Abstract
Background
Biomarkers based on detecting prostate cancer-specific transcripts are associated with inferior outcomes, but their validation in a clinical context is lacking.
Objective
To determine whether detecting prostate cancer enhanced transcripts in whole blood using an analytically valid assay has prognostic significance relative to circulating tumor cell (CTC) enumeration.
Design, Setting, and Participants
The predictive value for overall survival of the detection in whole blood by reverse transcription real-time polymerase chain reaction (RT-PCR) of KLK3, KLK2, HOXB13, GRHL2, and FOXA1 was studied in 97 men with metastatic castration-resistant prostate cancer (mCRPC).
Intervention
2.5ml of blood was collected in PAXgene tubes for total RNA extraction and 7.5 ml for CTC enumeration from patients with progressive mCRPC.
Outcome Measurements and Statistical Analysis
Prostate cancer enriched genes were detected using a sensitive RT-PCR assay in whole blood from patients with mCRPC. Analytical validity of the assay was established in a clinical laboratory environment. The frequency of detecting transcripts was compared to CTC enumeration using CellSearch® in an independent data set and survival associations were explored by concordance probability estimate (CPE).
Results and Limitations
Two or more genes were detected by PCR in 53% (51 of 97, 95% CI 43–63%) of patients, and unfavorable CTC counts (≥5cells) were seen in 46% (45 of 97, 95% CI 36–56%). Importantly, transcripts were detectable in 11 of 52 patients with favorable CTC counts (21%, 95% CI 8–35%). Transcript detection predicted overall survival in a proportional hazards model. Significantly, the predictive accuracy of RT-PCR detection in combination with CTC enumeration had a CPE of 0.752 (SE=0.038), although limited by the number of patients.
Conclusions
This validated RT-PCR assay detecting prostate-specific RNA in whole blood is prognostic for survival, and may assess patient risk complimentary with CellSearch CTC enumeration. Its clinical utility is being prospectively explored.
Keywords: biomarker, circulating tumor cells, prostate cancer, prostate-specific markers
INTRODUCTION
Cancer cells represent only a small proportion of the cells in the circulation but are ultimately the cells that attach and proliferate in distant sites to form metastases. First described in 1869 (1), there are now numerous technologies being evaluated for the isolation, and characterization of circulating tumor cells (CTC) from phlebotomy samples obtained in routine clinical practice. The results of the various CTC assays define different biomarkers that are being explored clinically as indicators of overall survival, treatment efficacy, and as predictors of sensitivity to specific therapeutics.
Early studies in prostate cancer used reverse-transcription qualitative polymerase chain reaction (RT-PCR)-based assays to detect CTC expressing prostate-specific antigen (PSA), also known as kallikrein-related peptidase 3 (KLK3), in the mononuclear cell fraction of the blood (2). Of particular interest was the detection of KLK3 mRNA in the blood of patients with no detectable PSA who were responding to hormonal therapy, suggesting that detecting CTC could provide information that was unique from changes in PSA (2). Other studies have shown variable results due in large part to the lack of standards for assay performance and for reporting results (3). Strategies to improve the sensitivity and specificity of the assays by assessing additional genes such as prostate-specific membrane antigen, markers of epithelial-mesenchymal transition, or stem-cell origin have been similarly unrewarding (4–7).
The situation changed with FDA-clearance of the Veridex CellSearch assay. This assay employs immunomagnetic capture and immunohistochemical identification to score CTC using rigorously defined criteria shown to be reproducible between different laboratories and personnel (8). The test measures the number of cells meeting the validated definition of a CTC per 7.5 ml of blood, which was subsequently shown to be prognostic for survival pretreatment and post-treatment (9–11), and is currently under study as a surrogate endpoint for survival in metastatic castration-resistant prostate cancer (mCRPC) (12). Limitations of the CellSearch assay include low detection rates in chemotherapy-naïve mCRPC patients, where the development of new therapies is hindered by the lack of approvable endpoints short of survival, and the uncertainty of reliably finding favorable counts (4 or fewer cells/7.5 ml). In this regard, while the survival times of patients with high cell numbers are uniformly poor, those with favorable counts vary widely (11). Assays that reliably detect more cells in a higher percentage of patients and/or which can refine the prognostic assessment of patients with favorable cell counts are needed (5, 6, 9–11, 13).
Here, we report the development and analytical validation of an RT-PCR assay to detect gene transcripts that are highly expressed in prostate tissue and in peripheral blood from patients with mCRPC. The gene expression assay was performed on blood samples collected in PAXgene tubes that stabilize intracellular RNA, require minimal on-site processing, and can be stored and shipped for analysis at a reference laboratory. We have demonstrated that detecting 2 or more transcripts, a positive test, can provide a more reliable and robust prediction of overall survival than that of CellSearch alone in mCRPC.
PATIENTS AND METHODS
Panel of prostate-specific transcripts
To select prostate cancer enriched gene transcripts for detection by RT-PCR, we interrogated the Tissue-specific Gene Expression and Regulation (TiGER) database (14), the Prostate Cancer Genomic Project (15), and the Novartis Gene Expression Database (16)(using Bio-GPS (17)) for genes that were overexpressed in prostate tissue relative to peripheral blood mononuclear cells (PBMC).
RT-PCR
TaqMan assays for the nominated transcripts were tested for primer-directed reverse transcription with CellDirect One Step (Invitrogen) using 1.5 µl of total RNA, followed by 14 cycles of real-time PCR (RT-PCR). The amplified product was multiplexed in 48×48 array PCR (Fluidigm) for 40 cycles (5). The constant threshold (Ct) for each replicate was recorded, and replicates without signal were attributed a value of 40.
Analytical validation
Analytical validation of the individual RT-PCR assays and clinical sample testing was conducted according to Clinical Laboratory Improvement Amendments (CLIA) guidelines (http://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/index.html) in the Department of Laboratory Medicine at Memorial Sloan-Kettering Cancer Center (MSKCC), according to our standard operating procedures (5), as described in Supplemental materials.
Protocol for sample collection, processing and analysis for RT-PCR in whole blood: Patients and volunteers
Signed informed consent was obtained on an Institutional Review Board–approved protocol. Patients included in the training and validation sets were men with progressive mCRPC per Prostate Cancer Working Group guidelines treated at MSKCC between August 2008 and May 2010(18). Duplicate patient samples were tested for the reproducibility of the gene panel. Control samples were obtained from volunteers with no evidence of prostate cancer. CTC were enumerated by CellSearch (Veridex) (19).
Blood sample collection
Blood samples were collected first in 7.5 ml CellSave tubes, followed by 2.5 ml in PAXgene Blood RNA tubes (Qiagen). PAXgene tubes were stored at −80 °C and batch-processed.
Sample processing for analysis
Total RNA was extracted according to manufacturer’s instructions. An RNA Integrity Number (RIN) of ≥6 using the RNA Nano Assay (Agilent) was considered satisfactory for subsequent analysis. Total RNA isolated from each sample was run in 6 replicates, and the median Ct was calculated.
Data reporting/Statistical methods
Receiver operator curves (ROC) were used to establish detection thresholds for each gene by comparing the median of 6 Ct replicates per subject from mCRPC patients in a training set to healthy volunteers. The threshold values derived from the ROC analysis define the transcript as present or absent, presented in supplemental Table S1.
Subsequently, an independent cohort of mCRPC patients was evaluated in a validation set to construct a composite prognostic factor from this gene panel. If at least 2 of the 5 gene transcripts were present, the patient was classified as positive. Internal controls for each PCR run were included for validation of the reaction. Results from CellSearch were reported as CTC number per 7.5 ml of blood.
To determine the discriminatory power of the gene panel and CTC enumeration, the concordance probability estimate (CPE) was used. The CPE measures the level of concordance between the survival time and the prognostic index, based on the linear combination of covariates in the Cox model, to provide an unbiased assessment of discrimination with survival data (20). CPE values range between 0.5 and 1.0, with 1.0 representing perfect concordance between the prognostic index and survival time. The kappa statistic was computed to measure the level of agreement between the gene panel and CTC number and to measure the reproducibility of the gene panel.
RESULTS
Nomination of genes for RT-PCR assay development
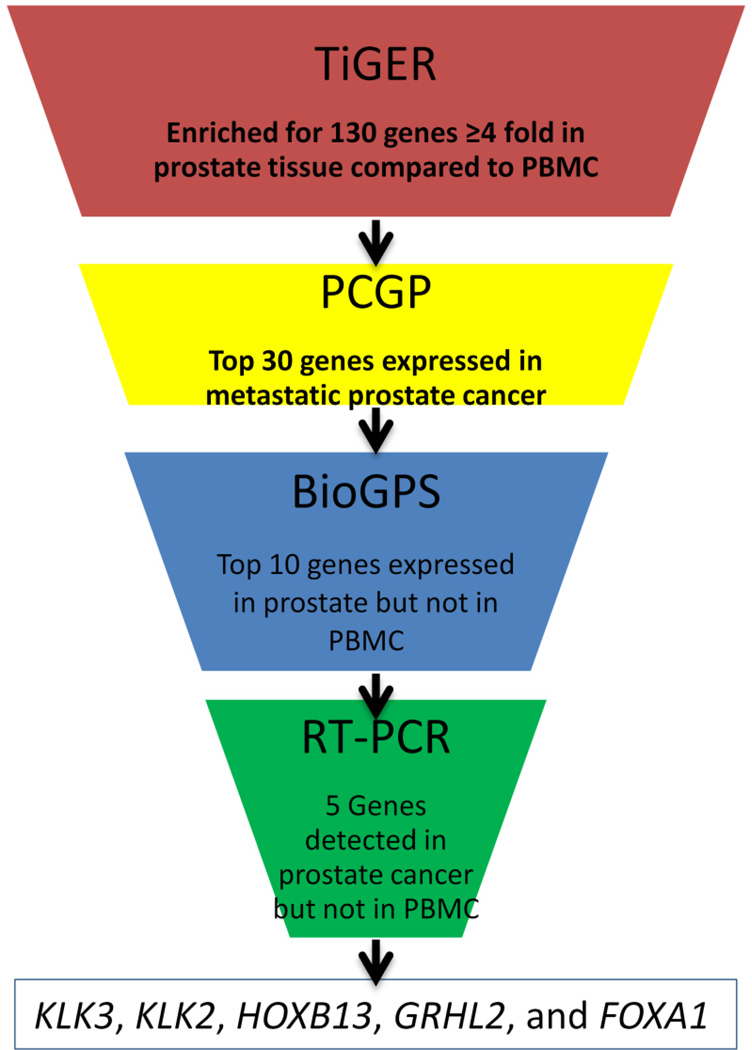
Genes were nominated for assay development based first on expression level in prostate tissue relative to PBMC (Figure 1). Mining the TiGER Database, we identified the 130 genes that are expressed 4-fold or higher in prostate tissue relative to PBMC. From this list of 130 genes, we selected the 30 genes with the highest expression in metastatic prostate cancer tissue samples in the Prostate Cancer Oncogenome Project database developed at MSKCC (15). We then compared the expression of the 30 genes to the Novartis Gene Expression Database and removed those that were (a)detectable in PBMC, (b)detectable in more than 2 tissues other than prostate, or (c)not detectable in any tissue. The result of this selection process left 11 genes: AR, NKX3.1, ACPP, NPY, HOXB13, GRHL2, FOXA1, SLC45A3, FOLH1, KLK2, and KLK3, for which individual TaqMan PCR assays (LifeTechnology) were tested for expression in whole blood PAX-gene specimens from healthy volunteers. Six genes detected in the volunteer specimens were removed from the 11 gene panel resulting in a final 5 gene panel: KLK3 (kallikrein-related peptidase 3) (catalog #Hs03063374_m1), KLK2 (kallikrein-related peptidase 2) (catalog #Hs00428383_m1), HOXB13 (home box B13) (catalog #Hs00197189_m1), GRHL2 (grainyhead-like 2) (catalog #Hs00227745_m1) and FOXA1 (forkhead box A1) (catalog #Hs00270129_m1). Supporting data for the filtering steps are listed in Table S2.
Figure 1. Selection of panel of prostate-specific genes highly expressed in prostate tissue and not expressed in PBMC.
The Tissue-specific Gene Expression and Regulation (TiGER),(14) Prostate Cancer Genomic Project,(15) and Novartis Gene Expression(16) (using Bio-GPS(17)) databases were queried for genes expressed in prostate tissue, but not expressed in PBMC or in >2 tissues other than prostate.
Analytical validation of prostate-specific transcript detection in blood
Multiplex RT-PCR assay performance
Intra- and inter-assay CV, dynamic linear correlation, and the reportable range of the multiplex panel are presented in Table S1 and Figure S1.
Patient data
The training set and the independent validation set included 56 and 97 mCRPC patients with progressive disease, respectively. The clinical characteristics are presented in Table 1. The median survival of the 97 patients in the validation group was 17 months (95% CI 13.8–23.5). Blood from 51 volunteers was collected as control samples.
Table 1.
Baseline patient clinical characteristics
| Training cohort | Validation cohort | |
|---|---|---|
| Characteristic | No. (%) or median (range) | No. (%) or median (range) |
| (n=56) | (n=97) | |
| Age, years | 73 (49–89) | 70 (47–90) |
| KPS | 80 (70–90) | 80 (70–90) |
| Chemistry | ||
| PSA, ng/ml | 171 (4.95–4865) | 67.51 (<0.05–3096.37) |
| Hgb, g/dl | 12 (8.4–14.8) | 12.1 (9.2–15.4) |
| ALK, unit/L | 135 (39–2323) | 102 (31–1818) |
| LDH, unit/L | 258 (115–1352) | 204 (87–845) |
| ALB, g/dl | 4 (3.3–4.8) | 4.3 (3.4–5) |
| Primary treatment | ||
| Surgery | 22 (39%) | 43 (44%) |
| Radiation | 22 (39%) | 27 (28%) |
| No primary therapy | 12 (22%) | 27 (28%) |
| Systemic treatment | ||
| Hormone therapy | ||
| 1–2 lines | 25 (45%) | 44 (45%) |
| 3 lines | 15 (27%) | 29 (30%) |
| ≥4 lines | 16 (28%) | 24 (25%) |
| Ketoconazole | 23 (41%) | 44 (45%) |
| Prior chemotherapy | ||
| None | 42 (75%) | 53 (55%) |
| Any | 14 (25%) | 44 (45%) |
| Site of metastatic disease | ||
| Bone | 51 (91%) | 88 (91%) |
| Lymph node | 30 (54%) | 49 (51%) |
| Liver | 7 (12%) | 13 (13%) |
| Lung | 1 (2%) | 13 (13%) |
| Other soft tissue | 4 (7%) | 3 (3%) |
| Deceased | 44 (79%) | 77 (79%) |
| Follow-up (months) | 34 (1–142) | 43.3 (6.8–154) |
Detection by multiplex RT-PCR
All blood samples passed RIN quality. The detection thresholds, defined by ROC analysis in the training set of 56 mCRPC patients relative to 51 healthy volunteers, showed specificity rates of 94–100%. The frequency of detection of each gene transcript in the mCRPC and volunteer cohorts is presented in Table S1.
At least 2 of the 5 genes were detected in 51 of the 97 patients tested (53%, 95% CI 43–63%). The reproducibility for detection of 2 or more genes in duplicate samples from the same patient was 85% (34 of 40), with the kappa statistic equal to 0.70 (95% CI: 0.39, 1.00).
Detection of CTC using CellSearch
Unfavorable (5 of more cells/7.5 ml of blood) CTC counts were found in 45 of the 97 patients (46%, 95% CI 36–56%), of whom 40 (89%, 95% CI 74–100%) had at least 2 transcripts present in blood. Interestingly, one patient with CTC CellSearch count of 26 cells/7.5ml of blood and no transcripts detected by RT-PCR had a serum PSA <0.05ng/ml. Two additional patients with CTC ≥5 cells/7.5ml had only KLK3 detected, and one patient had FOXA1 detected, alone. For the 52 patients with favorable (4 or fewer cells/7.5 of blood) counts, 11 (21%, 95% CI 8–35%) had at least 2 transcripts detectable. There was a moderate level of agreement with the kappa statistic equal to 0.67 (95% CI 0.47–0.87). The RT-PCR also detected CTC at a higher frequency in chemotherapy-naïve patients (Table S3).
Prognostic value of multiplex RT-PCR detection for patients with mCRPC
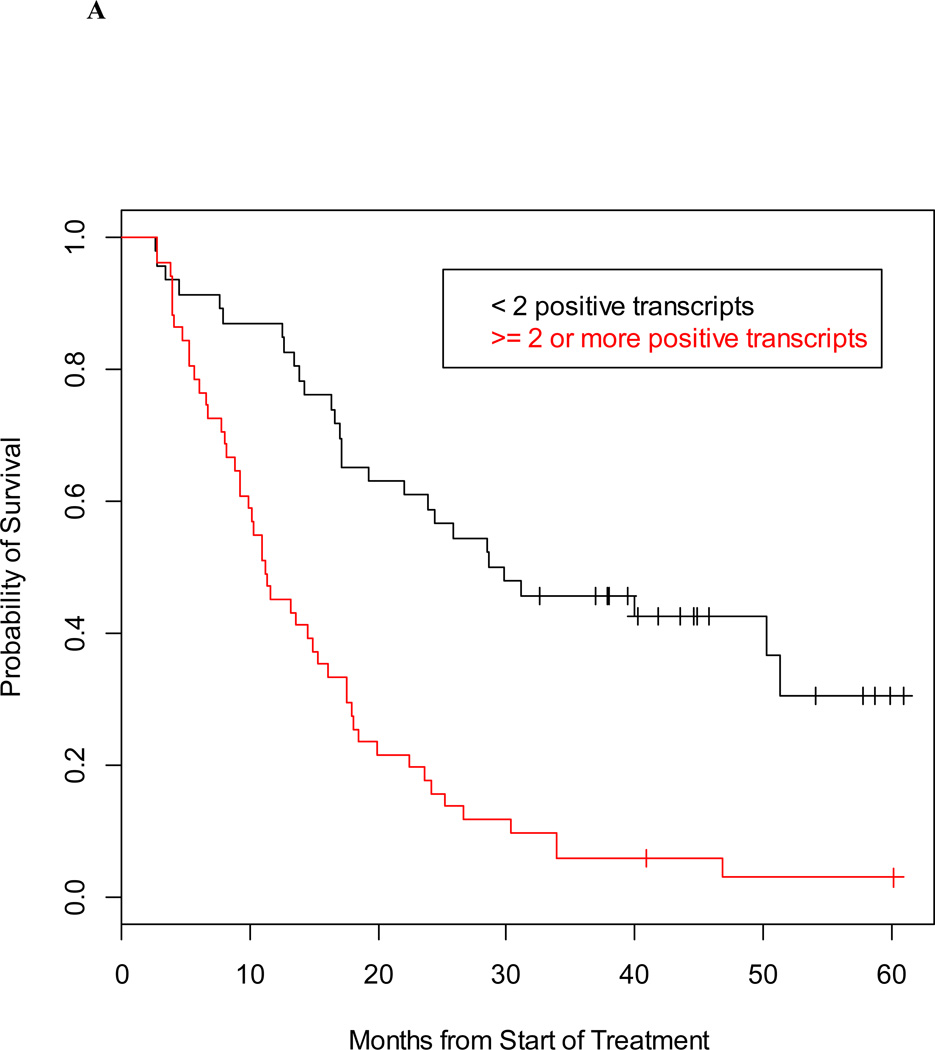
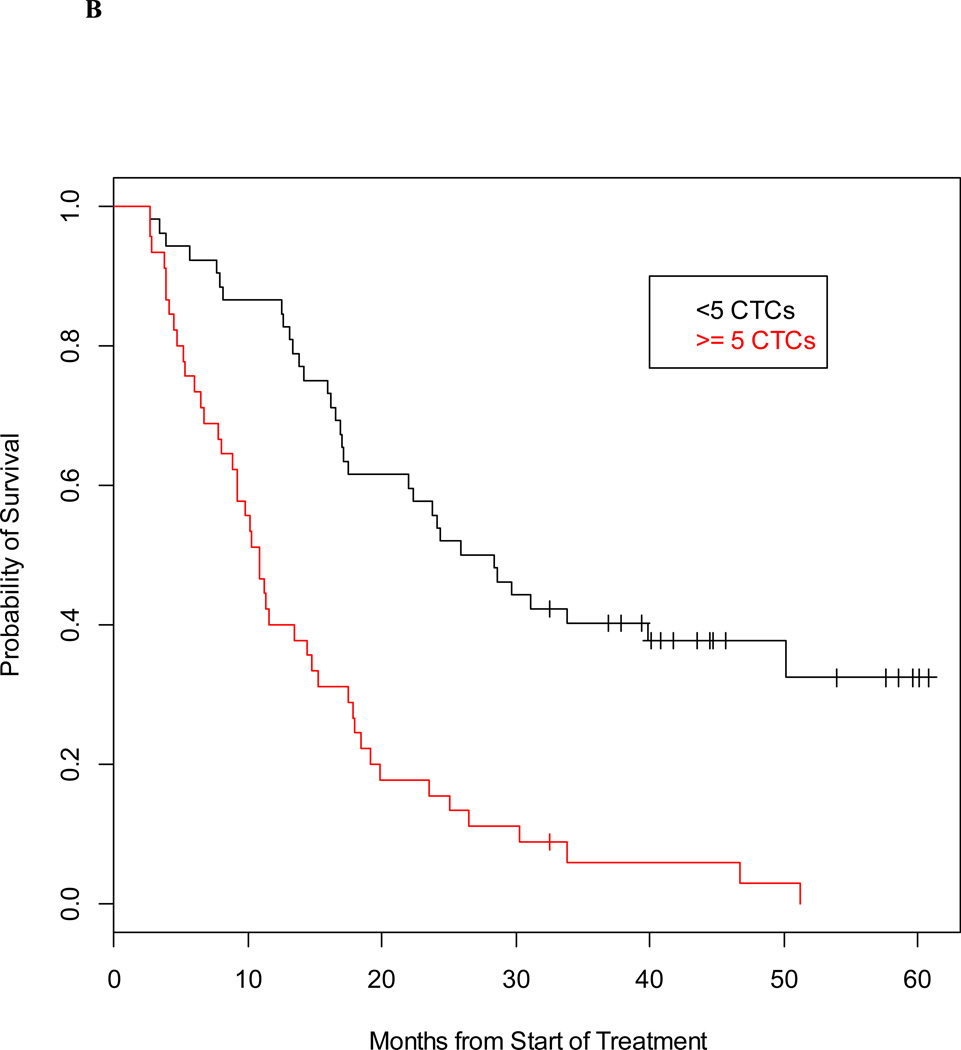
Individually, the 5-gene panel and CTC enumeration (CellSearch®) are strong prognostic factors that provide complementary survival information. Using separate proportional hazards models, the hazard ratio for the gene panel was 3.23 (95% CI 2.00–5.21) and the hazard ratio for CTC enumeration is 3.24 (95% CI 2.04–5.17). The median survival for the 97 mCRPC patients by detectable and undetectable classification from the gene panel was 11.2 months and 29.2 months, respectively; while the median survival based on favorable and unfavorable CellSearch counts was 27.1 months and 10.9 months (Figures 2A and 2B).
Figure 2. Prognostic value of detection of prostate-specific transcripts and CTC enumeration in men with mCRPC.
Kaplan-Meier estimate of survival, calculated from time of blood draw, and based on detection of ≥2 prostate-specific transcripts (A) and baseline CTC counts (B).
Combined prognostic model
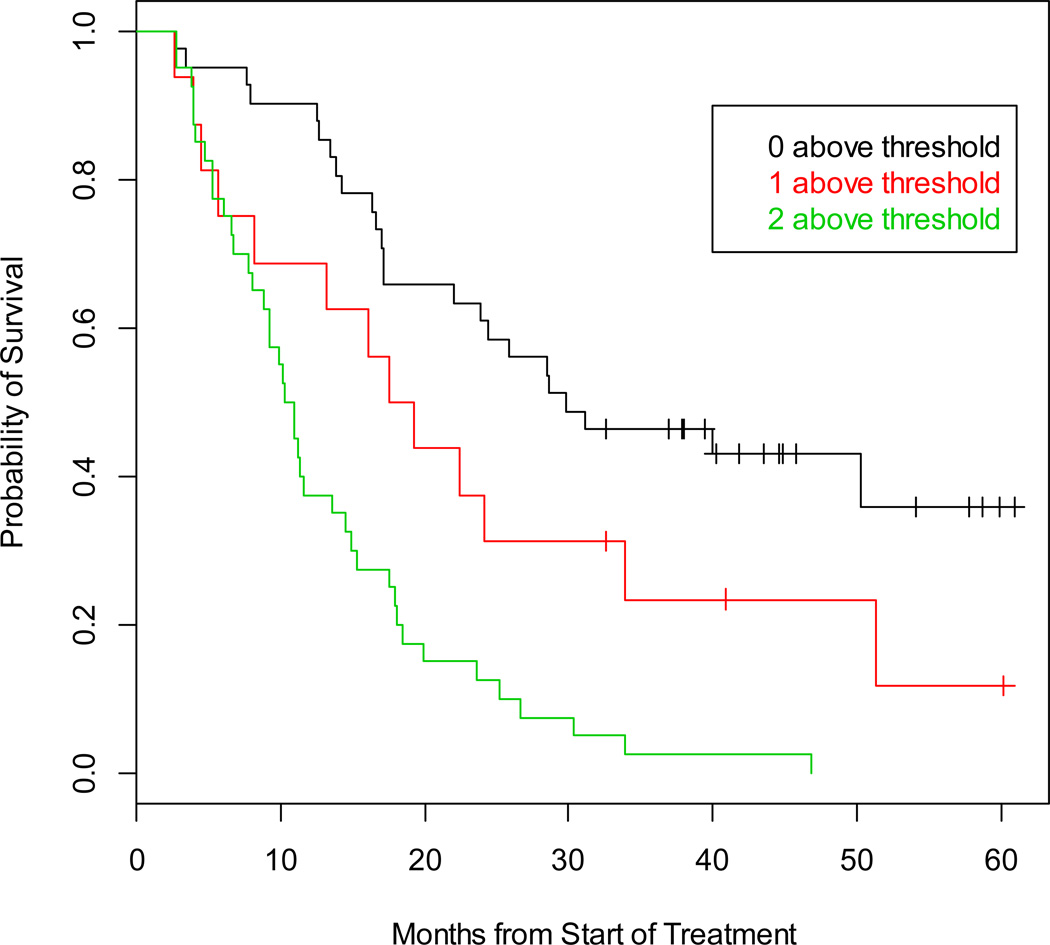
A model including both the gene panel and CellSearch results enabled a refinement of the risk assessment and in particular for patients with low (favorable) cell counts by Veridex, based on 0, 1, or 2 unfavorable risk factors as presented in Table 2. The CPE for this model is 0.752 (SE=0.038), indicating the model is a strong discriminator of patient risk. The Kaplan-Meier estimate of survival by number of unfavorable risk factors is depicted in Figure 3. Patients with 0 unfavorable risk factors were in the best risk group, with a median survival of 29.8 months, while those with 1 or 2 unfavorable factors had estimated median survival of 18.4 months and 10.6 months, respectively.
Table 2.
Estimates of the hazard ratio from the Cox proportional hazards model based on CTC enumeration (favorable: ≤4 cells vs. unfavorable: ≥5 cells) and the 5-gene panel (favorable: ≤ 1 prostate-specific transcript detected vs. unfavorable: ≥2 transcripts detected). LCL, lower confidence limit, UCL, upper confidence limit.
| Number of unfavorable risk factors |
Number of patients |
HR | 0.95 LCL | 0.95 UCL |
|---|---|---|---|---|
| 0 | 41 | 1.00 | ||
| 1 | 16 | 1.88 | 0.96 | 3.70 |
| 2 | 40 | 4.34 | 2.55 | 7.40 |
CPE = 0.752 (SE=0.038)
Figure 3. Prognostic value of detection based on the combination of prostate-specific transcripts and CTC enumeration in men with mCRPC.
The Kaplan-Meier estimate of survival, calculated from time of blood draw, based on whether 0, 1, or 2 unfavorable risk factors were present.
DISCUSSION
The dramatically changed therapeutic landscape of mCRPC has improved the outlook for patients but makes the development of future drugs more difficult. Factors to better enable drug development include an improved understanding of pre-treatment patient risk and the development of validated surrogates for survival to assess treatment effects more rapidly. Presently, CellSearch is the only analytically valid FDA-cleared CTC enumeration assay for use in clinical practice. Widespread adaptation in clinical practice has been limited by detection rates of only ~40% in patients with progressive mCRPC, and survival times that range from very short to very long for patients with favorable (4 or fewer cell counts). Validated blood based assays that can increase detection rates and refine patient risk are needed (7). The highly sensitive RT-PCR-based assay of prostate-enriched transcripts in whole blood presented here provides a similar assessment of patient risk relative to CellSearch while offering ease of collection and minimal on site processing. Used together, a markedly improved risk assessment is shown, in particular for patients with favorable counts.
The genes were selected based on enriched expression in prostate tissue, highest expression in metastatic prostate cancer and no detection in PBMC. The 5 genes identified have important biologic functions. KLK3 and KLK2 encode highly active serine proteases that convert pro-PSA, and activate growth factors in areas of metastatic spread (21). The recently described G84E variant of HOXB13 is associated with increased risk of hereditary prostate cancer offering new mechanistic insights (22). FOXA1 modulates steroid hormone receptor transactivation, and is frequently overexpressed in mCRPC. Notable is that FOXA1 levels are inversely related to androgen receptor expression and correlate with higher Gleason scores, increased cell proliferation and migration, and more rapid progression (23). FOXA1 mutations have also been shown to play a role in tumor sensitivity to enzalutamide and resistance to bicalutamide (24–26). GRHL2 is been involved in tumor development by regulating telomerase hTERT (27).
Importantly, the RT-PCR-based multiplex assay was analytically validated according to CLIA-guidelines by standard operating procedures in a clinical laboratory (5). Sample collection requires only a direct draw into PAXgene tubes to minimize RNA degradation at room temperature without additional on-site processing. The standard operating procedure developed followed recommended regulatory guidance (28), and includes relevant controls. The reproducibility of detection in duplicate samples from the same patient was statistically robust.
The risk groups defined based on the detection of 2 or more transcripts enabled separation of median survival times. Notable is that many patients with favorable CTC counts had at least 2 detectable transcripts in blood, while others did not, which is not surprising given the marked discordance of duplicate baseline counts in patients with CellSearch counts of 10 or less (, producing uncertainty in the classification of a patient as having a favorable or unfavorable risk (29). Used alone, the classification of the 5-gene panel test was prognostic for survival with a similar association as the unfavorable vs. favorable CellSearch classification. The combined results of the 5-gene panel with CellSearch enabled both the identification of patients with a markedly unfavorable prognosis, while improving the segregation of risk of patients with favorable counts.
In addition, the RT-PCR assay detected the presence of CTC at a higher frequency in chemotherapy-naïve patients than CellSearch®, a group now being studied intensively in mCRPC, which will enable this biomarker to be evaluated in a higher proportion of patients. Also of note is that as many of the selected genes are androgen regulated, the assay may provide an indication of androgen pathway output to predict the sensitivity to AR directed therapies.
CONCLUSIONS
This validated RT-PCR assay detecting prostate-specific RNA in whole blood is prognostic for survival, and enhances the power to discriminate between low- and high-risk patients relative to using CellSearch alone. Its clinical utility is being prospectively explored, with focus on chemotherapy-naïve mCRPC where detection rates with CellSearch® are low.
Supplementary Material
Acknowledgments
We thank Amy Plofker for assistance in editing the manuscript.
Grant Support: NCI SPORE in Prostate Cancer (P50 CA92629); the Department of Defense Prostate Cancer Research Program (PC051382); Prostate Cancer Foundation; Mr. William H. and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research and The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center; The Sidney Kimmel Center for Prostate and Urologic Cancers; ASCO YIA and DoD Prostate Cancer Research Program Physician Research Award W81XWH-09-1-0307 to DCD.
REFERENCES
- 1.Ashworth T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aus Med J. 1869;14:146–149. [Google Scholar]
- 2.Ghossein RA, Scher HI, Gerald WL, et al. Detection of circulating tumor cells in patients with localized and metastatic prostatic carcinoma: clinical implications. J Clin Oncol. 1995;13(5):1195–1200. doi: 10.1200/JCO.1995.13.5.1195. Epub 1995/05/01. [DOI] [PubMed] [Google Scholar]
- 3.McShane LM, Hayes DF. Publication of tumor marker research results: the necessity for complete and transparent reporting. J Clin Oncol. 2012;30(34):4223–4232. doi: 10.1200/JCO.2012.42.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Wang CY, Yang R, et al. Real-time quantitative RT-PCR assay of prostate-specific antigen and prostate-specific membrane antigen in peripheral blood for detection of prostate cancer micrometastasis. Urologic oncology. 2008;26(6):634–640. doi: 10.1016/j.urolonc.2007.07.016. Epub 2008/03/28. [DOI] [PubMed] [Google Scholar]
- 5.Danila DC, Anand A, Sung CC, et al. TMPRSS2-ERG status in circulating tumor cells as a predictive biomarker of sensitivity in castration-resistant prostate cancer patients treated with abiraterone acetate. Eur Urol. 2011;60(5):897–904. doi: 10.1016/j.eururo.2011.07.011. Epub 2011/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nature reviews Cancer. 2008;8(5):329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 7.Danila DC, Fleisher M, Scher HI. Circulating tumor cells as biomarkers in prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(12):3903–3912. doi: 10.1158/1078-0432.CCR-10-2650. Epub 2011/06/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaffer DR, Leversha MA, Danila DC, et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(7):2023–2029. doi: 10.1158/1078-0432.CCR-06-2701. [DOI] [PubMed] [Google Scholar]
- 9.Danila DC, Heller G, Gignac GA, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(23):7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. Epub 2007/12/07. [DOI] [PubMed] [Google Scholar]
- 10.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(19):6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. Epub 2008/10/03. [DOI] [PubMed] [Google Scholar]
- 11.Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10(3):233–239. doi: 10.1016/S1470-2045(08)70340-1. Epub 2009/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scher H, Heller G, Molina A, et al. Evaluation of circulating tumor cell (CTC) enumeration as an efficacy response biomarker of overall survival (OS) in metastatic castration-resistant prostate cancer (mCRPC): Planned final analysis (FA) of COU-AA-301, a randomized double-blind, placebo-controlled phase III study of abiraterone acetate (AA) plus low-dose prednisone (P) post docetaxel. In: ASCO, editor. J Clin Oncol. suppl. Vol. 29. 2011. abstr LBA4517^) 2011. [Google Scholar]
- 13.Danila DC, Pantel K, Fleisher M, Scher HI. Circulating tumors cells as biomarkers: progress toward biomarker qualification. Cancer J. 2011;17(6):438–450. doi: 10.1097/PPO.0b013e31823e69ac. Epub 2011/12/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Yu X, Zack DJ, Zhu H, Qian J. TiGER: a database for tissue-specific gene expression and regulation. BMC Bioinformatics. 2008;9:271. doi: 10.1186/1471-2105-9-271. Epub 2008/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. Epub 2010/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su AI, Cooke MP, Ching KA, et al. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci U S A. 2002;99(7):4465–4470. doi: 10.1073/pnas.012025199. Epub 2002/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu C, Orozco C, Boyer J, et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10(11):R130. doi: 10.1186/gb-2009-10-11-r130. Epub 2009/11/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scher HI, Eisenberger M, D'Amico AV, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004;22(3):537–556. doi: 10.1200/JCO.2004.07.099. Epub 2004/01/31. [DOI] [PubMed] [Google Scholar]
- 19.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(20):6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 20.Gonen M, Heller G. Concordance probability and discriminative power of proportional hazards regression. Biometrika. 2005;92:965–970. [Google Scholar]
- 21.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nature reviews Cancer. 2008;8(4):268–278. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 22.Ewing CM, Ray AM, Lange EM, et al. Germline mutations in HOXB13 and prostate-cancer risk. N Engl J Med. 2012;366(2):141–149. doi: 10.1056/NEJMoa1110000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerhardt J, Montani M, Wild P, et al. FOXA1 promotes tumor progression in prostate cancer and represents a novel hallmark of castration-resistant prostate cancer. Am J Pathol. 2012;180(2):848–861. doi: 10.1016/j.ajpath.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Barbieri CE, Baca SC, Lawrence MS, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nature genetics. 2012;44(6):685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belikov S, Oberg C, Jaaskelainen T, Rahkama V, Palvimo JJ, Wrange O. FoxA1 corrupts the antiandrogenic effect of bicalutamide but only weakly attenuates the effect of MDV3100 (Enzalutamide) Molecular and cellular endocrinology. 2012 doi: 10.1016/j.mce.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Kang X, Chen W, Kim RH, Kang MK, Park NH. Regulation of the hTERT promoter activity by MSH2, the hnRNPs K and D GRHL2 in human oral squamous cell carcinoma cells. Oncogene. 2009;28(4):565–574. doi: 10.1038/onc.2008.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FDA. Guidance for Industry. E16 Biomarkers Related to Drug or Biotechnology Product Development: Context, Structure, and Format of Qualification Submissions. 2011 Aug; [PubMed] [Google Scholar]
- 29.Veridex. 510k for CellSearch Veridex. 2008 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.