Abstract
Chemokines, a group of small chemotactic cytokines, and their G-protein-coupled receptors were originally identified for their ability to mediate various pro- and anti-inflammatory responses. Beyond the influence of chemokines and their cognate receptors in several inflammatory diseases, several malignancies have been shown to be dependent of chemokines for progression, tumor growth, cellular migration and invasion, and angiogenesis; those later facilitating the development of distant metastases. In hepatocellular carcinoma (HCC), chemokines were shown to affect leukocyte recruitment, neovascularization and tumor progression. CXCL12 (stromal-derived factor 1 alpha- SDF-1) is the primary ligand for the seven transmembrane G-protein coupled receptor CXCR4. The CXCR4/CXCL12 axis exerts a variety of functions at different steps of HCC tumor progression, using autocrine and/or paracrine mechanisms to sustain tumor cell growth, to induce angiogenesis and to facilitate tumor escape through evasion of immune surveillance. In this review, we have comprehensively described the role of CXCR4/CXCL12 in HCC and also investigated the role of CXCR7, an alternative receptors that also binds CXCL12 with potentially distinct downstream effects. Preclinical data converge to demonstrate that inhibition of the CXCR4/CXCL12 axis may lead to direct inhibition of tumor migration, invasion, and metastases. This pathway is under investigation to identify potential novel treatments in HCC and other cancers. However, one of the major challenges faced in this emerging field targeting the CXCR4/CXCL12 signaling pathway, is the translation of current knowledge into the design and development of effective inhibitors of CXCR4 and/or CXCL12 for cancer therapy.
Keywords: Chemokines, CXCR4 receptor, CXCL12 chemokine, CXCR7 receptor, hepatocellular carcinoma
Introduction
Primary liver cancer is the third leading cause of cancer-related mortality and the sixth most common cancer worldwide with approximately 750000 new cases every year [1]. Hepatocellular carcinoma (HCC) accounts for around 90% of primary liver cancers. The geographic distribution of liver cancer incidence is heterogeneous, with the highest rates in developing areas and a lower but growing incidence in developed countries. This distribution reflects the prevalence of known risk factors. While hepatitis B virus (HBV) is the primary cause of HCC in developing areas, in developed countries hepatitis C virus (HCV) and alcohol consumption are the main known causative factors. In the majority of HCC cases cirrhosis is present prior to malignant degeneration. Tumor resection, liver transplantation and percutaneous treatment are curative options, while transarterial chemoembolization and treatment with sorafenib, a small molecule inhibitor of several tyrosine protein kinase (vascular endothelial growth factor [VEGF] and platelet derived growth factor receptor [PDGFR]) and Raf kinases, are palliative treatments. The very poor prognosis of this disease is linked to the fact that most patients are diagnosed at advanced stages with very limited opportunities for implementing curative strategies [2].
Seeking for preventive strategies such as vaccination and/or improving early diagnosis are likely to provide long-term benefit in HCC. However, as most tumors often develop silently and are not amenable for curative surgical resection due to the development of multiples liver lesions and/or distant metastases, new systemic therapies for advanced disease are required. So far, sorafenib, a tyrosine kinase inhibitors that inhibits the activation of PDGFR, VEGFR and Raf, remains the only medical option for advanced diseases that unequivocally prolong survival [3,4]. Various other drugs also targeting VEGFR and PDGFR along with other kinases, including brivanib, erlotinib, linifanib and sunitinib have failed to demonstrate being more efficient than sorafenib in HCC [5]. Moreover, the increased toxicity of multikinase inhibitors in cirrhotic patients and the lack of predictive response biomarkers in a heterogeneous disease could explain these failures. Other therapeutic targets in HCC are clearly needed. For patients with tumors displaying primary resistance or acquired resistance to sorafenib, no validated option has yet emerged. Results from several preclinical studies have identified a number of signaling pathways involved in HCC, improving our understanding of its pathogenesis and offering targets for the development of systemic therapies. Pathways implicated in HCC development involve growth factors such as the epidermal growth factor (EGF), insulin-like growth factor (IGF) and hepatocyte growth factor (HGF); cytoplasmic intermediates (AKT/mTOR and RAS/MAPK signaling cascades); pro-angiogenic factors such as VEGF, fibroblast growth factor (FGF), PDGF; and signaling pathways involved in cell differentiation such as WNT/β-catenin, Hedgehog and Notch [6]. In addition, chemokines such as CXCL12 and its ligands that are often expressed in the inflammatory microenvironment of cancer cells in HCC were thought to be potentially used as targets for the design and the evaluation of new drugs in HCC.
Chemokines
Chemokines and cancer
Chemokines, a group of small (<15 kDa) chemotactic cytokines, and their G-protein-coupled receptors were originally reported to mediate different pro- and anti-inflammatory responses [7]. Chemokines are subdivided into four families based on the position of the cysteine residues located in the N-terminal region: CXC, CC, C and CX3C chemokines, in which the X represents any amino acid. CXC chemokines can be further subdivided depending on the presence or absence of an ELR (Glu, Leu, Arg) motif, located in front of the first conserved cysteine residue. ELR (+) CXC chemokines have been described to possess neutrophil chemotactic and angiogenic properties, whereas most ELR (-) CXC chemokines are angiostatic and attract lymphocytes and natural killer cells [8].
Chemokines are secreted by different cell types and were originally described as mediators in leukocyte migration to inflammatory sites and to secondary lymphoid organs [9]. They exert their functional effects by binding to G protein-coupled seven-transmembrane domain receptors (GPCRs) defined as CXCR, CCR, CR or CX3CR. To date, just over 20 human chemokine receptors have been identified, and while some ligands bind exclusively to one receptor, others may interact with several members of a receptor family.
Chemokines play an essential role in tumor progression, acting on endothelial, epithelial and tumor cells. They act either by autocrine or paracrine mechanism to sustain tumor cell growth, induce angiogenesis and facilitate tumor escape through evasion of immune surveillance. Some chemokines actively participate in initiation, promotion and tumor progression [10-12], being involved in cellular migration, invasion, angiogenesis/angiostatic activity, tumor growth and metastases development.
Chemokines and HCC
Although the etiology of HCC is diverse, with few exceptions, prior to HCC transformation, most patients have developed cirrhosis following liver inflammation and lymphocytic infiltration mediated by chemokines [13]. The role of inflammation and chemokines in the pathogenesis of HCC is supported by evidence that HCV patients responding to interferon (IFN) have a 20% lower risk of developing HCC [14].
Cytokines, and specifically chemokines, associated with type-1 immune response are major regulators of the liver inflammation present in HCV infection. CXCL 9, CXCL 10, CXCL 11, CCL3, CCL4, and CCL5 have a migration and activation effect on T lymphocytes in HCV patients expressing CXCR3 or CCR5 [15,16]. If the normal specific immune response fails in these patients, these chemokines attract non-specific T lymphocytes, which produce and perpetuate inflammation without removing the virus. Liu et al. showed that peripheral blood lymphocyte expression of the chemokine receptors CXCR3, CCR5 or CCR6 was decreased in HCC patients with a consequent increase in the recruitment of these lymphocytes to the liver [17,18].
Several other chemokines and their receptors appear to be directly implicated in HCC [19]. The CCL20-CCR6 axis may mediate the growth and progression of HCC through phosphorylation of MAPK [20,21] and is associated with intrahepatic metastasis of HCC and poor prognosis after resection [22,23]. Serum CCL20 is also a potential marker for detecting HCC in HCV-infected patients [24]. CCL5-CCR1 promotes metastasis and invasion of the HCC cell line Huh7 and CCL3-CCR1 contributes to the growth and progression of HCC, whereas the CX3CL1-CX3CR1 axis is believed to be involved in HCC tumor growth inhibition [19].
CXCL12, also known as stromal-derived factor 1 alpha (SDF1α), is the specific ligand for CXCR4. However, it can also activate CXCR7. These three proteins allow the migration of progenitor cells in embryonic development, and the knockout of any one of them in mice results in lethality [25,26]. CXCR4 is involved in other physiological functions (immunity, hematopoiesis, brain development, angiogenesis) and pathological processes (HIV infection, autoimmune diseases and cancer) [27]. After CXCL12 binding, CXCR4 exerts its activity via a heterotrimeric G-protein, which is dissociated into activated subunits. These subunits activate different pathways, notably calcium release and cellular migration, PI3K/AKT and cellular survival, Ras-MAPK, and cell proliferation [27,28]. β-arrestin is also recruited downstream of CXCR4 to control receptor trafficking and desensitization. Moreover, it has been shown that CXCL12, alone and in combination with VEGF-A, has a key role in tumor vasculature remodeling under hypoxic conditions [29].
Beyond their influence in several inflammatory diseases, chemokines and their receptors play diverse roles in HCC tumor biology affecting leukocyte recruitment, neovascularization, and tumor progression. The chemokine CXCL12-CXCR4 axis is attracting increasing attention with mounting evidence of its role in HCC progression.
CXCL12-CXCR4 axis
CXCL12-CXCR4 axis in pathological liver stroma remodeling
Much clinical and preclinical evidences point to a role of the CXCL12-CXCR4 axis in the pathogenesis of chronic hepatitis and fibrosis. CXCR4 mRNA is upregulated in the liver following HCV infection [30] and CXCR4 is overexpressed at active inflammatory foci resembling lymphoid structures formed in cirrhotic livers [31]. In addition, Asselah et al. concluded that genes involved in the immune response (including CXCR4) and in extracellular matrix turnover are implicated in the transition from mild to moderate fibrosis in patients with chronic hepatitis C [32].
The ligand CXCL12 was also overexpressed in inflammatory and cirrhotic liver samples of HCV, HBV infected patients or primary sclerosing cholangitis compared to normal human liver tissue [31,33]. Furthermore, a significant elevation in plasma CXCL12 levels has been described in patients with high fibrosis compared to patients with healthy liver and low fibrotic tissues. CXCL12 may play a role in fibrosis by directing recruitment of endothelial progenitor cells. In addition, liver-infiltrating lymphocytes overexpressing CXCR4 migrate and adhere to the extracellular matrix in response to CXCL12 [31]. Moreover, after massive liver injury, hepatocytes [34,35] and/or oval cells [36] upregulated CXCL12 expression, which exerts its biological effect on oval cells in a paracrine or autocrine manner. Thus, oval cells migrate, following a CXCL12 gradient, from the periportal to the pericentral liver parenchyma, where the microenvironment is favorable for proliferation or differentiation [34-36]. Nonetheless, these findings must be interpreted with caution given recent discordant data suggesting that CXCL12-CXCR4 signaling contributes not only to hepatic protection and liver regeneration, but also prevents the progression of liver fibrosis [37]. Differences in the animal models, hematopoietic cells populations, liver damage intensity or drugs used could explain this discrepancy.
Taken together, these data support the role of the CXCL12-CXCR4 pathway in hepatic stroma remodeling and potential therapeutic applications of CXCR4 inhibitors as anti-fibrotic agents [38].
CXCL12-CXCR4 axis and chemotaxis
Cancer cells that express CXCR4 can migrate via chemotaxis through a CXCL12 gradient to target tissues, supporting a chemotactic role for this axis in the development of metastases. Chemotaxis is recapitulating physiological functions that have been observed during embryogenesis and hepatic regeneration after liver injury. Evidences of the role of CXCL12/CXCR4 on chemotaxis in cancer has been provided by reports showing that high levels of CXCL12 in bones, lymph nodes, and lungs [39] can attract CXCR4-expressing breast cancer cells, promoting metastasis and supporting the “seed and soil” hypothesis proposed by Paget in the past century [40]. This report in breast cancer more than a decade ago resulted in extensive research in this area, with the consequent implication of this signaling pathway in many other human cancers including prostate, esophageal, gastric, colon, pancreas, renal, lung, and HCC [41-43], as discussed below.
CXCL12-CXCR4 axis in HCC
The CXCL12-CXCR4 axis has aroused recent interest in HCC pathogenesis and progression. A number of studies have demonstrated immunohistochemical staining of CXCR4 in HCC tissue but not in normal hepatic tissue [44,45]. Reports of CXCR4 mRNA expression are contrasting; Liu et al. found expression in HCC tumor-tissues and negative expression in normal tissues [45], while others report reduced expression in HCC tissue [43] or no differences [44]. Nonetheless, the majority of studies showed correlations between high CXCR4 expression and aggressive tumor behavior, metastasis development, and poor prognosis (Table 1) [44,46,47]. Xiang et al. identified CXCR4 overexpression as an independent risk factor for reduced disease-free survival (relative risk [RR]=5.440; p=0.023) and overall survival (RR=7.082; p=0.001) in 181 HCC patients [44]. Likewise, another study showed that nuclear immunohistochemical CXCR4 expression was an independent prognostic factor for overall survival (p=0.038) in 268 HCC patients [46]. Although other studies failed to correlate CXCR4 expression with survival in HCC patients, a significantly decreased 3-year-survival rate was observed in patients with tumor strongly expressing CXCR4, the small patient sample size may explain these results [47].
Table 1.
Studies showing the relationship between CXCR4 expression and prognosis in HCC patients
| Study (Reference) | Date | N patients | Metastasis (p-value) | DFS (p-value) | OS months (p-value) | 3-year OS (p-value) |
|---|---|---|---|---|---|---|
| Schimanski et al. [51] | 2006 | 39 | Lymphatic (0.005) | - | - | Decreased (0.01) |
| Distant (0.009) | ||||||
| Xiang et al. [44] | 2009 | 181 | Bone (<0.001) | 0.023 | 18 vs 36 (p<0.001) | - |
| Xiang et al.[46] | 2009 | 268 | Lymphatic (0.001) | - | 15.1 vs 24.5 (p=0.002) | - |
Expression of the CXCR4 ligand, CXCL12, has been reported in tumor ascites fluid and mRNA has been detected in HCC lymph node metastases, but was undetectable in HCC and normal hepatic tissues [45]. In addition, evaluation of CXCL12 and CXCR4 polymorphisms identified an association between a CXCL12 gene variant and HCC; individuals bearing the CXCL12 (RS1801157) polymorphism having higher risk of developing stage III or IV HCC, while no significant association with the CXCR4 polymorphism (RS2228014) was observed [48]. In other studies, no correlation between CXCL12 polymorphisms and HCC development has been identified in HCV or alcoholic cirrhosis patients [49,50].
In summary, it is now known that injured hepatocytes, oval cells, biliary epithelial cells, sinusoidal endothelial cells, tumor-associated leucocytes and HCC cells can release CXCL12, which in turn activates CXCR4-expressing cells, such as HCC cells, lymphocytes or endothelial cells in an autocrine or paracrine manner (Figure 1). Furthermore, tissues expressing high levels of CXCL12 can also attract malignant HCC cells expressing CXCR4.
Figure 1.
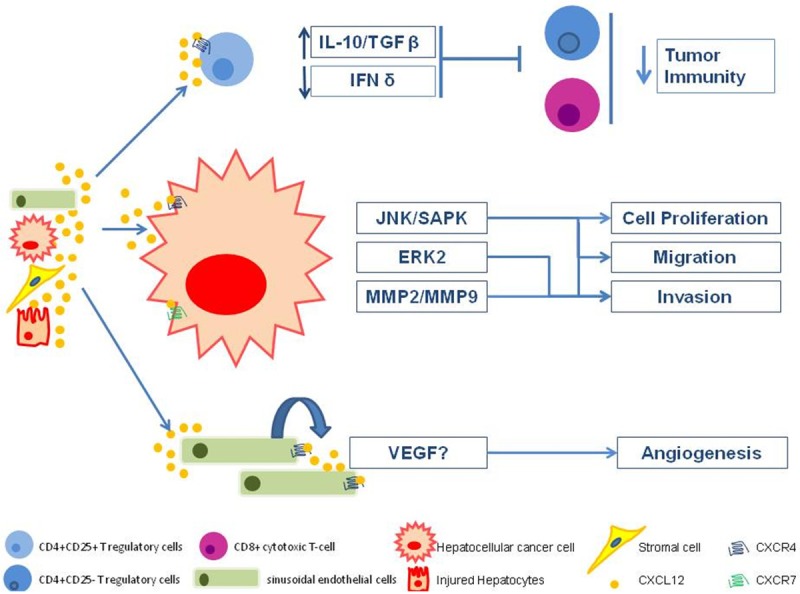
Role of the CXCR4-CXCL12 axis in HCC progression.
CXCL12-CXCR4 axis in HCC cells: proliferation, survival, invasion, and migration
Evidence of the involvement of the CXCL12-CXCR4 axis in cell growth, migration, and invasion of HCC cells comes from the over-expression of CXCR4 in several HCC cell lines, including the Huh7 and Hep3B human hepatoma cell lines, which also secrete CXCL12 under basal conditions. In addition, CXCL12 induces translocation of CXCR4 from the membrane and cytoplasm to the perinuclear region in Huh7 and Hep3B cells [51].
Interestingly, it has been shown that both chemotherapy and loss of phosphatase and tensin homolog (PTEN) function promote cell invasiveness through a reactive oxygen species (ROS)-dependent upregulation of the CXCL12/CXCR4 axis in pancreatic or prostate cancer cells [52,53]. Therefore, a tumor microenvironment rich in ROS and pro-inflammatory molecules as occurs during HCC progression may critically influence CXCR4-mediated expression and functions, ultimately encouraging cancer progression and metastasis development.
CXCL12 can stimulate cell proliferation through its association with CXCR4 and glycosaminoglycans, such as syndecan-4 (SDC-4) present in the plasma membrane. It has a strong effect on migration and invasion of Huh7 cells, which is dependent on MAPK-ERK1/2 and JNK/SAPK activation. CXCL12 also induces activation of MMP-9, which is involved in cell invasion acting on the degradation of various components of the basement membrane [54]. Zhang et al. identified that osteopontin upregulates MMP-2 and invasion by activating the CXCL12-CXCR4 pathway in HepG2 and SMMC7721 HCC cell lines [55]. In Hca-F and Hca-P mouse HCC cell lines, CXCL12-CXCR4 also mediates MMP-9 and MMP-2 secretion, facilitating lymph node metastasis [56].
Nonetheless, conflicting data were reported indicating that the CXCL12-CXCR4 axis does not operate in other HCC cell lines. Mitra et al. described that in HepG2 cells, CXCR4 does not response to CXCL12 stimulation because of a defect in the receptor [57] and Kim et al. reported that despite abundant CXCR4 expression in five cell lines (HepG2, Hep3B, SK-HEP-1, NCL-H630 and PCL/PRF5), the protein remained trapped in the cytoplasm with a negligible response to CXCL12. These authors also showed that CXCL12 bound to all cell lines, and while it induced the phosphorylation of AKT and ERK1/2 in some cell lines (PCL/PRF5, Hep3B), neither migration nor proliferation were seen [58]. These findings suggest that the biological effect of CXCL12 in HCC cell lines depends on the cellular subtype, and those additional factors such as CXCR7 receptors and their ligands (CXCL11) may modulate the final outcome of this pathway.
CXCL12-CXCR4 axis in T lymphocytes: tumor immunity suppression
Reported cases of spontaneous HCC regression suggest that immune mechanisms are also important to control HCC progression [59,60]. However, HCC cells may favor the development of a microenvironment, which often impairs tumor immunity. As such, a high prevalence of CD4+CD25+ T regulatory cells (T reg) has been reported in peripheral blood, tumor tissue, and peritumor regions of HCC patients [61,62].
Secretion of CXCL12 appears to play an important role in the migration and accumulation of CD4+CD25+ T reg cells in HCC tissue through the activation of CXCL12-CXCR4 signaling. T reg cells can inhibit cytokine secretion and proliferation of CD4+CD25- T cells in HCC patients causing potential immune tolerance for tumor cells. Immunosuppression could be due to the modification of cytokine secretion in the tumor microenvironment, such as increased secretion of IL-10 and TGF-beta (TGF-β) and decreased IFN-gamma (IFNγ) [61]. The loss of IFNγ promotes HCC formation [63], since IFNγ produced by cytotoxic T cells induces growth inhibition and death in HCC cells [64,65] as well as the stimulation of chemokines such as CXCL9 and CXCL10, which can attract lymphocytes to HCC tissue [66]. Furthermore, CD4+CD25+ T reg cells could regulate CD8+ cytotoxic T-cell activity, allowing HCC progression [67].
CXCL12-CXCR4 axis in endothelial cells: angiogenesis
Participation of the CXCL12-CXCR4 axis in tumor neovascularization through VEGF-dependent and independent mechanisms has been reported. CXCR4 upregulates VEGF expression through the Akt pathway in breast cancer [68]. In addition, CXCR4 is upregulated under hypoxic conditions in glioblastoma, via the hypoxia inducible factor 1-alfa (HIF1-α) and VEGF [69]. Other models showed that response to VEGF and hypoxia may be more variable depending of the model, as evidence has been reported that VEGF was not inducing CXCR4 expression in some HCC cell lines, whereas hypoxia only increased the cytoplasmic and cell surface expression of CXCR4 in PLC/PRF5 cells [58]. Nonetheless, it has been demonstrated that osteopontin, a potent tumor angiogenic factor [70] induced expression and activation of MMP-2, mediated by the CXCL12/CXCR4 axis, which is associated with increased HCC cell invasion [55].
Beyond direct angiogenic activity on HCC tumor cells, the CXCL12/CXCR4 axis may also be involved in the induction of neoangiogenesis via the activation of sinusoidal endothelial cells. Li et al. reported that CXCL12 and CXCR4 were significantly overexpressed in sinusoidal endothelial cells of HCC tissue, suggesting a role in HCC neoangiogenesis by means of an autocrine mechanism [71]. Furthermore, in two phase II trials in HCC patients, reduction in circulating VEGF-A and CXCL12 levels was observed after bevacizumab treatment [72], and an increase in circulating plasma CXCL12 levels correlated with progression of HCC patients under sunitinib treatment [73].
CXCL12-CXCR4 axis and metastasis
Liu et al detected CXCL12 in portal lymph nodes and tumor ascites fluid, and CXCR4 in HCC tissues, suggesting that this signaling pathway plays an important role in metastasis of HCC by promoting the migration of tumor cells [45]. Additional supportive evidence was accumulated including a report that CXCL12 secreted by hepatic stellate cells (Ito cells) favors liver metastasis [74]. Several studies have confirmed the relation between CXCR4 overexpression and metastasis development in HCC [75]. CXCR4 was shown to be involved in the process of metastasis in HCC, contributing to the formation of portal vein tumor thrombus through cell invasion and migration [76]. Other studies have shown that the location of CXCR4 expression in the HCC cell may impact its biological significance in this setting. According to the findings of Xiang et al. there is a close correlation between increased expression of nuclear CXCR4 and lymph node metastasis in HCC patients, while this was not the case with cytoplasmatic CXCR4 expression [44]. However, other studies show mostly cytoplasmatic expression of CXCR4 [46,51].
CXCR7 and HCC
CXCR7 has recently been discovered as a new player in the CXCL12-CXCR4 axis, described as binding to CXCL12 with even greater affinity than CXCR4. CXCL11 the natural ligand for CXCR3, is also a ligand of CXCR7 but displays 10-fold lower affinity than CXCL12 [77]. CXCR7 is overexpressed in tumor cells, including breast, prostate, lung, and pancreatic cancer cells, as well as in tumoral endothelial cells [78-80]. New data propose that CXCR7 promotes development and progression in cancer [81,82]. Various in vitro and in vivo studies suggest that alterations in CXCR7 expression are associated with proliferation, apoptosis, migration, invasion and tumor growth [83-85]. Moreover, CXCR7 expression regulated the expression of the proangiogenic factors interleukin 8 (IL-8) and VEGF, which may regulate tumor angiogenesis [86].
Interestingly, in contrast to CXCR4, CXCR7 exerts its biological effect mediated by β-arrestin, independently of G protein activation. Hence, the CXCR7-CXCL12 complex is internalized and CXCL12 degraded, regulating both extracellular CXCL12 levels and CXCL12-CXCR4 signaling [87]. When coexpressed, CXCR4 and CXCR7 can form homo- and heterodimers, with heterodimerization seemingly playing an important role in the modulation of the downstream signaling of CXCR4 [88].
As a result, CXCR7 was initially regarded as a decoy receptor, acting as either a scavenger of extracellular CXCL12 or a modulator of CXCR4 signaling and effects [89]. Subsequent findings revealed that CXCL12 mediated activation of CXCR7 alone evoking other intracellular signaling events such as activation of the MAPK pathway [83] in a β-arrestin-dependent manner [90]. Over the last few years, several reports have been published showing that calcium influx and inhibition of the adenylyl cyclase are exclusively mediated by CXCR4, whereas CXCR4 and CXCR7 together or CXCR7 alone can activate intracellular signaling pathways including phosphorylation of Akt and MAPK and activation of JAK2/STAT3. The PI3k/Akt activation predominantly results in cytoskeleton rearrangement and therefore influences cell migration and chemotaxis, whereas activation of MAPK and Akt signaling pathways promotes cell survival and proliferation, and transcriptional regulation of multiple genes involved in angiogenesis, invasion and adhesion of cells [83,91,92].
Overexpression of CXCR7 protein and mRNA has been described in human HCC compared to normal liver tissue [93,94]. Furthermore, expression of CXCR7 in 116 specimens from HCC patients was significantly higher in patients with lung metastasis (p=0.0017) [93]. CXCR7 effects might be mediated through osteopontin upregulation [95]. In vivo studies confirmed that HCC cells with high expression of CXCR7 were prone to metastasize to the lungs [93]. Interestingly, the authors showed that CXCR4 expression was lower in the tumor than in para-cancerous tissues. The location of CXCR7 has been reported in both HCC cells [93,94] and endothelial cells [96], which may influence the mechanisms mediating the role of this axis in HCC. Moreover, CXCR7/CXCL12 induce invasion, proliferation and VGEF secretion in HCC cells lines (SMMC-7721, HCCLM3) [93,94].
The initial view that CXCL12 only targets CXCR4 and that CXCR7 is a decoy receptor limits the role of CXCR4 activity and clearly needs to be redefined. It has been proposed that CXCL12 scavenging by CXCR7 may reduce retention of CXCR4-positive tumor cells within the tumor, increasing the dissemination of CXCR4-positive tumor cells, however this hypothesis needs to be verified in preclinical models. Furthermore, despite broad knowledge of CXCR4 expression in different tumor types, co-expression patterns of both receptors and their regulation in the context of the tumor microenvironment are lacking.
Anti-CXCR4 drugs and HCC clinical trials
In this review, we have shown that the metastatic process is influenced by CXCL12 and its receptor CXCR4 in HCC patients. Thus, identification of novel agents that can down regulate CXCR4 expression and its associated functions have great potential in the treatment of metastatic HCC. A wide variety of strategies, such as peptides, small molecules, antibodies, and small interfering RNA, have been employed to target this pathway.
Nevertheless, to date few compounds have advanced to early stages of clinical development. Plerixafor (AMD3100; Genzyme), which is known to block CXCR4 is approved by the FDA for mobilization of hematopoietic CD34+ cells and has been extensively studied in hematological malignances. A clinical study evaluating Plerixafor in combination with mitoxantrone, etoposide and cytarabine in acute myeloid leukemia patients (sponsored by the Washington University School of Medicine) has recently been completed. In addition, clinical studies with Plerixafor in combination with bevacizumab in recurrent high-grade glioma patients or with bortezomib in relapsed or refractory multiple myeloma patients are ongoing. However, the phase I study of an oral CXCR4 antagonist, MSX-122 (Metastatix, Inc) in patients with refractory metastatic or locally advanced solid tumors has suspended participant recruitment.
A phase Ib, open-label, multicenter study evaluating BMS-936564 (Bristol-Myers Squibb), a fully human IgG(4) monoclonal antibody that specifically recognizes human CXCR4, in monotherapy and combined with lenalidomide/dexamethasone or bortezomib/dexamethasone in relapsed or refractory multiple myeloma patients will be completed in February 2015. A nanobody inhibiting CXCR4 (ALX-0651; Ablynx) has been recently studied in a first-in-man phase I study in healthy male volunteers.
There are currently no reports of clinical studies evaluating the activity of CXCR4-inhibitors in HCC. In a rectal cancer study, it was proposed that this axis could work as an escape mechanism after antiangiogenic therapies [97]. Taking into account that hypoxia upregulates CXCR4 and CXCR7 [98] and that neoangiogenesis play an important role in HCC, the axis could work as an escape mechanism to antiangiogenic drugs such as sorafenib, considered as standard of care in advanced HCC. Considering that chemokines activation may be primed in response to sorafenib and other antiangiogenic agents, blocking the CXCL12-CXCR4 axis using CXCR4 inhibitor in HCC could be considered either monotherapy or in combination for patients with tumors that relapsed after sorafenib treatment. High circulating levels of CXCL12 were indeed observed in HCC patients with progression during treatment with the antiangiogenic agent, sunitinib [73]. In any case, better knowledge of the biological complexity of the axis, its regulation and the exact relationship existing between the different components in HCC are warranted before developing treatment strategies.
Conclusions
The poor prognosis of advanced HCC along with the lack of effective treatments highlights the urgent need for research into new therapeutic approaches and the identifications of novel targets. The underlying inflammatory environment in HCC with a previous context of cirrhosis renders chemokines an area with good potential for investigation. This review has focused on current literature reporting on the role of the CXCL12-CXCR4 axis in liver inflammation and HCC pathogenesis acting directly in HCC cells or indirectly in the microenvironment cells such as leucocytes or endothelial cells. The chemokine CXCL12 and its receptors CXCR4 and CXCR7 exert a variety of effects on tumor cells in vitro and in vivo, promoting growth, invasion, angiogenesis and progression of HCC tumors. However, publications addressing novel pharmacologic approaches for CXCR4/CXCL12 targeting therapies in HCC are few and far between, and are mostly in the preclinical or early clinical developmental stage. Further studies will certainly reveal new aspects of the interaction or role of other chemokines and its receptors such as CXCL11 or CXCR7, which should be analyzed in conjunction with CXCR4 and CXCL12, and will hopefully result in the design and development of promising clinical candidates for the inhibition of this axis in HCC.
Acknowledgements
The Foundation Nelia & Amadeo Barleta (FNAB) supported this work as well as the Association d’Aide à la Recherche & l’Enseignement en Cancérologie (AAREC).
Disclosure of conflict of interest
None to disclose.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734–745. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 4.Furuse J. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Biologics. 2008;2:779–788. doi: 10.2147/btt.s3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol. 2013;10:34–42. doi: 10.1038/nrgastro.2012.199. [DOI] [PubMed] [Google Scholar]
- 6.Sia D, Villanueva A. Signaling pathways in hepatocellular carcinoma. Oncology. 2011;81(Suppl 1):18–23. doi: 10.1159/000333254. [DOI] [PubMed] [Google Scholar]
- 7.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 8.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 9.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 10.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 11.Mehrad B, Keane MP, Strieter RM. Chemokines as mediators of angiogenesis. Thromb Haemost. 2007;97:755–762. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Q, Han X, Peng J, Qin H, Wang Y. The role of CXC chemokines and their receptors in the progression and treatment of tumors. J Mol Histol. 2012;43:699–713. doi: 10.1007/s10735-012-9435-x. [DOI] [PubMed] [Google Scholar]
- 13.Marra F. Chemokines in liver inflammation and fibrosis. Front Biosci. 2002;7:d1899–1914. doi: 10.2741/A887. [DOI] [PubMed] [Google Scholar]
- 14.Castello G, Costantini S, Scala S. Targeting the inflammation in HCV-associated hepatocellular carcinoma: a role in the prevention and treatment. J Transl Med. 2010;8:109. doi: 10.1186/1479-5876-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larrubia JR, Benito-Martinez S, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Role of chemokines and their receptors in viral persistence and liver damage during chronic hepatitis C virus infection. World J Gastroenterol. 2008;14:7149–7159. doi: 10.3748/wjg.14.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang W, Shin EC. Clinical implications of chemokines in acute and chronic hepatitis C virus infection. Yonsei Med J. 2011;52:871–878. doi: 10.3349/ymj.2011.52.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Poon RT, Feng X, Yu WC, Luk JM, Fan ST. Reduced expression of chemokine receptors on peripheral blood lymphocytes in patients with hepatocellular carcinoma. Am J Gastroenterol. 2004;99:1111–1121. doi: 10.1111/j.1572-0241.2004.30265.x. [DOI] [PubMed] [Google Scholar]
- 18.Roberts LR. Chemokines as attractive targets in liver carcinogenesis. Am J Gastroenterol. 2005;100:499–501. doi: 10.1111/j.1572-0241.2005.t01-4-41219.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang F, Geng XP. Chemokines and hepatocellular carcinoma. World J Gastroenterol. 2010;16:1832–1836. doi: 10.3748/wjg.v16.i15.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujii H, Itoh Y, Yamaguchi K, Yamauchi N, Harano Y, Nakajima T, Minami M, Okanoue T. Chemokine CCL20 enhances the growth of HuH7 cells via phosphorylation of p44/42 MAPK in vitro. Biochem Biophys Res Commun. 2004;322:1052–1058. doi: 10.1016/j.bbrc.2004.07.207. [DOI] [PubMed] [Google Scholar]
- 21.Rubie C, Frick VO, Wagner M, Rau B, Weber C, Kruse B, Kempf K, Tilton B, Konig J, Schilling M. Enhanced expression and clinical significance of CC-chemokine MIP-3 alpha in hepatocellular carcinoma. Scand J Immunol. 2006;63:468–477. doi: 10.1111/j.1365-3083.2006.001766.x. [DOI] [PubMed] [Google Scholar]
- 22.Uchida H, Iwashita Y, Sasaki A, Shibata K, Matsumoto T, Ohta M, Kitano S. Chemokine receptor CCR6 as a prognostic factor after hepatic resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2006;21:161–168. doi: 10.1111/j.1440-1746.2005.04157.x. [DOI] [PubMed] [Google Scholar]
- 23.Ding X, Wang K, Wang H, Zhang G, Liu Y, Yang Q, Chen W, Hu S. High expression of CCL20 is associated with poor prognosis in patients with hepatocellular carcinoma after curative resection. J Gastrointest Surg. 2012;16:828–836. doi: 10.1007/s11605-011-1775-4. [DOI] [PubMed] [Google Scholar]
- 24.Soliman HH, Nagy H, Kotb N, Alm El-Din MA. The role of chemokine CC ligand 20 in patients with liver cirrhosis and hepatocellular carcinoma. Int J Biol Markers. 2012;27:e125–131. doi: 10.5301/JBM.2012.9097. [DOI] [PubMed] [Google Scholar]
- 25.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 26.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 27.Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG, Walenkamp AM. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer. 2013;49:219–230. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O, Levis M, Rubin JB, Negrin RR, Estey EH, Konoplev S, Andreeff M, Konopleva M. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113:6215–6224. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–9064. doi: 10.1158/0008-5472.CAN-05-3759. [DOI] [PubMed] [Google Scholar]
- 30.Mitra P, Shibuta K, Mathai J, Shimoda K, Banner BF, Mori M, Barnard GF. CXCR4 mRNA expression in colon, esophageal and gastric cancers and hepatitis C infected liver. Int J Oncol. 1999;14:917–925. doi: 10.3892/ijo.14.5.917. [DOI] [PubMed] [Google Scholar]
- 31.Wald O, Pappo O, Safadi R, Dagan-Berger M, Beider K, Wald H, Franitza S, Weiss I, Avniel S, Boaz P, Hanna J, Zamir G, Eid A, Mandelboim O, Spengler U, Galun E, Peled A. Involvement of the CXCL12/CXCR4 pathway in the advanced liver disease that is associated with hepatitis C virus or hepatitis B virus. Eur J Immunol. 2004;34:1164–1174. doi: 10.1002/eji.200324441. [DOI] [PubMed] [Google Scholar]
- 32.Asselah T, Bieche I, Laurendeau I, Paradis V, Vidaud D, Degott C, Martinot M, Bedossa P, Valla D, Vidaud M, Marcellin P. Liver gene expression signature of mild fibrosis in patients with chronic hepatitis C. Gastroenterology. 2005;129:2064–2075. doi: 10.1053/j.gastro.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Terada R, Yamamoto K, Hakoda T, Shimada N, Okano N, Baba N, Ninomiya Y, Gershwin ME, Shiratori Y. Stromal cell-derived factor-1 from biliary epithelial cells recruits CXCR4-positive cells: implications for inflammatory liver diseases. Lab Invest. 2003;83:665–672. doi: 10.1097/01.lab.0000067498.89585.06. [DOI] [PubMed] [Google Scholar]
- 34.Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF-1alpha/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339–351. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]
- 35.Zheng D, Oh SH, Jung Y, Petersen BE. Oval cell response in 2-acetylaminofluorene/partial hepatectomy rat is attenuated by short interfering RNA targeted to stromal cell-derived factor-1. Am J Pathol. 2006;169:2066–2074. doi: 10.2353/ajpath.2006.060211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mavier P, Martin N, Couchie D, Preaux AM, Laperche Y, Zafrani ES. Expression of stromal cell-derived factor-1 and of its receptor CXCR4 in liver regeneration from oval cells in rat. Am J Pathol. 2004;165:1969–1977. doi: 10.1016/S0002-9440(10)63248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuchiya A, Imai M, Kamimura H, Takamura M, Yamagiwa S, Sugiyama T, Nomoto M, Heike T, Nagasawa T, Nakahata T, Aoyagi Y. Increased susceptibility to severe chronic liver damage in CXCR4 conditional knock-out mice. Dig Dis Sci. 2012;57:2892–2900. doi: 10.1007/s10620-012-2239-8. [DOI] [PubMed] [Google Scholar]
- 38.Hong F, Tuyama A, Lee TF, Loke J, Agarwal R, Cheng X, Garg A, Fiel MI, Schwartz M, Walewski J, Branch A, Schecter AD, Bansal MB. Hepatic stellate cells express functional CXCR4: role in stromal cell-derived factor-1alpha-mediated stellate cell activation. Hepatology. 2009;49:2055–2067. doi: 10.1002/hep.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 40.Ribatti D, Mangialardi G, Vacca A. Stephen Paget and the ‘seed and soil’ theory of metastatic dissemination. Clin Exp Med. 2006;6:145–149. doi: 10.1007/s10238-006-0117-4. [DOI] [PubMed] [Google Scholar]
- 41.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- 42.Kaifi JT, Yekebas EF, Schurr P, Obonyo D, Wachowiak R, Busch P, Heinecke A, Pantel K, Izbicki JR. Tumor-cell homing to lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. J Natl Cancer Inst. 2005;97:1840–1847. doi: 10.1093/jnci/dji431. [DOI] [PubMed] [Google Scholar]
- 43.Zagzag D, Krishnamachary B, Yee H, Okuyama H, Chiriboga L, Ali MA, Melamed J, Semenza GL. Stromal cell-derived factor-1alpha and CXCR4 expression in hemangioblastoma and clear cell-renal cell carcinoma: von Hippel-Lindau loss-of-function induces expression of a ligand and its receptor. Cancer Res. 2005;65:6178–6188. doi: 10.1158/0008-5472.CAN-04-4406. [DOI] [PubMed] [Google Scholar]
- 44.Xiang ZL, Zeng ZC, Tang ZY, Fan J, Zhuang PY, Liang Y, Tan YS, He J. Chemokine receptor CXCR4 expression in hepatocellular carcinoma patients increases the risk of bone metastases and poor survival. BMC Cancer. 2009;9:176. doi: 10.1186/1471-2407-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu H, Pan Z, Li A, Fu S, Lei Y, Sun H, Wu M, Zhou W. Roles of chemokine receptor 4 (CXCR4) and chemokine ligand 12 (CXCL12) in metastasis of hepatocellular carcinoma cells. Cell Mol Immunol. 2008;5:373–378. doi: 10.1038/cmi.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiang Z, Zeng Z, Tang Z, Fan J, Sun H, Wu W, Tan Y. Increased expression of vascular endothelial growth factor-C and nuclear CXCR4 in hepatocellular carcinoma is correlated with lymph node metastasis and poor outcome. Cancer J. 2009;15:519–525. doi: 10.1097/PPO.0b013e3181c6aa6b. [DOI] [PubMed] [Google Scholar]
- 47.Li W, Gomez E, Zhang Z. Immunohistochemical expression of stromal cell-derived factor-1 (SDF-1) and CXCR4 ligand receptor system in hepatocellular carcinoma. J Exp Clin Cancer Res. 2007;26:527–533. [PubMed] [Google Scholar]
- 48.Lin GT, Tseng HF, Yang CH, Hou MF, Chuang LY, Tai HT, Tai MH, Cheng YH, Wen CH, Liu CS, Huang CJ, Wang CL, Chang HW. Combinational polymorphisms of seven CXCL12-related genes are protective against breast cancer in Taiwan. OMICS. 2009;13:165–172. doi: 10.1089/omi.2008.0050. [DOI] [PubMed] [Google Scholar]
- 49.Nahon P, Sutton A, Rufat P, Simon C, Trinchet JC, Gattegno L, Beaugrand M, Charnaux N. Chemokine system polymorphisms, survival and hepatocellular carcinoma occurrence in patients with hepatitis C virus-related cirrhosis. World J Gastroenterol. 2008;14:713–719. doi: 10.3748/wjg.14.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nahon P, Sutton A, Rufat P, Faisant C, Simon C, Barget N, Trinchet JC, Beaugrand M, Gattegno L, Charnaux N. Lack of association of some chemokine system polymorphisms with the risks of death and hepatocellular carcinoma occurrence in patients with alcoholic cirrhosis: a prospective study. Eur J Gastroenterol Hepatol. 2007;19:425–431. doi: 10.1097/MEG.0b013e3280120e2b. [DOI] [PubMed] [Google Scholar]
- 51.Schimanski CC, Bahre R, Gockel I, Muller A, Frerichs K, Horner V, Teufel A, Simiantonaki N, Biesterfeld S, Wehler T, Schuler M, Achenbach T, Junginger T, Galle PR, Moehler M. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br J Cancer. 2006;95:210–217. doi: 10.1038/sj.bjc.6603251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chetram MA, Don-Salu-Hewage AS, Hinton CV. ROS enhances CXCR4-mediated functions through inactivation of PTEN in prostate cancer cells. Biochem Biophys Res Commun. 2011;410:195–200. doi: 10.1016/j.bbrc.2011.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arora S, Bhardwaj A, Singh S, Srivastava SK, McClellan S, Nirodi CS, Piazza GA, Grizzle WE, Owen LB, Singh AP. An undesired effect of chemotherapy: gemcitabine promotes pancreatic cancer cell invasiveness through reactive oxygen species-dependent, nuclear factor kappaB- and hypoxia-inducible factor 1alpha-mediated up-regulation of CXCR4. J Biol Chem. 2013;288:21197–21207. doi: 10.1074/jbc.M113.484576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutton A, Friand V, Brule-Donneger S, Chaigneau T, Ziol M, Sainte-Catherine O, Poire A, Saffar L, Kraemer M, Vassy J, Nahon P, Salzmann JL, Gattegno L, Charnaux N. Stromal cell-derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol Cancer Res. 2007;5:21–33. doi: 10.1158/1541-7786.MCR-06-0103. [DOI] [PubMed] [Google Scholar]
- 55.Zhang R, Pan X, Huang Z, Weber GF, Zhang G. Osteopontin enhances the expression and activity of MMP-2 via the SDF-1/CXCR4 axis in hepatocellular carcinoma cell lines. PLoS One. 2011;6:e23831. doi: 10.1371/journal.pone.0023831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chu H, Zhou H, Liu Y, Liu X, Hu Y, Zhang J. Functional expression of CXC chemokine recepter-4 mediates the secretion of matrix metalloproteinases from mouse hepatocarcinoma cell lines with different lymphatic metastasis ability. Int J Biochem Cell Biol. 2007;39:197–205. doi: 10.1016/j.biocel.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 57.Mitra P, De A, Ethier MF, Mimori K, Kodys K, Shibuta K, Mori M, Madison JM, Miller-Graziano C, Barnard GF. Loss of chemokine SDF-1alpha-mediated CXCR4 signalling and receptor internalization in human hepatoma cell line HepG2. Cell Signal. 2001;13:311–319. doi: 10.1016/s0898-6568(01)00156-5. [DOI] [PubMed] [Google Scholar]
- 58.Kim SW, Kim HY, Song IC, Jin SA, Lee HJ, Yun HJ, Kim S, Jo DY. Cytoplasmic trapping of CXCR4 in hepatocellular carcinoma cell lines. Cancer Res Treat. 2008;40:53–61. doi: 10.4143/crt.2008.40.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin TJ, Liao LY, Lin CL, Shih LS, Chang TA, Tu HY, Chen RC, Wang CS. Spontaneous regression of hepatocellular carcinoma: a case report and literature review. Hepatogastroenterology. 2004;51:579–582. [PubMed] [Google Scholar]
- 60.Oquinena S, Inarrairaegui M, Vila JJ, Alegre F, Zozaya JM, Sangro B. Spontaneous regression of hepatocellular carcinoma: three case reports and a categorized review of the literature. Dig Dis Sci. 2009;54:1147–1153. doi: 10.1007/s10620-008-0447-z. [DOI] [PubMed] [Google Scholar]
- 61.Shen X, Li N, Li H, Zhang T, Wang F, Li Q. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136:1745–1754. doi: 10.1007/s00432-010-0833-8. [DOI] [PubMed] [Google Scholar]
- 62.Yang XH, Yamagiwa S, Ichida T, Matsuda Y, Sugahara S, Watanabe H, Sato Y, Abo T, Horwitz DA, Aoyagi Y. Increase of CD4+ CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma. J Hepatol. 2006;45:254–262. doi: 10.1016/j.jhep.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 63.Eferl R. A dual role for interferon gamma signalling in hepatocellular carcinoma. J Hepatol. 2012;57:940–942. doi: 10.1016/j.jhep.2012.07.040. [DOI] [PubMed] [Google Scholar]
- 64.Li P, Du Q, Cao Z, Guo Z, Evankovich J, Yan W, Chang Y, Shao L, Stolz DB, Tsung A, Geller DA. Interferon-gamma induces autophagy with growth inhibition and cell death in human hepatocellular carcinoma (HCC) cells through interferon-regulatory factor-1 (IRF-1) Cancer Lett. 2012;314:213–222. doi: 10.1016/j.canlet.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang L, Hu HD, Hu P, Lan YH, Peng ML, Chen M, Ren H. Gene therapy with CX3CL1/Fractalkine induces antitumor immunity to regress effectively mouse hepatocellular carcinoma. Gene Ther. 2007;14:1226–1234. doi: 10.1038/sj.gt.3302959. [DOI] [PubMed] [Google Scholar]
- 66.Hirano S, Iwashita Y, Sasaki A, Kai S, Ohta M, Kitano S. Increased mRNA expression of chemokines in hepatocellular carcinoma with tumor-infiltrating lymphocytes. J Gastroenterol Hepatol. 2007;22:690–696. doi: 10.1111/j.1440-1746.2006.04551.x. [DOI] [PubMed] [Google Scholar]
- 67.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, Yao J, Jin L, Wang H, Yang Y, Fu YX, Wang FS. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 68.Liang Z, Brooks J, Willard M, Liang K, Yoon Y, Kang S, Shim H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359:716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zagzag D, Lukyanov Y, Lan L, Ali MA, Esencay M, Mendez O, Yee H, Voura EB, Newcomb EW. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86:1221–1232. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 70.Gupta A, Zhou CQ, Chellaiah MA. Osteopontin and MMP9: Associations with VEGF Expression/Secretion and Angiogenesis in PC3 Prostate Cancer Cells. Cancers. 2013;5:617–638. doi: 10.3390/cancers5020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li W, Gomez E, Zhang Z. Immunohistochemical expression of stromal cell-derived factor-1 (SDF-1) and CXCR4 ligand receptor system in hepatocellular carcinoma. J Exp Clin Cancer Res. 2007;26:527–533. [PubMed] [Google Scholar]
- 72.Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, Chen H, Clark-Garvey S, Weinberg A, Mandeli J, Christos P, Mazumdar M, Popa E, Brown RS Jr, Rafii S, Schwartz JD. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J. Clin. Oncol. 2008;26:2992–2998. doi: 10.1200/JCO.2007.15.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS, Lahdenranta J, Bhargava P, Meyerhardt J, Clark JW, Kwak EL, Hezel AF, Miksad R, Abrams TA, Enzinger PC, Fuchs CS, Ryan DP, Jain RK. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J. Clin. Oncol. 2009;27:3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsusue R, Kubo H, Hisamori S, Okoshi K, Takagi H, Hida K, Nakano K, Itami A, Kawada K, Nagayama S, Sakai Y. Hepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis. Ann Surg Oncol. 2009;16:2645–2653. doi: 10.1245/s10434-009-0599-x. [DOI] [PubMed] [Google Scholar]
- 75.Xiang ZL, Zeng ZC, Fan J, Wu WZ, He J, Zeng HY, Tang ZY. A clinicopathological model to predict bone metastasis in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2011;137:1791–1797. doi: 10.1007/s00432-011-1060-7. [DOI] [PubMed] [Google Scholar]
- 76.Li N, Guo W, Shi J, Xue J, Hu H, Xie D, Wu M, Cheng S. Expression of the chemokine receptor CXCR4 in human hepatocellular carcinoma and its role in portal vein tumor thrombus. J Exp Clin Cancer Res. 2010;29:156. doi: 10.1186/1756-9966-29-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Costantini S, Raucci R, De Vero T, Castello G, Colonna G. Common structural interactions between the receptors CXCR3, CXCR4 and CXCR7 complexed with their natural ligands, CXCL11 and CXCL12, by a modeling approach. Cytokine. 2013;64:316–321. doi: 10.1016/j.cyto.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 78.Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, Kleer CG, Essner JJ, Nasevicius A, Luker GD, Howard MC, Schall TJ. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci U S A. 2007;104:15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iwakiri S, Mino N, Takahashi T, Sonobe M, Nagai S, Okubo K, Wada H, Date H, Miyahara R. Higher expression of chemokine receptor CXCR7 is linked to early and metastatic recurrence in pathological stage I nonsmall cell lung cancer. Cancer. 2009;115:2580–2593. doi: 10.1002/cncr.24281. [DOI] [PubMed] [Google Scholar]
- 80.Gebauer F, Tachezy M, Effenberger K, von Loga K, Zander H, Marx A, Kaifi JT, Sauter G, Izbicki JR, Bockhorn M. Prognostic impact of CXCR4 and CXCR7 expression in pancreatic adenocarcinoma. J Surg Oncol. 2011;104:140–145. doi: 10.1002/jso.21957. [DOI] [PubMed] [Google Scholar]
- 81.Deutsch AJ, Steinbauer E, Hofmann NA, Strunk D, Gerlza T, Beham-Schmid C, Schaider H, Neumeister P. Chemokine receptors in gastric MALT lymphoma: loss of CXCR4 and upregulation of CXCR7 is associated with progression to diffuse large B-cell lymphoma. Mod Pathol. 2013;26:182–194. doi: 10.1038/modpathol.2012.134. [DOI] [PubMed] [Google Scholar]
- 82.Hou KL, Hao MG, Bo JJ, Wang JH. CXCR7 in tumorigenesis and progression. Chin J Cancer. 2010;29:456–459. doi: 10.5732/cjc.009.10404. [DOI] [PubMed] [Google Scholar]
- 83.Hattermann K, Held-Feindt J, Lucius R, Muerkoster SS, Penfold ME, Schall TJ, Mentlein R. The chemokine receptor CXCR7 is highly expressed in human glioma cells and mediates antiapoptotic effects. Cancer Res. 2010;70:3299–3308. doi: 10.1158/0008-5472.CAN-09-3642. [DOI] [PubMed] [Google Scholar]
- 84.Meijer J, Ogink J, Roos E. Effect of the chemokine receptor CXCR7 on proliferation of carcinoma cells in vitro and in vivo. Br J Cancer. 2008;99:1493–1501. doi: 10.1038/sj.bjc.6604727. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Dai X, Tan Y, Cai S, Xiong X, Wang L, Ye Q, Yan X, Ma K, Cai L. The role of CXCR7 on the adhesion, proliferation and angiogenesis of endothelial progenitor cells. J Cell Mol Med. 2011;15:1299–1309. doi: 10.1111/j.1582-4934.2011.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hao M, Zheng J, Hou K, Wang J, Chen X, Lu X, Bo J, Xu C, Shen K. Role of chemokine receptor CXCR7 in bladder cancer progression. Biochem Pharmacol. 2012;84:204–214. doi: 10.1016/j.bcp.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 87.Sanchez-Alcaniz JA, Haege S, Mueller W, Pla R, Mackay F, Schulz S, Lopez-Bendito G, Stumm R, Marin O. Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron. 2011;69:77–90. doi: 10.1016/j.neuron.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 88.Luker KE, Gupta M, Luker GD. Imaging chemokine receptor dimerization with firefly luciferase complementation. FASEB J. 2009;23:823–834. doi: 10.1096/fj.08-116749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Naumann U, Cameroni E, Pruenster M, Mahabaleshwar H, Raz E, Zerwes HG, Rot A, Thelen M. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS One. 2010;5:e9175. doi: 10.1371/journal.pone.0009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Decaillot FM, Kazmi MA, Lin Y, Ray-Saha S, Sakmar TP, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J Biol Chem. 2011;286:32188–32197. doi: 10.1074/jbc.M111.277038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh S, Srivastava SK, Bhardwaj A, Owen LB, Singh AP. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br J Cancer. 2010;103:1671–1679. doi: 10.1038/sj.bjc.6605968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kumar R, Tripathi V, Ahmad M, Nath N, Mir RA, Chauhan SS, Luthra K. CXCR7 mediated Gialpha independent activation of ERK and Akt promotes cell survival and chemotaxis in T cells. Cell Immunol. 2012;272:230–241. doi: 10.1016/j.cellimm.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 93.Xue TC, Chen RX, Han D, Chen J, Xue Q, Gao DM, Sun RX, Tang ZY, Ye SL. Down-regulation of CXCR7 inhibits the growth and lung metastasis of human hepatocellular carcinoma cells with highly metastatic potential. Exp Ther Med. 2012;3:117–123. doi: 10.3892/etm.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zheng K, Li HY, Su XL, Wang XY, Tian T, Li F, Ren GS. Chemokine receptor CXCR7 regulates the invasion, angiogenesis and tumor growth of human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2010;29:31. doi: 10.1186/1756-9966-29-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xue TC, Chen RX, Ren ZG, Zou JH, Tang ZY, Ye SL. Transmembrane receptor CXCR7 increases the risk of extrahepatic metastasis of relatively well-differentiated hepatocellular carcinoma through upregulation of osteopontin. Oncol Rep. 2013;30:105–110. doi: 10.3892/or.2013.2442. [DOI] [PubMed] [Google Scholar]
- 96.Monnier J, Boissan M, L’Helgoualc’h A, Lacombe ML, Turlin B, Zucman-Rossi J, Theret N, Piquet-Pellorce C, Samson M. CXCR7 is up-regulated in human and murine hepatocellular carcinoma and is specifically expressed by endothelial cells. Eur J Cancer. 2012;48:138–148. doi: 10.1016/j.ejca.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 97.Xu L, Duda DG, di Tomaso E, Ancukiewicz M, Chung DC, Lauwers GY, Samuel R, Shellito P, Czito BG, Lin PC, Poleski M, Bentley R, Clark JW, Willett CG, Jain RK. Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1alpha, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res. 2009;69:7905–7910. doi: 10.1158/0008-5472.CAN-09-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu H, Xue W, Ge G, Luo X, Li Y, Xiang H, Ding X, Tian P, Tian X. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1alpha in MSCs. Biochem Biophys Res Commun. 2010;401:509–515. doi: 10.1016/j.bbrc.2010.09.076. [DOI] [PubMed] [Google Scholar]


