Abstract
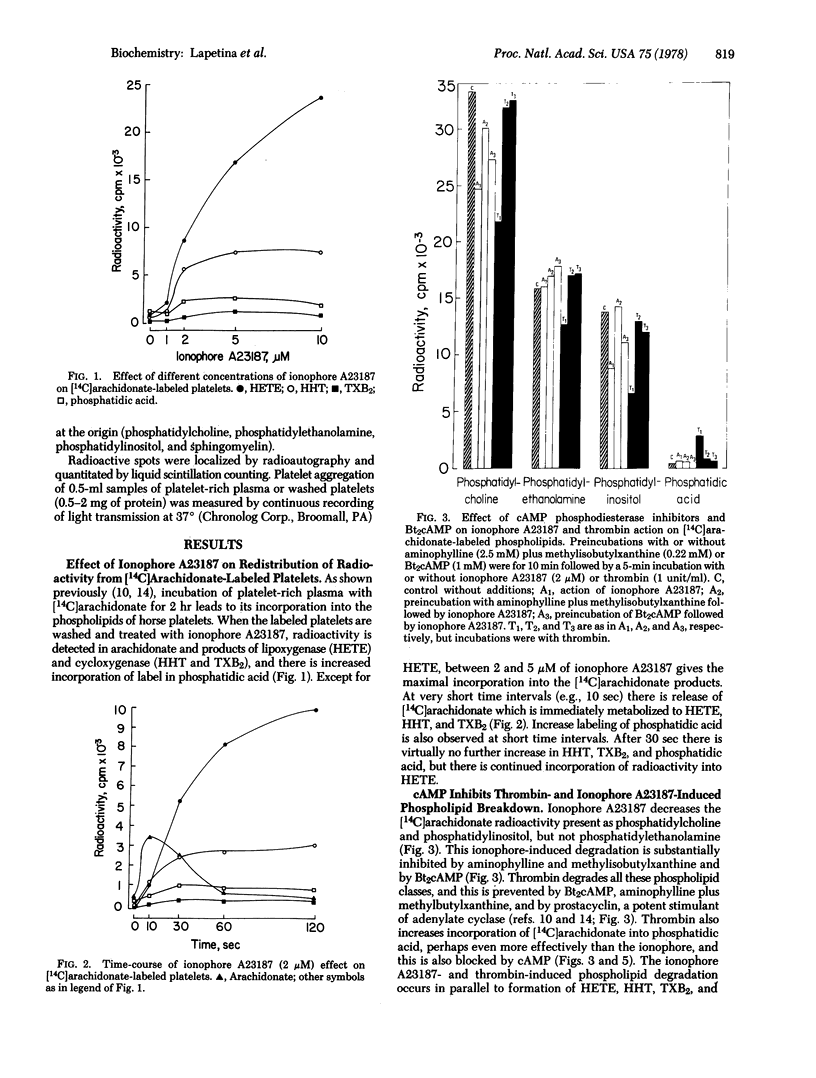
Stimulation of platelets labeled with [14C]-arachidonate by ionophore A23187 or thrombin produces rapid degradation of specific membrane phospholipids. This is also reflected by the release of [14C]archidonate, which is immediately transformed into products of the cycloxygenase and lipoxygenase enzyme systems, and by increased labeling of phosphatidic acid. Arachidonate metabolism can be effectively prevented by preincubation with indomethacin and eicosatetraynoic acid, but platelet aggregation induced by ionophore A23187 or trombin is not blocked under these conditions. Nevertheless, in the virtually total absence of metabolism of arachidonate, platelet aggregation still occurs concomitantly with phospholipid breakdown and with increased labeling of phosphatidic acid. Increased levels of cyclic AMP block both phospholipase activation and aggregation induced by ionophore A23187 and trombin. These data suggest that some early consequence of phospholipase activation, independent of a metabolic product of arachidonate but possibly related to the production of phosphatidic acid, may play a central, causative role in mediating platelet aggregation.
Full text
PDF
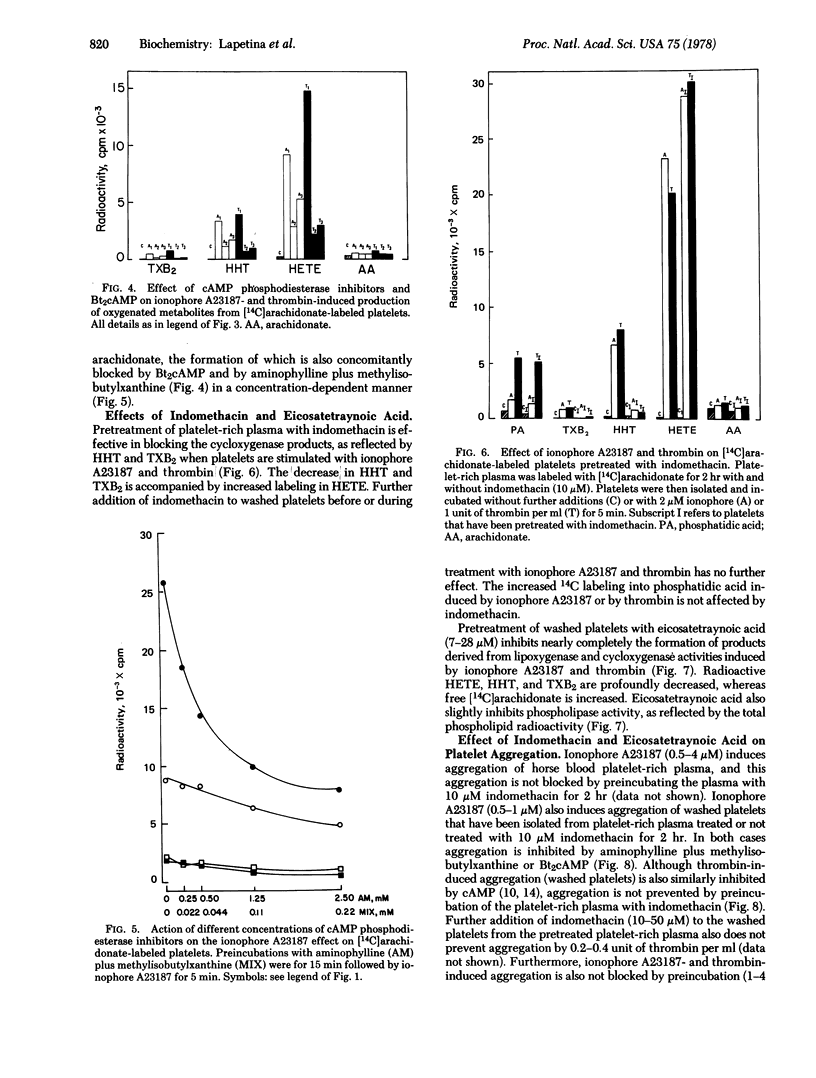

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bills T. K., Smith J. B., Silver M. J. Metabolism of [14C]arachidonic acid by human platelets. Biochim Biophys Acta. 1976 Feb 23;424(2):303–314. doi: 10.1016/0005-2760(76)90198-3. [DOI] [PubMed] [Google Scholar]
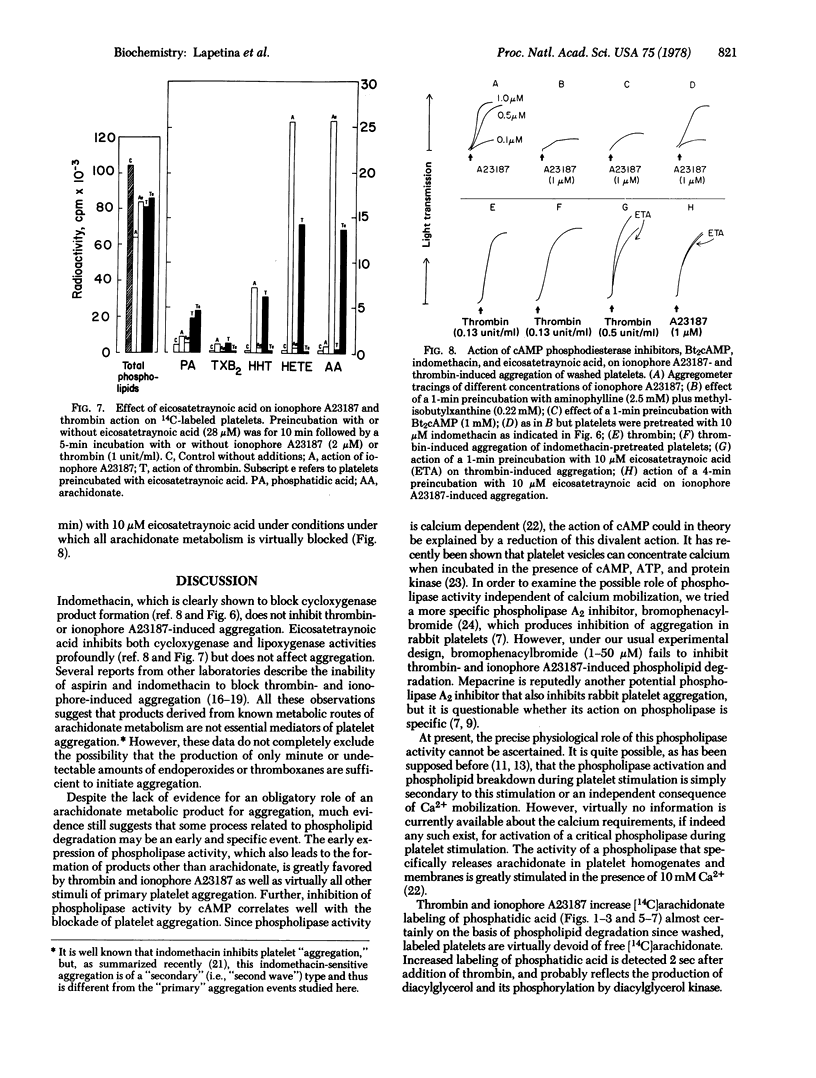
- Blackwell G. J., Duncombe W. G., Flower R. J., Parsons M. F., Vane J. R. The distribution and metabolism of arachidonic acid in rabbit platelets during aggregation and its modification by drugs. Br J Pharmacol. 1977 Feb;59(2):353–366. doi: 10.1111/j.1476-5381.1977.tb07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult H., Bonta I. L. Rat platelets aggregate in the absence of endogenous precursors of prostaglandin endoperoxides. Nature. 1976 Dec 2;264(5585):449–451. doi: 10.1038/264449a0. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Feinman R. D., Detwiler T. C. Interrelations of platelet aggregation and secretion. J Clin Invest. 1977 Oct;60(4):866–873. doi: 10.1172/JCI108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen A., Cohen P. Patterns of fatty acid release from endogenous substrates by human platelet homogenates and membranes. J Biol Chem. 1975 Dec 25;250(24):9342–9347. [PubMed] [Google Scholar]
- Feinstein M. B., Fraser C. Human platelet secretion and aggregation induced by calcium ionophores. Inhibition by PGE1 and dibutyryl cyclic AMP. J Gen Physiol. 1975 Nov;66(5):561–581. doi: 10.1085/jgp.66.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., Peller J. D., Krick T. P., White J. G. Cyclic AMP and platelet prostaglandin synthesis. Prostaglandins. 1977 Jul;14(1):39–50. doi: 10.1016/0090-6980(77)90155-1. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Detection and isolation of an endoperoxide intermediate in prostaglandin biosynthesis. Proc Natl Acad Sci U S A. 1973 Mar;70(3):899–903. doi: 10.1073/pnas.70.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käser-Glanzmann R., Jakäbovä M., George J. N., Lüscher E. F. Stimulation of calcium uptake in platelet membrane vesicles by adenosine 3',5'-cyclic monophosphate and protein kinase. Biochim Biophys Acta. 1977 May 2;466(3):429–440. doi: 10.1016/0005-2736(77)90336-4. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Schmitges C. J., Chandrabose K., Cuatrecasas P. Regulation of phospholipase activity in platelets. Adv Prostaglandin Thromboxane Res. 1978;3:127–135. [PubMed] [Google Scholar]
- Lapetina E. G., Schmitges C. J., Chandrabose K., Cuatrecases P. Cyclic adenosine 3',5'-monophosphate and prostacyclin inhibit membrane phospholipase activity in platelets. Biochem Biophys Res Commun. 1977 Jun 6;76(3):828–835. doi: 10.1016/0006-291x(77)91575-3. [DOI] [PubMed] [Google Scholar]
- Lloyd J. V., Mustard J. F. Changes in 32P-content of phosphatidic acid and the phosphoinositides of rabbit platelets during aggregation induced by collagen or thrombin. Br J Haematol. 1974 Feb;26(2):243–253. doi: 10.1111/j.1365-2141.1974.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Lloyd J. V., Nishizawa E. E., Joist J. H., Mustard J. F. Effect of ADP-induced aggregation on 32 PO 4 incorporation into phosphatidic acid and the phosphoinositides of rabbit platelets. Br J Haematol. 1973 May;24(5):589–604. doi: 10.1111/j.1365-2141.1973.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Minkes M., Stanford N., Chi M. M., Roth G. J., Raz A., Needleman P., Majerus P. W. Cyclic adenosine 3',5'-monophosphate inhibits the availability of arachidonate to prostaglandin synthetase in human platelet suspensions. J Clin Invest. 1977 Mar;59(3):449–454. doi: 10.1172/JCI108659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman P., Minkes M., Raz A. Thromboxanes: selective biosynthesis and distinct biological properties. Science. 1976 Jul 9;193(4248):163–165. doi: 10.1126/science.945611. [DOI] [PubMed] [Google Scholar]
- Needleman P., Moncada S., Bunting S., Vane J. R., Hamberg M., Samuelsson B. Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature. 1976 Jun 17;261(5561):558–560. doi: 10.1038/261558a0. [DOI] [PubMed] [Google Scholar]
- Needleman P., Raz A., Ferrendelli J. A., Minkes M. Application of imidazole as a selective inhibitor thromboxane synthetase in human platelets. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1716–1720. doi: 10.1073/pnas.74.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. R. Effects of salicylates on human platelets. Lancet. 1968 Apr 13;1(7546):779–783. doi: 10.1016/s0140-6736(68)92228-9. [DOI] [PubMed] [Google Scholar]
- Pickett W. C., Jesse R. L., Cohen P. Initiation of phospholipase A2 activity in human platelets by the calcium ion ionophore A23187. Biochim Biophys Acta. 1976 Jan 18;486(1):209–213. doi: 10.1016/0005-2760(77)90086-8. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Deykin D. The mobilization of arachidonic acid in platelets exposed to thrombin or ionophore A23187. Effects of adenosine triphosphate deprivation. J Clin Invest. 1977 Aug;60(2):495–498. doi: 10.1172/JCI108801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Willis A. L. Aspirin selectively inhibits prostaglandin production in human platelets. Nat New Biol. 1971 Jun 23;231(25):235–237. doi: 10.1038/newbio231235a0. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B. Carrageenan and thrombin trigger prostaglandin synthetase-independent aggregation of rabbit platelets: inhibition by phospholipase A2 inhibitors. J Pharm Pharmacol. 1977 Apr;29(4):222–228. doi: 10.1111/j.2042-7158.1977.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Volwerk J. J., Pieterson W. A., de Haas G. H. Histidine at the active site of phospholipase A2. Biochemistry. 1974 Mar 26;13(7):1446–1454. doi: 10.1021/bi00704a020. [DOI] [PubMed] [Google Scholar]
- White J. G., Rao G. H., Gerrard J. M. Effects of the lonophore A23187 on blood platelets I. Influence on aggregation and secretion. Am J Pathol. 1974 Nov;77(2):135–149. [PMC free article] [PubMed] [Google Scholar]