Abstract
Brain metastases from primary breast cancer are difficult to treat and associated with poor prognosis. Our understanding of the molecular basis for the development of such cancers is sparse. We hypothesized that the pro-metastatic microRNA-10b (miR-10b) plays a role in breast cancer brain metastasis. The study cohort comprised of twenty patients with breast cancer and brain metastasis as well as ten control patients (age, stage, and follow-up matched) with breast cancer without brain metastasis. All cases were microscopically reviewed to select tumor blocks with >50% tumor cells. RNA was extracted from formalin fixed paraffin embedded (FFPE) tumor tissue blocks. Expression of miR-10b was analyzed using qRT-PCR. The relevance of miR-10b expression was also tested using human breast cancer cell lines. An increased expression of miR-10b was noted in the primary breast cancer specimens of patients who subsequently developed brain metastasis, compared to those who did not. miR-10b also increased the invasive potential of breast cancer cells in vitro. Wilcoxon signed rank test revealed a statistically significant difference between the paired tumors from breast cancers and brain metastasis (p <0.001). Increased expression of miR-10b appears to be associated with breast cancer brain metastasis. These findings are clinically relevant since miR-10b could serve as a prognostic and/or therapeutic target for anti-metastatic therapy. Identifying molecular signatures of primary breast cancers which have a propensity for brain metastasis is critical for designing novel therapies to counter the development of brain metastasis in patients diagnosed with breast cancer.
Keywords: MicroRNAs, breast cancer, brain metastasis, miR-10b, antagomirs
Introduction
Breast cancer remains the leading cause of cancer-related deaths worldwide among women and it is expected that 1 in 8 women in the United States will develop breast cancer in her lifetime [1]. Breast cancer mortality is primarily connected to metastase to distant organs; up to 30% of patients with breast cancer will develop a metastatic brain tumor. Breast cancer is the second leading cause of brain metastases and significantly impacts patient’s quality of life [2]. Patients often present with significant cognitive impairment or focal neurological deficits and the median survival is around 7 months [3]. The incidence of breast cancer brain metastasis is increasing due to several factors including improved neuroimaging techniques, an aging population, and more effective systemic treatment for the primary disease [3-5].
The molecular mechanisms of brain metastasis of breast cancer remain largely unknown. Finding molecular signatures which can identify patients with greater propensity for brain metastasis of breast cancer would represent a significant step toward developing optimal targeted therapies as well as designing novel approaches to prevent, delay and eliminate brain metastasis [6]. In recent years, microRNAs (miRNAs) have emerged as promising prognostic and therapeutic targets for metastatic breast cancers [7-11]. In particular, miR-7 [12] and miR-1258 [13] have been reported to play a role in brain metastasis from primary breast cancer.
The present study was undertaken to determine the role, if any, of miR-10b in breast cancer brain metastasis. miR-10b was previously proposed to determine metastatic potential of breast cancer cells where it was shown to be positively correlated with invasion and metastasis [14] but no clinical results are available. Another study by the same group reported silencing of miR-10b to reduce pulmonary metastases in an experimental metastasis model [15]. More recently, increased miR-10b levels have been associated with bone metastases [16] as well as spread to the lymph nodes [17] in patients with breast cancer. While these studies point to an involvement of miR-10b, in general, in metastasis of breast cancer to distant organs, there is no evidence for the role of this miRNA specifically in brain metastases of breast cancers. To fill this void, we investigated the endogenous expression levels of miR-10b in breast cancer patients-derived primary tumor specimens as a pilot study. We compared the levels of miR-10b in breast cancer patients with and without brain metastasis.
Materials and methods
Breast cancer patients
A retrospective search was done through the computerized database at the Department of Pathology, Karmanos Cancer Institute, Wayne State University, for breast cancer cases with brain metastasis. The period of analysis was December 2011. Only cases with a primary breast cancer diagnosis with available follow-up and specimens (from both breast and brain sites) were included in the study. Additionally, age, stage and follow-up matched breast cancer cases without brain metastasis were also retrieved from the database. The study was approved by the Institutional Review Board.
Tumor selection
In each case, the histopathological glass slides were microscopically reviewed to select the tumor block with preserved, viable tumor tissue comprising over 50% of tumors in the paraffin block, and tumor area was marked. Slides with large areas of necrosis were excluded from the study. Ten sections of 10 μM thickness were cut from each selected block marked for tumor areas and avoiding normal tissues.
Cell lines
Human breast cancer cell lines MDA-MB-231 and MDA-MB-468 were cultured in DMEM and RPMI media with 10% fetal bovine serum and penicillin/streptomycin, respectively. All cells were cultured in 5% CO2-humidified atmosphere at 37°C. The cell lines have been tested and authenticated in core facility (Applied Genomics Technology Center at Wayne State University) by short tandem repeat profiling using the PowerPlex 16 System (Promega, Madison, WI).
RNA extraction and Real-Time RT-PCR
RNA was extracted from the FFPE tumor samples using the RNeasy Mini Kit and RNase-free DNase Set (Qiagen, Valencia, CA) as per the vendor’s protocol. Expression of miR-10b was assessed using quantitative RT-PCR, as described previously [9]. For the cultured cells, total RNA was isolated using mirVana miRNA Isolation Kit (Ambion, Austin, TX). The miRNA levels were determined using miRNA-specific Taqman MGB probes from the Taqman MicroRNA Assay (Applied Biosystems, Foster City, CA). The relative amounts of miRNA were normalized to the endogenous control miRNA RNU48.
Cell invasion assay
Cell invasion assays were performed using 24-well Transwell Permeable Supports with 8 μM pores (Corning, Lowell, MA). Cells (control and pre-miR-10b-transfected) were suspended in serum-free medium and seeded into the Transwell inserts coated with growth factor reduced Matrigel (BD Biosciences, Bedford, MA). Bottom wells were filled with media containing complete media. After 24 h, cells were stained with 4 μg/ml calcein AM (Invitrogen, Carlsbad, CA) in PBS at 37°C for 1 h and detached from inserts by trypsinization and fluorescence of the invaded cells was read in ULTRA Multifunctional Microplate Reader (TECAN, San Jose, CA).
miR-10b transfections
Cells were seeded at 2.5 × 105 cells per well in six-well plates and transfected with pre-miR-10b or miRNA-negative controls (Ambion) at a final concentration of 200 nM using Dharma FECT1 transfection reagent (Dharmacon, Lafayette, CO). After 2 days of transfection, cells were passaged and transfected twice again before using these cells for invasion assays.
Data analysis
The data are presented as the mean values ± SE. Values of p ≤ 0.05 and lower were considered to be statistically significant.
Results
Patients
Over the 15 year study period, 30 breast cancer cases met the study criteria. These included 20 breast cancer cases with brain metastasis and 10 age, stage and follow-up matched breast cancer cases without brain metastasis (Table 1). The median age was 53 years (range: 31 to 83 years). The time of developing brain metastasis from the initial diagnosis of breast cancer ranged from 4 to 67 months. Figure 1 shows the typical radiographic features and gross appearance of a patient who underwent resection of a solitary breast cancer brain metastasis.
Table 1.
Clinico-pathological details of patients in the study cohort
| Serial # | Age at initial diagnosis (years) | Tumor size (maximum dimension in cm) | Pathological diagnosis | Time to development of brain metastasis (months) |
|---|---|---|---|---|
| Breast cancers with brain metastasis | ||||
|
| ||||
| 1 | 65 | 2.0 | Ductal Carcinoma in-situ | 65 |
| 2 | 43 | 0.1 | Invasive Ductal Carcinoma | 20 |
| 3 | 47 | 4.0 | Invasive Ductal Carcinoma | 19 |
| 4 | 39 | 1 | Invasive Ductal Carcinoma | 5 |
| 5 | 31 | 1 | Invasive Ductal Carcinoma | 4 |
| 6 | 52 | 1 | Invasive Ductal Carcinoma | 21 |
| 7 | 55 | 0.6 | Invasive Ductal Carcinoma | 19 |
| 8 | 52 | 1 | Invasive Ductal Carcinoma | 37 |
| 9 | 38 | 8 | Invasive Ductal Carcinoma | 67 |
| 10 | 44 | 4.0 | Invasive Ductal Carcinoma | 11 |
| 11 | 44 | 8.7 | Invasive Ductal Carcinoma | 11 |
| 12 | 36 | 1.4 | Invasive Lobular Carcinoma | 12 |
| 13 | 53 | 3.3 | Ductal Adenocarcinoma in-situ | 29 |
| 14 | 62 | 4.5 | Invasive Ductal Carcinoma | 29 |
| 15 | 60 | 2.1 | Invasive Ductal Carcinoma | 55 |
| 16 | 80 | 1.7 | Invasive Ductal Carcinoma | 21 |
| 17 | 49 | 0.5 | Invasive Ductal Carcinoma | 50 |
| 18 | 44 | 2.3 | Invasive Ductal Carcinoma | 43 |
| 19 | 46 | 0.1 | Ductal Carcinoma In-Situ | 36 |
| 20 | 58 | 5.1 | Invasive Ductal Carcinoma | 5 |
|
| ||||
| Breast cancers without brain metastasis | ||||
|
| ||||
| 1 | 51 | 2.0 | Ductal Carcinoma In-Situ | |
| 2 | 71 | 3.0 | Invasive Ductal Carcinoma | |
| 3 | 70 | 1.7 | Invasive Ductal Carcinoma | |
| 4 | 44 | 3.2 | Invasive Ductal Carcinoma | |
| 5 | 51 | 3.5 | Invasive Ductal Carcinoma | |
| 6 | 63 | 1.6 | Invasive Ductal Carcinoma | |
| 7 | 49 | 0.5 | Invasive Ductal Carcinoma | |
| 8 | 83 | 1.7 | Invasive Ductal Carcinoma | |
| 9 | 74 | 6.5 | Invasive Ductal Carcinoma | |
| 10 | 46 | 1.8 | Invasive Ductal Carcinoma | |
Figure 1.
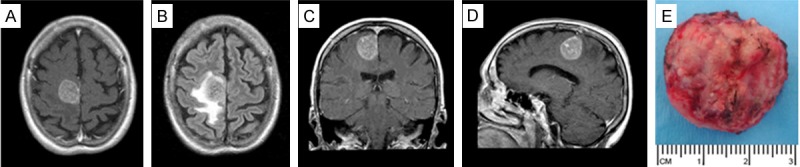
Patient with solitary breast cancer brain metastasis involving the posterior aspect of the right superior frontal gyrus. Post-contrast T1-weighted axial (A), coronal (B), and sagittal (D) images and fluid-attenuated inversion recovery (FLAIR) axial image (C) showing a 2.6 × 3.0 × 3.2 cm mass with surrounding vasogenic edema. (E) Intraoperative photograph following en-bloc microsurgical resection of the tumor.
miR-10b and invasion of breast cancer cells
To study the role of miR-10b in invasion, an in vitro analysis of the effect of miR-10b levels on invasiveness of breast cancer cells was performed. We chose two cell lines, MDA-MB-231 and MDA-MB-468, representing moderate to low endogenous levels of miR-10b. First, we transfected these cells with pre-miR-10b oligonucleotides, and found increased expression of miR-10b, suggesting efficient transfections (Figure 2A). Next, we tested the invasion potential of miR-10b transfected cells, relative to control cells, and found ~18% increase in invasiveness of MDA-MB-231 cells and ~29% increase in invasiveness of MDA-MB-468 that were transfected with pre-miR-10b (Figure 2B). These results clearly suggest that higher levels of miR-10b lead to increased invasive potential of breast cancer cells.
Figure 2.
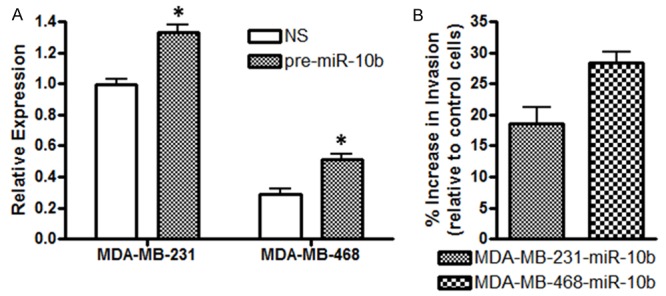
A. MDA-MB-231 and MDA-MB-468 breast cancer cells were transfected with non-specific pre-miRNA controls (NS) or pre-miR-10b, as described under Methods section. Relative expression of miR-10b was determined by quantitative RT-PCR. RNU48 was used as miRNA control against which the data was normalized. B. miR-10b-trasfected MDA-MB-231 (MDA-MB-231-miR-10b) and MDA-MB-468 (MDA-MB-468-miR-10b) cells exhibited significantly increased invasiveness, compared to control (non-specific pre-miR-transfected cells). *p <0.05
miR-10b in patients with brain metastases
Next, we measured the levels of miR-10b in patient samples. As shown in Figure 3, miR-10b levels were significantly higher in pooled samples representing patients with brain metastases, when compared to the pooled samples representing patients without brain metastases. Data was statistically analyzed to determine the clinical relevance of miRNAs in breast cancer brain metastasis using the Wilcoxon signed rank test. It was observed that there was a statistically significant difference in the expression of miR-10b between the paired tumors from breast cancers and brain metastasis (p <0.001). We also analyzed the expression levels of miR-10b in all individual patients’ samples and the results are shown as Figure 4. It is evident that the breast cancer patients with brain metastasis exhibit elevated levels of miR-10b although there are patient-specific differences in the expression of miR-10b.
Figure 3.
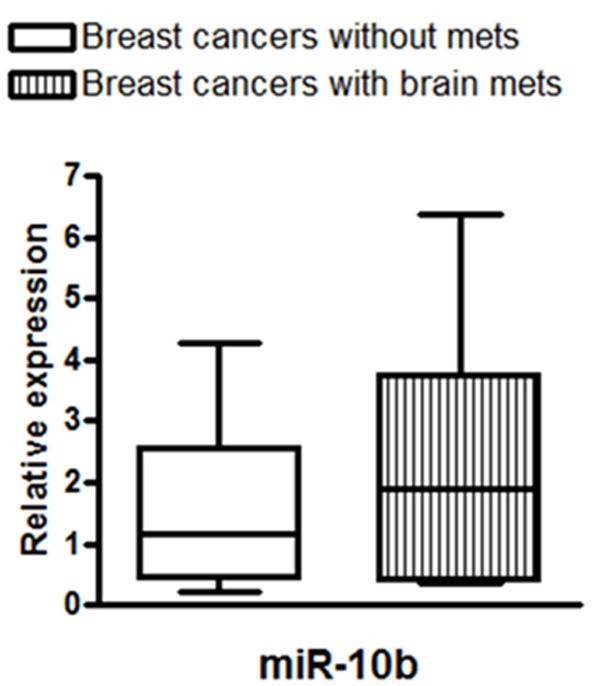
Relative expression of miR-10b in pooled patient samples. The miRNAs extracted from FFPE samples of breast cancer patients with or without breast cancer brain metastasis were pooled as separate groups, and analyzed for expression of miR-10b. RNU48 was used as miRNA control against which the data was normalized.
Figure 4.
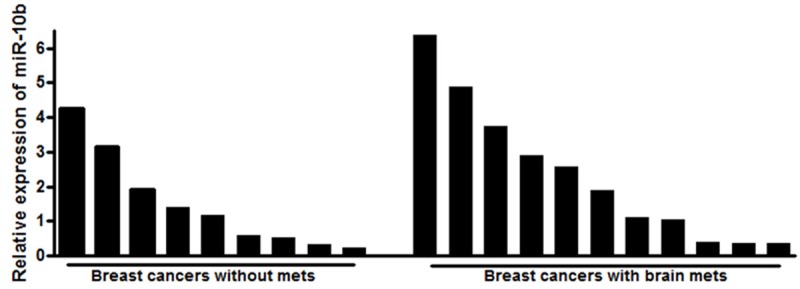
Relative expression of miR-10b in individual patient samples. The miRNAs extracted from FFPE samples of individual breast cancer patients with or without breast cancer brain metastasis were analyzed for expression of miR-10b. RNU48 was used as miRNA control against which the data was normalized.
Discussion
miRNAs, the small non-coding RNAs, have been implicated as key regulators of several physiological and pathological processes, including cancer [10,18]. These tiny molecules (20-25 nucleotides in length) are critical regulators of cancer progression, invasion and metastasis. This is mainly because a single miRNA can affect several downstream genes and signaling pathways. Due to this multimodal downstream signaling effect, miRNAs hold great promise in prognosis as well as treatment of human cancers.The expression of miRNAs in the primary tumor could be silenced using antagomirs (chemically modified anti-miRNA oligonucleotides) which can potentially prevent the development of metastasis. Therefore, development of miRNA-based therapies could serve as personalized medicine that might ultimately improve patients’ quality of life, reduce healthcare costs and improve overall survival.
Breast cancer continues to affect the lives of millions of women worldwide. Over 90% of cancer-related mortality is caused by metastatic disease. Advanced breast cancer patients suffer from metastases to several organs, especially bones. While brain is not the primary site for metastasis, it occurs in 10-16% breast cancer patients with metastatic disease and is linked to one of the worst prognosis. Brain metastasis of breast cancers and the underlying molecular processes are largely unknown. In recent years, the role of miRNAs in the regulation of cancer metastases has been advocated, which prompted us to evaluate the functional role of miR-10b in breast cancer brain metastasis. We shortlisted miR-10b for initial investigation because of the reported association of miR-10b with breast cancer cells’ invasive and metastatic capabilities [19]. It is one of the most significantly up-regulated miRNA in the human breast cancer cells [14]. The expression levels of this miRNA are especially elevatedin metastatic cell lines and tumors, as compared to non-metastatic or poorly-metastatic cell lines and tumors. The ability of miR-10b to influence invasion and metastasis has also been demonstrated in several other solid tumors [20-25]. Translational studies performed on clinical human breast cancer samples have revealed elevated miR-10b levels in extracranial metastatic tumors, compared to metastasis-free tumors [14,26].
In the present study, we observed high expression of miR-10b in breast cancer patients with brain metastasis, relative to those without brain metastasis. These findings are in clear agreement with several other reports referenced above. We also studied the in vitro effects of miR-10b expression on the invasive potential of breast cancer cells. Over-expression of miR-10b significantly increased the invasiveness of breast cancer cells. Combined together, our results established a positive correlation of miR-10b expression with in vitro invasive capacity and the development of brain metastases in patients with breast cancer. It is important to point out that the levels of miR-10b reported by us are those detected in the breast tumors of patients with brain metastases. We trust that the increased expression of miR-10b appears to be associated with metastatic potential of the primary breast cancer; however, once the cancer metastasized to the brain (homing), miR-10b levels could be altered, which needs further in-depth investigation.
miRNAs exert their mechanistic function through regulation of several target genes by either activating them or suppressing them. The reported downstream targets for miR-10b include HOXD10 [14], T-lymphoma invasion and metastasis-1 factor [27], stress-induced cell surface molecule MICB [28], tat-interacting protein 30 [24], etc. The mechanisms of tumor metastasis and invasive functions of miR-10b appear to be tissue specific and depend upon the expression pattern of its target mRNAs and gene targets in a given cell type. It is possible that the targets of miR-10b in breast cancer brain metastasis might be distinct from those in other cancer types or even other breast cancer subtypes. Over-expression of miR-10b leading to cancer metastasis has been correlated with the metastasis-promoting transcription factor Twist which induces epithelial-to-mesenchymal transition (EMT) [14]. miR-10b requires Twist to induce EMT and the resulting cell motility and invasiveness in the breast epithelial cells. E-cadherin, another important determinant of EMT has also been proposed to be a target of miR-10b [29]. Thus, it appears that miR-10b influences metastasis through a complex regulation of multiple factors that determine EMT. We have demonstrated the mechanisticimportance of EMT phenomenon in the metastasis of human cancers including breast and prostate carcinomas [9,30,31]. However, a correlation of EMT phenomenon with miR-10b in our study cohort would be an area of future investigation.
Our findings are clinically very relevant since miR-10b could serve as a potential target for anti-metastatic therapy. The expression of miR-10b could be silenced using miR-10b antagomirs for the prevention of brain metastasis in early stage patients. Clinically, it is important to investigate the role of miR-10b in metastasis of breast cancers since the expression could underlie the basis and selection criteria for future clinical trials for miRNA-based, anti-metastasis agents. Identification of molecular alterations in patients with metastasis may help determine prognosis, assist in risk stratification and would help design novel molecular targeted therapies to prevent, delay and/or eliminate metastasis.
Disclosure of conflict of interest
None of the authors report any conflict of interest or financial disclosure.
References
- 1.De Santis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Jaboin JJ, Ferraro DJ, Dewees TA, Rich KM, Chicoine MR, Dowling JL, Mansur DB, Drzymala RE, Simpson JR, Magnuson WJ, Patel AH, Zoberi I. Survival following gamma knife radiosurgery for brain metastasis from breast cancer. Radiat Oncol. 2013;8:131. doi: 10.1186/1748-717X-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang H, Lee HW, Kim Y, Lee Y, Choi YS, Kim KH, Jin J, Lee J, Joo KM, Nam DH. Radiosensitization of brain metastasis by targeting c-MET. Lab Invest. 2013;93:344–353. doi: 10.1038/labinvest.2012.180. [DOI] [PubMed] [Google Scholar]
- 4.Arslan C, Dizdar O, Altundag K. Systemic treatment in breast-cancer patients with brain metastasis. Expert Opin Pharmacother. 2010;11:1089–1100. doi: 10.1517/14656561003702412. [DOI] [PubMed] [Google Scholar]
- 5.Altundag K, Bondy ML, Mirza NQ, Kau SW, Broglio K, Hortobagyi GN, Rivera E. Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with central nervous system metastasis. Cancer. 2007;110:2640–2647. doi: 10.1002/cncr.23088. [DOI] [PubMed] [Google Scholar]
- 6.Bollig-Fischer A, Michelhaugh SK, Ali-Fehmi R, Mittal S. The molecular genomics of metastatic brain tumours. OA Molecular Oncology. 2013;1:6. doi: 10.13172/2052-9635-1-1-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferracin M, Querzoli P, Calin GA, Negrini M. MicroRNAs: toward the clinic for breast cancer patients. Semin Oncol. 2011;38:764–775. doi: 10.1053/j.seminoncol.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Wang J. MicroRNA-mediated breast cancer metastasis: from primary site to distant organs. Oncogene. 2012;31:2499–2511. doi: 10.1038/onc.2011.444. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad A, Aboukameel A, Kong D, Wang Z, Sethi S, Chen W, Sarkar FH, Raz A. Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Res. 2011;71:3400–3409. doi: 10.1158/0008-5472.CAN-10-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed A, Ali AS, Ali S, Ahmad A, Philip PA, Sarkar FH. MicroRNAs in breast cancer research: progress and promise. In: Ahmad A, editor. Breast Cancer Metastasis and Drug Resistance. New York: Springer; 2012. pp. 399–413. [Google Scholar]
- 11.Tang J, Ahmad A, Sarkar FH. The Role of MicroRNAs in Breast Cancer Migration, Invasion and Metastasis. Int J Mol Sci. 2012;13:13414–13437. doi: 10.3390/ijms131013414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuda H, Xing F, Pandey PR, Sharma S, Watabe M, Pai SK, Mo YY, Iiizumi-Gairani M, Hirota S, Liu Y, Wu K, Pochampally R, Watabe K. miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Res. 2013;73:1434–1444. doi: 10.1158/0008-5472.CAN-12-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Sullivan PS, Goodman JC, Gunaratne PH, Marchetti D. MicroRNA-1258 suppresses breast cancer brain metastasis by targeting heparanase. Cancer Res. 2011;71:645–654. doi: 10.1158/0008-5472.CAN-10-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA- 10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 15.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW, Weinberg RA. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28:341–347. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao FL, Hu GD, Wang XF, Zhang XH, Zhang YK, Yu ZS. Serum overexpression of microRNA-10b in patients with bone metastatic primary breast cancer. J Int Med Res. 2012;40:859–866. doi: 10.1177/147323001204000304. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Cai F, Zhang B, Barekati Z, Zhong XY. The level of circulating miRNA-10b and miRNA-373 in detecting lymph node metastasis of breast cancer: potential biomarkers. Tumour Biol. 2013;34:455–462. doi: 10.1007/s13277-012-0570-5. [DOI] [PubMed] [Google Scholar]
- 18.Seema S, Fazlul HS. Evolving Concept of Cancer Stem Cells: Role of Micro-RNAs and their Implications in Tumor Aggressiveness. J Carcinog Mutagen. 2011;S1:005. [Google Scholar]
- 19.Ma L. Role of miR-10b in breast cancer metastasis. Breast Cancer Res. 2010;12:210. doi: 10.1186/bcr2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li G, Wu Z, Peng Y, Liu X, Lu J, Wang L, Pan Q, He ML, Li XP. MicroRNA-10b induced by Epstein-Barr virus-encoded latent membrane protein-1 promotes the metastasis of human nasopharyngeal carcinoma cells. Cancer Lett. 2010;299:29–36. doi: 10.1016/j.canlet.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Li QJ, Zhou L, Yang F, Wang GX, Zheng H, Wang DS, He Y, Dou KF. MicroRNA-10b promotes migration and invasion through CADM1 in human hepatocellular carcinoma cells. Tumour Biol. 2012;33:1455–1465. doi: 10.1007/s13277-012-0396-1. [DOI] [PubMed] [Google Scholar]
- 22.Wang YY, Ye ZY, Zhao ZS, Li L, Wang YX, Tao HQ, Wang HJ, He XJ. Clinicopathologic significance of miR-10b expression in gastric carcinoma. Hum Pathol. 2013;44:1278–1285. doi: 10.1016/j.humpath.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Jikuzono T, Kawamoto M, Yoshitake H, Kikuchi K, Akasu H, Ishikawa H, Hirokawa M, Miyauchi A, Tsuchiya S, Shimizu K, Takizawa T. The miR-221/222 cluster, miR-10b and miR-92a are highly upregulated in metastatic minimally invasive follicular thyroid carcinoma. Int J Oncol. 2013;42:1858–1868. doi: 10.3892/ijo.2013.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouyang H, Gore J, Deitz S, Korc M. microRNA-10b enhances pancreatic cancer cell invasion by suppressing TIP30 expression and promoting EGF and TGF-beta actions. Oncogene. 2013 doi: 10.1038/onc.2017.190. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun XJ, Liu H, Zhang P, Zhang XD, Jiang ZW, Jiang CC. miR-10b Promotes Migration and Invasion in Nasopharyngeal Carcinoma Cells. Asian Pac J Cancer Prev. 2013;14:5533–5537. doi: 10.7314/apjcp.2013.14.9.5533. [DOI] [PubMed] [Google Scholar]
- 26.Baffa R, Fassan M, Volinia S, O’Hara B, Liu CG, Palazzo JP, Gardiman M, Rugge M, Gomella LG, Croce CM, Rosenberg A. MicroRNA expression profiling of human metastatic cancers identifies cancer gene targets. J Pathol. 2009;219:214–221. doi: 10.1002/path.2586. [DOI] [PubMed] [Google Scholar]
- 27.Moriarty CH, Pursell B, Mercurio AM. miR-10b targets Tiam1: implications for Rac activation and carcinoma migration. J Biol Chem. 2010;285:20541–20546. doi: 10.1074/jbc.M110.121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsukerman P, Stern-Ginossar N, Gur C, Glasner A, Nachmani D, Bauman Y, Yamin R, Vitenshtein A, Stanietsky N, Bar-Mag T, Lankry D, Mandelboim O. MiR-10b downregulates the stress-induced cell surface molecule MICB, a critical ligand for cancer cell recognition by natural killer cells. Cancer Res. 2012;72:5463–5472. doi: 10.1158/0008-5472.CAN-11-2671. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Zhao J, Zhang PY, Zhang Y, Sun SY, Yu SY, Xi QS. MicroRNA-10b targets E-cadherin and modulates breast cancer metastasis. Med Sci Monit. 2012;18:BR299–308. doi: 10.12659/MSM.883262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sethi S, Sarkar FH, Ahmed Q, Bandyopadhyay S, Nahleh ZA, Semaan A, Sakr W, Munkarah A, Ali-Fehmi R. Molecular markers of epithelial-to-mesenchymal transition are associated with tumor aggressiveness in breast carcinoma. Transl Oncol. 2011;4:222–226. doi: 10.1593/tlo.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sethi S, Macoska J, Chen W, Sarkar FH. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res. 2010;3:90–99. [PMC free article] [PubMed] [Google Scholar]


