Abstract
Objective:
To investigate the potential links between thinning of retinal ganglion cell axons in eyes of patients with multiple sclerosis (MS) without past optic neuritis (ON) and MS-related inflammatory damage of the posterior visual pathway.
Methods:
Temporal retinal nerve fiber layer (tRNFL) thickness was analyzed in eyes with no history of ON (NON) from 53 patients with relapsing-remitting MS. Fifty normal age- and sex-matched controls were examined with optical coherence tomography. Low-contrast visual acuity charts were used for functional assessment of vision. The optic tract (OT) and optic radiation (OR) were identified using probabilistic tractography, and volume of T2 fluid-attenuated inversion recovery lesions and diffusion tensor imaging (DTI) indices were measured within both structures. Cross-sectional diameter of the OT was also calculated.
Results:
tRNFL thickness was significantly reduced in NON eyes and was associated with reduced low-contrast visual acuity. Lesions within the OR were detected in the majority of patients. There was a significant correlation between thinning of the tRNFL and OR lesion volume (adjusted for non-OR lesion volume, age, sex, and disease duration). tRNFL thickness also correlated with OR DTI indices. No OT lesions were identified in any of the patients and no relationship between retinal nerve fiber layer loss and potential markers of OT lesions was found.
Conclusion:
The results demonstrate a strong tract-specific association between loss of tRNFL fibers and MS-related inflammation within OR.
Susceptibility of the visual system to damage in multiple sclerosis (MS)1–3 and its hierarchical organization coupled with recent technological advances make it an ideal model to study pathophysiology of the disease. Retinal ganglion cells (RGC) are of particular interest since their unique position and accessibility to direct in vivo measurement may be applied to study MS-related neurodegeneration, including the possible effect of pathologic changes in neighboring cellular elements.
Optic neuritis (ON) typically results in loss of a significant number of RGC axons,4 which can be quantified by measuring retinal nerve fiber layer (RNFL) thickness.
In addition, thinning of RNFL in patients with MS without a history of ON (MS-NON) has been reported recently.5–16 However, the pathologic basis for this thinning is uncertain. A number of explanations, such as subclinical inflammation of the optic nerve, primary retinal pathology, or retrograde degeneration, have been offered, but no convincing evidence has been produced to support any of those hypotheses.6,8,15
We recently reported an association between RNFL thinning and latency delay of the multifocal visual evoked potentials (mfVEP) in NON eyes of patients with MS.5 In view of the binocular character of the mfVEP delay and RNFL thinning, we hypothesized that this relationship may be attributed to retrograde degeneration of axons damaged by acute inflammation in the retro-chiasmal part of the visual pathway at the level of the optic tracts (OT) or optic radiations (OR).
As a result of recent technological advances in tractography, both the OT and the OR can now be isolated from surrounding white matter tissue and closely studied in individual patients.17 In addition, coregistration of diffusion tensor imaging (DTI) with T2 fluid-attenuated inversion recovery (FLAIR) images allows direct measurement of lesional load and diffusivity indices within those structures.
Therefore, in order to verify our hypothesis, in the current study we investigated the association between axonal thinning of the RGC in NON eyes of patients with MS and markers of primary MS-related inflammation of the retro-chiasmal visual pathway.
METHODS
Subjects.
Fifty-three consecutive patients with relapsing-remitting MS (RRMS) without a history of clinical ON in at least one eye were enrolled. Patients with any other systemic or ocular disease were excluded. Fifty normal age- and sex-matched controls were examined with optical coherence tomography (OCT).
Clinical assessments.
Best-corrected visual acuity (VA) was measured using low-contrast letter acuity Sloan charts (LCLA) (1.25% and 2.5%) at 4 meters. The numbers of letters correctly identified (maximum 60/chart) were recorded for each eye.
Optical coherence tomography.
OCT was performed using Spectralis (Heidelberg Engineering, Carlsbad, CA). Global (gRNFL) and temporal (tRNFL) RNFL thickness was assessed using the axonal RNFL protocol. In addition, a radial protocol using a star-like pattern of line scans centered on the macula was used. A detailed description of the analysis and segmentation procedure can be found elsewhere.18 The inner nuclear layer (INL), combined ganglion cell layer and inner plexiform layer (GCL/IPL), and combined outer plexiform layer, outer nuclear layer, and photoreceptor inner segment layer (OPL/ONL/PIS) were analyzed.
MRI.
MRI data were collected using 3.0T scanner with a 32-channel head coil (GE Healthcare, Little Chalfont, UK). MRI sequences are described in the supplemental data on the Neurology® Web site at Neurology.org. Probabilistic tractography17 was used to reconstruct OT and OR fibers (figure 1A). The reconstruction procedure is described in the supplemental data. MS lesions were identified on coregistered T2 FLAIR images and segmented automatically using ITK-SNAP software (figure 1B). Lesions were then intersected with visual pathway fibers (figure 1C) to calculate OT and OR lesion volume (figure 1D). Averaged (between left and right side) lesion volume was used for main analysis. OR lesion volume asymmetry was calculated as a difference between left and right OR lesion volume. DTI indices (fractional anisotropy [FA], mean diffusivity [MD], axial diffusivity [AD], and radial diffusivity [RD]) were calculated along OT and OR using MrVISTA software (Stanford University, Stanford, CA) (figure 1E). Asymmetry of DTI indices was calculated as a difference between left and right side.
Figure 1. Consecutive steps of MRI analysis.
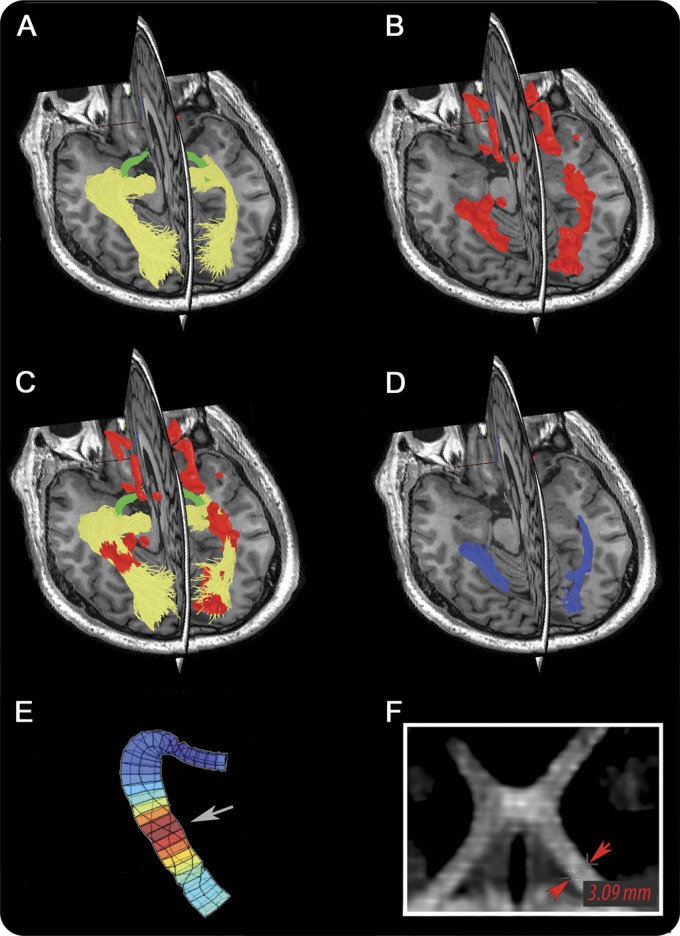
Quantification of the visual pathway lesions and diffusion tensor imaging indices. (A) 3D posterior visual pathway reconstruction using probabilistic tractography. Green = optic tract, yellow = optic radiation (OR). (B) Brain T2 fluid-attenuated inversion recovery lesions. (C) Coregistration of T2 lesions with visual pathway fibers. (D) Visual pathway lesions (blue). (E) Example of OR radial diffusivity from the same patients. Note significant diffusivity increase at the location of the lesion (arrow). (F) MRI of the optic tract was rotated using 3DSlicer software to provide a parallel view of chiasm and optic tracts. Red arrows indicate the site where diameter of the optic tract was measured. For A and B, T1 is used to display structural brain image.
Two indirect ways to assess primary OT damage were also used: asymmetry between left and right OT diameter and asymmetry between left and right OT diffusivity indices. Both methods were based on the assumption that, similar to the optic nerve, MS lesions in the OT are unlikely to be bilateral. The diameter of OT was measured using high-resolution volumetric T1 images (see supplemental data) (figure 1F).
Statistics.
Statistical analysis was performed using SPSS 21.0 (SPSS, Chicago, IL).
Pearson correlation coefficient was used for bivariate correlation. Where partial correlation was used, it was adjusted for age, sex, and duration of the disease. Student t test was used to assess difference between patients with MS and normal controls. Univariate general linear model adjusted for age and sex was used to analyze differences between multiple groups.
Standard protocol approvals, registrations, and patient consents.
All procedures followed the tenets of the Declaration of Helsinki and written informed consent was obtained from all participants.
RESULTS
Fifty-three patients with RRMS were enrolled (age 40.2 ± 11.6 years, 39 women and 14 men). Average time from diagnosis of MS was 4.8 ± 3.1 years. There were 22 patients with a history of ON in 1 eye (at least 12 months prior to the study) and 31 patients without ON in either eye. One eye was randomly selected for patients without a history of ON; the NON eye was used in patients with a history of previous ON.
RNFL analysis.
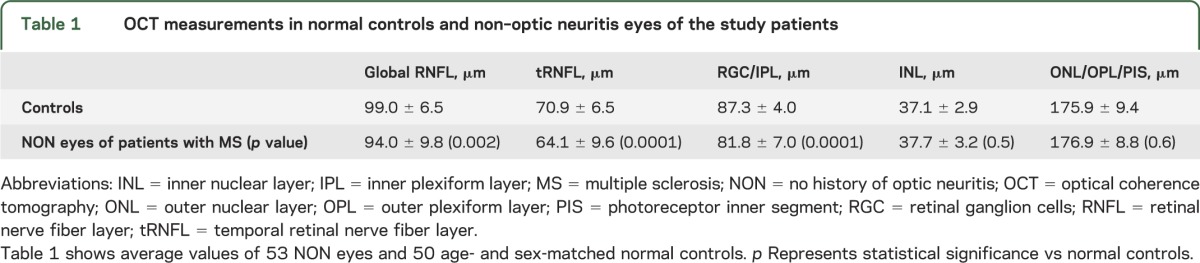
There was a significant reduction of global and temporal RNFL thickness and GCL/IPL thickness in MS-NON eyes compared to normal controls, whereas thickness of INL and OPL/ONL/PIS remained unchanged (table 1). Since tRNFL demonstrated considerably larger thinning compared to gRNFL and GCL/IPL (10% thinning of tRNFL vs 5% thinning of gRNFL and 6% of GCL/IPL thinning compared to mean values of normal controls), only results for tRNFL are reported here to simplify further analysis.
Table 1.
OCT measurements in normal controls and non–optic neuritis eyes of the study patients
There was a significant correlation between tRNFL thickness and LCLA at 2.5% and 1.25% luminance contrast (r = 0.45, p = 0.003 and r = 0.41, p = 0.01, respectively).
tRNFL thickness, however, did not correlate with age (p = 0.9) or disease duration (p = 0.1). There was also no difference in tRNFL thickness between male and female patients (p = 0.7).
Binocular nature of RNFL thinning in patients without a history of ON in either eye has previously been suggested.5 However, in order to exclude potential effect of the inter-eye correlation, in this study we performed analysis of inter-eye relationship only in NON patients with pathologically reduced RNFL thickness (below 95th percentile of RNFL thickness in normal controls). The correlation of tRNFL thickness between the 2 eyes remained high (r = 0.78, p < 0.001), confirming the binocular nature of RNFL thinning.
MRI analysis.
Fifty-two patients (98%) had detectable T2 FLAIR brain lesions. The average volume of brain lesions was 8,493 mm2. After intersecting T2 FLAIR images with OT fibers, no visible lesions of the OT were found in any of the patients. Lesions within OR were detected in 38 patients (72%). The average OR lesion volume was 743 mm2 and the correlation between brain lesion volume and OR lesion volume was high (r = 0.85, p < 0.001).
The occurrence of OR lesions did not differ among patients without prior ON and those with prior ON in our MS cohort (χ2 test, Fisher exact test, p = 0.7). Male patients had significantly larger OR lesion load compared to female patients (1,390 mm2 vs 511 mm2, p = 0.01), and OR lesion volume displayed a tendency for an association with disease duration (p = 0.054), but did not correlate with age of the patients (p = 0.2). The OR lesion volume demonstrated significant negative correlation with LCLA at 2.5% and 1.25% luminance contrast (r = −0.4, p = 0.01 and r = −0.38, p = 0.02, respectively). Combination of RNFL thickness and OR lesion volume explained 19% of variation in 1.25% LCVA (analysis of variance [ANOVA] = 0.02) and 16% of variation in 2.5% LCVA (ANOVA = 0.03).
Linear regression analysis revealed significant correlation between OR lesion volume and tRNFL thickness (r = −0.64, p < 0.001) (figure 2). Correction for non-OR white matter lesion volume (which is necessary to determine tract-specific relationship), as well as age, sex, and disease duration had minimal effect on this association (partial correlation r = −0.57, p < 0.001).
Figure 2. Partial correlation between temporal retinal nerve fiber layer thickness and optic radiation lesion volume.
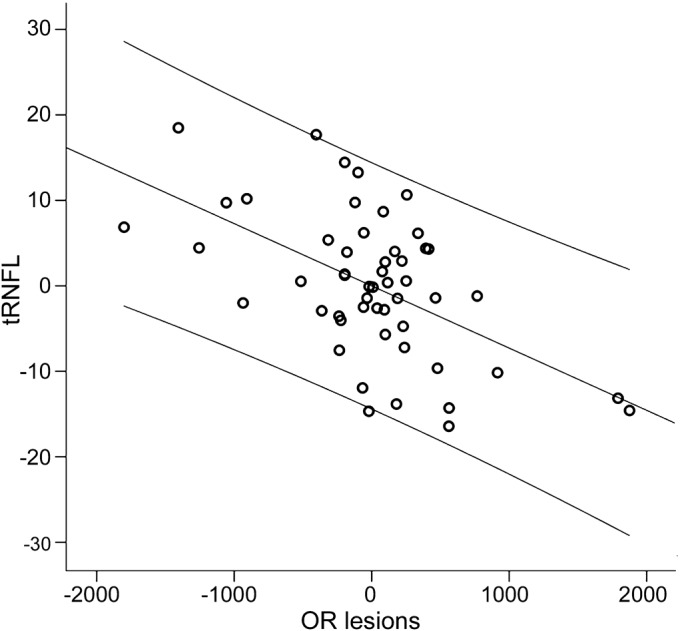
Partial regression plot between temporal retinal nerve fiber layer (tRNFL) thickness and optic radiation (OR) lesion volume. Dependent variable = tRNFL thickness. Independent variable = OR lesion volume adjusted for lesion volume outside of OR, disease duration, sex, and age. Axes represent residuals. Linear fit and 95% individual confidence intervals are shown.
Analysis of patients without history of ON.
Potential expansion of a lesion from the ON eye into the chiasm may cause RNFL thinning of the fellow eye. In order to exclude this possibility, patients were separated into 2 groups based on the presence of a history of ON. There was no difference in disease duration (p = 0.5), total (p = 0.4), or OR lesion volume (p = 0.3) between the groups.
The univariate general linear model adjusted for age and sex demonstrated significant reduction of tRNFL thickness compared to control eyes in fellow eyes of ON patients (63.4 ± 9.6, p = 0.002) and study eyes of NON patients (64.4 ± 9.6, p = 0.004). There was, however, no difference in tRNFL thickness between the groups (p = 0.9).
The correlation between tRNFL thickness and OR lesion volume, adjusted for non-OR lesion volume, age, sex, and disease duration, remained highly significant for both groups. Furthermore, tRNFL thickness of the study eyes of the patients without history of ON in either eye demonstrated noticeably larger correlation with OR lesion volume (r = −0.59, p = 0.001) when compared to tRNFL thickness of the fellow eyes of ON patients (r = −0.51, p = 0.02).
Analysis of OR DTI indices.
Diffusion indices (FA, MD, AD, and RD) represent another measure of brain tissue integrity. It has been demonstrated that DTI indices are significantly more abnormal in lesions than in normal-appearing white matter.19,20 Accordingly, we found DTI indices correlated highly with OR lesion volume (in order to determine tract-specific relationship, the correlation was corrected for lesion volume outside of the OR) (table 2).
Table 2.
Correlation between optic radiation DTI indices and optic radiation lesion volume and tRNFL thickness
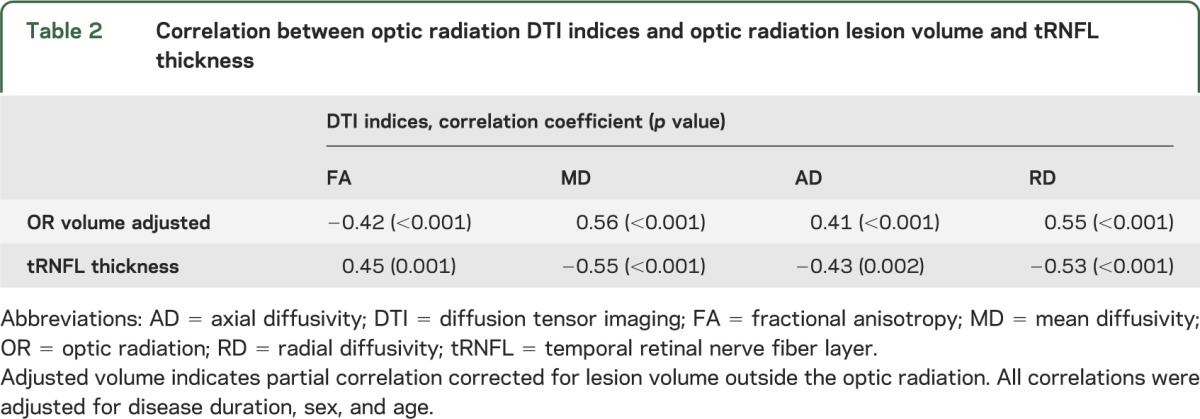
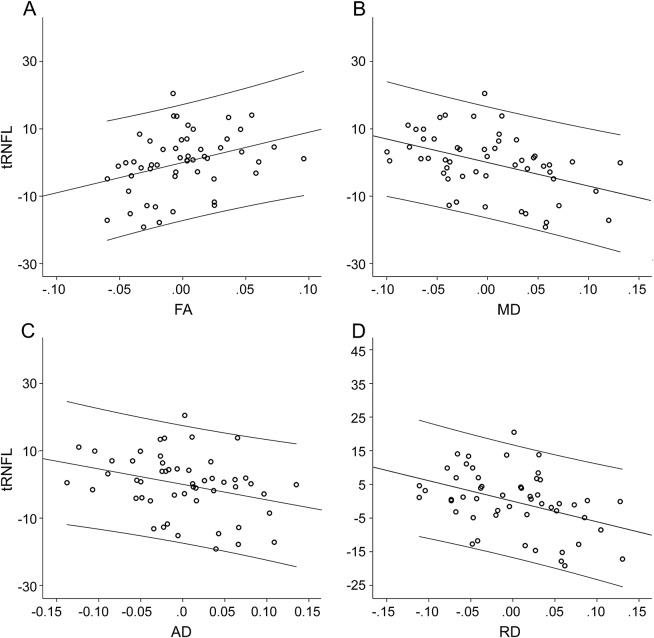
The correlation of DTI indices with tRNFL thickness revealed highly significant associations (table 2). FA demonstrated a positive correlation with tRNFL thickness, while all diffusivity measures were inversely associated with tRNFL. All correlations were adjusted for disease duration, sex, and age (figure 3).
Figure 3. Partial correlations between temporal retinal nerve fiber layer thickness and optic radiation diffusion tensor imaging indices.
Partial correlations between temporal retinal nerve fiber layer (tRNFL) thickness and optic radiation diffusion tensor imaging indices adjusted for disease duration, sex, and age. Axes represent residuals. Linear fit and 95% individual confidence intervals are shown. (A) Fractional anisotropy (FA). (B) Mean diffusivity (MD). (C) Axial diffusivity (AD). (D) Radial diffusivity (RD).
Optic tract analysis.
There were no OT lesions detected in our study cohort, but as the identification of lesions can be challenging due to the small size and convoluted shape of the OT, we also used left/right asymmetry of the OT diameter as an indirect measure of OT primary damage. Since OT fibers are partially crossing at the chiasm, OT lesions are likely to affect both eyes. Therefore, to investigate possible effect of OT lesions on loss of RNFL fibers, the correlation between tRNFL thickness of the study eye and asymmetry of the OT diameter was studied. Analysis of OT asymmetry, however, demonstrated significant association with left/right asymmetry of the OR lesion volume (r = −0.42, p = 0.001), which may (at least theoretically) be a result of trans-neuronal degeneration of OT fibers caused by OR lesions. Therefore, in order to correct for this factor, correlation between RNFL thickness and OT thickness asymmetry was adjusted for OR lesion volume asymmetry. The resulting partial correlation was not significant.
Similarly, left/right asymmetry of the OT DTI indices may indirectly indicate presence of OT lesions. Therefore, tRNFL thickness was correlated with absolute values of left/right asymmetry of the OT DTI indices, and when the correlations were adjusted for left/right asymmetry of OR DTI indices none of the partial correlations were significant.
DISCUSSION
The results of this study confirmed previous reports of RNFL and GCL/IPL thinning in NON eyes of patients with MS.5,6,21–23 Preferential loss of temporal RNFL is also consistent with earlier studies5,6,21–23 and may be related to the fact that tRNFL subserves the central retina including the macula and is represented by small-diameter fibers, which are reported to be more susceptible to damage in MS.24,25 Furthermore, the functional significance of the tRNFL thinning is supported by its correlation with low-contrast VA, as demonstrated in this study. Relatively small thinning of GCL/IPL may be related to the fact that current segmentation of the retinal layer does not allow separation of RGC layer from IPL (which constitutes almost 50% of combined GCL/IPL thickness but is not affected by degeneration of the RGC).
We have also reinforced our previous observation that in patients with MS without history of ON in either eye, tRNFL thinning tends to be binocular and symmetrical. The binocular nature of RNFL thinning implies its retro-chiasmal origin. Since the OT consist of the posterior part of the RGC axons themselves, lesions in the OT may result in retrograde degeneration of RGC axons and subsequent RNFL thinning. However, we did not find OT lesions in any of the patients, which is in agreement with previous studies demonstrating that lesions of the OT are rare in MS.26,27 There was also no relationship between tRNFL loss and potential markers of OT primary damage (both OT thickness asymmetry and OT DTI indices asymmetry), making the OT an unlikely site of primary RGC axonal damage.
In contrast, OR lesions were detected in the majority of patients and there was evidence to suggest a link between the status of the OR and thinning of the tRNFL. Thus, this association is strongly supported by significant tract-specific relationship between the RNFL thinning and the volume of OR lesions.
Subanalysis of patients without history of ON in either eye revealed an even higher correlation between tRNFL thickness and OR lesion load. This finding confirmed the strong association between RGC axonal thinning in NON eyes with inflammatory MS-related damage of the OR and suggested a potential masking effect on this relationship by previous ON in the other eye.
In addition, significant correlations were found between tRNFL loss and DTI indices in the OR. FA was inversely related to tRNFL thickness, while other diffusivity indices demonstrated a positive relationship with RNFL. In particular, we found that RD demonstrated the highest degree of correlation with tRNFL thickness.
This is in agreement with a previous report19 that found a significant tract-specific association between RNFL thickness with FA and RD (but not MD or AD). The higher degree of correlation between DTI indices and RNFL thickness reported in our study may be related to the fact that, contrary to the previous report,19 only patients with RRMS were included in our study. In addition, ON eyes were specifically excluded from the analysis.
The nature of diffusivity changes in MS remains elusive. Despite the fact that earlier studies linked axial diffusivity to axonal loss and radial diffusivity with myelin content, underlying pathologic specificity of DTI-derived parameters is speculative.28,29 What is understood, however, is that decreased FA and increased diffusivity are markers of tissue damage in MS and those changes are more prominent in lesions compared to normal-appearing white matter.19,20 Increased RD, in particular, was found to be related to overall tissue integrity within chronic MS lesions.30 Therefore, a close relationship between tRNFL loss and DTI metrics, described in this study, further links axonal thinning of RGC with primary damage of OR white matter.
The topographic correlation found between OR lesion volume and OT thickness also strengthens connection between primary lesional damage of the OR and RGC axonal loss. Since it is highly unlikely that inflammatory demyelination of the OT will cause change in OR lesion volume or that MS lesions are side-selective, i.e., preferentially affect the left or right side of the visual pathway, we hypothesize that this association may be driven by MS-related inflammatory damage of the OR.
Finally, preferential damage of tRNFL fibers supplying the central part of the visual field is also consistent with the potential role of OR lesions in RGC axonal thinning. More than 50% of visual cortex subserves just the central 10 degrees of the retina.31 This overrepresentation of the central visual field is largely formed at the retinal level and preserved in the OR.32 Assuming a uniform distribution of MS lesions within the OR, it is likely that OR fibers subserving central vision are damaged more frequently, which in turn may cause more extensive thinning of tRNFL fibers.
Taken together, our analysis of the posterior visual pathway indicates a close tract-specific association between MS-related inflammatory damage of the OR and thinning of RGC axons. However, the OR is formed by axons of more proximal neurons located in the lateral geniculate nucleus. Therefore, a damaging effect of OR lesions would require trans-neuronal transmission to reach RGC axons.
The phenomenon of trans-synaptic degeneration in the visual pathway was first described in196033 and compelling evidence advocating its existence has emerged from animal and human studies since then.24,34–39 Moreover, evidence of trans-synaptic retrograde degeneration has recently been reported in the visual system of patients with MS.19,20 However, while the observed relationship between tRNFL thinning and damage of the OR may suggest involvement of the trans-neuronal degeneration, several limitations of this study prevent us from drawing a definite causative link.
First, the size of the OR was determined based on currently existing fibers. Considering the substantial disease duration, it is reasonable to assume that a significant number of axons within the OR have already been lost at the time of examination. Therefore, the true volume of the OR and, consequently, the true size of OR lesions are likely to be underestimated. Similar reasoning would apply to DTI indices. Therefore, both structural and functional measures only partially reflect axonal loss in the OR caused by previous lesional activity, which may explain the moderate degree of correlation.
Second, it is difficult to eliminate the possibility of subclinical ON in the study eye. However, the symmetrical nature of the binocular RNFL thinning would argue against this. In addition, while subclinical ON may potentially cause some loss of tRNFL, it does not explain its tract-specific association with OR lesion volume. It may be theoretically possible that both structures, by virtue of sharing the same pathway, may also share similar burden of the disease, but the lack of association between the incidence of the OR lesions and occurrence of acute ON makes it unlikely.
Third, the potential impact of cortical lesions has not been assessed in this study, but it should be noted that rate of in vivo detection of the cortical lesion is very low even using the best currently available MRI sequences.
Finally, the cross-sectional design of the study is only able to demonstrate association, not causation. A longitudinal study, which is now under way, may provide a more realistic assessment of the relationship between RGC axonal thinning and inflammatory OR damage by analyzing changes in chronological order.
Supplementary Material
GLOSSARY
- AD
axial diffusivity
- ANOVA
analysis of variance
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FLAIR
fluid-attenuated inversion recovery
- GCL
ganglion cell layer
- gRNFL
global retinal nerve fiber layer
- INL
inner nuclear layer
- IPL
inner plexiform layer
- LCLA
low-contrast letter acuity
- MD
mean diffusivity
- mfVEP
multifocal visual evoked potentials
- MS
multiple sclerosis
- NON
no history of optic neuritis
- OCT
optical coherence tomography
- ON
optic neuritis
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- OR
optic radiations
- OT
optic tracts
- PIS
photoreceptor inner segment
- RD
radial diffusivity
- RGC
retinal ganglion cells
- RNFL
retinal nerve fiber layer
- RRMS
relapsing-remitting multiple sclerosis
- tRNFL
temporal retinal nerve fiber layer
- VA
visual acuity
Footnotes
Editorial, page 2152
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
A. Klistorner: study concept or design, acquisition of data, analysis or interpretation of data, drafting/revising the manuscript for content, including medical writing for content. P. Sriram: acquisition of data, analysis or interpretation of data. N. Vootakuru: analysis or interpretation of data. C. Wang: analysis or interpretation of data. M.H. Barnett: study concept or design, drafting/revising the manuscript for content. R. Garrick: study concept or design, drafting/revising the manuscript for content. J. Parratt: study concept or design, drafting/revising the manuscript for content. N. Levin: analysis or interpretation of data. N. Raz: analysis or interpretation of data. A. van der Walt: analysis or interpretation of data, drafting/revising the manuscript for content. L. Masters: analysis or interpretation of data. S.L. Graham: study concept or design, drafting/revising the manuscript for content. C. Yiannikas: study concept or design, analysis or interpretation of data, drafting/revising the manuscript for content.
STUDY FUNDING
Supported by Save Neuron grants from Novartis, Sydney Medical Foundation (grant number E34), and Sydney Hospital Foundation.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Sorensen TL, Frederiksen JL, Bronnum-Hansen H, Petersen HC. Optic neuritis as onset manifestation of multiple sclerosis. Neurology 1999;53:473–478 [DOI] [PubMed] [Google Scholar]
- 2.Hornabrook RS, Miller D, Newton MR, et al. Frequent involvement of optic radiation in patients with acute isolated optic neuritis. Neurology 1992;42:77–79 [DOI] [PubMed] [Google Scholar]
- 3.Jenkins T, Ciccarelli O, Toosy A, et al. Dissecting structure-function interactions in acute optic neuritis to investigate neuroplasticity. Hum Brain Mapp 2010;31:276–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petzold A, de Boer JF, Schippling S, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 2010;9:921–932 [DOI] [PubMed] [Google Scholar]
- 5.Klistorner A, Garrick R, Barnett MH, et al. Axonal loss in non-optic neuritis eyes of patients with multiple sclerosis linked to delayed visual evoked potential. Neurology 2013;15:242–245 [DOI] [PubMed] [Google Scholar]
- 6.Pueyo V, Martin J, Fernandez J, et al. Axonal loss in the retinal fiber layer in patients with multiple sclerosis. Mult Scler 2008;14:609–614 [DOI] [PubMed] [Google Scholar]
- 7.Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, Garcia-Layana A, Bejarana B, Villoslada P. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology 2007;66:1488–1494 [DOI] [PubMed] [Google Scholar]
- 8.Talman LS, Bisker ER, Sackel BS, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol 2010;67:749–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson AP, Trip SA, Schlottmann PG, et al. A preliminary longitudinal study of the retinal nerve fiber layer in progressive multiple sclerosis. J Neurol 2010;257:1083–1091 [DOI] [PubMed] [Google Scholar]
- 10.Gordon-Lipkin E, Chodkowski B, Reich DS, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 2007;69:1603–1609 [DOI] [PubMed] [Google Scholar]
- 11.Fisher JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 2006;113:324–332 [DOI] [PubMed] [Google Scholar]
- 12.Siger M, Dziegiewski K, Jasek L, et al. Optical coherence tomography in multiple sclerosis. J Neurol 2008;255:1555–1560 [DOI] [PubMed] [Google Scholar]
- 13.Costello F, Hodge W, Pan YI, Freedman M, DeMeulemeester C. Differences in retinal nerve fiber layer atrophy between multiple sclerosis subtypes. J Neurol Sci 2009;281:74–79 [DOI] [PubMed] [Google Scholar]
- 14.Parisi V, Manni G, Centofanti M, Gandolfi SA, Olzi D, Bucci MG. Correlation between optical coherence tomography, pattern electroretinogram, and visual evoked potentials in open-angle glaucoma patients. Ophthalmology 2001;108:905–912 [DOI] [PubMed] [Google Scholar]
- 15.Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman EM, Cutter GR, Calabresi PA. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology 2007;69:2085–2092 [DOI] [PubMed] [Google Scholar]
- 16.Bock M, Brandt AU, Dorr J, et al. Pattern of retinal nerve fiber layer loss in multiple sclerosis patients with or without optic neuritis and glaucoma patients. Clin Neurol Neurosurg 2010;112:647–652 [DOI] [PubMed] [Google Scholar]
- 17.Sherbondy AJ, Dougherty RF, Napel S, Wandell BA. Identifying the human optic radiation using diffusion imaging and fiber tractography. J Vis 2008;8:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sriram P, Graham SL, Wang C, Yiannikas C, Garrick R, Klistorner A. Transsynaptic retinal degeneration in optic neuropathies: optical coherence tomography study. Invest Ophthalmol Vis Sci 2012;53:1271–1275 [DOI] [PubMed] [Google Scholar]
- 19.Reich DS, Smith SA, Gordon-Lipkin EM, et al. Damage to optic radiation in multiple sclerosis is associated with retinal injury and visual disability. Arch Neurol 2009;66:998–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocca MA, Mesaros S, Preziosa P, et al. Wallerian and trans-synaptic degeneration contribute to optic radiation damage in multiple sclerosis: a diffusion tensor MRI study. Mult Scler 2013;19:1610–1617 [DOI] [PubMed] [Google Scholar]
- 21.Gundogan FC, Demirkaya S, Sobaci G. Is optical coherence tomography really a new biomarker candidate in multiple sclerosis? Invest Ophthalmol Vis Sci 2007;48:5773–5781 [DOI] [PubMed] [Google Scholar]
- 22.Henderson AP, Trip SA, Schlottmann PG, et al. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain 2008;131:277–287 [DOI] [PubMed] [Google Scholar]
- 23.Gelfand JM, Goodin DS, Boscardin WJ, Nolan R, Cuneo R, Green AJ. Retinal axonal loss begins early in the course of multiple sclerosis and is similar between progressive phenotypes. PloS ONE 2012;7:e36847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Size-selective neuronal changes in the anterior optic pathways suggest a differential susceptibility to injury in multiple sclerosis. Brain 2001;124:1813–1820 [DOI] [PubMed] [Google Scholar]
- 25.Ganter P, Prince C, Esiri MM. Spinal cord axonal loss in multiple sclerosis: a post-mortem study. Neuropathol Appl Neurobiol 1999;25:459–467 [DOI] [PubMed] [Google Scholar]
- 26.Dasenbrock HH, Smith SA, Ozturk A, Farrell SK, Calabresi PA, Reich DS. Diffusion tensor imaging of the optic tracts in multiple sclerosis: association with retinal thinning and visual disability. J Neuroimaging 2011;21:e41–e49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenblatt MA, Behrens MM, Zweifach PH, et al. Magnetic resonance imaging of optic tract involvement in multiple sclerosis. Am J Ophthalmol 1987;104:74–79 [DOI] [PubMed] [Google Scholar]
- 28.Wheeler-Kingshott CAM, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med 2009;61:1255–1260 [DOI] [PubMed] [Google Scholar]
- 29.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002;17:1429–1436 [DOI] [PubMed] [Google Scholar]
- 30.Klawiter EC, Schmidt RB, Trinkaus K, et al. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage 2011;55:1454–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horton JC, Hoyt WF. The representation of the visual field in the human striate cortex. Arch Ophthalmol 1991;109:816–824 [DOI] [PubMed] [Google Scholar]
- 32.Chaplin TA, Yu HH, Rosa MG. Representation of the visual field in the primary visual area of the marmoset monkey: magnification factors, point-image size, and proportionality to retinal ganglion cell density. J Comp Neurol 2013;521:1001–1019 [DOI] [PubMed] [Google Scholar]
- 33.Matthews MR, Cowan WM, Powell TP. Transneuronal cell degeneration in the lateral geniculate nucleus of the Macaque monkey. J Anat 1960;94:145–168 [PMC free article] [PubMed] [Google Scholar]
- 34.Weller RE, Kaas JH. Parameters affecting the loss of ganglion cells of the retina following ablation of striate cortex in primates. Vis Neurosci 1989;3:327–349 [DOI] [PubMed] [Google Scholar]
- 35.Ciccarelli O, Toosy AT, Hickman SJ, et al. Optic radiation changes after optic neuritis detected by tractography-based group mapping. Hum Brain Mapp 2005;25:308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta JS, Plant GT. Optical coherence tomography findings in congenital/long-standing homonymous hemianopia. Am J Ophthalmol 2005;140:727–729 [DOI] [PubMed] [Google Scholar]
- 37.Cowey A, Alexander I, Stoerig P. Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in hemianopic monkeys and humans. Brain 2011;134:2149–2157 [DOI] [PubMed] [Google Scholar]
- 38.Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain 2009;132:628–634 [DOI] [PubMed] [Google Scholar]
- 39.Bridge H, Jindahra P, Barbur J, Plant GT. Imaging reveals optic tract degeneration in hemianopia. Invest Ophthalmol Vis Sci 2011;52:382–388 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.