Abstract
Objective:
We hypothesized that in participants with a history of hypertension, lower late-life blood pressure (BP) will be associated with more brain pathology.
Methods:
Participants are 4,057 older men and women without dementia with midlife (mean age 50 ± 6 years) and late-life (mean age 76 ± 5 years) vascular screening, cognitive function, and brain structures on MRI ascertained as part of the Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study.
Results:
The association of late-life BP to brain measures depended on midlife hypertension history. Higher late-life systolic and diastolic BP (DBP) was associated with an increased risk of white matter lesions and cerebral microbleeds, and this was most pronounced in participants without a history of midlife hypertension. In contrast, in participants with a history of midlife hypertension, lower late-life DBP was associated with smaller total brain and gray matter volumes. This finding was reflected back in cognitive performance; in participants with midlife hypertension, lower DBP was associated with lower memory scores.
Conclusion:
In this large population-based cohort, late-life BP differentially affects brain pathology and cognitive performance, depending on the history of midlife hypertension. Our study suggests history of hypertension is critical to understand how late-life BP affects brain structure and function.
The traditional general practice teaches us that “the lower the blood pressure (BP), the better the prognosis of the patient.” However, evidence from both observational and randomized studies suggests that the favorable effects of low BP in preventing cerebrovascular disease1–4 are not reflected in consistent preservation of cognitive functioning.5–7
Whereas most studies report that higher midlife BP increases risk for late-life cognitive impairment,6,8–10 the effects of late-life BP on brain function are less clear.6 In fact, several studies in older individuals showed that lower BP might have detrimental effects on the brain.6,11,12 Such findings could suggest that chronic hypertension since midlife leads to structural and functional cerebrovascular changes, disrupting vasoregulatory mechanisms.13–15 This will leave the surviving brain tissue unprotected against the potentially harmful effects of low BP, resulting in microvascular and neurodegenerative changes leading to brain tissue loss16 and subsequent cognitive impairment. It is also possible that low late-life BP reflects a vascular system already damaged by comorbidities, such as chronic hypertension, which subsequently could lead to reduced cerebral blood flow and its sequela.16–19
Based on these factors, we hypothesized that history of midlife hypertension would modulate the association of late-life BP to markers of brain aging in an older population-based sample of participants without dementia.
METHODs
AGES-Reykjavik Study.
Subjects are participants in the Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study, which is a continuation of the Reykjavik Study. The Reykjavik Study (1967–1996) was initiated by the Icelandic Heart Association and included men and women born in 1907–1935 and living in the Reykjavik area. From 2002 to 2006, new data were collected for the AGES-Reykjavik Study. The study design and initial assessments of the cohort were described previously.20
Standard protocol approvals, registrations, and patient consents.
The AGES-Reykjavik Study was approved by the Icelandic National Bioethics Committee (VSN 00-063) and by the Institutional Review Board of the US National Institute on Aging, NIH. All participants signed an informed consent.
Brain MRI.
MRI including T1-, proton density–, T2*-, and T2-weighted and fluid-attenuated inversion recovery (FLAIR) images was acquired on a 1.5T Signa Twinspeed Excite system (General Electric Medical Systems, Waukesha, WI).21 Brain volumes (mL), including gray matter (GM), white matter (WM), CSF, and WM lesions (WML), were segmented automatically with an AGES-Reykjavik Study–modified algorithm based on the Montreal Neurological Institute pipeline as described previously.21,22 Total brain volume (TBV) was calculated as the sum of GM, WM, and WML volumes. Total intracranial volume (ICV) was computed as the sum of TBV and CSF volumes and TBV, GM volume (GMV), and WM volume (WMV) were expressed relative to ICV (% ICV). WML volume was dichotomized into severe WML (highest quintile) and the rest. Brain infarcts were identified by trained radiographers as defects in the brain parenchyma with associated hyperintensity on T2 and FLAIR. Subcortical brain infarcts had a diameter of at least 4 mm. For infarcts in the cerebellum and brainstem or with cortical involvement, no size criterion was required. Cerebral microbleeds were defined as focal areas of signal void within the brain parenchyma that met the following criteria: visible on T2* images, smaller or invisible on T2 images, not abutting a parenchymal defect, and not showing any other structure in the signal void area.23 Microbleeds were classified based on their frequency in each subject as 0–1 and ≥2.
Cognition.
A battery of 6 different cognitive tests was administered to all participants as described earlier.23 From these tests, 3 cognitive domain composite scores were calculated: (1) memory composite score included immediate and delayed recall of a modified version of the California Verbal Learning Test; (2) processing speed composite included the Figure Comparison Test, Digit Symbol Substitution Test, and Stroop 1 and 2; and (3) executive function composite included a short version of the CANTAB Spatial Working Memory test, the Digits Backward test, and Stroop 3. Composite measures were computed by converting raw scores to standardized Z scores and averaging them across the tests in each composite. Interrater reliability for all tests was excellent (Spearman correlations range 0.96–0.99).
Midlife and late-life blood pressure.
BP control in Iceland during the Reykjavik and AGES-Reykjavik Study followed the World Health Organization and European guidelines. At midlife and late life, systolic BP (SBP) and diastolic BP (DBP) were measured 2 times in a sitting position to the nearest 2 mm Hg with a mercury sphygmomanometer using a standard-sized cuff (bladder width × length: 15 × 22 cm). Hypertension was defined as antihypertensive treatment or BP >140/90 mm Hg. Late-life SBP and DBP were categorized based on the BP distribution of the population and following clinical guidelines as much as possible; SBP: ≤120, 121–140, >140 mm Hg, DBP: ≤70, 71–80, >80 mm Hg. Pulse pressure (PP) was calculated as the difference between SBP and DBP.
Other variables.
Participants were asked to fill out a standardized questionnaire including information on medical history, medication use, and lifestyle factors and a fasting blood sample was drawn.20 Education was classified into college/university, secondary school, or primary school (low). Weight and height were measured and body mass index (BMI) (kg/m2) was calculated. Fasting cholesterol and glucose levels were measured and diabetes was defined as having a history, using glucose-modifying medication, or fasting glucose of ≥126 mg/dL. Smoking was defined as current/former vs no smoking. Coronary heart disease was defined as a history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass surgery based on hospital records, angina pectoris on the Rose Angina Questionnaire, or evidence on ECG of possible/probable myocardial infarction. Congestive heart failure was defined as having one or more of the following ICD-10 codes: I50*, I11.0, I13.2, I97.13, I09.81.
Analytical sample.
Of the 5,764 participants in the AGES-Reykjavik Study, 953 had no MRI and 112 participants had missing brain volume data due to acquisition artifacts or postprocessing failures. Of the remaining 4,614, we excluded individuals with dementia (n = 260) since BP could be influenced by the neurodegenerative pathology.24 In addition, we excluded those with dementia assessments where no diagnosis could be made (n = 48), with incomplete cognitive testing (n = 227), and with missing midlife data (n = 22). This resulted in a total of 4,057 individuals without dementia with complete data on late-life brain volumes and cognitive function for analysis. Compared to the total AGES-Reykjavik sample, the individuals in the analytical sample were younger, were higher educated, had lower BP at midlife and late life, and had larger TBV (data not shown).
Data analysis.
Subject characteristics at midlife and late life were calculated for categories of midlife hypertension status and for combined categories of midlife hypertension and late-life DBP. Linear and logistic regression analyses, adjusted for demographics (age, sex, education) and midlife cardiovascular risk factors (BMI, smoking, diabetes, cholesterol), were used to assess the association of midlife hypertension with measures of cerebrovascular lesions (severe WML, brain infarcts ≥1, microbleeds ≥2) and measures of brain atrophy (TBV, GMV, WMV). Next, the joint association of midlife hypertension status and late-life BP with brain structure was investigated. Using logistic and linear regression analysis, we examined the association of late-life SBP or DBP (continuous and categorical) to measures of cerebrovascular lesions and brain atrophy stratified by midlife hypertension status. Interaction terms between midlife hypertension and late-life BP measures were incorporated in the regression models. Analysis of covariance (ANCOVA) was used to calculate mean (95% confidence interval [CI]) adjusted brain volumes across categories of late-life SBP or DBP stratified by midlife hypertension status. Several models were investigated: model 1 included demographics; model 2 also included midlife and late-life cardiovascular risk factors and cerebrovascular lesions (when investigating measures of brain atrophy) to assess whether these lesions mediated the relation between BP and brain atrophy. To assess the mediating effect of cardiovascular disease, models were additionally adjusted for coronary artery disease, heart failure, and high PP. p Values for trend were calculated by repeating the regression models by using BP categories as a continuous variable. Finally, we assessed the combined effect of midlife hypertension and late-life BP on cognitive performance (memory, processing speed, executive functioning) using ANCOVA, adjusting for demographics and cardiovascular risk factors, and tested the interaction (midlife hypertension × late-life BP).
RESULTS
Characteristics of the study population.
In the study sample of 4,057, mean (range) age at midlife and late life was 50 years (33–75) and 76 years (66–95) and 59% were women. The prevalence of hypertension at midlife was 34% (n = 1,365) and 6% (n = 251) used antihypertensive treatment at midlife. Mean (10th–90th percentile range) late-life SBP and DBP levels were 142 mm Hg (119–170) and 74 mm Hg (62–86).
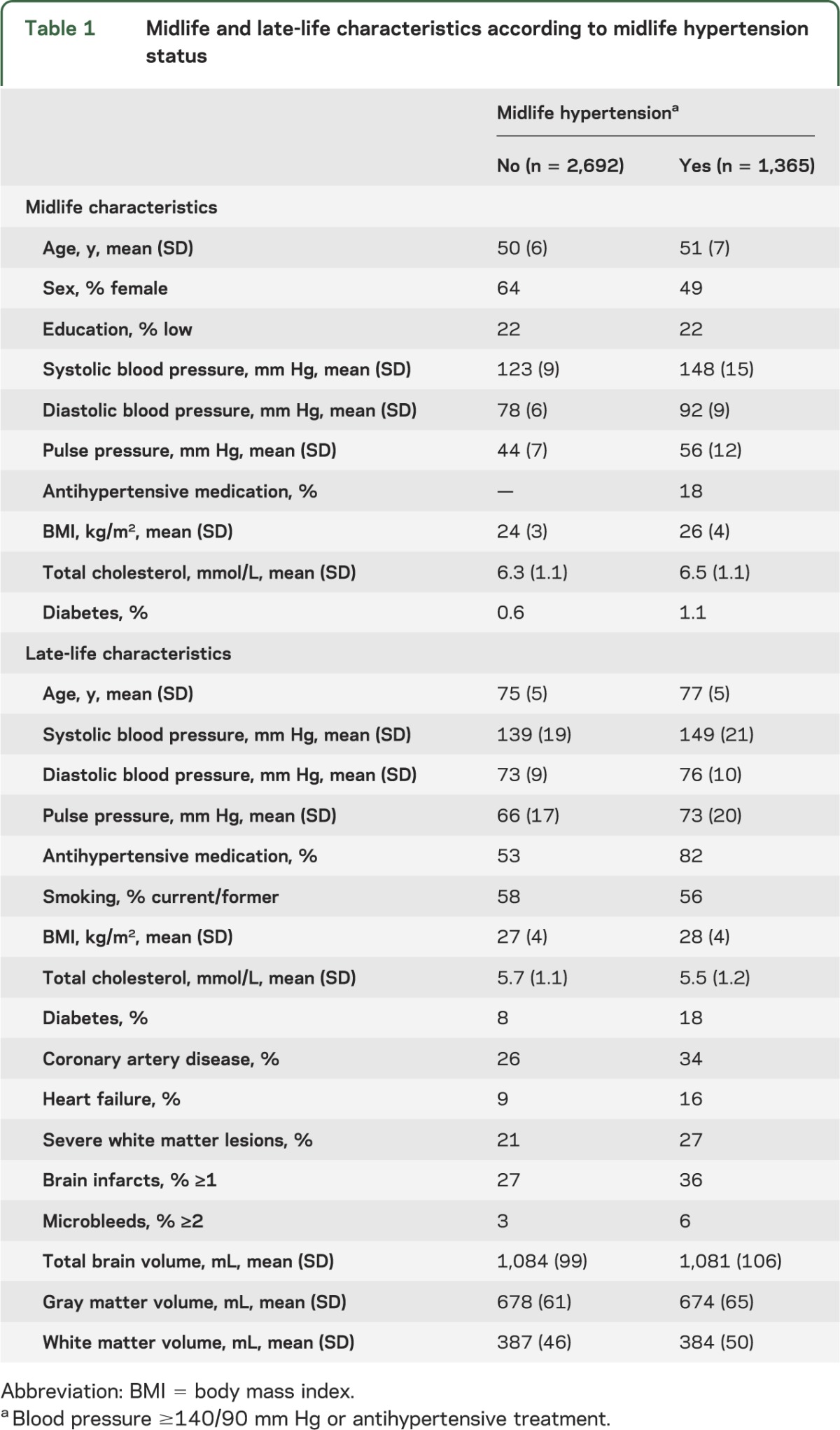
Participants with a history of midlife hypertension were significantly older at midlife and late life, were more often male, had higher PP at midlife and late life, used more antihypertensive drugs at late life, and had more diabetes, more hypertension-related comorbidities such as coronary heart disease and heart failure, more cerebrovascular lesions, and smaller brain volumes on MRI (table 1).
Table 1.
Midlife and late-life characteristics according to midlife hypertension status
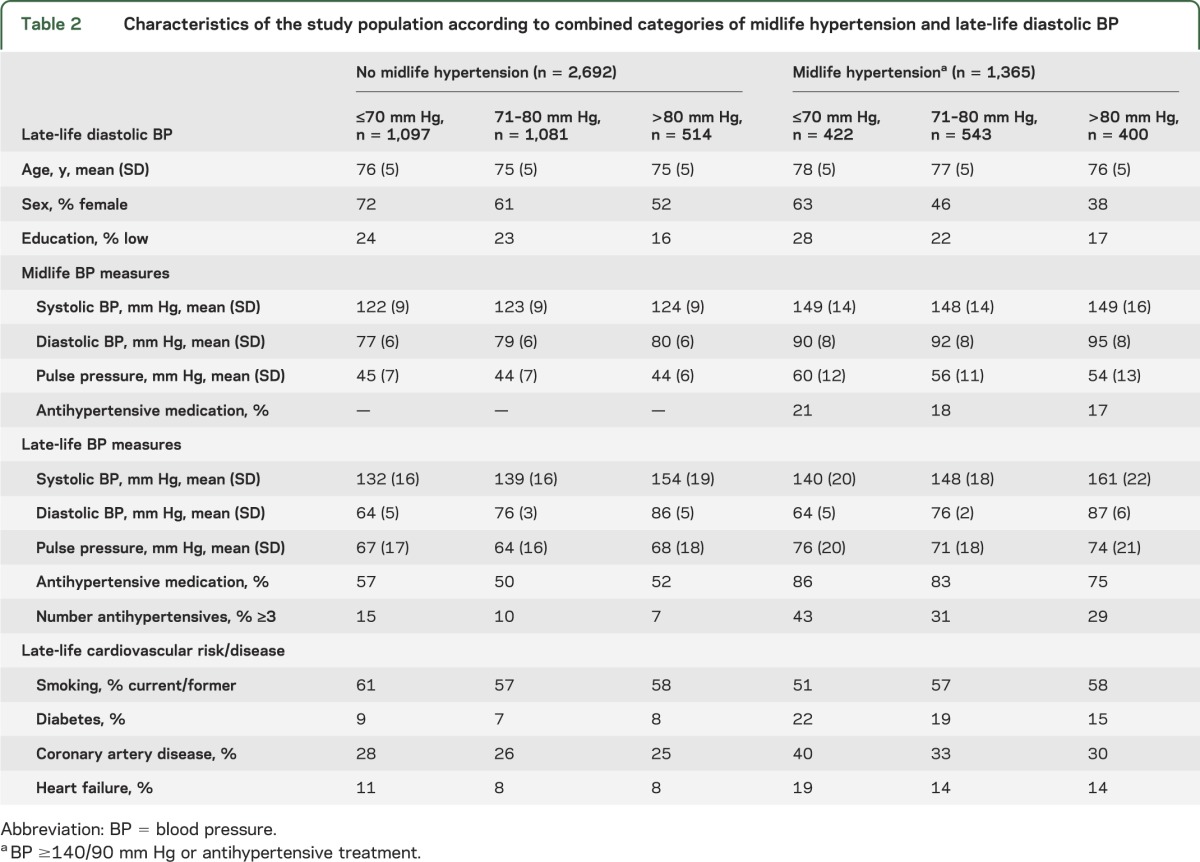
In participants with midlife hypertension, those with lower late-life DBP were less educated, had higher PP, used more antihypertensive drugs, and had the highest prevalence of diabetes, coronary heart disease, and heart failure. In contrast, in participants without midlife hypertension, those with lower DBP had a similar cardiovascular risk profile compared to those with higher DBP (table 2).
Table 2.
Characteristics of the study population according to combined categories of midlife hypertension and late-life diastolic BP
Midlife hypertension and late-life brain structure and function.
Compared to participants without midlife hypertension, those with midlife hypertension had higher adjusted odds (95% CI) of having severe WMLs 1.3 (1.1–1.6), brain infarcts 1.2 (1.1–1.4), or multiple microbleeds 1.4 (1.0–1.9). Furthermore, brain volumes (% ICV) were significantly lower in those with midlife hypertension, compared to those without midlife hypertension; mean differences (95% CI) in TBV, GMV, and WMV were −0.5% ICV (−0.7; −0.2), −0.4% ICV (−0.6; −0.2), and −0.2% ICV (−0.3; −0.1). Finally, those with midlife hypertension had lower cognitive scores than those without midlife hypertension, however, only statistically significant for executive functioning; mean differences in (95% CI) memory, processing speed, and executive functioning scores were −0.032 (−0.085; 0.020), −0.017 (−0.060; 0.026), and −0.041 (−0.082; −0.000). These results were independent of demographics and midlife cardiovascular risk factors.
Joint association of midlife hypertension and late-life BP on cerebrovascular lesions.
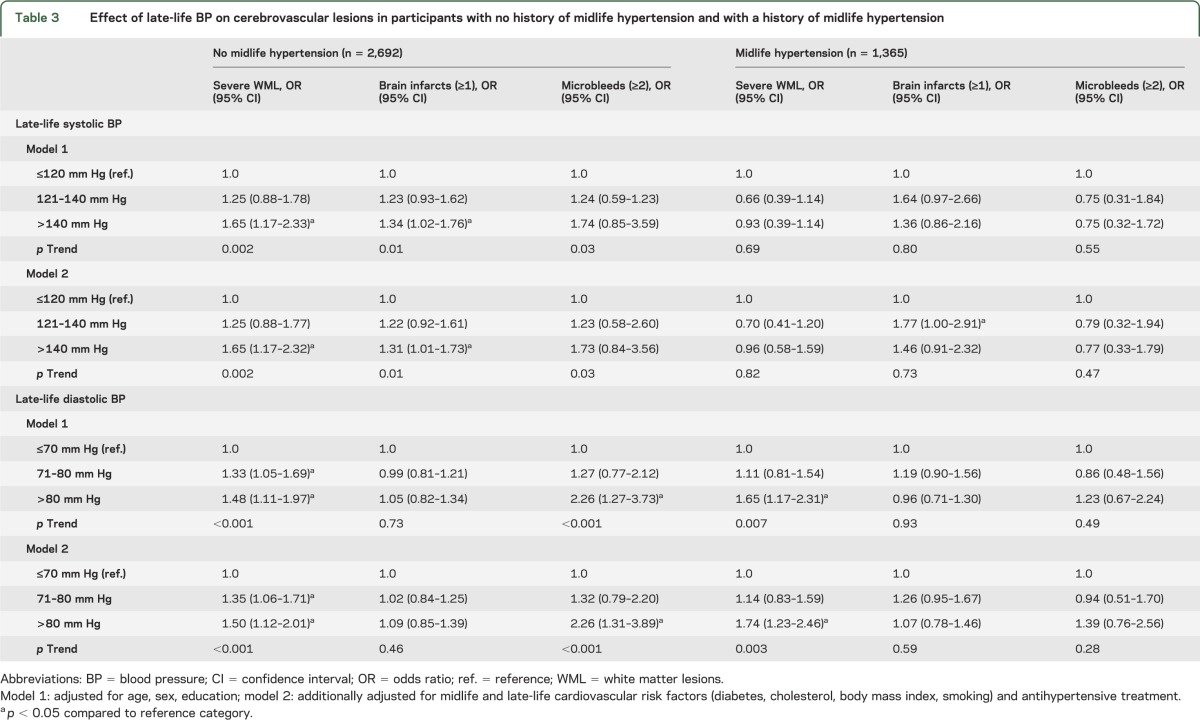
Higher late-life SBP was associated with an increased risk of WMLs, brain infarcts, and microbleeds, but only in those without a history of hypertension (table 3). Higher DBP was associated with an increased risk for severe WMLs, regardless of hypertension history, and with an increased risk of multiple microbleeds in participants without a history of midlife hypertension (table 3). Late-life DBP was not associated with the presence of brain infarcts. These results were independent of demographics and midlife and late-life cardiovascular risk factors. Also, further adjustment for markers of cardiovascular disease yielded similar results as presented in table 3 (data not shown).
Table 3.
Effect of late-life BP on cerebrovascular lesions in participants with no history of midlife hypertension and with a history of midlife hypertension
Joint association of midlife hypertension and late-life BP on brain tissue volumes.
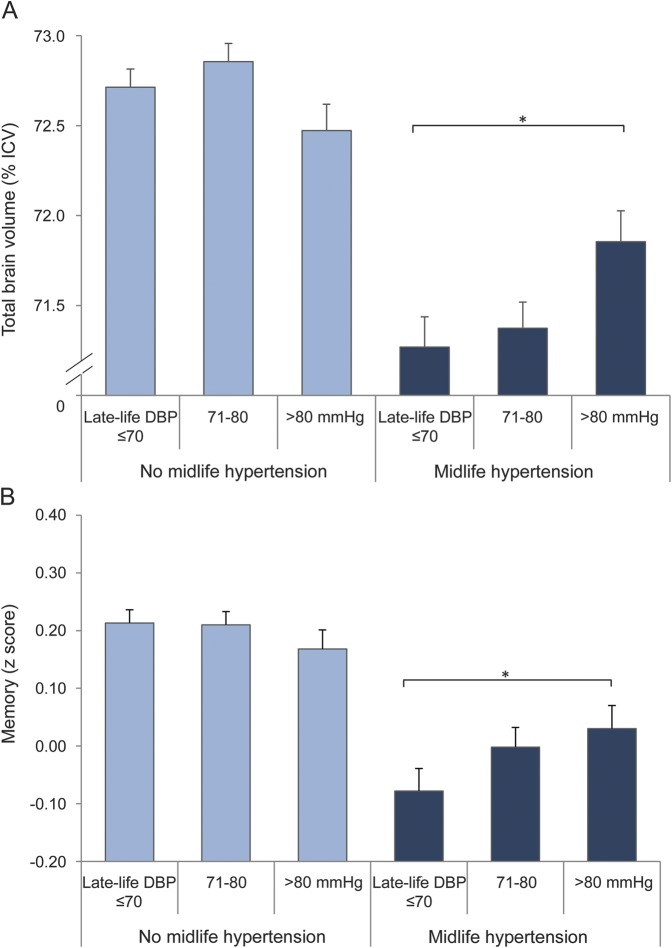
Higher late-life SBP and DBP were associated with smaller WMV, particularly in those without a history of hypertension. However, these results were largely explained by presence of WMLs, brain infarcts, and microbleeds (table 4). In contrast, lower late-life DBP (and to a lesser extent lower late-life SBP) was associated with smaller TBV and GMV in participants with a history of hypertension (table 4). Participants with both midlife hypertension and low late-life DBP (≤70 mm Hg) had the smallest TBV and GMV, independent of demographics, cardiovascular risk factors, and cerebrovascular lesions (figure, A); p interactions (midlife hypertension × late-life DBP) for TBV and GM were 0.01 and 0.009. Further adjustment for cardiovascular disease only partly explained our findings; in participants with a history of hypertension, mean differences (95% CI) between low and high DBP for TBV and GMV were −0.59% ICV (−1.07; −0.11) and −0.55% ICV (−0.96; −0.15).
Table 4.
Effect of late-life BP on brain tissue volumes in participants with no history of midlife hypertension and with a history of midlife hypertension
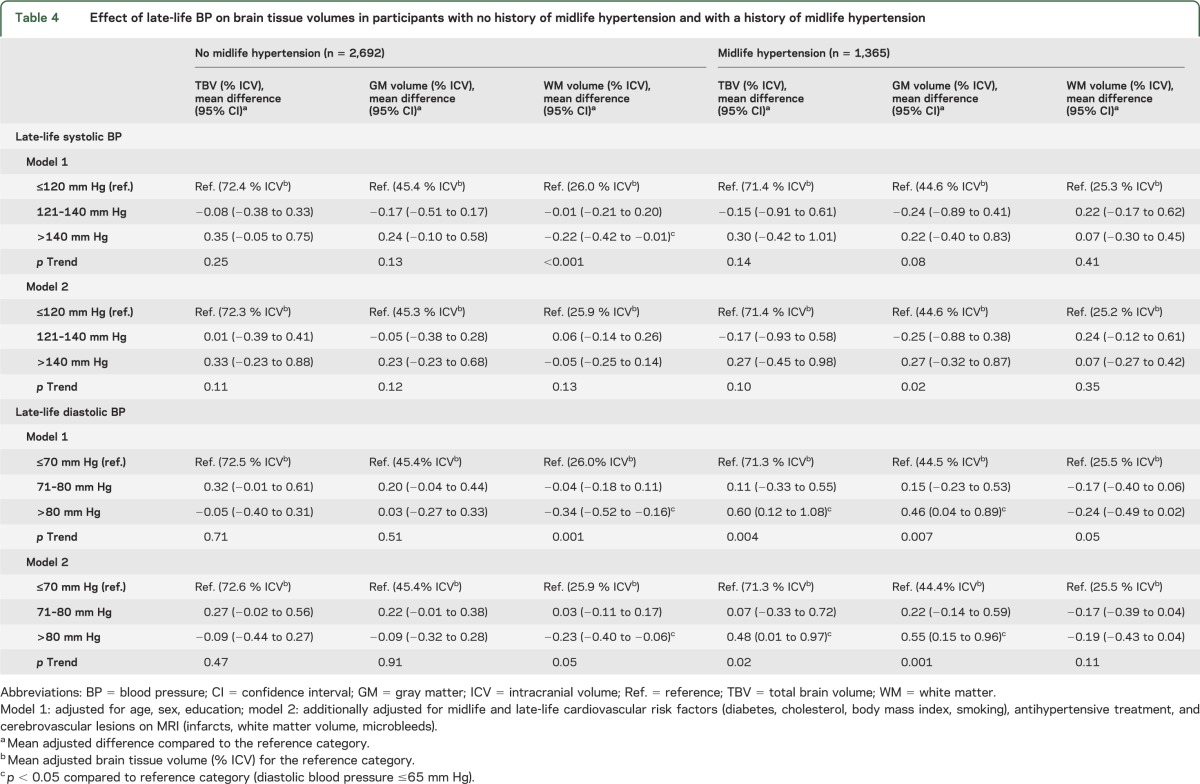
Figure. Total brain volume and memory performance across combined categories of midlife hypertension and late-life DBP.
(A) Mean (SE) total brain volume (% intracranial volume [ICV]) and (B) mean (SE) memory performance (z score). Values are adjusted for age, sex, education, midlife and late-life cardiovascular risk factors, antihypertensive treatment, and cerebrovascular lesions on MRI (when studying total brain volume). *p < 0.05. DBP = diastolic blood pressure.
Joint association of midlife hypertension and late-life BP on cognition.
The associations between higher late-life SBP and DBP and presence of cerebrovascular lesions in participants without midlife hypertension were not reflected in the association of late-life BP with cognitive performance (figure, B for DBP). However, the effects of late-life DBP on brain volume were reflected in the association of late-life DBP with memory performance; in participants with midlife hypertension, those with lower late-life DBP had the worst memory performance; mean difference between low and high DBP was −0.11 (95% CI −0.24; −0.00). p Interaction (midlife hypertension × late-life DBP) was 0.01. These findings were independent of demographics and cardiovascular risk factors. Late-life BP was not significantly associated with processing speed or executive functioning (data not shown).
Several sensitivity analyses were performed: (1) including participants with dementia (n = 260) and with incomplete cognitive testing (n = 227), (2) repeating analyses in the 3,806 participants without antihypertensive treatment at midlife, (3) excluding participants who were older than 65 at midlife (n = 27), and (4) excluding participants with a Mini-Mental State Examination <24 (n = 303) (since lower BP might be the result of a neurodegenerative process) did not change the presented results.
DISCUSSION
Previous research has consistently reported an association between midlife hypertension and brain lesions,8–10 but the findings on the association of late-life BP to brain outcomes has been inconsistent.6 Our study suggests history of midlife hypertension is critical to understand how late-life BP affects brain structure and function. First, higher late-life SBP and DBP increases risk for diffuse small-vessel disease, particularly in participants without a history of hypertension. Second, in participants with a history of hypertension, lower late-life DBP is associated with more TBV and GM atrophy. These findings were reflected back in cognitive performance; in participants with midlife hypertension, lower DBP was associated with lower memory scores.
Our findings bring new insight into the effects of late-life BP on vascular architecture and brain function. Of interest, in these analyses, higher late-life SBP and DBP has the strongest effect on small vessels, represented by an increased risk of severe WMLs or multiple microbleeds, which is in line with several large-scale studies in aging populations.4,25–28 However, it is likely that large-vessel events led to death, so that large-vessel pathology is not well-represented in this sample.25 That this relation was less strong in participants with a history of hypertension could be due to fact that antihypertensive treatment, started between midlife and late life, influenced the deteriorating effects of high BP on the cerebrovasculature. Our finding that the relation between higher late-life BP and risk of small-vessel disease was not reflected back in cognitive performance needs further investigation.
On the other hand, lower late-life DBP has a significant association with brain volume, particularly GMV. These findings are in concordance with previous observations showing that declining BP or low late-life BP were associated with more global brain atrophy and cognitive impairment.11,18,29,30 Also, former studies in cardiovascular patients showed that lower BP was related to more brain atrophy, particularly in those with lower cerebral perfusion levels.16,31 These and our findings provide evidence of a cumulative effect of early hypertension and late hypotension that leads to certain subgroups in old age with increased susceptibility to brain atrophy and cognitive impairment.
This relation between low late-life DBP and brain pathology in participants with a long-term history of hypertension might be explained by several mechanisms. First, a causal mechanism explaining low BP–related brain pathology should be considered. Chronic hypertension can cause a reduction in resting cerebral blood flow,14,15 which in combination with impaired cerebrovascular autoregulation could lead to insufficient cerebral tissue perfusion during lower systemic BP levels, subsequently triggering the formation of brain pathology.15,16,32 Since the participants who had the smallest brain volumes and the lowest cognitive scores were more intensively treated for their hypertension (see table 2), it could be that aiming at less stringent BP targets through reduction of antihypertensive drugs prevents further brain pathology. Second, low DBP could be a risk indicator of comorbidities, such as coronary artery disease, heart failure, or increased arterial stiffness, and the relation between low BP and brain pathology might be due to an epiphenomenon that is not necessarily causal. Since high PP was not strongly related to brain structure and function (data not shown), it is unlikely that arterial stiffness is an important explanatory factor in the relation between low BP and the brain. However, adjusting for presence of heart failure explained a large part of the relation between lower SBP and brain atrophy, suggesting that low SBP in our study is an indicator of reduced cardiac output.33 Future studies including more detailed information on cardiac function and arterial stiffness are required to better understand the underlying mechanisms. Finally, low BP could be the consequence of brain atrophy, once it is severe enough. Although it is unlikely that in this sample without dementia the atrophy is so severe that it will cause a decline in BP, future analysis should focus on the longitudinal association of late-life BP and brain volumes.
Strengths of this study are the availability of both midlife and late-life data, the population-based setting, the large sample size, and the extensive range of cognitive tests. Also, the use of volumetric assessment of total and tissue-specific brain volumes allowed us to obtain precise estimates of brain structures. As with all studies of older participants, the results should be interpreted in the context of survivorship. In this case study, participants are survivors of the Reykjavik Study, and we know that the prevalence of midlife hypertension in our sample is lower than in the complete Reykjavik Study sample (34% vs 50%). This may lead to underrepresentation of those with hypertension at midlife, but there is no reason to think those people would have a different risk for altered brain structure and function than what we report here. Also, no information is available on the hypertension status between mid and late life. A large part of the participants without midlife hypertension developed hypertension throughout their life course, since approximately 50% use antihypertensive medication at late life. It is possible that these participants developed hypertension shortly after their midlife examination, and consequently had hypertension for a long time while classified as “no midlife hypertension.” This possible misclassification, however, would most likely have attenuated our findings in the “no midlife hypertension” group.
Our results indicate that older participants without a history of hypertension but who currently have high BP are at risk for cerebrovascular lesions, suggesting that lowering of BP in these participants might be beneficial. In contrast, older participants with a history of hypertension but who currently have lower BP might have more extensive target organ damage and are at risk of brain atrophy and cognitive impairment. Future studies should aim to better define patients who may be at higher risk to develop cerebral complications of lower BP and should investigate whether less stringent BP targets than currently recommended will prevent further brain damage in these patients so that more tailored advice for BP control could be given.
Supplementary Material
GLOSSARY
- AGES-Reykjavik Study
Age, Genee/Environment Susceptibility–Reykjavik Study
- ANCOVA
analysis of covariance
- BMI
body mass index
- BP
blood pressure
- CI
confidence interval
- DBP
diastolic blood pressure
- FLAIR
fluid-attenuated inversion recovery
- GM
gray matter
- GMV
gray matter volume
- ICD-10
International Classification of Diseases-10
- ICV
intracranial volume
- PP
pulse pressure
- SBP
systolic blood pressure
- TBV
total brain volume
- WM
white matter
- WML
white matter lesions
- WMV
white matter volume
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Muller: analysis and interpretation, writing manuscript, critical revision of the manuscript for important intellectual content. Dr. Sigurdsson: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Kjartansson: critical revision of the manuscript for important intellectual content. Dr. Aspelund: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Lopez: critical revision of the manuscript for important intellectual content. Dr. Jonnson: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Harris: study concept and design, critical revision of the manuscript for important intellectual content. Prof. van Buchem: critical revision of the manuscript for important intellectual content. Dr. Gudnason: study concept and design, critical revision of the manuscript for important intellectual content. Dr. Launer: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
Supported by a grant from the NIH (N01-AG-1-2100), National Institute on Aging Intramural Research Program, the Hjartavernd (the Icelandic Heart Association), the Althingi (the Icelandic Parliament), and Alzheimer Nederland (M.M.; WE.15-2011-02).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–1898 [DOI] [PubMed] [Google Scholar]
- 2.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation 2005;112:1644–1650 [DOI] [PubMed] [Google Scholar]
- 4.Godin O, Tzourio C, Maillard P, Mazoyer B, Dufouil C. Antihypertensive treatment and change in blood pressure are associated with the progression of white matter lesion volumes: the Three-City (3C)–Dijon Magnetic Resonance Imaging Study. Circulation 2011;123:266–273 [DOI] [PubMed] [Google Scholar]
- 5.Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial Cognitive Function Assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 2008;7:683–689 [DOI] [PubMed] [Google Scholar]
- 6.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 2005;4:487–499 [DOI] [PubMed] [Google Scholar]
- 7.Muller M, Smulders YM, de Leeuw PW, Stehouwer CD. Treatment of hypertension in the oldest old: a critical role for frailty? Hypertension 2014;63:433–441 [DOI] [PubMed] [Google Scholar]
- 8.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function: The Honolulu-Asia Aging Study. JAMA 1995;274:1846–1851 [PubMed] [Google Scholar]
- 9.Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension 2004;44:29–34 [DOI] [PubMed] [Google Scholar]
- 10.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011;77:461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Euser SM, van BT, Schram MT, et al. The effect of age on the association between blood pressure and cognitive function later in life. J Am Geriatr Soc 2009;57:1232–1237 [DOI] [PubMed] [Google Scholar]
- 12.Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012;60:2014–2019 [DOI] [PubMed] [Google Scholar]
- 13.Strandgaard S, Paulson OB. Cerebrovascular consequences of hypertension. Lancet 1994;344:519–521 [DOI] [PubMed] [Google Scholar]
- 14.Muller M, van der Graaf Y, Visseren FL, Mali WP, Geerlings MI. Hypertension and longitudinal changes in cerebral blood flow: the SMART-MR study. Ann Neurol 2012;71:825–833 [DOI] [PubMed] [Google Scholar]
- 15.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab 2008;7:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller M, van der Graaf Y, Visseren FL, Vlek AL, Mali WP, Geerlings MI. Blood pressure, cerebral blood flow, and brain volumes: The SMART-MR study. J Hypertens 2010;28:1498–1505 [DOI] [PubMed] [Google Scholar]
- 17.Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain 2011;134:3398–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med 2006;166:1003–1008 [DOI] [PubMed] [Google Scholar]
- 19.Muller M, van der Graaf Y, Algra A, Hendrikse J, Mali WP, Geerlings MI. Carotid atherosclerosis and progression of brain atrophy: the SMART-MR study. Ann Neurol 2011;70:237–244 [DOI] [PubMed] [Google Scholar]
- 20.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility–Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007;165:1076–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sigurdsson S, Aspelund T, Forsberg L, et al. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik Study. Neuroimage 2012;59:3862–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging 2002;21:1280–1291 [DOI] [PubMed] [Google Scholar]
- 23.Vidal JS, Sigurdsson S, Jonsdottir MK, et al. Coronary artery calcium, brain function and structure: the AGES-Reykjavik Study. Stroke 2010;41:891–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart R, Xue QL, Masaki K, et al. Change in blood pressure and incident dementia: a 32-year prospective study. Hypertension 2009;54:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veldink JH, Scheltens P, Jonker C, Launer LJ. Progression of cerebral white matter hyperintensities on MRI is related to diastolic blood pressure. Neurology 1998;51:319–320 [DOI] [PubMed] [Google Scholar]
- 26.Poels MM, Vernooij MW, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam Scan Study. Stroke 2010;41:S103–S106 [DOI] [PubMed] [Google Scholar]
- 27.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke 2002;33:21–25 [DOI] [PubMed] [Google Scholar]
- 28.de Leeuw FE, de Groot JC, Oudkerk M, et al. A follow-up study of blood pressure and cerebral white matter lesions. Ann Neurol 1999;46:827–833 [DOI] [PubMed] [Google Scholar]
- 29.Den Heijer T, Skoog I, Oudkerk M, et al. Association between blood pressure levels over time and brain atrophy in the elderly. Neurobiol Aging 2003;24:307–313 [DOI] [PubMed] [Google Scholar]
- 30.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet 1996;347:1141–1145 [DOI] [PubMed] [Google Scholar]
- 31.Jochemsen HM, Muller M, Visseren FL, et al. Blood pressure and progression of brain atrophy: the SMART-MR Study. JAMA Neurol 2013;70:1046–1053 [DOI] [PubMed] [Google Scholar]
- 32.Suter OC, Sunthorn T, Kraftsik R, et al. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke 2002;33:1986–1992 [DOI] [PubMed] [Google Scholar]
- 33.van Bemmel T, Holman ER, Gussekloo J, Blauw GJ, Bax JJ, Westendorp RG. Low blood pressure in the very old, a consequence of imminent heart failure: the Leiden 85-plus study. J Hum Hypertens 2009;23:27–32 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.