Abstract
In the Drosophila oogenesis, germline stem cells (GSCs) continuously self-renew and differentiate into daughter cells for consecutive germline lineage commitment. This developmental process has become an in vivo working platform for studying adult stem cell fate regulation. An increasing number of studies have shown that while concerted actions of extrinsic signals from the niche and intrinsic regulatory machineries control GSC self-renewal and germline differentiation, epigenetic regulation is implicated in the process. Here, we report that Brahma (Brm), the ATPase subunit of the Drosophila SWI/SNF chromatin-remodeling complexes, is required for maintaining GSC fate. Removal or knockdown of Brm function in either germline or niche cells causes a GSC loss, but does not disrupt normal germline differentiation within the germarium evidenced at the molecular and morphological levels. There are two Drosophila SWI/SNF complexes: the Brm-associated protein (BAP) complex and the polybromo-containing BAP (PBAP) complex. More genetic studies reveal that mutations in polybromo/bap180, rather than gene encoding Osa, the BAP complex-specific subunit, elicit a defect in GSC maintenance reminiscent of the brm mutant phenotype. Further genetic interaction test suggests a functional association between brm and polybromo in controlling GSC self-renewal. Taken together, studies in this paper provide the first demonstration that Brm in the form of the PBAP complex functions in the GSC fate regulation.
Introduction
Drosophila oogenesis begins with the asymmetric division of the germline stem cells (GSCs) at the anterior tip of the germarium in the ovary [1]. This division produces one daughter cell retaining the stem cell identity, and another differentiating progeny called cystoblast (CB). Each CB subsequently proceeds with four incomplete mitotic divisions to consecutively form interconnected 2-cell, 4-cell, 8-cell and 16-cell germline cysts. Within the 16-cell cyst, only one germ cell differentiates as oocyte, whereas the remaining 15 become supportive nurse cells [2]. After encapsulated by a monolayer of epithelial follicle cells, the cyst moves out of the germarium to form an egg chamber [3]. Continuous generation of self-renewing GSCs and their differentiating descendant cells for the cyst development are essential for fertility throughout the female fly's lifetime.
In the oogenesis, GSC cell fate is maintained by both extrinsic signals from the niche and intrinsic regulatory machineries. Cap cells (CpCs) in the niche produce BMP-like signal molecule Dpp for activating BMP signaling pathway in GSCs. Active BMP signaling maintains GSC fate by repressing differentiation via transcriptional silence of the differentiation promoting gene, bag-of-marbles (bam) [4], [5], [6], [7], [8], [9], [10]. In addition to the BMP/Bam pathway, the Nano/Pumilio complex and miRNA pathway are cell-autonomously required for GSC maintenance [11], [12], [13], [14], [15], [16], [17]. Likewise, regulation of stepwise germline differentiation derived from GSCs within the germarium involves intrinsic and extrinsic mechanisms. In the case of cell autonomous mode, a number of molecular markers such as Sex lethal, Bam, Nanos, A2BP1, Bruno and Orb have been identified as key regulators for specific transitions within the differentiation process [4], [12], [18], [19], [20], [21]. Recently, Escort cells (ECs) physically interacting with differentiating germ cells in the germarium were found to promote germline differentiation in a non-cell autonomous manner through restricting BMP signaling inside the GSC niche [22], [23]. Both GSCs and germline cyst development in the germarium have become in vivo working platforms for addressing how adult stem cell fate and stem cell-derived cell lineage commitment are regulated [1].
A growing number of evidences have indicated that GSC fate regulation can also occur at epigenetic level. We and others have identified a number of epigenetic factors involving histone modification or chromatin remodeling as regulators for GSC maintenance and germ cell differentiation [24], [25], . In the present study, we extended the investigation to the Drosophila SWI/SNF chromatin-remodeling complex. There exists two subtypes of the SWI/SNF complexes in Drosophila: the Brahma (Brm)-associated protein (BAP) complex and the polybromo-containing BAP (PBAP) complex [34], [35]. Genetic studies revealed that Brm, the ATPase subunit of the SWI/SNF complexes, is required intrinsically and extrinsically for maintaining GSCs, but not for germline differentiation within the germarium. Mutations in gene polybromo/bap180, rather than osa, caused a defect in GSC maintenance reminiscent of the brm mutant phenotype. We further showed a genetic interaction of brm with polybromo/bap180 in sustaining the GSC population. Thus, we propose that Brm acts in the form of the PBAP complex to control GSC self-renewal in the Drosophila oogenesis.
Material and Methods
Drosophila stocks and genetics
All Drosophila strains were maintained and crossed at 25°C unless otherwise stated. The following fly stocks were used in this study:
Canton S (CS) and w1118 strain was used as wild type.
Mutant alleles and transgene: brmT362 [36], UAS-brmK804R [37], bap180Δ86 [38] (from Jessica E. Treisman), brm2, osa308, osa2, hsFlp;FRT80B,ubi-GFP (Bloomington Drosophila Stock Center, BDSC), yw122;FRT82B,ubi-GFP (gift from Zhao-hui Wang).
RNAi: UAS-brm-RNAi-B35211, UAS-brm-RNAi-B34520, UAS-bap180-RNAi-B32840, UAS-bap170-RNAi-B26308 (BDSC), UAS-bap180-RNAi-V108618 (Vienna Drosophila RNAi Center, VDRC). The on-target effects of the above RNAi transgenes were molecularly validated based on the RT-PCR quantative assay (Figure S2)
Gal4/UAS: nos-Gal4.NGT, bab1-Gal4 and tubP-Gal80ts (BDSC), c587-Gal4 [39] (gift from Yu Cai)
Mosaic clones were generated by mitotic recombination using FLP/FRT system. To generate GSC or germline cyst clones mutant for brm or bap180, hsFLP; FRT80B ubiGFP was crossed to FRT80B, FRT80B brmT362, hsFLP; FRT82B ubiGFP was crossed to FRT82B, FRT82B bap180Δ86. Two-day-old female adult progenies with appropriate genotype (hsFLP; FRT80B ubiGFP/FRT80B brmT362 or hsFLP; FRT82B ubiGFP/FRT82B bap180Δ86) were heat-shocked at 37°C twice a day on three consecutive days for one hour each time. Meanwhile, the progenies from the cross of hsFLP; FRT80B ubiGFP and FRT80B or hsFLP; FRT82B ubiGFP and FRT82B were used as FRT controls respectively. Ovaries were then dissected at day 2, 7,14 and 21 after the last heat-shock treatment for the clonal analysis. The FRT clones were identified by the absence of GFP expression.
RNAi-based knockdown experiments were performed by Gal4/UAS binary system [40]. For the spatial-temporally controlled study, the RNAi transgenic line was crossed to tubP-Gal80ts and bab1-Gal4. The females were raised at 18°C until 2 days after eclosion and then shifted to 29°C for a number of days.
Antibodies and immunofluorescence
Antibody staining was carried out as described previously [41]. The following primary antibodies were used: guinea pig anti-Brm (1∶1000, from Feng Tie) [42], mouse anti- α -Spec (1∶20, DSHB 3A9(323 or M10-2)), mouse anti-Bam (1∶5, DSHB Fly Bag-of-Marbles), rabbit anti-Nanos (1∶1000, from Akira Nakamura), mouse anti-Sxl (DSHB, M114), guinea pig anti-A2BP1 (from Michael Buszczak) [19], mouse anti-Orb (DSHB, orb 4H8), rabbit anti-Bruno (1∶1000, from Mary A. Lilly) [43], rabbit anti-pMad (1∶2000 gift from E. Laufer) [44], rabbit anti-Vasa (1∶200, Santa Cruz sc-30210). Secondary antibodies conjugated with Alexa Fluor 488, 546 (Invitrogen) were used at 1∶1000 dilutions. DAPI (Invitrogen) was used to visualize nuclei.
Confocal images were captured on Leica TCS SP5 laser confocal microscope.
GSC and UGC identification and statistical analysis
GSCs were identified by the presence of a spectrosome anchored to the CpC contact site. The spectrosome-containing single germ cells in the germarium which are located away from terminal filaments and cap cells were classified as UGCs.
Student's t test and Mann–Whitney test were chosen to calculate p-values.
Results and Discussion
The polybromo-containing BAP (PBAP) complex is required intrinsically for GSC maintenance in the Drosophila ovary
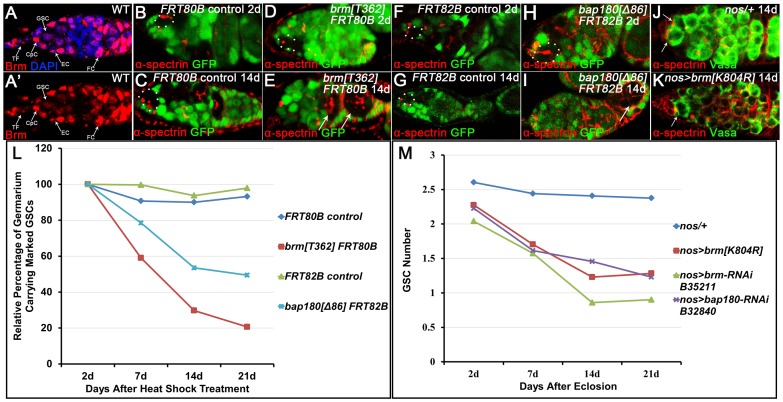
Recent studies have shown that the BAP complex, one of the Drosophila SWI/SNF chromatin-remodeling complexes, regulates stem cell lineage commitment and stem cell proliferation in the adult Drosophila intestine [45], [46]. These findings prompted us to investigate if the Drosophila SWI/SNF complexes function in regulating the GSC fate in the ovary. For this purpose, we first examined whether Brm, the ATPase subunit of the Drosophila SWI/SNF complexes, has a role in GSC maintenance. The immunofluorescence assay using anti-Brm serum revealed that brm is ubiquitously expressed in almost all cell types in the wild type germaria including GSCs, the niche and follicle cells (Figure 1A and A′). To determine if loss-of-function mutations in brm perturb GSC self-renewal, we performed a clonal analysis in which GSC clones homozygous for either the mutant allele of brm or wild type control are generated by FLP/FRT-based mitotic recombination. The GSC clones can be identified by loss of GFP expression and the presence of an anteriorly anchored spectrosome in the germaria (Figure 1B–D). Statistic analysis showed that in contrast to that of the controls, the rate of GSC clones homozygous for brmT362, a null mutant allele with embryonic lethality, declines rapidly in a 21-day time course after clonal induction (ACI) (Figure 1L), suggesting a cell-autonomous role of Brm in sustaining the GSC population. To validate this observation, we knocked down brm in the germline by expressing either UAS-brm RNAi (B35211 from Bloomington Drosophila Stock Center, BDSC) or UAS-brmK804R, a dominant negative form of brm transgene under the control of nos-Gal4 driver. As depicted in figure 1M, reduced expression of brm in the germline caused a marked decrease in GSC number per germarium during three weeks after fly eclosion. Combined with the clonal analysis above, the Gal4/UAS binary system-based assay indicates that Brm is required intrinsically for maintaining GSCs.
Figure 1. Mutation or reduced expression of brm or polybromo/bap180 in the germline causes a defective GSC maintenance.
(A, A′) In the wild type germarium, brm is ubiquitously expressed in almost all cell types, predominantly in TFs, CpCs, ECs and follicle cells (FCs). (B–I) Germaria with the control (B, C, F, G) or brmT362 (D, E) or bap180Δ86 (H, I) mutant GSC clones (broken circles) marked by the absence of GFP and the presence of an anteriorly anchored spectrosome (α-spectrin staining). In the wild type controls, marked GSCs are evident at 2 days and 14 days ACI (B, C, F, G). Conversely, marked GSCs mutant for brm (D) or bap180 (H) are only detected at 2 days ACI, but lost at 14 days ACI (E, I). Instead, the mutant cyst clones are present in the germaria (arrows in E and I). (J, K) The control (J) and brm knockdown (K) germarium stained for α-spectrin and Vasa. While two GSCs are present in the control germarium, the mutant one contains only one GSC. GSCs are indicated by arrows. (L) Graph showing the relative percentage of germaria containing marked wild type control or brm or bap180 mutant GSCs over a 3-week period ACI. Note that all initial percentages at day 2 ACI are normalized to 100%. (M) Graph showing that a gradual GSC loss is elicited by knocking down either brm or bap180 in the germline.
It is established that Brm functions in the form of either BAP or PBAP complex. We then sought to characterize the Brm-containing SWI/SNF complex in the context of GSC maintenance. To define the complex subtype functioning in the ovary, we first tested Osa, the BAP complex-specific subunit, for a potential role in maintaining GSCs. As adult escapers of female flies transheterozygous for osa2/osa308 can be collected for an assay, we examined if loss of osa function impairs GSC self-renewal by directly counting GSC number at different time points after fly eclosion. Clearly, osa2/osa308 females had a relatively constant number of GSCs per germarium with a 14 day test period, similar to the wild type counterparts (Table 1). Thus, these results do not support a possibility that Brm in the form of the BAP complex is involved in sustaining the GSC population. Polybromo/Bap180 is a signature subunit of the PBAP complex. To test the alternative that the PBAP, rather than BAP complex is required for GSC maintenance, we then analyzed how polybromo mutant GSCs are maintained using the clonal analysis described above (Figure 1F–I). As shown in figure 1L, the rate of GSC clones mutant for bap180Δ86, a null allele of polybromo, remarkedly decreased in a 21 day period ACI. Similarly, a gradual GSC loss was observed in a RNAi-based knock down experiment (RNAi transgenic line B32840 from the BDSC) (Figure 1M). All data above indicate that polybromo in the germline is essential for maintaining GSCs. To functionally link Brm to Polybromo, we further tested for possible genetic interactions of brm with bap180 in controlling GSC self-renewal. In these experiments (Table 2), brm2/+ or bap180Δ86/+ single heterozygous flies had a relatively constant number of GSCs per germarium during a 14 day test period, but brm2/bap180Δ86 female flies that are double heterozygotes for both brm and bap180 displayed a defect in sustaining the GSC population. In parallel, GSC maintenance within the test period remained normal in double heterozygotes for both brm and osa (Table 2). Thus, these studies identified a genetic interaction of brm with bap180, suggesting that the germline Brm functions in the form of the PBAP complex during GSC maintenance.
Table 1. osa mutations do not disrupt GSC maintenance in the ovary.
| Phenotype | GSC number per germarium | ||
| 2 day | 7 day | 14 day | |
| CS/w1118 | 2.45 (n = 110) | 2.45 (n = 104) | 2.44 (n = 104) |
| osa2/osa308 | 2.51 (n = 109) | 2.49 (n = 105) | 2.45 (n = 96) |
Table 2. brm genetically interacts with bap180 but not osa in maintaining GSCs.
| Phenotype | GSC number per germarium | ||
| 2 day | 7 day | 14 day | |
| brm2/+ | 2.33 (n = 108) | 2.27 (n = 85) | 2.11 (n = 83) |
| bap180Δ86/+ | 2.52 (n = 108) | 2.36 (n = 105) | 2.5 (n = 88) |
| osa2/+ | 2.45 (n = 106) | 2.35 (n = 89) | 2.31 (n = 87) |
| brm2/bap180Δ86 | 2.20 (n = 87) | 1.39 (n = 82)* | 1.45 (n = 84)* |
| brm2/osa2 | 2.47 (n = 108) | 2.21 (n = 83) | 2.21 (n = 82) |
*p<0.05.
GSC maintenance defects elicited by the PBAP complex deficiency could be attributable to promoted cell death or precocious differentiation. To differentiate these possibilities, we performed an immunostaining assay using anti-Cleaved-Caspase 3 antibody. The experiment failed to detect any apoptotic signals in the marked brm mutant GSCs (data not shown). Given that the lost brm mutant GSCs are able to develop into differentiated germline cysts (Figure 1E), we argue that brm mutation-induced GSC loss might result from increased stem cell differentiation. BMP signaling plays a pivotal role in controlling GSC self-renewal through repressing differentiation via modulating transcriptional silencing of bam, a GSC differentiation promoting gene. To understand mechanisms underlying the defective GSC maintenance, we examined BMP signaling activities, as well as bam expression pattern in brm mutant GSC clones. The indirect immunofluorescence analyses revealed that while the mutant GSCs respond to BMP signals at the same magnitude as the neighboring wild type controls, repression of bam expression remains in the mutant GSCs (Figure S1A, B and data not shown). These data suggest that the cell-autonomous role of the PBAP complex in GSC maintenance is independent of BMP/Bam pathway.
PBAP complex in the niche has a non-cell autonomous role in maintaining GSCs
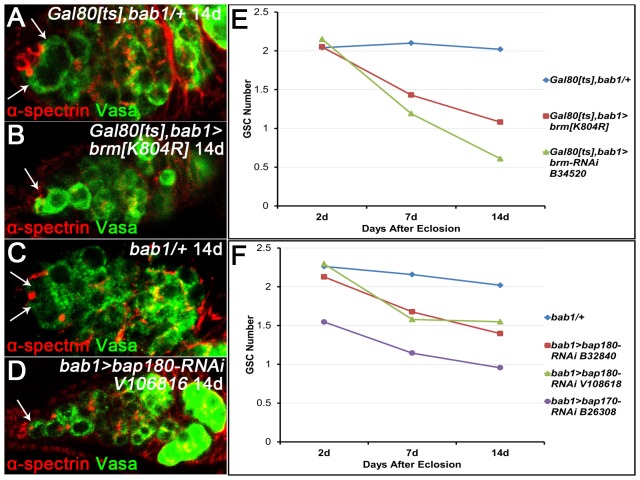
Given that epigenetic regulation of GSC maintenance involves both intrinsic and extrinsic mechanisms [31], [32] and Brm expression is evident in the GSC niche (Figure 1A and A′), we assumed that the PBAP complex has also a non-cell autonomous role in controlling GSC self-renewal. To discern the possibility, we first combined tubP-Gal80ts with UAS-brm RNAi (B34520 from the BDSC) or UAS-brmK804R and bab1-Gal4, a GSC niche-specific driver, and performed a temperature shift assay. In the experiments, the females were raised at 18°C until 2 days after eclosion and then shifted to 29°C for a number of days. Cell number counting at different time points revealed that reduced expression of brm in the niche cells only at adulthood causes a gradual GSC loss (Figure 2A, B and E). Thus, this spatial-temporally controlled study supports the notion that Brm is required extrinsically for maintaining GSCs.
Figure 2. Knock down of the PBAP complex subunits in the niche leads to a gradual GSC loss.
(A–D) The control germaria (A, C) and mutant ones expressing brm-Dominant-Negative (brm[K804R]) (B) or bap180-RNAi transgene (D) under the control of bab1-gal4, stained for Vasa and α-spectrin. Only one GSC is present in the knockdown germarium at 14 days after eclosion (B, D), whereas the control germarium contains two GSCs (A, C). GSCs are indicated by arrows in all panels. (E, F) Graphs show that compared with the controls, knocking down brm (E) or the PBAP specific subunit encoding gene (bap180 or bap170) (F) in the niche causes a significant drop of GSC number per germarium over a 2-week period after eclosion.
To verify if Brm in the niche acts in the form of the PBAP complex to control GSC self-renewal, we next tested polybromo and bap170, another specific subunit of the PBAP complex, for the non-cell autonomous roles in sustaining the GSC population. As depicted in figure 2C, D and F, RNAi-based knock down of either polybromo (B32840 from the BDSC, V108618 from Vienna Drosophila RNAi Center, VDRC) or bap170 (B26308 from the BDSC) in the niche cells elicited a significant decrease in GSC number per germarium in a 14 day time course, albeit reduced GSC number at eclosion was observed in the case of bap170 knock down. These data not only indicate extrinsic roles of polybromo and bap170 in GSC maintenance, but also provide more evidence that the PBAP complex controls GSC self-renewal at multiple levels.
CpCs in the niche produce BMP signals for keeping GSCs in an undifferentiated and self-renewing state. To determine whether knocking down the PBAP complex perturbs the niche signaling output, we expressed UAS-brm RNAi (B35211 from the BDSC) in the niche cells and then examined the expression levels of pMad in GSCs located in the knockdown germaria. As evident in supplementary figure S1C and S1D, pMad expression was remarkedly reduced in a significantly higher percentage of the mutant GSCs than that of the control ones (31.21%, n = 141 vs 9.29%, n = 140), indicative of a compromised BMP signaling emitted from the knock down niche. These results suggest that the niche-specific brm knockdown impairs BMP signaling output, presumably eliciting a defective GSC maintenance phenotype.
The PBAP complex is dispensable for germline differentiation within the germarium
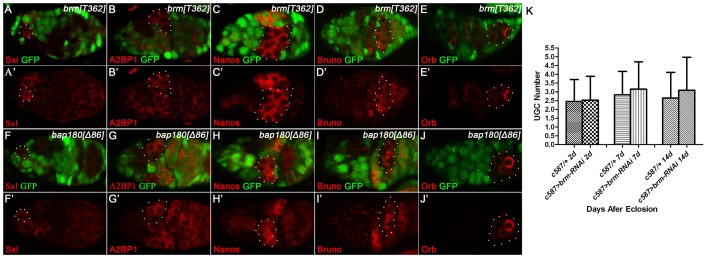
It has been shown that germline lineage commitment in the ovary proceeds with stepwise GSC-derived germ cell differentiation involving germline cyst development within the germarium, and epigenetic regulation is implicated in this process [30], [32]. In order to clarify whether the PBAP complex acts in the germline differentiation, we first generated germline clones homozygous for the brmT362 allele using the FRT/FLP technique and analyzed how differentiation of GSCs and their derivatives occurs in brm mutant germaria at both molecular and morphological levels. As shown in figure 3A–E′, the expression pattern of all tested molecular markers for ranging from the early to late differentiation remained unchanged (Sex lethal, 100%, n = 23; A2BP1, 100%, n = 27; Nanos, 100%, n = 19; Bruno, 100%, n = 29; Orb, 95.65%, n = 23). In addition, we observed that the mature egg chambers in which the entire germ line is mutated for brm appear normal with respective to the cyst development (Figure 1E). Similarly, loss-of-function mutations in the germline bap180 did not cause any molecular and morphological abnormality in the differentiation (Figure 1I, 3F–J′ and data not shown). Taken together, these studies indicate that the PBAP complex is not required cell-autonomously for controlling germline lineage commitment within the germarium.
Figure 3. Removal or knock down of brm or bap180 function does not disrupt germline differentiation within the germarium.
(A–J′) Germaria containing brmT362 (A–E′) or bap180Δ86 (F–J′) mutant germ cell clones (broken circles) marked by the absence of nuclear GFP, stained for Sxl (A, A′, F, F′), A2BP1 (B, B′, G, G′), Nanos (C, C′, H, H′), Bruno (D, D′, I, I′) or Orb (E, E′, J, J′). GSC-derived germline differentiation within the germarium proceeds with dynamic expression of a number of molecular markers such as Sxl in GSCs/CBs (A, A′, F, F′), A2BP1 in germ cells starting from the 4-cell cysts (B, B′, G, G′), Nanos in 16-cell germline cysts (C, C′, H, H′), Bruno in germ cells of the 16-cell cysts (D, D′, I, I′) and Orb in oocyte of the 16-cell cysts (E, E′, J, J′). The expression pattern of all tested differentiation markers remains unchanged in the germline clones homozygous for either brmT362 (A–E′) or bap180Δ86 (F–J′). (K) Graph shows that compared with the controls, brm knockdown in ECs does not cause the accumulation of UGCs in the germarium over a 14-day time course after eclosion.
Escort cells (ECs) in the germarium have recently been defined as the germline stem cell differentiation niche. Considering that brm is predominantly expressed in ECs, we next sought to exclude a possibility that the PBAP complex has a non-cell autonomous role in the germline differentiation. For this purpose, UAS-brm-RNAi transgene (B35211 from BDSC) was expressed under the control of c587-Gal4, restricting brm down-regulation to ECs and early follicle cells in adults. Same as the control group, reduced expression of brm in the somatic cells did not block GSC differentiation, as indicated by the absence of accumulation of the undifferentiated germ cells (UGCs) (Figure 3K). Thus, these data provide more evidence that the PBAP complex is dispensable for germline lineage differentiation within the germarium.
Previous studies have shown that the core subunits of the Drosophila SWI/SNF complexes, Brm, Mor and Snr1, are required in the germline for oogenesis [38], [47], [48], [49]. However, it remains to be determined how the Brm-containing complex functions in this process. In this paper, we identified a regulatory role for Brm in maintaining GSCs, while excluding its involvement in germline differentiation within the germarium. Moreover, our studies revealed that mutations in polybromo/bap180, rather than osa, cause a similar phenotype to that in brm, and brm genetically interacts with bap180 in controlling GSC self-renewal. These genetic data lead us to propose that Brm acts in the form of the PBAP complex to sustain the GSC population. Given that requirements for the core Brm complex in oogenesis are independent of either BAP-specific subunit Osa or PBAP-specific subunits Bap180/Bap170 [38], we provide here the first demonstration that Brm functions in concert with Polybromo/Bap180 in early oogenesis, defining the Drosophila SWI/SNF complex subtype in the specific developmental context.
Like other epigenetic factors [31], [32], the PBAP complex controls GSC self-renewal both intrinsically and extrinsically. In the case of cell-autonomous manner, GSCs lacking Brm activity are able to respond properly to BMP signals emitted from the niche, sustaining the repression of the bam expression. This observation rules out the possibility that the canonical BMP/Bam pathway is implicated in Brm-controlled GSC maintenance. On the contrary, knocking down brm in the niche cells impairs BMP signaling output, probably eliciting a non-cell autonomous defect in GSC maintenance. Emerging evidence indicates that the chromatin-remodeling complexes execute unique developmental roles through interacting with a variety of transcription factors and/or other co-factors such as histone-modifying enzymes in a cell-type specific or developmental-stage specific manner [50]. Therefore, identification and functional characterization of the PBAP complex-binding partners, as well as epigenetically regulated target genes in the ovary would be beneficial for elucidating the above distinct mechanisms underlying intrinsic and extrinsic function of the PBAP complex in controlling GSC self-renewal. Given that the evolutionarily conserved SWI/SNF complexes have crucial roles in regulating both embryonic and adult stem cell fate in invertebrates, vertebrates and plants [24], [45], [50], mechanistic studies on PBAP complex-controlled GSC maintenance may shed lights on stem cell regulation in higher organisms.
Supporting Information
brm knock down in the niche, rather than loss of brm function in GSCs perturbs BMP signaling. (A–D) Germaria with the control (A) or brmT362 homozygous (B) GSC clone (broken circles) labeled by the absence of the nuclear GFP, or expressing bab1-gal4 alone (C) or brm-RNAi with bab1-gal4 (D), stained for pMad (A, B) or pMad and α-spectrin (C, D). Clearly, high levels of pMad expression are evident in both marked wild type control and brm mutant GSC (broken circles in A and B). By contrast, pMad expression in GSCs is remarkably reduced in the brm knock down germarium (arrowhead in D), compared with that in the control (arrow in C).
(TIF)
Molecular validation of on-targeting effects of the RNAi transgenic strains. (A, B) RT-PCR analysis shows that actin-gal4 or hs-gal4 induced expression of the UAS-brm-RNAi (A) or UAS-bap180-RNAi (B) transgene in the 3rd instar larvae leads to a reduction in the expression of endogenous brm (A) or bap180 (B) at mRNA levels. The presented gels are representative of three independent experiments.
(TIF)
Acknowledgments
We deeply thank Jessica E. Treisman, Edward Laufer, Feng Tie, Akira Nakamura, Michael Buszczak, Mary A. Lilly, Zhao-hui Wang, Yu Cai, the Bloomington Drosophila Stock Center, the Vienna Drosophila RNAi Center and the Developmental Studies Hybridoma Bank for providing fly stocks and antibodies.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Basic Research Program of China (2012CB966903), National Natural Science Foundation of China (J1210047), Innovation Project of Shanghai Municipal Education Commission (12ZZ015) and Postdoctoral Research Foundation of Shanghai Jiao Tong University (13X100030030).The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kirilly D, Xie T (2007) The Drosophila ovary: an active stem cell community. Cell Research 17: 15–25. [DOI] [PubMed] [Google Scholar]
- 2.Spradling AC, editor(1993) Developmental genetics of oogenesis. New York: Cold Spring Harbor Laboratory Press. 1–70 p. [Google Scholar]
- 3. Horne-Badovinac S, Bilder D (2005) Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Developmental Dynamics 232: 559–574. [DOI] [PubMed] [Google Scholar]
- 4. McKearin D, Ohlstein B (1995) A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development 121: 2937–2947. [DOI] [PubMed] [Google Scholar]
- 5. Xie T, Spradling A (1998) decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94: 251–260. [DOI] [PubMed] [Google Scholar]
- 6. Chen D, McKearin D (2003) Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Current Biology 13: 1786–1791. [DOI] [PubMed] [Google Scholar]
- 7. Chen D, McKearin D (2003) A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development 130: 1159–1170. [DOI] [PubMed] [Google Scholar]
- 8. Song X, Wong MD, Kawase E, Xi R, Ding BC, et al. (2004) Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 131: 1353–1364. [DOI] [PubMed] [Google Scholar]
- 9. Rojas-Ríos P, Guerrero I, González-Reyes A (2012) Cytoneme-Mediated Delivery of Hedgehog Regulates the Expression of Bone Morphogenetic Proteins to Maintain Germline Stem Cells in Drosophila . PLoS Biology 10: e1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xie (2008) Germline stem cell niches. StemBook [PubMed] [Google Scholar]
- 11. Forbes A, Lehmann R (1998) Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development 125: 679–690. [DOI] [PubMed] [Google Scholar]
- 12. Wang Z, Lin H (2004) Nanos Maintains Germline Stem Cell Self-Renewal by Preventing Differentiation. Science 303: 2016–2019. [DOI] [PubMed] [Google Scholar]
- 13. Bhat KM (1999) The posterior determinant gene nanos is required for the maintenance of the adult germline stem cells during Drosophila oogenesis. Genetics 151: 1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin Z, Xie T (2007) Dcr-1 Maintains Drosophila Ovarian Stem Cells. Current Biology 17: 539–544. [DOI] [PubMed] [Google Scholar]
- 15. Lin H, Spradling AC (1997) A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development 124: 2463–2476. [DOI] [PubMed] [Google Scholar]
- 16. Park JK, Liu X, Strauss TJ, McKearin DM, Liu Q (2007) The miRNA Pathway Intrinsically Controls Self-Renewal of Drosophila Germline Stem Cells. Current Biology 17: 533–538. [DOI] [PubMed] [Google Scholar]
- 17. Yang L, Chen D, Duan R, Xia L, Wang J, et al. (2007) Argonaute 1 regulates the fate of germline stem cells in Drosophila. Development 134: 4265–4272. [DOI] [PubMed] [Google Scholar]
- 18. Chau J, Kulnane LS, Salz HK (2012) Sex-lethal enables germline stem cell differentiation by down-regulating Nanos protein levels during Drosophila oogenesis. Proceedings of the National Academy of Sciences 109: 9465–9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tastan OY, Maines JZ, Li Y, McKearin DM, Buszczak M (2010) Drosophila Ataxin 2-binding protein 1 marks an intermediate step in the molecular differentiation of female germline cysts. Development 137: 3167–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parisi MJ, Deng W, Wang Z, Lin H (2001) The arrest gene is required for germline cyst formation during Drosophila oogenesis. genesis 29: 196–209. [DOI] [PubMed] [Google Scholar]
- 21. Christerson LB, McKearin DM (1994) orb is required for anteroposterior and dorsoventral patterning during Drosophila oogenesis. Genes & Development 8: 614–628. [DOI] [PubMed] [Google Scholar]
- 22. Decotto E, Spradling AC (2005) The Drosophila Ovarian and Testis Stem Cell Niches: Similar Somatic Stem Cells and Signals. Developmental Cell 9: 501–510. [DOI] [PubMed] [Google Scholar]
- 23. Kirilly D, Wang S, Xie T (2011) Self-maintained escort cells form a germline stem cell differentiation niche. Development 138: 5087–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xi R, Xie T (2005) Stem cell self-renewal controlled by chromatin remodeling factors. Science 310: 1487–1489. [DOI] [PubMed] [Google Scholar]
- 25. Maines JZ, Park JK, Williams M, McKearin DM (2007) Stonewalling Drosophila stem cell differentiation by epigenetic controls. Development 134: 1471–1479. [DOI] [PubMed] [Google Scholar]
- 26. Yin H, Lin H (2007) An epigenetic activation role of Piwi and a Piwi-associated piRNA in Drosophila melanogaster. Nature 450: 304–308. [DOI] [PubMed] [Google Scholar]
- 27. Buszczak M, Paterno S, Spradling AC (2009) Drosophila stem cells share a common requirement for the histone H2B ubiquitin protease scrawny. Science 323: 248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ables ET, Drummond-Barbosa D (2010) The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila . Cell Stem Cell 7: 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eliazer S, Shalaby NA, Buszczak M (2011) Loss of lysine-specific demethylase 1 nonautonomously causes stem cell tumors in the Drosophila ovary. Proceedings of the National Academy of Sciences of the United States of America 108: 7064–7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang X, Pan L, Wang S, Zhou J, McDowell W, et al. (2011) Histone H3K9 trimethylase eggless controls germline stem cell maintenance and differentiation. PLoS Genetics 7: e1002426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xin T, Xuan T, Tan J, Li M, Zhao G, et al. (2013) The Drosophila putative histone acetyltransferase Enok maintains female germline stem cells through regulating Bruno and the niche. Developmental Biology 384: 1–12. [DOI] [PubMed] [Google Scholar]
- 32. Xuan T, Xin T, He J, Tan J, Gao Y, et al. (2013) dBre1/dSet1-dependent pathway for histone H3K4 trimethylation has essential roles in controlling germline stem cell maintenance and germ cell differentiation in the Drosophila ovary. Developmental Biology 379: 167–181. [DOI] [PubMed] [Google Scholar]
- 33. Clough E, Tedeschi T, Hazelrigg T (2014) Epigenetic Regulation of Oogenesis and Germ Stem Cell Maintenance by the Drosophila Histone Methyltransferase Eggless/dSetDB1. Developmental Biology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mohrmann L, Langenberg K, Krijgsveld J, Kal AJ, Heck AJ, et al. (2004) Differential targeting of two distinct SWI/SNF-related Drosophila chromatin-remodeling complexes. Molecular and Cellular Biology 24: 3077–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moshkin YM, Mohrmann L, van Ijcken WF, Verrijzer CP (2007) Functional differentiation of SWI/SNF remodelers in transcription and cell cycle control. Molecular and Cellular Biology 27: 651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Collins RT, Treisman JE (2000) Osa-containing Brahma chromatin remodeling complexes are required for the repression of wingless target genes. Genes & Development 14: 3140–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elfring LK, Daniel C, Papoulas O, Deuring R, Sarte M, et al. (1998) Genetic analysis of brahma: the Drosophila homolog of the yeast chromatin remodeling factor SWI2/SNF2. Genetics 148: 251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carrera I, Zavadil J, Treisman JE (2008) Two subunits specific to the PBAP chromatin remodeling complex have distinct and redundant functions during drosophila development. Molecular and Cellular Biology 28: 5238–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kai T, Spradling A (2003) An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proceedings of the National Academy of Sciences of the United States of America 100: 4633–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- 41. Li Q, Xin T, Chen W, Zhu M, Li M (2008) Lethal(2)giant larvae is required in the follicle cells for formation of the initial AP asymmetry and the oocyte polarity during Drosophila oogenesis. Cell Research 18: 372–384. [DOI] [PubMed] [Google Scholar]
- 42. Tie F, Banerjee R, Conrad PA, Scacheri PC, Harte PJ (2012) Histone demethylase UTX and chromatin remodeler BRM bind directly to CBP and modulate acetylation of histone H3 lysine 27. Molecular and Cellular Biology 32: 2323–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sugimura I, Lilly MA (2006) Bruno Inhibits the Expression of Mitotic Cyclins during the Prophase I Meiotic Arrest of Drosophila Oocytes. Developmental Cell 10: 127–135. [DOI] [PubMed] [Google Scholar]
- 44. Gancz D, Lengil T, Gilboa L (2011) Coordinated regulation of niche and stem cell precursors by hormonal signaling. PLoS Biology 9: e1001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jin Y, Xu J, Yin M-X, Lu Y, Hu L, et al. (2013) Brahma is essential for Drosophila intestinal stem cell proliferation and regulated by Hippo signaling. eLife 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zeng X, Lin X, Hou SX (2013) The Osa-containing SWI/SNF chromatin-remodeling complex regulates stem cell commitment in the adult Drosophila intestine. Development 140: 3532–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brizuela BJ, Elfring L, Ballard J, Tamkun JW, Kennison JA (1994) Genetic analysis of the brahma gene of Drosophila melanogaster and polytene chromosome subdivisions 72AB. Genetics 137: 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brizuela BJ, Kennison JA (1997) The Drosophila homeotic gene moira regulates expression of engrailed and HOM genes in imaginal tissues. Mechanisms of Development 65: 209–220. [DOI] [PubMed] [Google Scholar]
- 49. Zraly CB, Marenda DR, Nanchal R, Cavalli G, Muchardt C, et al. (2003) SNR1 is an essential subunit in a subset of drosophila brm complexes, targeting specific functions during development. Developmental Biology 253: 291–308. [DOI] [PubMed] [Google Scholar]
- 50. Ho L, Crabtree GR (2010) Chromatin remodelling during development. Nature 463: 474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
brm knock down in the niche, rather than loss of brm function in GSCs perturbs BMP signaling. (A–D) Germaria with the control (A) or brmT362 homozygous (B) GSC clone (broken circles) labeled by the absence of the nuclear GFP, or expressing bab1-gal4 alone (C) or brm-RNAi with bab1-gal4 (D), stained for pMad (A, B) or pMad and α-spectrin (C, D). Clearly, high levels of pMad expression are evident in both marked wild type control and brm mutant GSC (broken circles in A and B). By contrast, pMad expression in GSCs is remarkably reduced in the brm knock down germarium (arrowhead in D), compared with that in the control (arrow in C).
(TIF)
Molecular validation of on-targeting effects of the RNAi transgenic strains. (A, B) RT-PCR analysis shows that actin-gal4 or hs-gal4 induced expression of the UAS-brm-RNAi (A) or UAS-bap180-RNAi (B) transgene in the 3rd instar larvae leads to a reduction in the expression of endogenous brm (A) or bap180 (B) at mRNA levels. The presented gels are representative of three independent experiments.
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.