.1093/ndt/12.9.1908. [DOI] [PubMed] [Google Scholar]
- 2.Schaeffner ES, Kurth T, Curhan GC, Glynn RJ, Rexrode KM, Baigent C, Buring JE, Gaziano JM. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003;14:2084–2091. doi: 10.1681/ASN.V1482084. [DOI] [PubMed] [Google Scholar]
- 3.Hsu CY, Bates DW, Kuperman GJ, Curhan GC. Diabetes, hemoglobin A(1c),cholesterol, and the risk of moderate chronic renal insufficiency in an ambulatory population. Am J Kidney Dis. 2000;36:272–281. doi: 10.1053/ajkd.2000.8971. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Paka L, Kako Y, Singhal P, Duan W, Pillarisetti S. A protective role for kidney apolipoprotein E. Regulation of mesangial cell proliferation and matrix expansion. J Biol Chem. 2001;276:49142–49147. doi: 10.1074/jbc.M104879200. [DOI] [PubMed] [Google Scholar]
- 5.Doran AC, Lehtinen AB, Meller N, Lipinski MJ, Slayton RP, Oldham SN, Skaflen MD, Yeboah J, Rich SS, Bowden DW, McNamara CA. Id3 is a novel atheroprotective factor containing a functionally significant single-nucleotide polymorphism associated with intima-media thickness in humans. Circ Res. 2010;106:1303–1311. doi: 10.1161/CIRCRESAHA.109.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagavant H, Scindia Y, Nackiewicz D, Nandula SR, Doran A, Cutchins A, Oldham S, Deshmukh U, McNamara C. Deficiency of a transcriptional regulator, inhibitor of differentiation 3, induces glomerulonephritis in apolipoprotein E-deficient mice: a model linking hyperlipidemia and renal disease. Am J Pathol. 2011;179:651–660. doi: 10.1016/j.ajpath.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen M, Segerer S, Dantas M, Brown PA, Hudkins KL, Goodpaster T, Kirk E, LeBoeuf RC, Alpers CE. Renal injury in apolipoprotein E-deficient mice. Lab Invest. 2002;82:999–1006. doi: 10.1097/01.lab.0000022222.03120.d4. [DOI] [PubMed] [Google Scholar]
- 8.Forrest S, McNamara C. Id family of transcription factors and vascular lesion formation. Arterioscler Thromb Vasc Biol. 2004;24:2014–2020. doi: 10.1161/01.ATV.0000143932.03151.ad. [DOI] [PubMed] [Google Scholar]
- 9.Kee BL. E and ID proteins branch out. Nat Rev Immunol. 2009;9:175–184. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- 10.Doran AC, Meller N, Cutchins A, Deliri H, Slayton RP, Oldham SN, Kim JB, Keller SR, McNamara CA. The helix-loop-helix factors Id3 and E47 are novel regulators of adiponectin. Circ Res. 2008;103:624–634. doi: 10.1161/CIRCRESAHA.108.175893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamanna VS, Pai R, Roh DD, Kirschenbaum MA. Oxidative modification of low-density lipoprotein enhances the murine mesangial cell cytokines associated with monocyte migration, differentiation, and proliferation. Lab Invest. 1996;74(6):1067–79. [PubMed] [Google Scholar]
- 12.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002 Sep;161(3):799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagavant H, Deshmukh US, Wang H, Ly T, Fu SM. Role for nephritogenic T cells in lupus glomerulonephritis: progression to renal failure is accompanied by T cell activation and expansion in regional lymph nodes. J Immunol. 2006;177:8258–8265. doi: 10.4049/jimmunol.177.11.8258. [DOI] [PubMed] [Google Scholar]
- 14.Glaser JR, Glaser EM. Stereology, morphometry and mapping: the whole is greater than the sum of the parts. J Chem Neuroanat. 2000;20:115–126. doi: 10.1016/s0891-0618(00)00073-9. [DOI] [PubMed] [Google Scholar]
- 15.Ishiyama A, Agena J, Lopez I, Tang Y. Unbiased stereological quantification of neurons in the human spiral ganglion. PNeuroscience Lett. 2001;304:93–96. doi: 10.1016/s0304-3940(01)01774-8. [DOI] [PubMed] [Google Scholar]
- 16.Mene P, Stoppaciaro A. Isolation and propogation of glomerular mesangial cells. In: Hewitson, Becker, editors. Kidney research Experimental protocols. Humana Press; New York, NY: 2009. pp. 1–17. [Google Scholar]
- 17.Watson AD, Leitinger N, Navab M, Faull KF, Hörkkö S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte / endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 18.Deshmukh US, Nandula SR, Thimmalapura PR, Scindia YM, Bagavant H. Activation of innate immune responses through Toll-like receptor 3 causes a rapid loss of salivary gland function. J Oral Pathol Med. 2009;38:42–47. doi: 10.1111/j.1600-0714.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30:396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varghese Z, Fernando R, Moorhead JF, Powis SH, Ruan XZ. Effects of sirolimus on mesangial cell cholesterol homeostasis: a novel mechanism for its action against lipid-mediated injury in renal allografts. Am J Physiol Renal Physiol. 2005;289:F43–F48. doi: 10.1152/ajprenal.00181.2004. [DOI] [PubMed] [Google Scholar]
- 21.Boisvert WA, Rose DM, Johnson KA, Fuentes ME, Lira SA, Curtiss LK, Terkeltaub RA. Up-regulated expression of the CXCR2 ligand KC/GRO-alpha in atherosclerotic lesions plays a central role in macrophage accumulation and lesion progression. Am J Pathol. 2006;168:1385–1395. doi: 10.2353/ajpath.2006.040748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huo Y, Weber C, Forlow SB, Sperandio M, Thatte J, Mack M, Jung S, Littman DR, Ley K. The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J Clin Invest. 2001;108:1307–1314. doi: 10.1172/JCI12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo Y, Lloyd C, Gutierrez-Ramos JC, Dorf ME. Chemokine amplification in mesangial cells. J Immunol. 1999;163(7):3985–3992. [PubMed] [Google Scholar]
- 24.Lynn DJ, Chan C, Naseer M, Yau M, Lo R, Sribnaia A, Ring G, Que J, Wee K, Winsor GL, Laird MR, Breuer K, Foroushani AK, Brinkman FS, Hancock RE. Curating the innate immunity interactome. BMC Syst Biol. 2010;4:117. doi: 10.1186/1752-0509-4-117. [ http://www.innatedb.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tenger C, Zhou X. Apolipoprotein E modulates immune activation by acting on the antigen-presenting cell. Immunology. 2003;109(3):392–397. doi: 10.1046/j.1365-2567.2003.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Declèves AE, Mathew AV, Cunard R, Sharma K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol. 2011;22:1846–1855. doi: 10.1681/ASN.2011010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


