Abstract
Background
Early fetuses heal wounds without the formation of a scar. Many studies have attempted to explain this remarkable phenomenon. However, the exact mechanism remains unknown. Herein, we examine the predominant cell types of the epidermis and dermis—the keratinocyte and fibroblast—during different stages of fetal development to better understand the changes that lead to scarring wound repair versus regeneration.
Materials and Methods
Keratinocytes and fibroblasts were harvested and cultured from the dorsal skin of time-dated BALB/c fetuses. Total RNA was isolated and microarray analysis was performed using chips with 42,000 genes. Significance analysis of microarrays (SAM) was utilized to select genes with greater than 2-fold expression differences with a false discovery rate (FDR) of less than 2. Enrichment analysis was performed on significant genes to identify differentially expressed pathways.
Results
By comparing the gene expression profile of keratinocytes from E16 versus E18 fetuses, we identified 24 genes that were downregulated at E16. Analysis of E16 and E18 fibroblasts revealed 522 differentially expressed genes. Enrichment analysis showed the top 20 signaling pathways that were downregulated in E16 keratinocytes and upregulated or downregulated in E16 fibroblasts.
Conclusions
Our data reveal 546 differentially expressed genes in keratinocytes and fibroblasts between the scarless and scarring transition. Additionally, a total of 60 signaling pathways have been identified to be either upregulated or downregulated in these cell types. The genes and pathways recognized by our study may prove to be essential targets that may discriminate between fetal wound regeneration and adult wound repair.
Keywords: Wound healing, Scarless repair, Regeneration, Microarray
Introduction
In 1979 a landmark paper reported that early human fetuses are able to heal wounds without the appearance of a scar (1): whereas scar formation is a physiological process in adult skin and postnatal skin responds to injury with a fibrotic repair process to form scar, embryonic tissue responds with scarless skin regeneration (2). Since then, a number of original articles have attempted to unveil the biological mechanisms underlying this remarkable phenomenon. While the key pathways responsible remain unclear, these studies successfully uncovered a number of important differences between embryonic and adult tissues, all of which may contribute to the observed differences in wound healing. For instance, fetal skin and wounds have been found to differ in terms of both extracellular matrix makeup and response to inflammation (3). In addition, fetal wound research has demonstrated that early to midgestational fetal cutaneous wounds undergo complete scarless regeneration across a number of mammalian species, as well as in ex vivo models (4–6). In mice, wounds up to 1.5 mm in size at a gestational age 16.5 days (E16.5) or younger have been shown to heal scarlessly with normal restitution of the extracellular matrix and demonstrable regeneration of dermal appendages (7). While this ability to heal scarlessly has been attributed to a variety of intrinsic factors within fetal tissue, no specific cytokines, factors, or cells, however, have yet been identified to be the major driving force for this regeneration.
In order to identify the unknown mediators of fetal regeneration, we performed microarray transcriptional profiling on fetal and postnatal keratinocytes and fibroblasts harvested from E16 and E18 murine fetuses to detect transcriptome differences that occur during scarless versus scarring repair. Fibroblasts were chosen due to their role as prominent sources of connective tissue crucial in both the proliferative and remodeling phases of wound healing (8), and keratinocytes for their ability to regulate the development of fibrosis in the skin (9). Using microarray analysis we found a number of genes with differential transcription in E16 versus E18 cells and mapped them to integrated gene networks via functional genomics. This enabled the identification of relevant pathways that are potential targets for inducing regenerative-type repair with improved collagen architecture and potential epidermal appendage restoration in the postnatal scarring wound.
Materials and Methods
Animals
Six-week-old wild-type BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA). For timed gestations, the mice were bred overnight and the day of vaginal plug was considered E0.5 day of gestation. Animals were maintained in the Stanford Animal Care Laboratory and all procedures were conducted in accordance with university-approved protocols according to National Institutes of Health guidelines.
Primary Cell Culture
Pregnant mice at gestational age E16.5 and E18.5 were euthanized using CO2 and cervical dislocation. Fetal mice were then removed from uteri. Dorsal skin was collected using a dissecting microscope under sterile conditions. In order to obtain sufficient cells to achieve primary culture, skin from E16.5 (n = 10) and postnatal day E18.5 mice (n = 10) was pooled for subsequent keratinocyte and fibroblast harvest.
For keratinocyte primary cell culture, tissue was incubated in defined keratinocyte-SFM (serum-free media) (Gibco Life Technologies, Carlsbad, CA) with 5 mg/mL dispase (BD Biosciences, San Jose, CA) and 10X antibiotic-antimycotic (Gibco Life Technologies) at 4 degrees for 7 hours. Skin was then cut and treated with 0.25% trypsin for 10 min. The cells were subsequently seeded in keratinocyte-SFM.
For fibroblast primary cell culture, tissue was minced and treated with 0.25% trypsin/EDTA in 37 degrees with gentle agitation for 10 min. Cells were plated with mouse embryonic fibroblast (MEF) culture medium consisting of DMEM (Dulbecco’s Modified Eagle Medium), GlutaMAX Supplement (Gibco Life Technologies), 10% fetal bovine serum (Omega Scientific, Tarzana, CA), 0.1 mM 2-mercaptoethanol (Sigma, St. Louis, MO), and 1% penicillin/streptomycin (Gibco Life Technologies).
Keratinocytes and fibroblasts were kept at 37°C in a humid incubator with 5% CO2. Passage one keratinocytes and fibroblasts from both male and female fetuses were used for all experiments.
RNA Extraction and Amplification
RNA from keratinocytes and fibroblasts was extracted using the Trizol protocol (Invitrogen, Carlsbad, CA) per manufacturer’s instructions. One microgram of RNA from each experimental sample was amplified using the MessageAmp aRNA kit (Ambion, Austin, TX). At the same time, 1 µg aliquots of universal mouse RNA were amplified in individual reaction mixtures and utilized as an internal amplification control to allow comparisons between different arrays.
Preparation of Fluorescent cDNA Probes
Four micrograms of RNA and 2 µg of random hexamer were heated at 65°C for 10 minutes and reverse transcribed in a 30 µL total reaction volume containing 2 mM of each deoxyribose nucleotide triphosphate, 1X first-strand buffer, 0.5 µL RNAse inhibitor, 200 U superscript II, 10 mM dithiolthretol, and 3 µL Cy3-deoxyribose uridine triphosphate (dUTP) (for experimental samples) or Cy5-dUTP (for universal mouse control samples) at 42°C for 1 hour. An additional 200 U superscript II was added to boost the reaction and incubated for 1 hour at 42°C. Fluorescent Cy3- or Cy5-labeled probes were washed with TE buffer (10 mM tris, 1 mM EDTA) through a microcon mini column (Millipore, Billerica, MA), concentrated with 450 µL TE buffer, and recovered by spinning the inverted mini column into a fresh tube. Probes were immediately hybridized to microarray chips.
Pretreatment of Microarray Chips
Mouse microarray chips were printed in the Stanford Microarray Database Center with 42,000 specific cDNAs printed onto each lysine-coated slide. These cDNAs represent single accession numbers from Genbank. Sequences and accession numbers of the cDNAs can be found on http://genome-www5.stanford.edu//index.shtml. Before hybridization, microarray chips were first rehydrated (held face down briefly over boiling distilled water) and then snap-dried on a 100°C heating block for 5–10 seconds. DNA was then cross-linked using an ultraviolet cross-linker (300 mJ).
Microarray Hybridization
After heating at 100°C for 2 minutes, fluorescent-labeled probes were denatured and subsequently incubated at 37°C for 20 minutes. A hybridization mixture containing 32 µL of the recovered probe, 6.8 µL of 20X saline sodium citrate (SSC), and 1.2 µL of 10% sodium dodecyl sulfate (SDS) was dropped onto prewarmed microarray slides, and a cover slip applied. Slides were then placed in a sealed moisture chamber for 16 hours at 65°C for hybridization. Following this step, slides were immediately washed once with 1X SSC with 0.03% SDS, twice with 0.5% SSC, and twice with 0.06% SSC. After washing, slides were centrifuged at 84 x g for 2 minutes and scanned immediately using an Axon microarray scanner (Molecular Devices, Sunnyvale, CA).
Microarray Data Analysis
Scanned images were analyzed using the Genepix Pro 4.0 software (Molecular Devices). The densitometry data was up-loaded into the Stanford Microarray Database for gene identification and analysis. The log (base 2) of red/green normalized ratio (mean) was found and the data filtered based on a regression correlation of 0.6. Individual genes and arrays were centered by median. Genes were included in the analysis if they passed the filter criterion of >80% good data and subsequently clustered using Pearson correlation.
Following gene clustering, significance analysis of microarrays (SAM) was used to select genes with significant expression differences between the E16 and E18 transcriptomes for each time point. SAM identifies statistically significant changes in gene expression through the assimilation of a set of gene-specific t tests. Each gene is assigned a score based on its change in expression relative to the standard deviation of repeated measurements for that gene. SAM uses permutations of the repeated measurements to estimate the false discovery rate (FDR), equivalent to chance, for those genes. Genes that had at least a 2-fold expression difference with FDR less than 2 were selected.
Functional Analysis of Differentially Expressed Genes
To identify functional connections among significantly regulated genes, both network and pathway analyses of the probes filtered by microarray were performed as previously described by Jovov et al., using Ingenuity Pathways Analysis (IPA; www.ingenuity.com, Ingenuity Systems, Redwood City, CA). The significance of networks was calculated by IPA’s integrated Ingenuity algorithm, which calculates p-values based on the right-tailed Fisher’s exact test for each canonical pathway, evaluating the likelihood that the association between a subset of genes from the whole experimental data set and a related function/pathway is due to random association.
Results
Differential Gene Expression Between Scarless E16 and Scarring E18 Keratinocytes and Fibroblasts
Transcriptomes from E16 keratinocytes and fibroblasts were directly compared to transcriptomes from E18 keratinocytes and fibroblasts. SAM identified 546 genes differentially expressed with greater than 2-fold difference between E16 and E18 keratinocytes and fibroblasts. Of these 546 genes, for keratinocytes, 24 genes were found to be downregulated in E16 as compared to E18 (Table 1). For fibroblasts, 198 genes were found to be downregulated in E16 as compared to E18 (Table 2). Conversely, 324 genes were found to be upregulated in E16 fibroblasts in comparison to E18 fibroblasts (Table 3).
Table 1.
Genes downregulated in E16 keratinocytes.
| Symbol | Gene Name |
|---|---|
| Srp72 | signal recognition particle 72 |
| Tpm2 | tropomyosin 2, beta |
| Por | P450 (cytochrome) oxidoreductase |
| Aco1 | aconitase 1 |
| Mrpl35 | mitochondrial ribosomal protein L35 |
| Xab2 | XPA binding protein 2 |
| Rpl13a | ribosomal protein L13A |
| Dstn | destrin |
| Klraq1 | KLRAQ motif containing 1 |
| Ube2s | ubiquitin-conjugating enzyme E2S |
| Gtf2h5 | general transcription factor IIH, polypeptide 5 |
| Lphn2 | latrophilin 2 |
| Trappc5 | trafficking protein particle complex 5 |
| Znrf2 | zinc and ring finger 2 |
| Pin1 | protein (peptidyl-prolyl cis/trans isomerase) NIMA-interacting 1 |
| Mark2 | MAP/microtubule affinity-regulating kinase 2 |
| Fahd2a | fumarylacetoacetate hydrolase domain containing 2A |
| Cd320 | CD320 antigen |
| Pnrc2 | Proline-rich nuclear receptor coactivator 2 |
| Rps15a | ribosomal protein S15A |
| Kpna6 | karyopherin (importin) alpha 6 |
| Eif3d | eukaryotic translation initiation factor 3, subunit D |
| Snrpg | small nuclear ribonucleoprotein polypeptide G |
| Efna5 | ephrin A5 |
Table 2.
Genes downregulated in E16 fibroblasts.
| Symbol | Gene Name | Symbol | Gene Name |
|---|---|---|---|
| Rps29 | ribosomal protein S29 | Cdk20 | cyclin-dependent kinase 20 |
| Gapdh | glyceraldehyde-3-phosphate dehydrogenase |
Pcdhg@ | protocadherin gamma cluster |
| Hmgn1 | high mobility group nucleosomal binding domain 1 |
Sun1 | Sad1 and UNC84 domain containing 1 |
| Lyrm4 | LYR motif containing 4 | Ilf3 | interleukin enhancer binding factor 3 |
| Dcun1d1 | DCN1, defective in cullin neddylation 1, domain containing 1 (S. cerevisiae) |
Akr1c19 | aldo-keto reductase family 1, member C19 |
| Fkbp1a | FK506 binding protein 1a | Mphosph8 | M-phase phosphoprotein 8 |
| Pfkm | phosphofructokinase, muscle | Pop5 | processing of precursor 5, ribonuclease P/MRP family (S. cerevisiae) |
| Adcy7 | adenylate cyclase 7 | Mpv17 | MpV17 mitochondrial inner membrane protein |
| Krt83 | keratin 83 | Ulk1 | Unc-51 like kinase 1 (C. elegans) |
| Rrp1 | ribosomal RNA processing 1 homolog (S. cerevisiae) |
Hspa5 | heat shock protein 5 |
| Lrrcc1 | leucine rich repeat and coiled- coil domain containing 1 |
Pax3 | paired box gene 3 |
| Ldb3 | LIM domain binding 3 | Ubr5 | ubiquitin protein ligase E3 component n-recognin 5 |
| Hadh | hydroxyacyl-Coenzyme A dehydrogenase |
Sorl1 | sortilin-related receptor, LDLR class A repeats-containing |
| Gls | glutaminase | Son | Son DNA binding protein |
| Msi2 | Musashi homolog 2 (Drosophila) |
Dnajc19 | DnaJ (Hsp40) homolog, subfamily C, member 19 |
| Rbfox2 | RNA binding protein, fox-1 homolog (C. elegans) 2 |
Lyl1 | lymphoblastomic leukemia 1 |
| Myo9a | myosin IXa | Csf2 | colony stimulating factor 2 (granulocyte-macrophage) |
| Cdk4 | cyclin-dependent kinase 4 | Tmem184a | transmembrane protein 184a |
| Gja1 | gap junction protein, alpha 1 | Plrg1 | pleiotropic regulator 1, PRL1 homolog (Arabidopsis) |
| Lck | lymphocyte protein tyrosine kinase |
Prl8a2 | prolactin family 8, subfamily a, member 2 |
| Rpl37a | ribosomal protein L37a | Stim1 | stromal interaction molecule 1 |
| Sh2b2 | SH2B adaptor protein 2 | Klhdc10 | kelch domain containing 10 |
| Spnb2 | spectrin beta 2 | 2700097O09Rik | RIKEN cDNA 2700097O09 gene |
| Arpp19 | cAMP-regulated phosphoprotein 19 |
Tmx2 | thioredoxin-related transmembrane protein 2 |
| Rela | v-rel reticuloendotheliosis viral oncogene homolog A (avian) |
Nip7 | nuclear import 7 homolog (S. cerevisiae) |
| H2-DMb1 | histocompatibility 2, class II, locus Mb1 |
Xdh | xanthine dehydrogenase |
| Wfdc5 | WAP four-disulfide core domain 5 |
Nynrin | NYN domain and retroviral integrase containing |
| Tyrp1 | tyrosinase-related protein 1 | Rmnd5a | required for meiotic nuclear division 5 homolog A (S. cerevisiae) |
| 0610031J06Rik | RIKEN cDNA 0610031J06 gene |
Ube2b | ubiquitin-conjugating enzyme E2B, RAD6 homology (S. cerevisiae) |
| septin 9 | Stoml3 | stomatin (Epb7.2)-like 3 | |
| Grina | glutamate receptor, ionotropic, N-methyl D-aspartate-associated protein 1 (glutamate binding) |
S100a9 | S100 calcium binding protein A9 (calgranulin B) |
| Mtch1 | mitochondrial carrier homolog (C. elegans) |
1 Ing5 | inhibitor of growth family, member 5 |
| Cpt2 | carnitine palmitoyltransferase 2 | B3galnt2 | UDP-GalNAc:betaGlcNAc beta 1,3-galactosaminyltransferase, polypeptide 2 |
| Ctnnb1 | catenin (cadherin associated protein), beta 1 |
Rabif | RAB interacting factor |
| Egfl6 | EGF-like-domain, multiple 6 | Pex11b | peroxisomal biogenesis factor 11 beta |
| Golph3 | golgi phosphoprotein 3 | Abhd16a | abhydrolase domain containing 16A |
| Tcea1 | transcription elongation factor A (SII) 1 |
Fkbpl | FK506 binding protein-like |
| Abcb10 | ATP-binding cassette, sub- family B (MDR/TAP), member 10 |
Calm2 | calmodulin 2 |
| Mtbp | Mdm2, transformed 3T3 cell double minute p53 binding protein |
Foxn3 | forkhead box N3 |
| Lias | lipoic acid synthetase | Kdm2b | lysine (K)-specific demethylase 2B |
| 2310011J03Rik | RIKEN cDNA 2310011J03 gene |
Gnpnat1 | glucosamine-phosphate N- acetyltransferase 1 |
| Cyth2 | cytohesin 2 | Bik | BCL2-interacting killer |
| Eif3h | eukaryotic translation initiation factor 3, subunit H |
Pde2a | phosphodiesterase 2A, cGMP- stimulated |
| Bcl2l2 | BCL2-like 2 | Rcc2 | regulator of chromosome condensation 2 |
| Tex2 | testis expressed gene 2 | Cd151 | CD151 antigen |
| Hnrnpa2b1 | heterogeneous nuclear ribonucleoprotein A2/B1 |
Zfp704 | zinc finger protein 704 |
| Tbx15 | T-box 15 | Sohlh1 | spermatogenesis and oogenesis specific basic helix-loop-helix 1 |
| Maoa | monoamine oxidase A | Antxr1 | anthrax toxin receptor 1 |
| Psmb9 | proteasome (prosome, macropain) subunit, beta type 9 (large multifunctional peptidase 2) |
Llph | LLP homolog, long-term synaptic facilitation (Aplysia) |
| Cdk18 | cyclin-dependent kinase 18 | Ywhae | tyrosine 3- monooxygenase/tryptophan 5- monooxygenase activation protein, epsilon polypeptide |
| Cd83 | CD83 antigen | Cdk16 | cyclin-dependent kinase 16 |
| Tns4 | tensin 4 | Adss | adenylosuccinate synthetase, non muscle |
| Slc38a10 | solute carrier family 38, member 10 |
Fam45a | family with sequence similarity 45, member A |
| Cdc42se1 | CDC42 small effector 1 | Fastkd2 | FAST kinase domains 2 |
| Smurf1 | SMAD specific E3 ubiquitin protein ligase 1 |
Heatr2 | HEAT repeat containing 2 |
| 1600002H07Rik | RIKEN cDNA 1600002H07 gene |
Tpr | translocated promoter region |
| Cpt1a | carnitine palmitoyltransferase 1a, liver |
Lasp1 | LIM and SH3 protein 1 |
| Cartpt | CART prepropeptide | Car2 | carbonic anhydrase 2 |
| Fam82b | family with sequence similarity 82, member B |
Taf10 | TAF10 RNA polymerase II, TATA box binding protein (TBP)-associated factor |
| Tdrd3 | tudor domain containing 3 | Stx2 | syntaxin 2 |
| Galt | galactose-1-phosphate uridyl transferase |
Commd10 | COMM domain containing 10 |
| Dync1i2 | dynein cytoplasmic 1 intermediate chain 2 |
Lap3 | leucine aminopeptidase 3 |
| Ctps2 | cytidine 5’-triphosphate synthase 2 |
Hacl1 | 2-hydroxyacyl-CoA lyase 1 |
| Naa35 | N(alpha)-acetyltransferase 35, NatC auxiliary subunit |
Scp2 | sterol carrier protein 2, liver |
| Dcun1d1 | DCN1, defective in cullin neddylation 1, domain containing 1 (S. cerevisiae) |
Iffo2 | intermediate filament family orphan 2 |
| Acd | adrenocortical dysplasia | Upf3b | UPF3 regulator of nonsense transcripts homolog B (yeast) |
| Cisd1 | CDGSH iron sulfur domain 1 | Lmbr1l | limb region 1 like |
| Btf3l4 | basic transcription factor 3-like 4 |
Tmco1 | transmembrane and coiled-coil domains 1 |
| Golph3l | golgi phosphoprotein 3-like | Klhl13 | kelch-like 13 (Drosophila) |
| Tbx15 | T-box 15 | Fbxo15 | F-box protein 15 |
| Rprd1a | regulation of nuclear pre-mRNA domain containing 1A |
Dnaja1 | DnaJ (Hsp40) homolog, subfamily A, member 1 |
| Cat | catalase | Mau2 | MAU2 chromatid cohesion factor homolog (C. elegans) |
| Dclk2 | doublecortin-like kinase 2 | Ywhah | tyrosine 3- monooxygenase/tryptophan 5- monooxygenase activation protein, eta polypeptide |
| Alx3 | aristaless-like homeobox 3 | 1110008L16Rik | RIKEN cDNA 1110008L16 gene |
| Myl6 | myosin, light polypeptide 6, alkali, smooth muscle and non- muscle |
Rbpms | RNA binding protein gene with multiple splicing |
| Sra1 | steroid receptor RNA activator 1 | Tmem38b | transmembrane protein 38B |
| Tmc6 | transmembrane channel-like gene family 6 |
Plac1l | placenta-specific 1-like |
| Brd7 | bromodomain containing 7 | Dusp26 | dual specificity phosphatase 26 (putative) |
| Cxcl1 | chemokine (C-X-C motif) ligand 1 |
Mmp3 | matrix metallopeptidase 3 |
| Ap1g1 | adaptor protein complex AP-1, gamma 1 subunit |
Cisd2 | CDGSH iron sulfur domain 2 |
| St3gal1 | ST3 beta-galactoside alpha-2,3- sialyltransferase 1 |
Son | Son DNA binding protein |
| Mylk3 | myosin light chain kinase 3 | 2200002K05Rik | RIKEN cDNA 2200002K05 gene |
| Pdk4 | pyruvate dehydrogenase kinase, isoenzyme 4 |
Krt20 | keratin 20 |
| Rtkn | rhotekin | Fam126a | family with sequence similarity 126, member A |
| Rab11a | RAB11a, member RAS oncogene family |
Nynrin | NYN domain and retroviral integrase containing |
| Epdr1 | ependymin related protein 1 (zebrafish) |
Smg6 | Smg-6 homolog, nonsense mediated mRNA decay factor (C. elegans) |
| Krt83 | keratin 83 | 1700021C14Rik | RIKEN cDNA 1700021C14 gene |
| Pax3 | paired box gene 3 | Cdc34 | cell division cycle 34 homolog (S. cerevisiae) |
| 9030624J02Rik | RIKEN cDNA 9030624J02 gene |
Rpl17 | ribosomal protein L17 |
| Csnk1g2 | casein kinase 1, gamma 2 | Rpgrip1 | retinitis pigmentosa GTPase regulator interacting protein 1 |
| Stk30 | serine/threonine kinase 30 | Ube2v2 | ubiquitin-conjugating enzyme E2 variant 2 |
| Macf1 | microtubule-actin crosslinking factor 1 |
Apbb2 | amyloid beta (A4) precursor protein-binding, family B, member 2 |
| Ap3b2 | adaptor-related protein complex 3, beta 2 subunit |
Crtam | cytotoxic and regulatory T cell molecule |
| Fubp1 | far upstream element (FUSE) binding protein 1 |
Pon2 | paraoxonase 2 |
| Arrdc3 | arrestin domain containing 3 | Nudt7 | nudix (nucleoside diphosphate linked moiety X)-type motif 7 |
| Vim | vimentin | Smg6 | Smg-6 homolog, nonsense mediated mRNA decay factor (C. elegans) |
| Limd1 | LIM domains containing 1 | Ssr2 | signal sequence receptor, beta |
| Fubp1 | far upstream element (FUSE) binding protein 1 |
Parl | presenilin associated, rhomboid- like |
| Igfbp5 | insulin-like growth factor binding protein 5 |
Csnk2a1 | casein kinase 2, alpha 1 polypeptide |
| H2-Q8 | histocompatibility 2, Q region locus 8 |
Cdk20 | cyclin-dependent kinase 20 |
Table 3.
Genes upregulated in E16 fibroblasts.
| Symbol | Gene Name | Symbol | Gene Name |
|---|---|---|---|
| Lpar4 | lysophosphatidic acid receptor 4 | Bola1 | bolA-like 1 (E. coli) |
| Ankrd17 | ankyrin repeat domain 17 | Tssk2 | testis-specific serine kinase 2 |
| C330019G07Rik | RIKEN cDNA C330019G07 gene | Grin1 | glutamate receptor, ionotropic, NMDA1 (zeta 1) |
| Golt1b | golgi transport 1 homolog B (S. cerevisiae) |
Asb12 | ankyrin repeat and SOCS box- containing 12 |
| Adprh | ADP-ribosylarginine hydrolase | Mtdh | metadherin |
| Cstf2t | cleavage stimulation factor, 3’ pre- RNA subunit 2, tau |
Ankrd1 | ankyrin repeat domain 1 (cardiac muscle) |
| Ifrg15 | interferon alpha responsive gene | Slc27a3 | solute carrier family 27 (fatty acid transporter), member 3 |
| 4632428N05Rik | RIKEN cDNA 4632428N05 gene | Darc | Duffy blood group, chemokine receptor |
| Gsk3b | glycogen synthase kinase 3 beta | Ezh1 | enhancer of zeste homolog 1 (Drosophila) |
| Slc35a1 | solute carrier family 35 (CMP-sialic acid transporter), member 1 |
AU019823 | expressed sequence AU019823 |
| Mepce | methylphosphate capping enzyme | Hist1h2ae | histone cluster 1, H2ae |
| Ptges | prostaglandin E synthase | Bdh2 | 3-hydroxybutyrate dehydrogenase, type 2 |
| Slc9a6 | solute carrier family 9 (sodium/hydrogen exchanger), member 6 |
Mphosph10 | M-phase phosphoprotein 10 (U3 small nucleolar ribonucleoprotein) |
| BC016495 | cDNA sequence BC016495 | Prkag1 | protein kinase, AMP-activated, gamma 1 non-catalytic subunit |
| Mocs1 | molybdenum cofactor synthesis 1 | Lrat | lecithin-retinol acyltransferase (phosphatidylcholine-retinol-O- acyltransferase) |
| Pbk | PDZ binding kinase | Gtf3c6 | general transcription factor IIIC, polypeptide 6, alpha |
| Ficd | FIC domain containing | Aasdhppt | aminoadipate-semialdehyde dehydrogenase-phosphopantetheinyl transferase |
| Cdc42ep5 | CDC42 effector protein (Rho GTPase binding) 5 |
Ddx49 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 49 |
| 4930528F23Rik | RIKEN cDNA 4930528F23 gene | Ilk | integrin linked kinase |
| Myo10 | myosin X | Kcnab3 | potassium voltage-gated channel, shaker-related subfamily, beta member 3 |
| Coro1a | coronin, actin binding protein 1A | Krt13 | keratin 13 |
| Ppp1r2 | protein phosphatase 1, regulatory (inhibitor) subunit 2 |
Med11 | mediator of RNA polymerase II transcription, subunit 11 homolog (S. cerevisiae) |
| Fank1 | fibronectin type 3 and ankyrin repeat domains 1 |
Appl1 | adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper containing 1 |
| S100a11 | S100 calcium binding protein A11 (calgizzarin) |
B4galt1 | UDP-Gal:betaGlcNAc beta 1,4- galactosyltransferase, polypeptide 1 |
| Zcchc3 | zinc finger, CCHC domain containing 3 |
5430437P03Rik | RIKEN cDNA 5430437P03 gene |
| Rsph9 | radial spoke head 9 homolog (Chlamydomonas) |
Lysmd4 | LysM, putative peptidoglycan- binding, domain containing 4 |
| Tex19.1 | testis expressed gene 19.1 | Atp4a | ATPase, H+/K+ exchanging, gastric, alpha polypeptide |
| Ift74 | intraflagellar transport 74 homolog (Chlamydomonas) |
Il10rb | interleukin 10 receptor, beta |
| Snap25 | synaptosomal-associated protein 25 | Dnajc10 | DnaJ (Hsp40) homolog, subfamily C, member 10 |
| Tipin | timeless interacting protein | 6-Sep | septin 6 |
| Rab3a | RAB3A, member RAS oncogene family |
Adal | adenosine deaminase-like |
| Foxn2 | forkhead box N2 | Tpm2 | tropomyosin 2, beta |
| Tmsb15l | thymosin beta 15b like | Ppp1r9a | protein phosphatase 1, regulatory (inhibitor) subunit 9A |
| Ppat | phosphoribosyl pyrophosphate amidotransferase |
Hspbp1 | HSPA (heat shock 70kDa) binding protein, cytoplasmic cochaperone 1 |
| Rbm34 | RNA binding motif protein 34 | Scnm1 | sodium channel modifier 1 |
| Mllt3 | translocated to, 3 | Ctnna2 | catenin (cadherin associated protein), alpha 2 |
| Cda | cytidine deaminase | Hao2 | hydroxyacid oxidase 2 |
| Plac9 | placenta specific 9 | Fam20c | family with sequence similarity 20, member C |
| Zfp821 | zinc finger protein 821 | Edn2 | endothelin 2 |
| Parp16 | poly (ADP-ribose) polymerase family, member 16 |
Rhoc | ras homolog gene family, member C |
| Uckl1 | uridine-cytidine kinase 1-like 1 | Cdkn2b | cyclin-dependent kinase inhibitor 2B (p15, inhibits CDK4) |
| Cct6a | chaperonin containing Tcp1, subunit 6a (zeta) |
Dhfr | dihydrofolate reductase |
| Acsl6 | acyl-CoA synthetase long-chain family member 6 |
Rabac1 | Rab acceptor 1 (prenylated) |
| Tspan31 | tetraspanin 31 | Scrn1 | secernin 1 |
| Spire1 | spire homolog 1 (Drosophila) | Slc33a1 | solute carrier family 33 (acetyl-CoA transporter), member 1 |
| Phtf2 | putative homeodomain transcription factor 2 |
Akirin2 | akirin 2 |
| Akap13 | A kinase (PRKA) anchor protein 13 | Wipi2 | WD repeat domain, phosphoinositide interacting 2 |
| Mdh2 | malate dehydrogenase 2, NAD (mitochondrial) |
Isoc1 | isochorismatase domain containing 1 |
| Mrpl49 | mitochondrial ribosomal protein L49 | Plekhf2 | pleckstrin homology domain containing, family F (with FYVE domain) member 2 |
| Efna2 | ephrin A2 | Tspan3 | tetraspanin 3 |
| Mbd4 | methyl-CpG binding domain protein 4 |
Ece2 | endothelin converting enzyme 2 |
| Vegfc | vascular endothelial growth factor C | Hspb7 | heat shock protein family, member 7 (cardiovascular) |
| Wrnip1 | Werner helicase interacting protein 1 | Atf4 | activating transcription factor 4 |
| Pikfyve | phosphoinositide kinase, FYVE finger containing |
D19Wsu162e | DNA segment, Chr 19, Wayne State University 162, expressed |
| Cyba | cytochrome b-245, alpha polypeptide | Srp14 | signal recognition particle 14 |
| Dstyk | dual serine/threonine and tyrosine protein kinase |
Il2 | interleukin 2 |
| Stmn3 | stathmin-like 3 | G3bp2 | GTPase activating protein (SH3 domain) binding protein 2 |
| Pepd | peptidase D | Inpp5d | inositol polyphosphate-5- phosphatase D |
| Chgb | chromogranin B | Slc30a4 | solute carrier family 30 (zinc transporter), member 4 |
| Thap7 | THAP domain containing 7 | Mmp14 | matrix metallopeptidase 14 (membrane-inserted) |
| 4833439L19Rik | RIKEN cDNA 4833439L19 gene | Lmbrd1 | LMBR1 domain containing 1 |
| Pdgfa | platelet derived growth factor, alpha | D10Wsu102e | DNA segment, Chr 10, Wayne State University 102, expressed |
| Pja1 | praja1, RING-H2 motif containing | Dock7 | dedicator of cytokinesis 7 |
| Car10 | carbonic anhydrase 10 | Camp | cathelicidin antimicrobial peptide |
| Trmt2a | TRM2 tRNA methyltransferase 2 homolog A (S. cerevisiae) |
Usp39 | ubiquitin specific peptidase 39 |
| Stat1 | signal transducer and activator of transcription 1 |
Ints4 | integrator complex subunit 4 |
| Trappc5 | trafficking protein particle complex 5 | Senp6 | SUMO/sentrin specific peptidase 6 |
| Zmat5 | zinc finger, matrin type 5 | Csnk2b | casein kinase 2, beta polypeptide |
| 1700012B15Rik | RIKEN cDNA 1700012B15 gene | Hras1 | Harvey rat sarcoma virus oncogene 1 |
| Dpep1 | dipeptidase 1 (renal) | Med19 | mediator of RNA polymerase II transcription, subunit 19 homolog (yeast) |
| Zyx | zyxin | Pgcp | plasma glutamate carboxypeptidase |
| Hpcal1 | hippocalcin-like 1 | Tmem41b | transmembrane protein 41B |
| Cdk5rap3 | CDK5 regulatory subunit associated protein 3 |
1700014N06Rik | RIKEN cDNA 1700014N06 gene |
| Ppp3cc | protein phosphatase 3, catalytic subunit, gamma isoform |
AI314180 | expressed sequence AI314180 |
| Mettl21a | methyltransferase like 21A | Prpsap1 | phosphoribosyl pyrophosphate synthetase-associated protein 1 |
| Tpd52l1 | tumor protein D52-like 1 | Gng13 | guanine nucleotide binding protein (G protein), gamma 13 |
| Hoxb2 | homeobox B2 | Snap23 | synaptosomal-associated protein 23 |
| Mobkl2a | MOB1, Mps One Binder kinase activator-like 2A (yeast) |
Mpp1 | membrane protein, palmitoylated |
| Txndc16 | thioredoxin domain containing 16 | Cnot1 | CCR4-NOT transcription complex, subunit 1 |
| Galntl5 | UDP-N-acetyl-alpha-D- galactosamine:polypeptide N- acetylgalactosaminyltransferase-like 5 |
Egf | epidermal growth factor |
| Mcm5 | minichromosome maintenance deficient 5, cell division cycle 46 (S. cerevisiae) |
Myo16 | myosin XVI |
| Tinf2 | Terf1 (TRF1)-interacting nuclear factor 2 |
Gatm | glycine amidinotransferase (L- arginine:glycine amidinotransferase) |
| Krt19 | keratin 19 | Krt24 | keratin 24 |
| Setd3 | SET domain containing 3 | Gas5 | growth arrest specific 5 |
| Bfar | bifunctional apoptosis regulator | Zfp318 | zinc finger protein 318 |
| Smarcd2 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily d, member 2 |
Rapgef4 | Rap guanine nucleotide exchange factor (GEF) 4 |
| Il12a | interleukin 12a | Carkd | carbohydrate kinase domain containing |
| Agpat3 | 1-acylglycerol-3-phosphate O- acyltransferase 3 |
2210012G02Rik | RIKEN cDNA 2210012G02 gene |
| Myc | myelocytomatosis oncogene | 1700001J03Rik | RIKEN cDNA 1700001J03 gene |
| Pon1 | paraoxonase 1 | Uqcrh | ubiquinol-cytochrome c reductase hinge protein |
| Slc44a3 | solute carrier family 44, member 3 | Sult1d1 | sulfotransferase family 1D, member 1 |
| Ccr5 | chemokine (C-C motif) receptor 5 | Paqr5 | progestin and adipoQ receptor family member V |
| Pex19 | peroxisomal biogenesis factor 19 | Syvn1 | synovial apoptosis inhibitor 1, synoviolin |
| Gadd45g | growth arrest and DNA-damage- inducible 45 gamma |
Cox17 | cytochrome c oxidase, subunit XVII assembly protein homolog (yeast) |
| 1700028P14Rik | RIKEN cDNA 1700028P14 gene | Crym | crystallin, mu |
| Cdh3 | cadherin 3 | Tbl2 | transducin (beta)-like 2 |
| Mettl21a | methyltransferase like 21A | Ogdh | oxoglutarate dehydrogenase (lipoamide) |
| Cenph | centromere protein H | Ypel1 | yippee-like 1 (Drosophila) |
| Khdc1b | KH domain containing 1B | Myl4 | myosin, light polypeptide 4 |
| Odz3 | odd Oz/ten-m homolog 3 (Drosophila) |
D2Ertd750e | DNA segment, Chr 2, ERATO Doi 750, expressed |
| Stab1 | stabilin 1 | Nme3 | non-metastatic cells 3, protein expressed in |
| Unkl | unkempt-like (Drosophila) | Sft2d1 | SFT2 domain containing 1 |
| Map1lc3b | microtubule-associated protein 1 light chain 3 beta |
Wwtr1 | WW domain containing transcription regulator 1 |
| Smurf2 | SMAD specific E3 ubiquitin protein ligase 2 |
Asb1 | ankyrin repeat and SOCS box- containing 1 |
| Ndufa13 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 13 |
Serpini2 | serine (or cysteine) peptidase inhibitor, clade I, member 2 |
| Rab11fip1 | RAB11 family interacting protein 1 (class I) |
Elk1 | ELK1, member of ETS oncogene family |
| Atp7a | ATPase, Cu++ transporting, alpha polypeptide |
Nubp1 | nucleotide binding protein 1 |
| Fcgbp | Fc fragment of IgG binding protein | Scaf11 | SR-related CTD-associated factor 11 |
| Josd2 | Josephin domain containing 2 | Acyp1 | acylphosphatase 1, erythrocyte (common) type |
| Irx5 | Iroquois related homeobox 5 (Drosophila) |
Rpl10 | ribosomal protein 10 |
| Stra13 | stimulated by retinoic acid 13 | Herc4 | hect domain and RLD 4 |
| Gys1 | glycogen synthase 1, muscle | Fam195b | family with sequence similarity 195, member B |
| Ccs | copper chaperone for superoxide dismutase |
Ubash3b | ubiquitin associated and SH3 domain containing, B |
| Slc1a5 | solute carrier family 1 (neutral amino acid transporter), member 5 |
Slc6a1 | solute carrier family 6 (neurotransmitter transporter, GABA), member 1 |
| Ngly1 | N-glycanase 1 | Angptl1 | angiopoietin-like 1 |
| Dcbld1 | discoidin, CUB and LCCL domain containing 1 |
Wdr54 | WD repeat domain 54 |
| Mast2 | microtubule associated serine/threonine kinase 2 |
Rbbp6 | retinoblastoma binding protein 6 |
| Crlf1 | cytokine receptor-like factor 1 | 1190007F08Rik | RIKEN cDNA 1190007F08 gene |
| Asnsd1 | asparagine synthetase domain containing 1 |
Dll1 | delta-like 1 (Drosophila) |
| Dcun1d5 | DCN1, defective in cullin neddylation 1, domain containing 5 (S. cerevisiae) |
Nppb | natriuretic peptide type B |
| Fam185a | family with sequence similarity 185, member A |
Nfatc3 | nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 3 |
| Hdac11 | histone deacetylase 11 | Lrch2 | leucine-rich repeats and calponin homology (CH) domain containing 2 |
| 1110034A24Rik | RIKEN cDNA 1110034A24 gene | Atp5b | ATP synthase, H+ transporting mitochondrial F1 complex, beta subunit |
| Rps4y2 | ribosomal protein S4, Y-linked 2 | Rrp12 | ribosomal RNA processing 12 homolog (S. cerevisiae) |
| Zfp868 | zinc finger protein 868 | Dhx16 | DEAH (Asp-Glu-Ala-His) box polypeptide 16 |
| Psmc2 | proteasome (prosome, macropain) 26S subunit, ATPase 2 |
Dmap1 | DNA methyltransferase 1-associated protein 1 |
| Rbm39 | RNA binding motif protein 39 | Slc1a4 | solute carrier family 1 (glutamate/neutral amino acid transporter), member 4 |
| Ctnna1 | catenin (cadherin associated protein), alpha 1 |
Smc4 | structural maintenance of chromosomes 4 |
| Cyth1 | cytohesin 1 | Camsap1 | calmodulin regulated spectrin- associated protein 1 |
| Sco1 | SCO cytochrome oxidase deficient homolog 1 (yeast) |
Cntln | centlein, centrosomal protein |
| Calb1 | calbindin 1 | Ckap2l | cytoskeleton associated protein 2- like |
| 2310001H18Rik | RIKEN cDNA 2310001H18 gene | Katna1 | katanin p60 (ATPase-containing) subunit A1 |
| Sh3d19 | SH3 domain protein D19 | Speg | SPEG complex locus |
| Acadsb | acyl-Coenzyme A dehydrogenase, short/branched chain |
Nsdhl | NAD(P) dependent steroid dehydrogenase-like |
| Map2k2 | mitogen-activated protein kinase kinase 2 |
Lsm6 | LSM6 homolog, U6 small nuclear RNA associated (S. cerevisiae) |
| Cd3g | CD3 antigen, gamma polypeptide | Tgm1 | transglutaminase 1, K polypeptide |
| Sepn1 | selenoprotein N, 1 | Notch1 | Notch gene homolog 1 (Drosophila) |
| Ndufa3 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 3 |
Ndufa11 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex 11 |
| B3gnt7 | UDP-GlcNAc:betaGal beta-1,3-N- acetylglucosaminyltransferase 7 |
Srsf3 | serine/arginine-rich splicing factor 3 |
| Stat6 | signal transducer and activator of transcription 6 |
Vps26b | vacuolar protein sorting 26 homolog B (yeast) |
| Nol4 | nucleolar protein 4 | Cetn3 | centrin 3 |
| Rpl37a | ribosomal protein L37a | Tprgl | transformation related protein 63 regulated like |
| Lhx6 | LIM homeobox protein 6 | Tgif2 | TGFB-induced factor homeobox 2 |
| Myof | myoferlin | BC016423 | cDNA sequence BC016423 |
| Cox5a | cytochrome c oxidase, subunit Va | Mycbp2 | MYC binding protein 2 |
| Rpl19 | ribosomal protein L19 | Jak2 | Janus kinase 2 |
| Cyth2 | cytohesin 2 | Metap2 | methionine aminopeptidase 2 |
| Chchd3 | coiled-coil-helix-coiled-coil-helix domain containing 3 |
Myh8 | myosin, heavy polypeptide 8, skeletal muscle, perinatal |
| Runx1t1 | translocated to, 1 (cyclin D-related) | Ehd3 | EH-domain containing 3 |
| Rpl3 | ribosomal protein L3 | Jak2 | Janus kinase 2 |
| Syf2 | SYF2 homolog, RNA splicing factor (S. cerevisiae) |
Lefty1 | left right determination factor 1 |
| Cyp26a1 | cytochrome P450, family 26, subfamily a, polypeptide 1 |
Dynll2 | dynein light chain LC8-type 2 |
| Agfg1 | ArfGAP with FG repeats 1 | Fam57a | family with sequence similarity 57, member A |
| Pin1 | protein (peptidyl-prolyl cis/trans isomerase) NIMA-interacting 1 |
Srp72 | signal recognition particle 72 |
| Tnfrsf12a | tumor necrosis factor receptor superfamily, member 12a |
Snhg11 | small nucleolar RNA host gene 11 |
| Ttc4 | tetratricopeptide repeat domain 4 | Celf4 | CUGBP, Elav-like family member 4 |
| G3bp2 | GTPase activating protein (SH3 domain) binding protein 2 |
1810043G02Rik | RIKEN cDNA 1810043G02 gene |
| Tm4sf20 | transmembrane 4 L six family member 20 |
Zrsr1 | zinc finger (CCCH type), RNA binding motif and serine/arginine rich 1 |
| Gosr2 | golgi SNAP receptor complex member 2 |
Il2ra | interleukin 2 receptor, alpha chain |
| Ndufaf1 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, assembly factor 1 |
Wnt4 | wingless-related MMTV integration site 4 |
| 8030474K03Rik | RIKEN cDNA 8030474K03 gene | Fam187b | family with sequence similarity 187, member B |
| Stoml2 | stomatin (Epb7.2)-like 2 | Bola1 | bolA-like 1 (E. coli) |
Functional Pathway Analysis
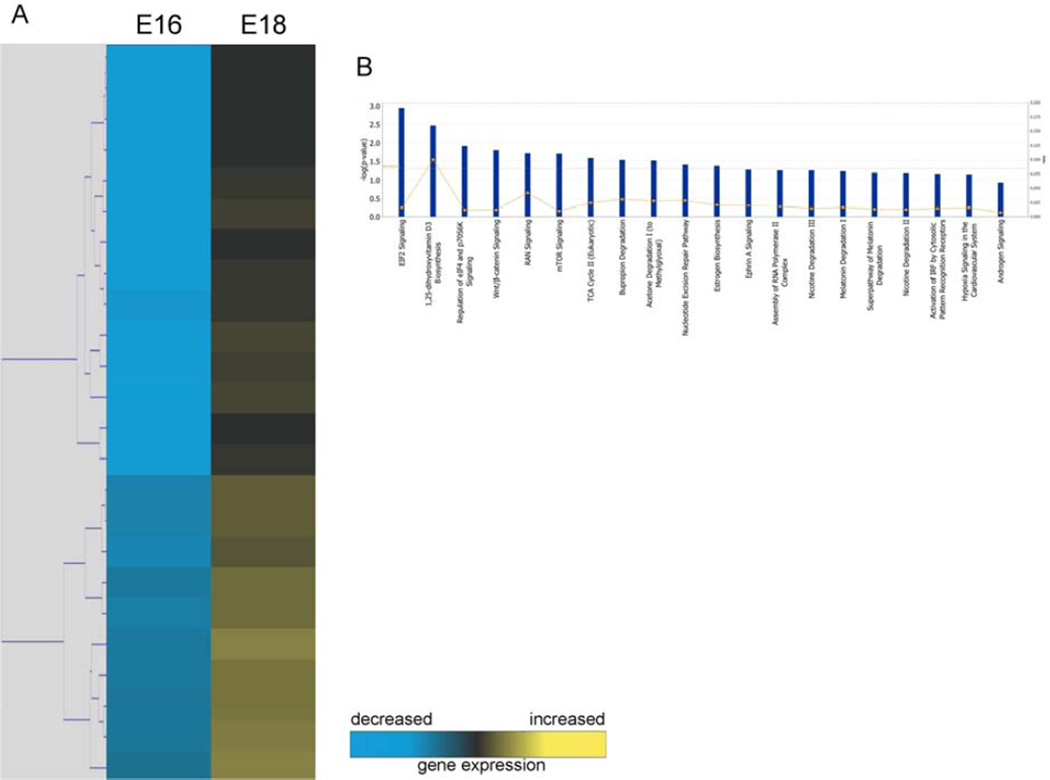
Out of the 24 genes found to be downregulated in E16 keratinocytes (Figure 1A), twenty functional pathways were identified (Figure 1B). The top five pathways were associated with: EIF2 signaling, 1,25-dihydroxyvitamin D3 biosynthesis, regulation of eIF4 and p7056K signaling, Wnt/β-catenin signaling, and RAN signaling.
Figure 1. Microarray analysis of E16 and E18 keratinocytes.
(A) Hierarchical clustering of differentially regulated genes from fetal keratinocytes at E16 vs. E18. Individual genes are clustering according to the dendrogram on the left, and expression levels are represented in the heatmap on the right. Yellow and blue indicate up- and down-regulation, respectively. (B) Canonical pathways significantly enriched for among genes whose expression was significantly downregulated in E16 samples compared to E18.
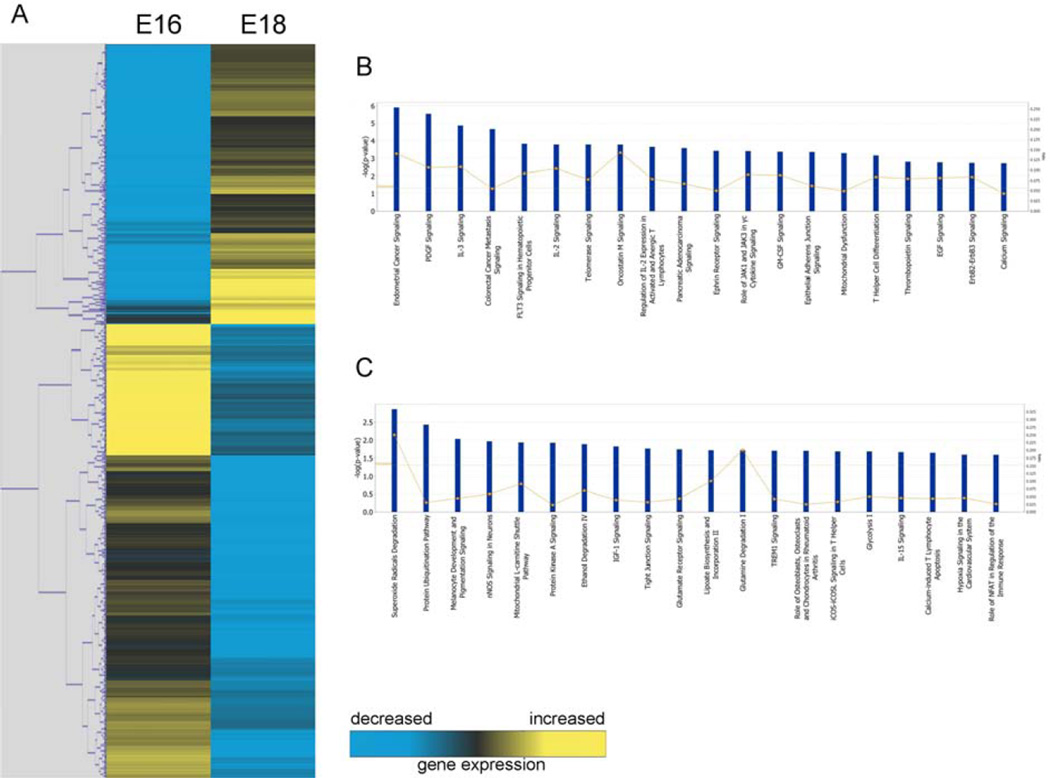
Of the 198 genes downregulated in E16 fibroblasts compared to E18 fibroblasts (Figure 2A), twenty functional pathways were again identified (Figure 2B). The top five pathways were: endometrial cancer signaling, PDGF signaling, IL-3 signaling, colorectal cancer metastasis signaling and FLT3 signaling in hematopoietic progenitor cells.
Figure 2. Microarray analysis of E16 and E18 fibroblasts.
(A) Hierarchical clustering of differentially regulated genes from fetal fibroblasts at E16 vs. E18. Individual genes are clustering according to the dendrogram on the left, and expression levels are represented in the heatmap on the right. Yellow and blue indicate up- and down-regulation, respectively. (B) Canonical pathways significantly enriched for among genes whose expression was significantly downregulated in E16 samples compared to E18. (C) Canonical pathways significantly enriched for among genes whose expression was significantly upregulated in E16 samples compared to E18.
From the 324 genes found to be upregulated in E16 fibroblasts in comparison to E18 fibroblasts (Figure 2C), the top five functional pathways were associated with: superoxide radicals degradation, protein ubiquination, melanocyte development and pigment signaling, nNOS signaling, and mitochondrial L-carnitine shuttling.
Discussion
Early gestational skin has the unique ability to regenerate following injury. However, during the later stages of fetal development, this ability gradually diminishes, culminating ultimately with an adult cutaneous wound healing process characterized by scarring. The goal of our study is to identify candidate pathways important to scarless wound healing that might be manipulated in adult wound healing to decrease scarring and promote regenerative healing. In order to achieve this goal, we performed microarray analysis on fetal keratinocytes and fibroblasts from fetal scarless and scarring time points. Furthermore, to better understand individual gene expression changes, we performed signal pathway analysis. This technique allowed us to identify gene cascades that are coordinately regulated during the transition period. In this section we will discuss in greater detail some of the especially relevant pathways found to be differentially activated in E16 versus E18 keratinocytes and fibroblasts.
Beta-Catenin Dependent Wnt Signaling
The Wnt family of glycoproteins is involved in proliferation, differentiation, migration, and carcinogenesis (10) as well as in dermal and epidermal maturation (11). Wnt proteins are expressed following cutaneous injury, and different Wnt signaling in response to injury has been found to occur pre- and post-natally. For instance, a 2010 study by Carre et al. revealed that β-catenin–dependent Wnt signaling expression is different between scarless fetal and scarring postnatal wound repair (7). This finding is corroborated by the results of our study. We demonstrated that Wnt signaling pathways are upregulated in nonwounded scarring fetal keratinocytes at E18 in comparison to nonscarring fetal keratinocytes at E16. While other studies in the past have compared Wnt signaling between fetal and adult skin, our study is the first to compare Wnt activation in early and late gestational age nonwounded skin. The differential activation of Wnt signaling pathways between E16 and E18 keratinocytes suggests that Wnt signaling may play an important role in regulating the different wound healing outcomes in early and late gestational skin. Moreover, Wnt is known to positively regulate TGF-β1, a key mediator of fibrosis implicated in hypertrophic scar formation (12, 13), transcription in postnatal skin cells, suggesting a possible mechanism by which upregulation of Wnt signaling in late gestational keratinocytes might contribute to loss of regenerative ability. Taken together, these data suggest that β-catenin–dependent Wnt pathways may be early and key regulators of embryonic wound healing.
PDGF Signaling
Whereas adult wounds are known to contain large quantities of PDGF, this growth factor is virtually absent in embryonic wounds (14). Similarly, a 2003 study by Song et al. found strong expression of PDGF in adult skin but not in unwounded fetal skin (15). However, no previous study has looked specifically at differential activation of PDGF signaling in early regenerative versus late scarring gestational fetal skin. Our functional pathway analysis data demonstrated for the first time increased activation of PDGF in E18 fibroblasts compared to E16 fibroblasts, suggesting an important role for increased PDGF signaling in loss of regenerative ability in late gestational fibroblasts. This finding is supported by published reports that have established a role for PDGF in regulating the proliferation and differentiation of keratinocytes and fibroblasts (16), as well as in upregulation of the expression of profibrotic TGF-β1 receptors (17). Furthermore, fetal wounds have been found to have lower concentrations of inflammatory cells such as neutrophils, which are recruited by PDGF, than adult wounds (2). This may provide a key mechanism for scarless fetal regeneration as neutrophils amplify the inflammatory response in wound beds, contributing to the formation of a scar.
Superoxide Radicals Degradation
Conversely, we found that E16 fibroblasts had upregulation of superoxide radicals degradation in comparison to E18 fibroblasts. During the early inflammatory phase following injury, inflammatory cells invade the wound bed. When active, these cells produce large amounts of reactive oxygen species (ROS) as part of their functional role in the wound healing process (18). While essential to debridement of the wound site, excess ROS can inhibit wound healing and lead to tissue damage. For instance, low levels of antioxidants accompanied by raised levels of markers of free radical damage led to decreased wound healing in aged and diabetic mice (19). Taken together, our findings suggest that enhanced detoxification of ROS may contribute to the regenerative ability of fetal skin.
Conclusion
Using functional pathway analysis, for the first time, we demonstrated differential pathway regulation in scarless and scarring fetal skin cells. Due to the large amount of data generated by both microarray and pathway analysis, we focused our discussion on a few pathways known to be particularly relevant to wound healing. However, our study reveals hundreds of genes and tens of pathways novel to the transition from scarless to scarring repair. We believe that identification of these pathways most likely to be proregenerative or profibrotic provides a valuable starting point for further experimental study aimed at elucidating mechanisms underlying the regenerative ability of early embryonic skin, with possible applications to other organ systems.
Acknowledgements
This work was supported in part by a grant from NIH grant R01 GM087609 (to H.P.L.), a Gift from Ingrid Lai and Bill Shu in honor of Anthony Shu (to H.P.L.), and the Hagey Laboratory for Pediatric Regenerative Medicine and Children’s Surgical Research Program (to M.T.L. and H.P.L.). Additional funding was provided by the Sarnoff Cardiovascular Research Foundation (to W.X.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Michael S. Hu: conception and design, data collection, analysis and interpretation, writing the article, critical revision of the article
Michael Januszyk: analysis and interpretation, writing the article, critical revision of the article
Wan Xing Hong: writing the article, analysis and interpretation, critical revision of the article
Graham G. Walmsley: conception and design, analysis and interpretation
Elizabeth R. Zielins: data collection, analysis and interpretation
David A. Atashroo: data collection, analysis and interpretation
Zeshaan N. Maan: data collection, analysis and interpretation
Adrian McArdle: data collection, analysis and interpretation
Danny M. Takanishi: analysis and interpretation, critical revision of the article
Geoffrey C. Gurtner: analysis and interpretation, critical revision of the article
Michael T. Longaker: critical revision of the article, obtaining funding
H. Peter Lorenz: conception and design, analysis and interpretation, critical revision of the article, obtaining funding
The authors have no conflicts of interest to disclose.
References
- 1.Rowlatt U. Intrauterine wound healing in a 20 week human fetus. Virchows Archiv. A, Pathological anatomy and histology. 1979;381(3):353–361. doi: 10.1007/BF00432477. [DOI] [PubMed] [Google Scholar]
- 2.Lo DD, Zimmermann AS, Nauta A, Longaker MT, Lorenz HP. Scarless fetal skin wound healing update. Birth defects research. Part C, Embryo today : reviews. 2012;96(3):237–247. doi: 10.1002/bdrc.21018. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan EP, Longaker MT, Lorenz HP. Fetal skin wound healing. Advances in clinical chemistry. 2009;48:137–161. doi: 10.1016/s0065-2423(09)48006-5. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz HP, et al. Scarless wound repair: a human fetal skin model. Development. 1992;114(1):253–259. doi: 10.1242/dev.114.1.253. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz HP, Whitby DJ, Longaker MT, Adzick NS. Fetal wound healing. The ontogeny of scar formation in the non-human primate. Annals of surgery. 1993;217(4):391–396. doi: 10.1097/00000658-199304000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenz HP, Adzick NS. Scarless skin wound repair in the fetus. The Western journal of medicine. 1993;159(3):350–355. [PMC free article] [PubMed] [Google Scholar]
- 7.Carre AL, et al. Interaction of wingless protein (Wnt), transforming growth factor-beta1, and hyaluronan production in fetal and postnatal fibroblasts. Plastic and reconstructive surgery. 2010;125(1):74–88. doi: 10.1097/PRS.0b013e3181c495d1. [DOI] [PubMed] [Google Scholar]
- 8.Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plastic and reconstructive surgery. 2010;126(4):1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varkey M, Ding J, Tredget EE. Fibrotic Remodeling of Tissue-Engineered Skin with Deep Dermal Fibroblasts Is Reduced by Keratinocytes. Tissue engineering. Part A. 2013 doi: 10.1089/ten.TEA.2013.0434. [DOI] [PubMed] [Google Scholar]
- 10.Colwell AS, Krummel TM, Longaker MT, Lorenz HP. Wnt-4 expression is increased in fibroblasts after TGF-beta1 stimulation and during fetal and postnatal wound repair. Plastic and reconstructive surgery. 2006;117(7):2297–2301. doi: 10.1097/01.prs.0000218708.16909.31. [DOI] [PubMed] [Google Scholar]
- 11.Reddy S, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mechanisms of development. 2001;107(1–2):69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 12.Lu L, et al. The temporal effects of anti-TGF-beta1, 2, and 3 monoclonal antibody on wound healing and hypertrophic scar formation. Journal of the American College of Surgeons. 2005;201(3):391–397. doi: 10.1016/j.jamcollsurg.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 13.Beanes SR, Dang C, Soo C, Ting K. Skin repair and scar formation: the central role of TGF-beta. Expert reviews in molecular medicine. 2003;5(8):1–22. doi: 10.1017/S1462399403005817. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson MW, O’Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2004;359(1445):839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song HF, et al. [A comparative study of PDGF and EGF expression in skin wound healing between human fetal and adult] Zhonghua zheng xing wai ke za zhi = Zhonghua zhengxing waike zazhi = Chinese journal of plastic surgery. 2003;19(3):199–202. [PubMed] [Google Scholar]
- 16.Tiede S, et al. Basic fibroblast growth factor: a potential new therapeutic tool for the treatment of hypertrophic and keloid scars. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 2009;191(1):33–44. doi: 10.1016/j.aanat.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Czuwara-Ladykowska J, Gore EA, Shegogue DA, Smith EA, Trojanowska M. Differential regulation of transforming growth factor-beta receptors type I and II by platelet-derived growth factor in human dermal fibroblasts. The British journal of dermatology. 2001;145(4):569–575. doi: 10.1046/j.1365-2133.2001.04443.x. [DOI] [PubMed] [Google Scholar]
- 18.Steiling H, Munz B, Werner S, Brauchle M. Different types of ROS-scavenging enzymes are expressed during cutaneous wound repair. Experimental cell research. 1999;247(2):484–494. doi: 10.1006/excr.1998.4366. [DOI] [PubMed] [Google Scholar]
- 19.Rasik AM, Shukla A. Antioxidant status in delayed healing type of wounds. International journal of experimental pathology. 2000;81(4):257–263. doi: 10.1046/j.1365-2613.2000.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]