Abstract
Objective:
We aimed to evaluate the combined effects of HIV and APOE ε4 allele(s) on glial metabolite levels, and on known cognitive deficits associated with either condition, across the ages.
Methods:
One hundred seventy-seven participants, primarily of white and mixed race (97 seronegative subjects: aged 44.7 ± 1.3 years, 85 [87.6%] men, 28 [28.9%] APOE ε4+; 80 HIV+ subjects: aged 47.3 ± 1.1 years, 73 [91.3%] men, 23 [28.8%] APOE ε4+), were assessed cross-sectionally for metabolite concentrations using proton magnetic resonance spectroscopy in 4 brain regions and for neuropsychological performance.
Results:
Frontal white matter myo-inositol was elevated in subjects with HIV across the age span but showed age-dependent increase in seronegative subjects, especially in APOE ε4+ carriers. In contrast, only seronegative APOE ε4+ subjects showed elevated myo-inositol in parietal cortex. All APOE ε4+ subjects had lower total creatine in basal ganglia. While all HIV subjects showed greater cognitive deficits, HIV+ APOE ε4+ subjects had the poorest executive function, fluency memory, and attention/working memory. Higher myo-inositol levels were associated with poorer fine motor function across all subjects, slower speed of information processing in APOE ε4+ subjects, and worse fluency in HIV+ APOE ε4+ subjects.
Conclusions:
In frontal white matter of subjects with HIV, the persistent elevation and lack of normal age-dependent increase in myo-inositol suggest that persistent glial activation attenuated the typical antagonistic pleiotropic effects of APOE ε4 on neuroinflammation. APOE ε4 negatively affects energy metabolism in brain regions rich in dopaminergic synapses. The combined effects of HIV infection and APOE ε4 may lead to greater cognitive deficits, especially in those with greater neuroinflammation. APOE ε4 allele(s) may be a useful genetic marker to identify white and mixed-race HIV subjects at risk for cognitive decline.
Combination antiretroviral therapy (cART) prolongs the life expectancy of HIV-infected individuals. However, the prevalence of HIV-associated neurocognitive disorders (HAND) continues to increase,1 in part because of the greater prevalence of HAND in older individuals (>50 years), who will comprise the majority of the infected population in the United States by 2015. HAND may result from the direct neurotoxic effects of HIV viral proteins and the host's neuroinflammatory responses. Some antiretrovirals (ARVs), many frequently abused substances (e.g., stimulants and cannabis), comorbid factors associated with aging (e.g., hypertension, diabetes), and certain genotypes may further exacerbate HAND. For example, the APOE ε4 allele, the strongest genetic risk factor for Alzheimer disease (AD) in older individuals,2 was associated with accelerated progression of HIV disease,3 and increased risk of HAND in some,4–8 but not all, studies.9,10 These conflicting findings may be partly attributable to antagonistic pleiotropy, because the APOE ε4 allele(s)5,11 has a negative effect on cognition only in older individuals.
Because HAND is often difficult to assess in the clinical setting,12 understanding how APOE ε4 allele(s) affects brain injury in patients with HIV may be useful for prognostication or early identification of individuals at risk for HAND. Therefore, we aimed to evaluate whether APOE ε4 allele(s) exacerbates brain metabolite or cognitive abnormalities in HIV+ subjects and whether age further interacts with these variables. We hypothesized that HIV+ subjects with APOE ε4 would have greater cognitive deficits and glial metabolite abnormalities on proton magnetic resonance spectroscopy (MRS) than subjects without ε4+ and that such abnormalities would be present even in the younger subjects.
METHODS
Standard protocol approvals, registrations, and patient consents.
The protocol was approved by the institutional review boards at the University of Hawaii and at the Queen's Medical Center. Three hundred ninety subjects from the local community were recruited between December 2004 and August 2012 and provided oral and written consents before the assessments.
Research participants.
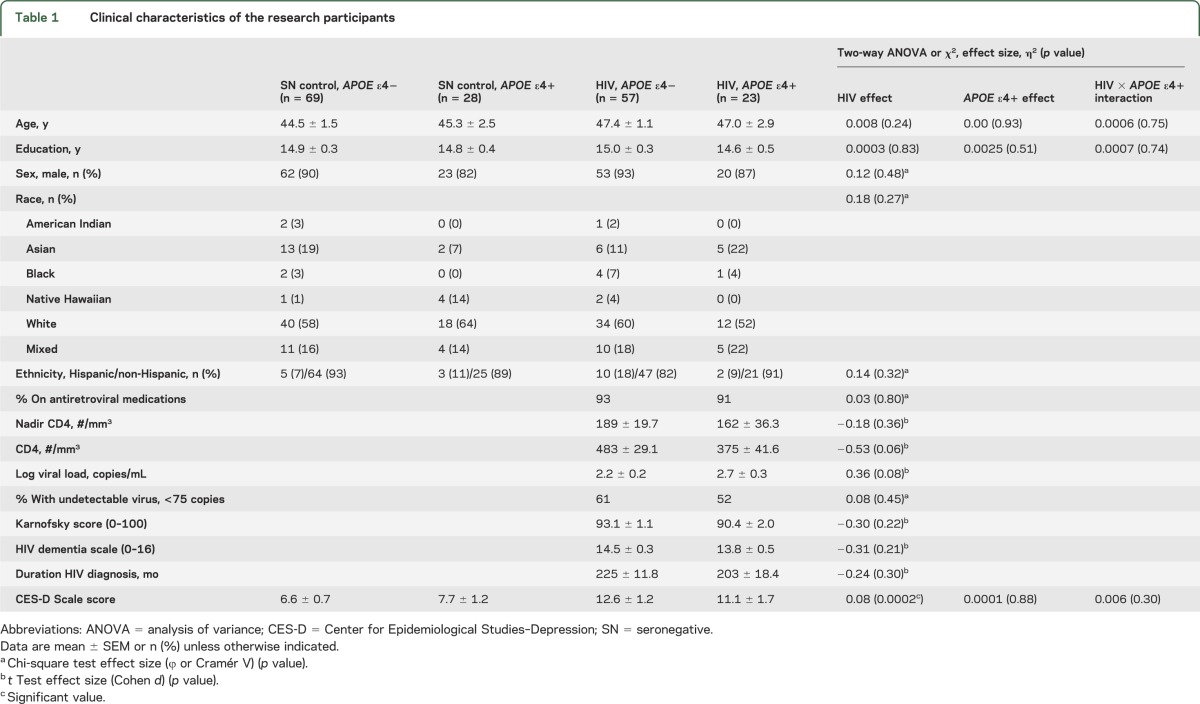
Three hundred thirty-eight participants fulfilled the study criteria and 177 with complete and acceptable datasets were included in this study (97 seronegative [SN] subjects: aged 44.7 ± 1.3 years, 85 [87.6%] men, 28 [28.9%] APOE ε4+; and 80 HIV+ subjects: aged 47.3 ± 1.1 years, 73 [91.3%] men, 23 [28.8%] APOE ε4+) (table 1). All subjects were 18 years or older and able to provide informed consent. All were tested to ensure HIV serostatus. HIV+ subjects were either not taking ARVs or stable on ARVs for >6 months (ARV-stable) and had a nadir CD4 cell count <500/mm3. Exclusion criteria for all subjects included the following: (1) chronic medical or neuropsychiatric illnesses that might confound the outcome variables; (2) significant abnormalities on laboratory measures that might indicate a severe metabolic disorder or organ failure; (3) history of head trauma with loss of consciousness >30 minutes; (4) history of drug dependence according to DSM-IV criteria, except for tobacco; (5) positive urine toxicology for common drugs of abuse, except for Δ9-tetrahydrocannabinol, because many were using medicinal marijuana; and (6) any contraindications for MRI.
Table 1.
Clinical characteristics of the research participants
Neuropsychological tests.
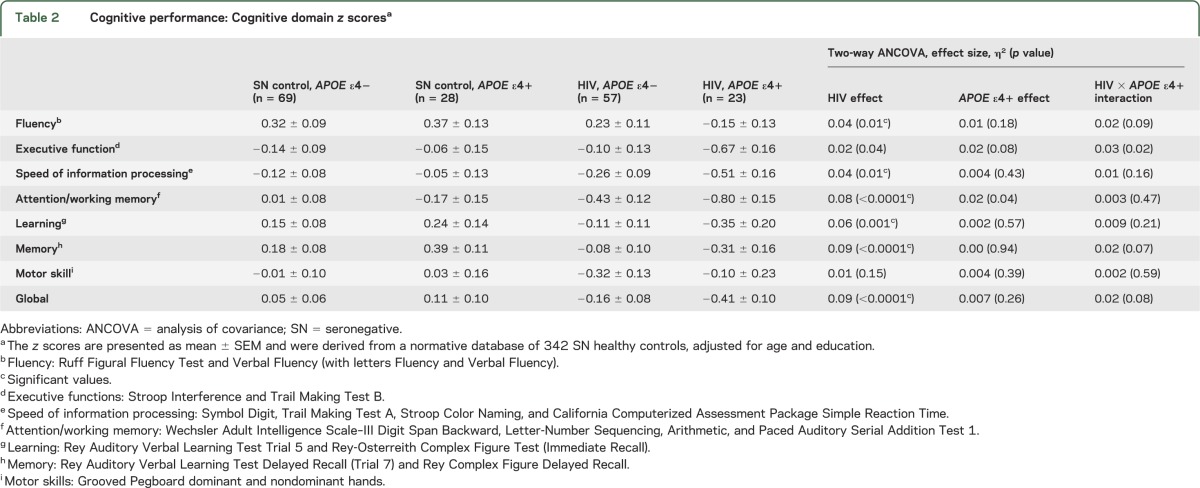
Each participant performed a battery of neuropsychological tests sensitive for detecting cognitive deficits in patients with HIV infection. The tests evaluated 7 cognitive domains (see table 2). In addition, depressive symptoms were assessed using the Center for Epidemiological Studies–Depression Scale. Twenty-nine (36.3%) of the HIV+ subjects were diagnosed with HAND according to the Updated Research Nosology,13 including clinical assessments with self-report, neurologic evaluations, Functional Activities Questionnaire, and Karnofsky score. Eleven had asymptomatic neurocognitive impairment, 17 minor neurocognitive disorder, and one had HIV-associated dementia; the remaining 51 HIV subjects had normal cognition. Conversely, only 15 (15.5%) SN controls had cognitive deficits comparable to HAND.
Table 2.
Cognitive performance: Cognitive domain z scoresa
APOE ε genotyping.
Genomic DNA was isolated from the human blood sample by using the DNeasy Blood and Tissue kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Genomic DNA concentration and purity were assessed with the Nanodrop 1000 (Thermo Scientific, West Palm Beach, FL). APOE ε genotypes were determined by PCR-based RFLP (restriction fragment length polymorphism). First, a 218–base pair fragment of the APOE gene was amplified using these primers: forward sequence (TCCAAGGAGCTGCAGGCGGCGCA) and reverse sequence (GCCCCGGCCTGGTACACTGCCA); afterward, the PCR fragment was digested with restriction enzymes AflIII (5,000 U/mL) and HaeII (20,000 U/mL), 10× buffer 3, and 10× bovine serum albumin (New England BioLabs, Ipswich, MA) for 12 hours at 37°C. Digest products were resolved on 4% agarose gel. APOE ε genotype was confirmed for each subject using the Illumina BeadXpress scanner (iGenix, Bainbridge Island, WA).
MRI and MRS.
All magnetic resonance studies were performed on a 3T MR scanner (Tim Trio; Siemens Medical Solutions, Erlangen, Germany) with an 8- or 12-channel head coil. The MRI protocol included a 3-plane localizer (repetition time [TR]/echo time [TE] = 20/5 milliseconds, 1-average), a sagittal 3-dimensional magnetization-prepared rapid-acquisition gradient echo (MP-RAGE) (TR/TE/inversion time = 2,200/4.91/1,000 milliseconds; 1-average; 208 × 256 × 160 matrix), and an axial fluid-attenuated inversion recovery sequence (TR/TE = 10,000/85 milliseconds; 1-average; 205 × 320 × 28 matrix). These structural images were evaluated for possible brain lesions and used to prescribe the MRS voxels and for gray–white matter segmentation. Proton MRS was acquired from 4 approximately 8-mL brain regions: basal ganglia, parietal gray matter, frontal white matter, and anterior cingulate gray matter (figure 1). A PRESS (point resolved spectroscopy) acquisition sequence was used (64 averages, TR/TE = 3,000/30 milliseconds, bandwidth 1,200 Hz, 4 dummy scans, 8-step phase cycling), and the T2 decay of the water signal was measured at 10 different echo times.
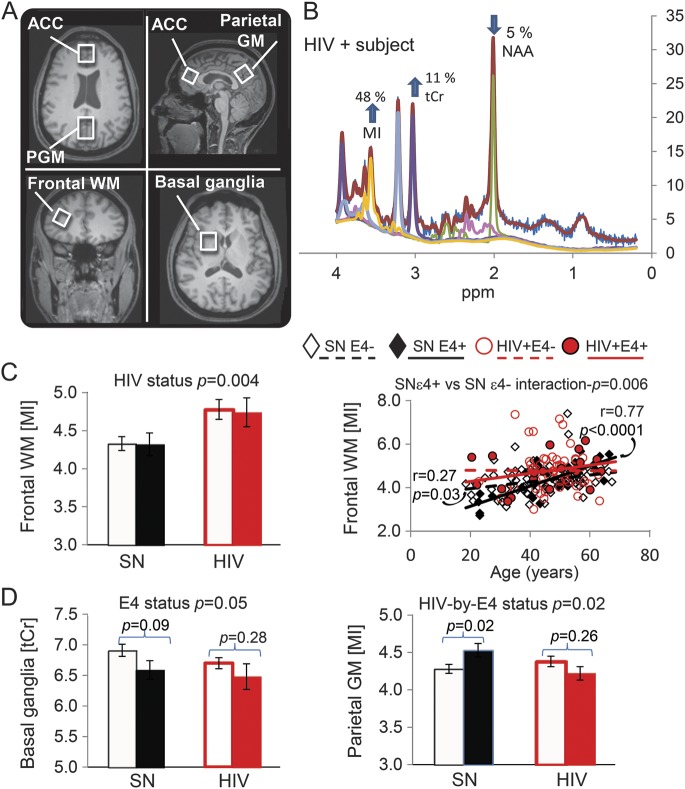
Figure 1. MRS and neurometabolite levels.
(A) T1-weighted structural MRI showing the 4 voxel locations. The ACC and PGM voxels were angled parallel to the skull line to minimize fat contamination from the skull. The frontal WM voxel is shown on a coronal image and the basal ganglia voxel on an axial image. (B) Representative magnetic resonance spectrum from the frontal WM of an HIV+ subject, with marked elevation of MI and minimally lower than normal NAA; the color lines are from fitting of the LCModel. (C) Compared with SN controls, subjects with HIV had higher frontal WM MI, with or without APOE ε4 allele(s) (bar graphs), and the HIV+ APOE ε4− subjects had the least age-dependent increase in the frontal MI level. (D) Regardless of HIV status, subjects with APOE ε4 had lower basal ganglia tCr levels, which persisted across the age span (see figure e-1). However, in the parietal cortex, only SN subjects but not HIV subjects with the APOE ε4 allele(s) showed higher MI levels. These group differences also persisted across the age span (figure e-1). ACC = anterior cingulate cortex; GM = gray matter; MI = myo-inositol; MRS = magnetic resonance spectroscopy; NAA = N-acetylaspartate; PGM = parietal gray matter; SN = seronegative; tCr = total creatine; WM = white matter.
MRS data were processed using a customized spectral analysis package of LCModel to determine concentrations of major metabolites, including the glial metabolites total creatine (tCr) and myo-inositol (MI). The metabolite concentrations were corrected for the variable percentages of CSF, gray matter, and white matter in each voxel, although no group differences were found for these variables. The water T2-decay data were used to calculate the %CSF in each voxel.14 The proportion of gray and white matter in each voxel was determined by segmenting the high-resolution MP-RAGE scans using FMRIB's Automated Segmentation Tool (FAST, version 4.1) for cortical voxels and FMRIB's Integrated Registration and Segmentation Tool (FIRST, version 1.2) for subcortical structures. Each spectrum was visually inspected and met the following criteria from the LCModel fit: (1) full width at half maximum <0.10 ppm (<0.12 ppm for basal ganglia), (2) signal-to-noise ratio >7, and (3) Cramér-Rao bounds for each metabolite ≤25%.
Statistical methods.
Statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC). Two-way analysis of variance and multiple contingency tables were used to compare clinical variables between HIV+ and SN subjects, with and without APOE ε4 allele(s). Two-way analyses of covariance, including age, education, or scanner software version as covariate, were used to test the independent and interactive effects of HIV status and APOE ε4 status (at least one APOE ε4+ allele) on brain metabolites and cognitive function. Relationships among age, cognitive performance, and brain metabolites that showed group differences were explored using linear regression. Because Bonferroni correction tends to be overly conservative and we focused our analyses based on prior knowledge of altered glial metabolites (i.e., MI and tCr) in 3 brain regions (frontal white matter, parietal cortex, and basal ganglia) and abnormal cognitive domains in HIV patients, p values <0.01 were considered statistically significant and p values <0.05 were considered trends for significance.
RESULTS
Our analysis focused on the effects of HIV and APOE ε4 status, their combined or interactive effects, and age-dependent changes on the glial metabolites (MI and tCr levels) and cognitive performance. Furthermore, we evaluated the relationships between MI levels that showed these effects in the 3 brain regions and cognitive performance.
Clinical characteristics.
The SN and HIV subject groups were similar in age, sex proportion, years of education, and proportion of APOE ε4 carriers (table 1). The 2 subject groups also had similar distributions of race and ethnicity, with predominantly white (52%–64% per group) or mixed-race (14%–22%) individuals and few blacks (0%–7%). Five subjects were APOE ε4 homozygous, and all were HIV-positive (3 had HAND and 2 had no cognitive deficits). The 2 HIV+ groups (APOE ε4− vs APOE ε4+) were not different in their CD4 counts, nadir CD4 count, log viral load, Karnofsky score, HIV dementia scale, or duration of HIV diagnosis. However, both HIV groups had more depressive symptoms on the Center for Epidemiological Studies–Depression Scale than the 2 SN groups, but they were not clinically depressed. Ten percent of the subjects (11 HIV, 6 SN) had nonsignificant incidental findings on MRI (data not shown).
Effects of HIV, APOE ε4, and age on brain metabolites.
HIV+ subjects had higher frontal white matter MI than SN subjects, regardless of their APOE ε4+ status (+10%, p = 0.004) (figure 1). However, individuals with APOE ε4+, both SN and HIV subjects, had slightly lower basal ganglia tCr (−4%, p = 0.05). In the parietal cortex, the effect of APOE ε4+ differed between SN controls (higher MI) and HIV subjects (nonsignificantly lower MI); interaction p value = 0.02. These findings remained unchanged after covarying for the percentage gray matter in each voxel.
Furthermore, age-dependent changes in the frontal white matter MI were different across the 4 subject groups (interaction p = 0.04, figure 1C, right). Both SN groups showed higher frontal white matter MI with older age. However, the slope is much steeper for those with ε4+ than ε4− (age × ε4+ status interaction p = 0.006), demonstrating an antagonistic pleiotropy effect only in APOE ε4+ SN subjects. In contrast, HIV subjects had elevated MI levels across the age span, and regardless of the APOE ε4+ status (age × APOE ε4+ status interaction p = 0.44). The glial metabolites in other brain regions did not show significant group × age interactions.
Effects of HIV, APOE ε4, and age on cognition.
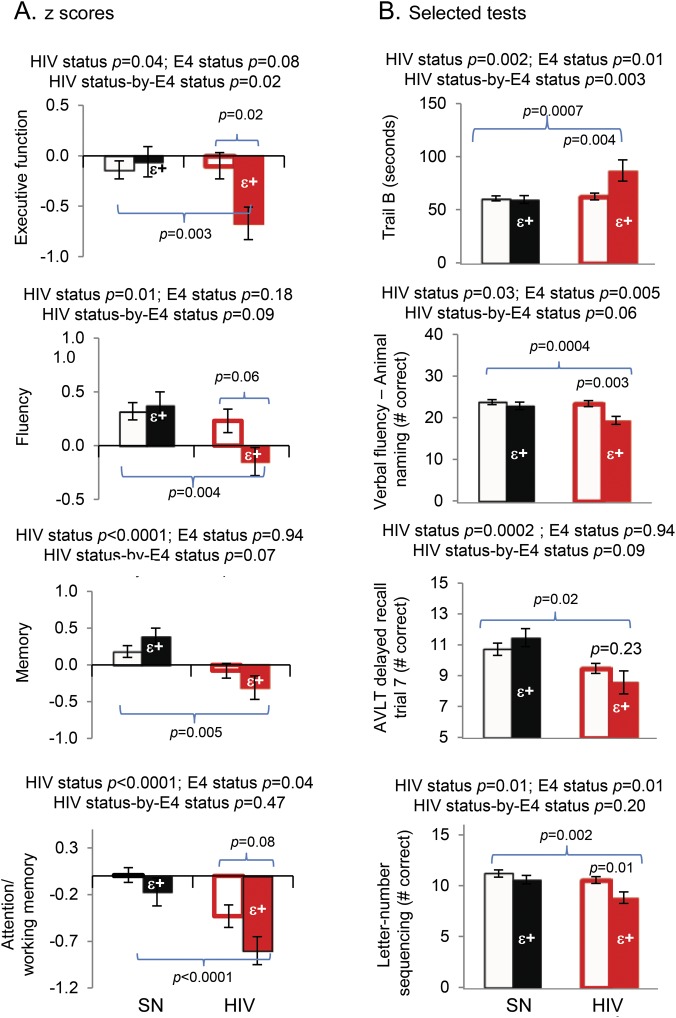
As expected, HIV subjects, regardless of APOE ε4+ status, performed significantly poorer than SN subjects on the majority of the cognitive domains, although the effect sizes were small in this cohort (table 2, figure 2). However, only HIV but not SN subjects with APOE ε4+ tended to perform worse than APOE ε4− subjects on executive function, as exemplified by the Trail B task (table 2; figure 2, top graphs). Similar trends were observed for the fluency and memory domains, and the corresponding tests that contributed to the poorest performance in the HIV ε4+ subjects (table 2; figure 2, middle graphs). Because APOE ε4+ subjects additionally tended to perform worse than APOE ε4− subjects on the attention/working memory domain (p = 0.04), this led to an additive effect with HIV status. Consequently, HIV+ APOE ε4+ subjects showed the poorest attention/working memory performance (p < 0.0001), especially in the letter-number sequencing task (figure 2, bottom graphs). Furthermore, HIV+ APOE ε4+ subjects had persistently lower performance on these tests across the age span, except for Trail Making Test B, in which slower performance was found primarily in the oldest HIV+ APOE ε4+ subjects (figure e-1 on the Neurology® Web site at Neurology.org).
Figure 2. Cognitive performance.
(A) On 2-way analysis of variance, subjects with HIV (red outlined and solid bars) performed more poorly than SN controls (black outlined and solid bars) on 4 cognitive domains: executive function, fluency, memory, and attention/working memory. On post hoc analyses, while no group difference was found in SN subjects with or without the APOE ε4 allele, HIV subjects with the APOE ε4 allele(s) (red solid bars) consistently performed more poorly on these tasks than SN subjects without the APOE ε4 allele (black outline bars). (B) Selected corresponding neuropsychological tests that contributed to the findings in the cognitive domains. Age and education were included as covariates for the 2-way analysis of covariance. AVLT = Auditory Verbal Learning Test; SN = seronegative.
Relationships between cognitive function and brain metabolite abnormalities.
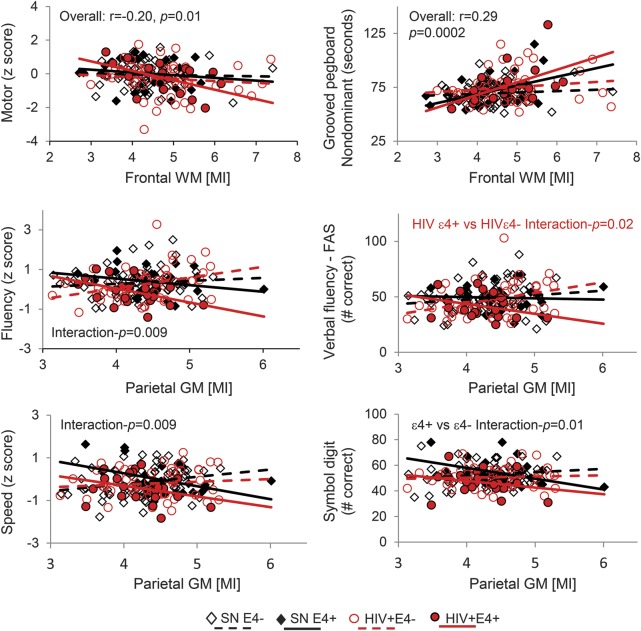
Subjects with higher frontal white matter MI had lower motor z scores, especially in the nondominant hand (figure 3). However, only APOE ε4+ subjects showed an association between higher parietal gray matter MI and poorer performance on the fluency domain (interaction p = 0.009), especially those also HIV+, and slower speed of information processing scores (interaction p = 0.009).
Figure 3. Correlations between MI levels and cognitive performance.
Higher levels of the glial marker MI in the frontal WM were associated with slower fine motor tasks, whereas higher MI levels in the parietal cortex were associated with poorer performance on fluency and speed of information processing domains, especially in subjects with APOE ε4 allele(s). FAS = Fluency and Verbal Fluency; GM = gray matter; MI = myo-inositol; SN = seronegative; WM = white matter.
DISCUSSION
This is the first study to evaluate the independent and combined effects of APOE ε4 genotype and HIV infection on brain metabolite abnormalities, including age-dependent changes, of our primarily (>90%) cART-treated HIV subjects. We also delineated the combined effects of HIV and APOE ε4 on cognitive performance and validated our hypothesis that HIV+ APOE ε4 carriers have the poorest cognitive performance throughout the lifespan. Lastly, the relationship between neuroinflammation (elevated MI) and cognitive performance across the subject groups is evaluated.
First, our primarily cART-treated HIV subjects showed persistent glial activation (with elevated MI) in the frontal white matter across the age span. This finding is consistent with that reported in prior MRS studies of patients with HIV,15 in postmortem brains of patients with HIV,16 and in Simian immunodeficiency virus–infected macaques.17 Persistent glial activation is also reflected by elevated CSF chemokines and cytokines (e.g., inducible protein-10, interleukin [IL]-8, and monocyte chemoattractant protein-1),18,19 which are released by astrocytes and microglia, especially in HIV patients with higher ratios of MI/tCr.19 However, APOE ε4 had little additional influence on the elevated frontal white matter MI across the lifespan of the HIV subjects. This contrasts with the antagonistic pleiotropy effect of APOE ε4+ in SN subjects: lower MI at younger age but higher MI at older age compared with APOE ε4− SN subjects. Furthermore, independent of HIV serostatus, subjects with APOE ε4 showed tendencies for lower levels of tCr in the basal ganglia but higher concentrations of MI in the parietal cortex across the age span. Collectively, these findings indicate that ε4+ allele(s) and HIV infection have independent and brain region–specific influence on energy metabolism and glial activation.
The elevated MI in younger HIV subjects also suggests premature brain aging, because MI increases with normal aging.20 Prematurely lower brain glutamate levels (possibly due to reduced reuptake and recycling of glutamate by activated astrocytes)21 and prematurely reduced cognitive reserve were also reported in younger subjects with HAND.22 However, in the parietal gray matter, elevated MI is found only in SN, but not HIV+, APOE ε4+ subjects. Elevated parietal MI is found in patients with AD,23 and likely reflects greater microglial activation, especially in ε4+ individuals.24 Likewise, ApoE4 mice displayed greater and more prolonged increases of cytokines (IL-1β, IL-6, tumor necrosis factor-α) than ApoE2- and ApoE3-expressing mice.25 The lack of an ε4+-mediated inflammatory response in the parietal cortex of ε4+ HIV subjects may reflect microglial dystrophy due to the premature aging. In addition, the lower basal ganglia tCr in all ε4+ subjects is similar to that found in basal ganglia of ARV-naive HIV patients with severe dementia26 and in occipital cortex of APOE ε4+ elderly without dementia.27 Lower or depleted tCr in these conditions might result from the increased metabolic demands with chronic glial activation, especially in the basal ganglia, which have dense dopaminergic synapses.
Second, APOE ε4+ subjects had poorer attention and working memory than APOE ε4− subjects, independent of HIV status. However, among the 4 groups, HIV+ APOE ε4+ subjects performed poorest on executive function, attention/working memory, fluency, and memory. APOE ε4+ negatively affects cognition in AD28 and in other brain injuries, such as traumatic brain injury,29 and younger individuals with MS.30 However, the effects of APOE ε4 allele on HAND were inconsistent among studies.4–10 Several studies found that HIV+ APOE ε4 carriers had increased risk of HAND,5–8 while others found increased risk only in older individuals (≥50 years)4 or no increased risk of HAND.9,10 These conflicting findings may in part be attributable to the antagonistic pleiotropy effect of the APOE ε4 allele(s),11 which negatively affects older individuals but may enhance cognitive function in younger individuals. Hence, the negative effects of APOE ε4 are evident only at older ages, as seen in most patients with AD. However, in some HIV-positive individuals, the negative effect of APOE ε4 may emerge earlier because younger HIV+ APOE ε4 carriers already showed brain atrophy and cognitive deficits.5 In the current study, APOE ε4+ exacerbated the cognitive deficits in our HIV subjects across all ages, except for the performance on Trail B, which was slower only in older ε4+ HIV+ subjects. The poorer performance on some of the cognitive tasks in older HIV APOE ε4+ patients likely resulted from the lower cognitive reserve in the aging HIV-infected brain, especially those with HAND.22 This finding is also consistent with the more widespread deficits in brain structural connectivity (on diffusion tensor imaging scans) in HIV+ APOE ε4 subjects.31
These discrepancies in the effects of APOE ε4 on HAND may also be partly attributable to racial differences among cohorts. A large longitudinal study found that only white but not black individuals with APOE ε4+ showed faster decline in semantic and working memory.32 Because the HIV subjects in the current study were primarily white, they might be more susceptible to the influence of APOE ε4+ allele(s) on attention/working memory.
The neuropathologic substrates for APOE ε4–mediated cognitive deficits in HIV patients are not well understood. However, APOE ε4+ HIV+ subjects showed greater atrophy in subcortical gray matter structures and white matter.5 In addition, in postmortem HIV-infected brains, both APOE ε4 and older age increased the likelihood of cerebral β-amyloid (Aβ) plaque deposition (as diffuse plaques and mild to moderate amyloid angiopathy, but sparse phospho-tau neurofibrillary tangles), and only APOE ε4 carriers with the Aβ plaques had greater probability of HAND.33 Furthermore, CSF ApoE protein was elevated only in HIV+ APOE ε4+ subjects, and the levels correlated with the severity of cognitive deficits,8 suggesting that the aberrant ApoE ε4 protein could not clear the Aβ and contributed to HAND. Therefore, both AD and HAND subjects with APOE ε4 may share similar mechanisms for excess accumulation of Aβ, which would elicit neuroinflammation and release of inflammatory molecules.34 Again, racial ancestry may also have an important role in how APOE ε4 might be expressed since brain samples from individuals with greater genetically determined African ancestry had fewer neuritic plaques.35
Lastly, neuroinflammation (higher MI) in the frontal white matter was associated with slower fine motor speed across all subjects, especially in the HIV+ APOE ε4+ subjects. Elevated glial marker MI has been associated with poorer cognitive performance in both ARV-naive26 and ARV-stable patients with HIV.15 Similarly, greater neuroinflammation (higher MI) in the parietal cortex was associated with poorer performance on verbal fluency, particularly in HIV+ APOE ε4+ subjects. These findings suggest that the neuroinflammatory responses resulting from HIV infection and APOE ε4 allele(s) may lead to synergistic or additive effects on cognitive deficits. Conversely, the antagonistic pleiotropic effect of APOE ε4 on MI in SN subjects suggests a protective or anti-inflammatory effect, with lower MI and better cognitive performance, in the younger SN APOE ε4+ subjects.
We had a moderately large sample size and a relatively higher proportion of APOE ε4 subjects (28.9% of the SN group and 28.8% of the HIV group) compared with prior reported frequencies across different ethnic groups (12%–20%).36 However, the small number of APOE ε4+ subjects led to only trends for HIV × APOE ε4 interactions for many tests and was insufficient to evaluate a gene-dose effect (1 copy or 2 copies of APOE ε4), the possible anti-inflammatory effects of ε2 and ε3 alleles, or a possible racial ancestry effect. Future studies with a larger sample size of APOE ε4 individuals are needed to validate our findings on the influence of APOE ε4 in HIV-associated brain injury and HAND. Although we excluded many common potential confounds (e.g., hepatitis C, drug dependence), some individuals still might have abused drugs, which might contribute to even more neuroinflammation with further elevation of MI37 and Alzheimer-like pathology (i.e., increased hyperphosphorylated tau).38
cART prolongs the lives of patients with HIV and is the cornerstone for HIV prevention. However, stably cART-treated patients with HIV continue to show persistent glial activation (elevated MI) that might contribute to their cognitive deficits. In younger subjects with HIV, the persistent glial activation attenuated the protective antagonistic pleiotropic effects of APOE ε4. HIV+ APOE ε4 subjects also had greater cognitive deficits, which may further increase the risk of HAND with older age. APOE ε4 allele(s) may be a useful genetic marker to identify white and mixed-race subjects with HIV at risk of cognitive decline.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful to the research participants in this study, and thank all of the clinical and technical research staff who helped with the data collection, and the community HIV physician providers (especially Drs. Drew Kovach, Cyril Goshima, Jennifer Frank, and Dominic Chow) who referred many of their patients to the study. The authors further thank Dr. Eric Miller for helping to classify the neuropsychological tests for the cognitive domains.
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- ARV
antiretroviral
- cART
combination antiretroviral therapy
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- HAND
HIV-associated neurocognitive disorders
- IL
interleukin
- MI
myo-inositol
- MP-RAGE
magnetization-prepared rapid-acquisition gradient echo
- MRS
magnetic resonance spectroscopy
- SN
seronegative
- tCr
total creatine
- TE
echo time
- TR
repetition time
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Chang: study concept, design, supervised all data collection, evaluated the subjects and all MRI scans, interpreted the data, wrote and critically revised the manuscript. Ms. Jiang: study design and analysis of the data, reviewed and approved final version of the paper. Mr. Cunningham and Dr. Buchthal: acquired and processed the MRS data, reviewed and approved final version of the manuscript. Drs. Douet and Andres: performed the genotyping of the subject samples, interpretation, reviewed and approved final version of the manuscript. Dr. Ernst: study concept, design, supervised the MRS acquisition and processing of the data, interpreted the data, critically revised the manuscript, reviewed and approved final version of the manuscript.
STUDY FUNDING
Supported by the National Institute on Mental Health (2R01-MH061427), the National Institute on Drug Abuse (K24-DA016170; K02-DA016991), the National Institute on Minority Health and Health Disparities (G12-MD007601-26), the National Institute of Neurological Diseases and Stroke (U54-NS056883), and the Office of National Drug Control Policy.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011;17:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harold D, Abraham R, Hollingworth P, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet 2009;41:1088–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burt TD, Agan BK, Marconi VC, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc Natl Acad Sci USA 2008;105:8718–8723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valcour V, Shikuma C, Shiramizu B, et al. Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J Neuroimmunol 2004;157:197–202 [DOI] [PubMed] [Google Scholar]
- 5.Chang L, Andres M, Sadino J, et al. Impact of apolipoprotein E epsilon4 and HIV on cognition and brain atrophy: antagonistic pleiotropy and premature brain aging. Neuroimage 2011;58:1017–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spector SA, Singh KK, Gupta S, et al. APOE epsilon4 and MBL-2 O/O genotypes are associated with neurocognitive impairment in HIV-infected plasma donors. AIDS 2010;24:1471–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corder EH, Robertson K, Lannfelt L, et al. HIV-infected subjects with the E4 allele for APOE have excess dementia and peripheral neuropathy. Nat Med 1998;4:1182–1184 [DOI] [PubMed] [Google Scholar]
- 8.Andres MA, Feger U, Nath A, Munsaka S, Jiang CS, Chang L. APOE epsilon 4 allele and CSF APOE on cognition in HIV-infected subjects. J Neuroimmune Pharmacol 2011;6:389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joska JA, Combrinck M, Valcour VG, et al. Association between apolipoprotein E4 genotype and human immunodeficiency virus-associated dementia in younger adults starting antiretroviral therapy in South Africa. J Neurovirol 2010;16:377–383 [DOI] [PubMed] [Google Scholar]
- 10.Morgan EE, Woods SP, Letendre SL, et al. Apolipoprotein E4 genotype does not increase risk of HIV-associated neurocognitive disorders. J Neurovirol 2013;19:150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuminello ER, Han SD. The apolipoprotein e antagonistic pleiotropy hypothesis: review and recommendations. Int J Alzheimers Dis 2011;2011:726197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morley D, McNamara P, Kennelly S, McMahon G, Bergin C. Limitations to the identification of HIV-associated neurocognitive disorders in clinical practice. HIV Med 2013;14:497–502 [DOI] [PubMed] [Google Scholar]
- 13.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007;69:1789–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain: I: compartments and water. J Magn Reson B 1993;102:1–8 [Google Scholar]
- 15.Chang L, Feger U, Ernst T. Brain imaging (CT, SPECT, PET, functional MRI & MRS) studies in HIV-associated brain disorders. In: Gendelman H, Grant I, Everall I, Lipton S, Swindells S, editors. The Neurology of AIDS, 3rd ed Oxford, UK: Oxford University Press; 2012 [Google Scholar]
- 16.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol 2005;64:529–536 [DOI] [PubMed] [Google Scholar]
- 17.Ratai EM, Pilkenton S, He J, et al. CD8+ lymphocyte depletion without SIV infection does not produce metabolic changes or pathological abnormalities in the rhesus macaque brain. J Med Primatol 2011;40:300–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cinque P, Vago L, Mengozzi M, et al. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS 1998;12:1327–1332 [DOI] [PubMed] [Google Scholar]
- 19.Letendre SL, Zheng JC, Kaul M, et al. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J Neurovirol 2011;17:63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang L, Ernst T, Poland R, Jenden D. In vivo proton magnetic resonance spectroscopy of the normal human aging brain. Life Sci 1996;58:2049–2056 [DOI] [PubMed] [Google Scholar]
- 21.Ernst T, Jiang C, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging 2010;32:1045–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang L, Holt JL, Yakupov R, Jiang CS, Ernst T. Lower cognitive reserve in the aging human immunodeficiency virus-infected brain. Neurobiol Aging 2013;34:1240–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernst T, Chang L, Melchor R, Mehringer CM. Frontotemporal dementia and early Alzheimer disease: differentiation with frontal lobe H-1 MR spectroscopy. Radiology 1997;203:829–836 [DOI] [PubMed] [Google Scholar]
- 24.Egensperger R, Kosel S, von Eitzen U, Graeber MB. Microglial activation in Alzheimer disease: association with APOE genotype. Brain Pathol 1998;8:439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y, Nwabuisi-Heath E, Dumanis SB, et al. APOE genotype alters glial activation and loss of synaptic markers in mice. Glia 2012;60:559–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang L, Ernst T, Witt M, et al. Relationships among cerebral metabolites, cognitive function and viral loads in antiretroviral-naïve HIV patients. Neuroimage 2002;17:1638–1648 [DOI] [PubMed] [Google Scholar]
- 27.Laakso MP, Hiltunen Y, Kononen M, et al. Decreased brain creatine levels in elderly apolipoprotein E epsilon 4 carriers. J Neural Transm 2003;110:267–275 [DOI] [PubMed] [Google Scholar]
- 28.Bracco L, Piccini C, Baccini M, et al. Pattern and progression of cognitive decline in Alzheimer's disease: role of premorbid intelligence and ApoE genotype. Dement Geriatr Cogn Disord 2007;24:483–491 [DOI] [PubMed] [Google Scholar]
- 29.Teasdale GM, Nicoll JA, Murray G, Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet 1997;350:1069–1071 [DOI] [PubMed] [Google Scholar]
- 30.Shi J, Zhao CB, Vollmer TL, Tyry TM, Kuniyoshi SM. APOE epsilon 4 allele is associated with cognitive impairment in patients with multiple sclerosis. Neurology 2008;70:185–190 [DOI] [PubMed] [Google Scholar]
- 31.Jahanshad N, Valcour VG, Nir TM, et al. Disrupted brain networks in the aging HIV+ population. Brain Connect 2012;2:335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnes LL, Arvanitakis Z, Yu L, Kelly J, De Jager PL, Bennett DA. Apolipoprotein E and change in episodic memory in blacks and whites. Neuroepidemiology 2013;40:211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soontornniyomkij V, Moore DJ, Gouaux B, et al. Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS 2012;26:2327–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnstone M, Gearing AJ, Miller KM. A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol 1999;93:182–193 [DOI] [PubMed] [Google Scholar]
- 35.Schlesinger D, Grinberg LT, Alba JG, et al. ; Brazilian Aging Brain Study Group. African ancestry protects against Alzheimer's disease-related neuropathology. Mol Psychiatry 2013;18:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA 1998;279:751–755 [DOI] [PubMed] [Google Scholar]
- 37.Chang L, Munsaka SM, Kraft-Terry S, Ernst T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol 2013;8:576–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anthony IC, Norrby KE, Dingwall T, et al. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain 2010;133:3685–3698 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.