Abstract
Objective: To evaluate the long-term results of the use of nerve growth factor (NGF)-loaded poly-D, L-lactide-co-glycolide (PLGA) microspheres for improve nerve regeneration with small gap tubulization. Methods: NGF microspheres were prepared by a modified W/O/W emulsion solvent evaporation method. Forty-eight male SD rats were separated into 4 groups and received a chitin conduit to bridge a sciatic nerve injury left a 2 mm gap. Saline (Group A), 20 ng/ml NGF solution (Group B), blank PLGA microspheres (Group C), or 40 ng/ml NGF-loaded microspheres (Group D) was injected in the gap. Each group had two study endpoints, 3 months subgroup and 1 year subgroup. Results: The myelinated fiber count at 2 mm distal to the conduit at 1 year was slightly less than at 3 months in all groups (P>0.05). However, the maturity of the myelinated nerves at 1 year was obviously improved. The fiber count, myelin sheath thickness, axon area of NGF microsphere group were significantly higher than the saline groups at 3 months (P=0.05, P<0.05, and P<0.05, respectively). The SFI was significantly improved in NGF microspheres group compared to the saline group and NGF solution group at 1 year (P<0.05, and P<0.05, respectively). Conclusions: The results demonstrated that the release of NGF microspheres in small gap tubulization benefit on peripheral nerve injury facilitated nerve regeneration histologically, especially for the maturity of early regenerative nerve fibers and also had an effect on functional recovery in the long term.
Keywords: Nerve growth factor (NGF), microsphere, nerve regeneration, small gap, tubulization
Introduction
Repair of peripheral nerve injuries is an intractable problem in the clinic. Epineurial neurorrhaphy has long been performed as a traditional repair method, but functional recovery is typically unsatisfactory. In our previous study, we observed a favorable effect of small gap tubulization over the epineurial neurorrhaphy approach [1-4]. However, functional recovery to the pre-injury level remains limited. Thus, enhancing the effect of the conduits has become a focus in the field of peripheral nerve regeneration.
The use of NGF for improving nerve regeneration has been well documented, but the observation time of prior study was rarely beyond 3 months [5-12]. The purpose of this study was to evaluate the long term results of the use of nerve growth factor for improve nerve regeneration with small gap tubulization. To solve the fast degradation and metabolism of NGF under physiological conditions, NGF loaded poly-D, L-lactide-co-glycolide (PLGA) microspheres were used to release NGF slowly and protect the bioactivity [7,13-15].
Materials and methods
Ethics statement
The study was approved by the Research Ethics Committee at Peking University People’s Hospital and met international biomedical ethics guidelines. The Biostatistics Department of Peking University Health Science Center supervised the acquisition of data.
Preparation of NGF-loaded microspheres
NGF-loaded microspheres (Figure 1) were prepared by a modified W/O/W emulsion solvent evaporation method as published previously [7,14-16]. Briefly, a 0.1-ml internal aqueous phase containing 5 μg of 2.5 S NGF (purified from male mouse submaxillary glands, Promega, USA) and 10 mg of ovalbumin (OVA, Sigma, USA), which was used as a protective additive, were emulsified in 2 ml of methylene chloride containing 50 mg of PLGA (50:50, eta=0.25 dL/g, DURECT, USA). The emulsion was sonicated for 30 seconds on an ice bath to create the primary emulsion. Then, under continuously stirring at 1500 rpm, the primary emulsion was added dropwise into 30 ml of a 3% (w/v) external aqueous solution of polyvinyl alcohol (PVA, Sigma, USA) to obtain a multiple emulsion. After 5 minutes, the resulting emulsion was poured into 300 ml of 0.3% w/v PVA and stirred with a magnetic stirrer for 3 hours at room temperature to evaporate the dichloromethane. Finally, the resulting suspension was centrifuged, and the collected microspheres were washed with deionized water for three times and freeze-dried to obtain a free-flowing powder. The mean size of the microspheres is 8.1±5.7 μm. The protein-loading (w/w) and encapsulation (%) efficiency achieved for NGF were 0.0024%, 13.85%, respectively. The initial burst release of NGF from microspheres was 18.77%, and 64.34% of the encapsulated NGF was released over a three-week period, as determined by an enzyme-linked immunosorbent assay (ELISA, Promega, USA).
Figure 1.
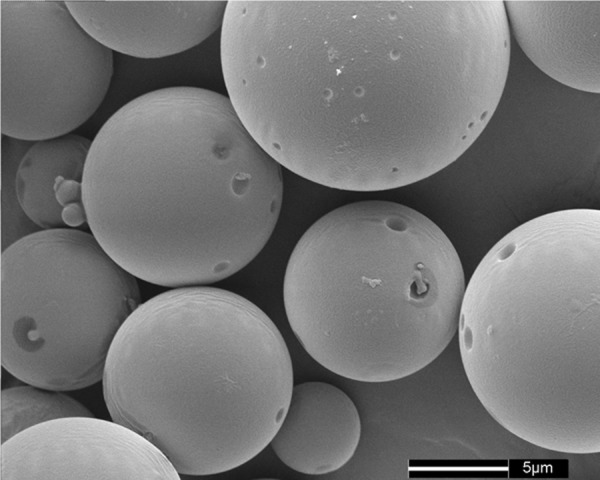
Scanning electron micrographs of nerve growth factor-loaded microspheres. The microspheres were fabricated by a modified W/O/W emulsion solvent evaporation method. Poly-D, L-lactide-co-glycolide was the encapsulation material, and ovalbumin was used as a protective additive.
Animals model and experimental design
A total of 48 male Sprague-Dawley rats, weighting 200-250 g, were used for this study. The animals were housed in transparent cages in a SPF facility and given a rodent diet and water ad libitum. Surgical procedures for the experimental animals were performed under a binocular surgical microscope using a microsurgical technique. SD rats were anesthetized with 2% pentobarbitone (0.2-0.3 ml/100 g) by intraperitoneal injection. After anesthesia, the right lower limbs were shaved and sterilized. The sciatic nerve and its crotch were exposed and freed from surrounding tissue. Sciatic nerve injury models were constructed by transecting the right sciatic nerve at 7 mm above the sciatic nerve fork (Figure 2). The animals received a chitin conduit [17,18] consisted of polysaccharide shell that demonstrated satisfactory biocompatibility and degradation characteristics to bridge a sciatic nerve injury left a 2 mm gap. Conduit size: tube length 4 mm, thickness 0.5 mm, inner diameter 1.5 mm. Both the distal and proximal ends of the nerve were inserted approximately 1 mm into the conduit; the distance between the nerve stumps was 2 mm [3,4]. All operations were performed by the same investigator.
Figure 2.
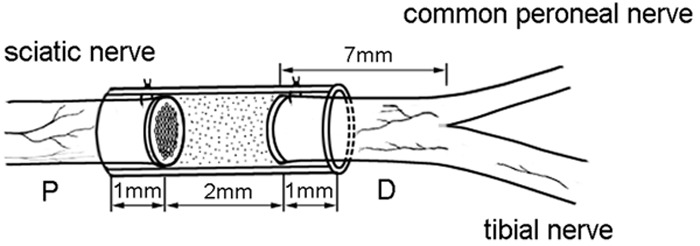
Animal model: chitin conduit bridging SD rat sciatic nerve left a 2 mm gap.
The animals were divided into 4 groups randomly (12 animals in each group). The animals were injected into the conduit cavity between the nerve stumps via microinjection with saline (Group A), 20 ng/ml NGF solution (Group B), saline containing blank PLGA microspheres (Group C), or 40 ng/ml NGF-loaded microspheres (Group D). Each group had two study endpoints, 3 months follow-up and 1 year follow-up.
Sciatic function index
At the two endpoints, the SFI was used to assess functional recovery. The index print length factor (PLF), toe spread factor (TSF), and intermediary toe spread factor (ITF) were recorded for the normal control foot (NPL, NTS, NIT) and the corresponding experimental foot (EPL, ETS, EIT) for each rat. The SFI was calculated as follows [19]: SFI=-38.3[(EPL-NPL)/NPL]+109.5[(ETS-NTS)/NTS]+13.3[(EIT-NIT)/NIT]-8.8. An SFI of nearly 0 is normal, whereas an SFI of -100 indicates total impairment of the sciatic nerve.
Electrophysiological examination
Electrophysiological assessment was performed at the corresponding endpoints. After anesthesia, the right repaired sciatic nerves were exposed and carefully isolated from the surrounding tissue. Stimulating bipolar electrodes were placed at the proximal and distal repair site in each group. The recording electrode was placed on the nerve shaft distally, whereas the ground electrode was placed subcutaneously. Rectangular pulses (duration 0.1 ms, 0.9 mA) were used to stimulate the repaired nerves. The compound muscle action potential (CMAP) was recorded, and the motor nerve conduction velocity (MNCV) was obtained simultaneously by dividing the distance between the two stimulating sites by the difference in the onset latency using an Oxford electrophysiologic instrument [20].
Weight of triceps surae muscles
After electrophysiological assessment, the animals were sacrificed by using an intra-arterial overdose of sodium pentobarbital. Triceps surae muscles from the operated and non-operated sides were dissected and detached from the bone at their origin and terminal point, and weighed immediately using an electronic scale. The conserved muscle-mass ratio was recorded for each animal by dividing the muscle mass on the experimental side by the muscle mass on the control side.
Histologic assessment
The sciatic nerve at 2 mm distal to the conduit was removed from each rat, and stained by osmium tetroxide. The dissected tissues were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 12 h at 4°C. The fixed nerves were rinsed with running water and then rinsed twice in phosphate buffer. The samples distal to the conduit were post-fixed in 1% osmium tetroxide for 12 h. After dehydration and embedding, the specimens were cut into 5-μm cross-sections. The images were obtained under a Leica microscope (Leica DM 4000B, Germany) at different magnifications. Five images from different parts of each section were analyzed, and data from five nerve sections were quantified. Finally, the average thickness of the myelin, total number of myelinated axons, and average area of the axons were evaluated by using a Leica Q550CW analytical system [17].
Statistical analysis
The results were expressed as the mean and standard deviation. Differences among groups were evaluated using t-tests. Analysis was performed by using the SPSS version 16.0 software (SPSS Inc., USA). P<0.05 was defined as statistically significant.
Results
General observations
The conduits in all 48 rats were well-tolerated. The wounds healed without infection, and no trophic ulceration appeared. Toe spread was observed in all animals, but autophagy was observed in the right feet of five animals (2 in saline group, 1 in NGF solution group, 1 in blank microspheres group and 1 in NGF microspheres group).
Sciatic functional index
The SFI of 1 year subgroups was significantly improved relative to that of 3 months in all groups (P<0.01). There was no statistically significant difference among the 3 months subgroups. However, the SFI was significantly improved in NGF microspheres group (-26.64±2.24) compared to the saline group (-30.96±3.20, P<0.05) and NGF solution group (-31.97±3.61, P<0.05) at 1 year (Figure 3A).
Figure 3.
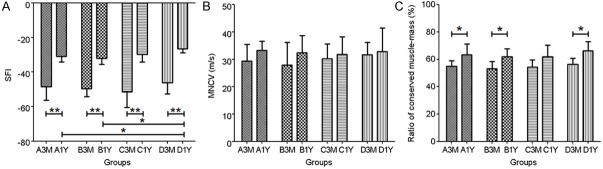
The functional parameters of the regenerated sciatic nerves at 3 months and 1 year. The sciatic functional index (A), the motor nerve conduction velocity (B) and the ratio of conserved muscle-mass (C). The conserved muscle mass ratio was recorded for each animal by dividing the muscle mass on the experimental side by the muscle mass on the control side. The bars represent the mean±SD. n=6, *P<0.05, **P<0.01.
Motor nerve conduction velocity
The results of MNCV at 1 year rats were higher than at 3 months ones in all groups (Figure 3B). The MNCV of NGF microsphere group (31.71±4.55 m/s) was a bit higher than the other groups (29.41±6.14 m/s, P>0.05; 27.90±8.35 m/s, P>0.05; 30.24±5.40 m/s, P>0.05; respectively) at 3 months. There was also no statistically difference at 1 year. The MNCV of saline group (33.24±3.44 m/s) was a bit higher than the other groups at 1 year (32.42±6.24 m/s, P>0.05; 31.76±6.40 m/s, P>0.05; 32.80±8.71 m/s, P>0.05; respectively).
Weight of triceps surae muscles
The ratio of conserved muscle mass at 1 year was significantly improved compared to that of 3 months (P<0.05), except in blank microspheres group (P>0.05). The ratio of conserved muscle mass in NGF microsphere group (56.41±4.40%) was higher than the others (54.81±4.15%, P>0.05; 53.05±5.47%, P>0.05; 54.47±5.23%, P>0.05; respectively) at 3 months (Figure 3C). The 1 year subgroup of NGF microsphere group (66.40±6.69%) also showed improvement relative to the other groups (63.31±7.81%, P>0.05; 61.91±5.97%, P>0.05; 61.94±8.55%, P>0.05; respectively).
Histomorphometry
The results of the morphometric analysis of each group are summarized in Tables 1 and 2. The myelinated fiber count at 2 mm distal to the conduit at 1 year was less than at 3 months in all groups (P>0.05). The myelin sheath thickness of myelinated nerves of the saline group, NGF solution group, blank microspheres group and NGF microspheres group at 1 year (1.33±0.21 μm, 1.27±0.12 μm, 1.38±0.12 μm, 1.41±0.13 μm, respectively) was obviously improved relative to that at 3 months (0.99±0.12 μm, P<0.01; 1.04±0.08 μm, P<0.01; 0.93±0.18 μm, P<0.01; 1.18±0.13 μm, P<0.05; respectively). The axon area of myelinated nerves in all groups at 1 year (12.15±0.55 μm2, 12.53±1.43 μm2, 12.47±0.92 μm2, respectively) was obviously improved than at 3 months (10.34±1.13 μm2, P<0.01; 10.29±0.62 μm2, P<0.01; 10.61±0.74 μm2, P<0.01; respectively), except in NGF microspheres group (13.02±1.17 μm2 vs. 11.98±1.4 μm2, p>0.05). The fiber count, myelin sheath thickness, axon area of NGF microsphere group were significantly higher than the saline group (P=0.05, P<0.05, and P<0.05, respectively) at 3 months. However, the morphological parameters of NGF microsphere group were slightly higher than the saline group (P>0.05, P>0.05, and P>0.05, respectively) at 1 year. Either in 3 months or 1 year subgroup, the results of NGF solution and blank microsphere groups had no statistically difference compared to the saline group (P>0.05) (Figure 4).
Table 1.
Morphometric analysis of the regenerated nerves at 2 mm distal to the conduits of groups (Mean±SD)
| Group | N | Fiber Count | Myelin Sheath Thickness (μm) | Axon Area (μm2) |
|---|---|---|---|---|
| A3M | 6 | 7159±1498 | 0.99±0.12 | 10.34±1.13 |
| A1Y | 6 | 6408±1166 | 1.33±0.21 | 12.15±0.55 |
| B3M | 6 | 7383±1136 | 1.04±0.08 | 10.29±0.62 |
| B1Y | 6 | 6186±1477 | 1.27±0.12 | 12.53±1.43 |
| C3M | 6 | 6820±1411 | 0.93±0.18 | 10.61±0.74 |
| C1Y | 6 | 6334±1511 | 1.38±0.12 | 12.47±0.92 |
| D3M | 6 | 9248±1746 | 1.18±0.13 | 11.98±1.4 |
| D1Y | 6 | 7899±1783 | 1.41±0.13 | 13.02±1.17 |
Table 2.
Morphometric analysis of the regenerated nerves at 2 mm distal to the conduits of groups (T-test for multiple comparisons in groups)
| Group | Fiber Count | Myelin Sheath Thickness | Axon Area |
|---|---|---|---|
| A3M vs. A1Y | NS | p<0.01 | p<0.01 |
| B3M vs. B1Y | NS | p<0.01 | p<0.01 |
| C3M vs. C1Y | NS | p<0.01 | p<0.01 |
| D3M vs. D1Y | NS | p<0.05 | NS |
| A3M vs. B3M | NS | NS | NS |
| A3M vs. C3M | NS | NS | NS |
| A3M vs. D3M | p=0.05 | p<0.05 | p<0.05 |
| A1Y vs. B1Y | NS | NS | NS |
| A1Y vs. C1Y | NS | NS | NS |
| A1Y vs. D1Y | NS | NS | NS |
Figure 4.

Light microscopy images of transverse sections of the sciatic nerve 2 mm distal to the conduit (stained by osmium tetroxide, ×400): Chitin conduit small gap tubulization at 3 months. (A: Saline; B: NGF solution; C: Saline containing blank microspheres; D: Saline containing NGF-loaded microspheres) and 1 year (E: Saline; F: NGF solution; G: Saline containing blank microspheres; H: Saline containing NGF-loaded microspheres).
Discussion
Small gap tubulization is based on the nerve-selective regeneration theory put forward by Cajal [21]. We tried different gap between the two ruptured stumps. After series of experiments, we found that the 2 mm was the most suitable gap in biological degradable conduit in rats 1, 3, 4 and confirmed that the repair effect was advantageous relative to the traditional epineurial neurorrhaphy [1-4,17-20]. Here, we only used this method to repair nerve injury, but not nerve defect. Based on these results, we were trying to load exogenous NGF in the conduit for further improvement of regeneration effect.
NGF is produced by the target organs of sensory and sympathetic nerves and retrogradely transported in the neurons [22]. When the peripheral nerve is injured, the injury serves as a signal to stimulate NGF synthesis [23] and promote retrograde transport to neurons [24]. NGF internalization and transport is mediated by high-affinity tyrosin kinase receptors (trkA) [25]. The receptors are express on the growth cones surface, and the expression of these receptors in neurons may be increased by NGF [26]. Sustained release bioactive NGF can increase the expression of high-affinity tyrosin kinase receptors and accelerate the retrograde transport of NGF to neurons to further support the survival of embryonic dorsal ganglion neurons [27].
NGF solution is not stable under physiological conditions, so direct administration of NGF into a conduit is difficult to maintain the bioactivity and the effective concentration during nerve regeneration. Polymeric microspheres were able to solve this problem. In several studies [14,28,29], NGF-loaded microspheres were prepared by different encapsulation material and combined with the nerve conduits. The results showed that NGF microspheres could improve nerve regeneration to a certain extent. But the object of those studies was a nerve injury, to explore the use of nerve conduit instead of autologous nerve graft, and they only obtained short-term results. The effect of small gap tubulization for peripheral nerve injury combined with NGF sustained release is not clear, especially in long-term effect. For sufficiently long postop observation endpoints for monitoring the progression of the morphological and functional predictors of recovery, we set two endpoints, the three months and 1 year respectively.
The dose of NGF has also been studied. Conti et al. [30] demonstrated that maximal neurite outgrowth was obtained at a concentration of 20 ng/ml. An in vitro study showed an effective concentration of NGF and found that overdosing would inhibit outgrowth [30]. So the concentration of NGF solution was set at 20 ng/ml. In vitro experiments confirmed that only approximately half of the encapsulated NGF was released from the NGF-loaded microspheres. Therefore, the NGF microspheres were set to provide a NGF concentration of 40 ng/ml.
In this study, the base material of the microspheres was PLGA, which is a biodegradable material that has been widely used in polymeric microspheres, and the ratio of lactic to glycolic acid can be modulated to alter the release characteristics [7]. In the study, the groups either with 3 months or 1 year follow-up, the result of the blank microsphere group was not worse than the saline group, demonstrating that the PLGA microsphere delivery system did not inhibit or obstruct the outgrowth of nerve fibers.
Myelin sheath thickness and myelinated nerve axon area provides a measure of the maturity of the regenerating nerve fibers. At 3 months, the maturity of the myelinated nerves with NGF sustained release was much better than the absence of exogenous NGF or direct administration of exogenous NGF. This result indicated that sustained release NGF was important to promote maturation of fibers in the distal nerve. This phenomenon may be due to that the released NGF binds to the p75 low-affinity NGF receptor on Schwann cells and up-regulates its expression [31,32], which promotes the migration of Schwann cells into the gap between the injured stumps32. This helps the formation of Büngner bands in basal lamina tubes and stimulates myelinization.
Maturity of myelinated nerve fibers at 1 year was superior to 3 months. The results were in agreement with those of Fox IK et al. [33] Obviously, with the extension of the time after operation, Myelin sheath thickness and axon area would be improved. In addition, those wrong matching nerve fibers which could not reach the target organ, often relatively immature and eventually pruned [2,18,20]. Consequently, the average value of the parameters of the effective nerve fibers retained even better. The myelin sheath thickness and axon area did not show significant differences among the four groups at 1 year, in contrast to outstanding performance of the NGF microspheres group at 3 months. In other words, even if there was no sustained release of NGF, nerve fibers could reach a certain maturity, but require more time.
It is interested to note that the total amount of myelinated nerve fibers number at 1 year postop was lower than the fibers number at 3 months. The results of this study were in agreement with those of Muratori L [34]. This observation could be interpreted as the result of multiple axonal sprouts during the early regeneration phases followed by a late pruning of the branches that were wrong matching and could not reach their target organ [34,35].
Because early myelinated nerve fibers are more mature, the SFI of NGF sustained release group was better than the other groups. With the extension of the time after operation, the better maturity of the nerve fibers establishing functional connections distally made SFI apparent recovery, especially in NGF sustained release group which indicated that sustained release NGF improved functional recovery in the long term. This phenomenon may be the result of the early NGF sustained release and play the role on regeneration. But earlier or later, the NGF microspheres had little or no influence on MNCV or the ratio of conserved muscle mass. It was not difficult to associate to previous studies found that NGF has little or no influence on motoneurons and their neurite outgrowth [31,36], that was the limitations of this study. Future studies could focus on microspheres associated with other various neurotrophic factors for nerve regeneration and the evaluation of relevant neurons and target organs.
In conclusion, the release of NGF microspheres in small gap tubulization benefit on peripheral nerve injury facilitated nerve regeneration histologically, especially for the maturity of early regenerative myelinated nerve fibers and also had an effect on functional recovery in the long term.
Acknowledgements
The study was supported by Chinese National Ministry of Science and Technology 973 Project Planning (No. 2014CB542200); The ministry of education innovation team (IRT1201); the National Natural Science Fund (No. 31271284, 31171150, 81171146, 30971526, 31100860, 31040043), and the Educational Ministry New Century Excellent Talents Support Project (No. BMU20110270). The authors thank Peiheng He for English language revision.
Disclosure of conflict of interest
None to disclose.
References
- 1.Zhang P, Han N, Wang T, Xue F, Kou Y, Wang Y, Yin X, Lu L, Tian G, Gong X, Chen S, Dang Y, Peng J, Jiang B. Biodegradable conduit small gap tubulization for peripheral nerve mutilation: a substitute for traditional epineurial neurorrhaphy. Int J Med Sci. 2013;10:171–175. doi: 10.7150/ijms.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kou Y, Peng J, Wu Z, Yin X, Zhang P, Zhang Y, Weng X, Qiu G, Jiang B. Small gap sleeve bridging can improve the accuracy of peripheral nerve selective regeneration. Artif Cells Nanomed Biotechnol. 2013;41:402–407. doi: 10.3109/21691401.2012.762007. [DOI] [PubMed] [Google Scholar]
- 3.Jiang B, Zhang P, Zhang D, Fu Z, Yin X, Zhang H. Study on small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:55–74. doi: 10.1080/10731190500430149. [DOI] [PubMed] [Google Scholar]
- 4.Jiang B, Zhang P, Jiang B. Advances in small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 2010;38:1–4. doi: 10.3109/10731190903495652. [DOI] [PubMed] [Google Scholar]
- 5.Barmpitsioti A, Konofaos P, Ignatiadis I, Papalois A, Zoubos AB, Soucacos PN. Nerve growth factor combined with an epineural conduit for bridging a short nerve gap (10 mm). A study in rabbits. Microsurgery. 2011;31:545–550. doi: 10.1002/micr.20925. [DOI] [PubMed] [Google Scholar]
- 6.He C, Chen Z, Chen Z. Enhancement of motor nerve regeneration by nerve growth factor. Microsurgery. 1992;13:151–154. doi: 10.1002/micr.1920130310. [DOI] [PubMed] [Google Scholar]
- 7.de Boer R, Knight AM, Borntraeger A, Hebert-Blouin MN, Spinner RJ, Malessy MJ, Yaszemski MJ, Windebank AJ. Rat sciatic nerve repair with a poly-lactic-co-glycolic acid scaffold and nerve growth factor releasing microspheres. Microsurgery. 2011;31:293–302. doi: 10.1002/micr.20869. [DOI] [PubMed] [Google Scholar]
- 8.Rich KM, Luszczynski JR, Osborne PA, Johnson EM Jr. Nerve growth factor protects adult sensory neurons from cell death and atrophy caused by nerve injury. J Neurocytol. 1987;16:261–268. doi: 10.1007/BF01795309. [DOI] [PubMed] [Google Scholar]
- 9.Rich KM, Alexander TD, Pryor JC, Hollowell JP. Nerve growth factor enhances regeneration through silicone chambers. Exp Neurol. 1989;105:162–170. doi: 10.1016/0014-4886(89)90115-5. [DOI] [PubMed] [Google Scholar]
- 10.Skaper SD. Nerve growth factor: a neurokine orchestrating neuroimmune-endocrine functions. Mol Neurobiol. 2001;24:183–199. doi: 10.1385/MN:24:1-3:183. [DOI] [PubMed] [Google Scholar]
- 11.Hontanilla B, Auba C, Gorria O. Nerve regeneration through nerve autografts after local administration of brain-derived neurotrophic factor with osmotic pumps. Neurosurgery. 2007;61:1268–1274. doi: 10.1227/01.neu.0000306106.70421.ed. discussion 1274-1265. [DOI] [PubMed] [Google Scholar]
- 12.Gravvanis AI, Tsoutsos DA, Tagaris GA, Papalois AE, Patralexis CG, Iconomou TG, Panayotou PN, Ioannovich JD. Beneficial effect of nerve growth factor-7S on peripheral nerve regeneration through inside-out vein grafts: an experimental study. Microsurgery. 2004;24:408–415. doi: 10.1002/micr.20055. [DOI] [PubMed] [Google Scholar]
- 13.Tsai CC, Lu MC, Chen YS, Wu CH, Lin CC. Locally administered nerve growth factor suppresses ginsenoside Rb1-enhanced peripheral nerve regeneration. Am J Chin Med. 2003;31:665–673. doi: 10.1142/S0192415X03001387. [DOI] [PubMed] [Google Scholar]
- 14.Xu X, Yee WC, Hwang PY, Yu H, Wan AC, Gao S, Boon KL, Mao HQ, Leong KW, Wang S. Peripheral nerve regeneration with sustained release of poly(phosphoester) microencapsulated nerve growth factor within nerve guide conduits. Biomaterials. 2003;24:2405–2412. doi: 10.1016/s0142-9612(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Yu H, Gao S, Ma HQ, Leong KW, Wang S. Polyphosphoester microspheres for sustained release of biologically active nerve growth factor. Biomaterials. 2002;23:3765–3772. doi: 10.1016/s0142-9612(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 16.Karagoz H, Ulkur E, Kerimoglu O, Alarcin E, Sahin C, Akakin D, Dortunc B. Vascular endothelial growth factor-loaded poly(lactic-co-glycolic acid) microspheres-induced lateral axonal sprouting into the vein graft bridging two healthy nerves: nerve graft prefabrication using controlled release system. Microsurgery. 2012;32:635–641. doi: 10.1002/micr.22016. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Zhang P, Wang Y, Yu K, Kou Y, Jiang B. Early spatiotemporal progress of myelinated nerve fiber regenerating through biological chitin conduit after injury. Artif Cells Blood Substit Immobil Biotechnol. 2010;38:103–108. doi: 10.3109/10731191003634836. [DOI] [PubMed] [Google Scholar]
- 18.Jianping P, Xiaofeng Y, Yanhua W, Zhenwei W, Yuhui K, Chungui X, Peixun Z, Baoguo J. Different multiple regeneration capacities of motor and sensory axons in peripheral nerve. Artif Cells Blood Substit Immobil Biotechnol. 2012;40:309–316. doi: 10.3109/10731199.2012.657205. [DOI] [PubMed] [Google Scholar]
- 19.Zhang P, He X, Zhao F, Zhang D, Fu Z, Jiang B. Bridging small-gap peripheral nerve defects using biodegradable chitin conduits with cultured schwann and bone marrow stromal cells in rats. J Reconstr Microsurg. 2005;21:565–571. doi: 10.1055/s-2005-922437. [DOI] [PubMed] [Google Scholar]
- 20.Jiang BG, Yin XF, Zhang DY, Fu ZG, Zhang HB. Maximum number of collaterals developed by one axon during peripheral nerve regeneration and the influence of that number on reinnervation effects. Eur Neurol. 2007;58:12–20. doi: 10.1159/000102161. [DOI] [PubMed] [Google Scholar]
- 21.Cajal SRY. Degeneration and Regeneration of the Nervous System. 1928 [Google Scholar]
- 22.Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 23.Rende M, Hagg T, Manthorpe M, Varon S. Nerve growth factor receptor immunoreactivity in neurons of the normal adult rat spinal cord and its modulation after peripheral nerve lesions. J Comp Neurol. 1992;319:285–298. doi: 10.1002/cne.903190208. [DOI] [PubMed] [Google Scholar]
- 24.DiStefano PS, Curtis R. Receptor mediated retrograde axonal transport of neurotrophic factors is increased after peripheral nerve injury. Prog Brain Res. 1994;103:35–42. doi: 10.1016/s0079-6123(08)61124-3. [DOI] [PubMed] [Google Scholar]
- 25.Frade JM, Barde YA. Nerve growth factor: two receptors, multiple functions. Bioessays. 1998;20:137–145. doi: 10.1002/(SICI)1521-1878(199802)20:2<137::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 26.Lindsay RM, Shooter EM, Radeke MJ, Misko TP, Dechant G, Thoenen H, Lindholm D. Nerve Growth Factor Regulates Expression of the Nerve Growth Factor Receptor Gene in Adult Sensory Neurons. Eur J Neurosci. 1990;2:389–396. doi: 10.1111/j.1460-9568.1990.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 27.Deckwerth TL, Johnson EM Jr. Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J Cell Biol. 1993;123:1207–1222. doi: 10.1083/jcb.123.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H, Peng J, Guo Q, Zhang L, Li Z, Zhao B, Sui X, Wang Y, Xu W, Lu S. Improvement of peripheral nerve regeneration in acellular nerve grafts with local release of nerve growth factor. Microsurgery. 2009;29:330–336. doi: 10.1002/micr.20635. [DOI] [PubMed] [Google Scholar]
- 29.Sun W, Sun C, Zhao H, Lin H, Han Q, Wang J, Ma H, Chen B, Xiao Z, Dai J. Improvement of sciatic nerve regeneration using laminin-binding human NGF-beta. PLoS One. 2009;4:e6180. doi: 10.1371/journal.pone.0006180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conti AM, Fischer SJ, Windebank AJ. Inhibition of axonal growth from sensory neurons by excess nerve growth factor. Ann Neurol. 1997;42:838–846. doi: 10.1002/ana.410420604. [DOI] [PubMed] [Google Scholar]
- 31.Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 32.Anton ES, Weskamp G, Reichardt LF, Matthew WD. Nerve growth factor and its low-affinity receptor promote Schwann cell migration. Proc Natl Acad Sci U S A. 1994;91:2795–2799. doi: 10.1073/pnas.91.7.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox IK, Brenner MJ, Johnson PJ, Hunter DA, Mackinnon SE. Axonal regeneration and motor neuron survival after microsurgical nerve reconstruction. Microsurgery. 2012;32:552–562. doi: 10.1002/micr.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muratori L, Ronchi G, Raimondo S, Giacobini-Robecchi MG, Fornaro M, Geuna S. Can regenerated nerve fibers return to normal size? A long-term post-traumatic study of the rat median nerve crush injury model. Microsurgery. 2012;32:383–387. doi: 10.1002/micr.21969. [DOI] [PubMed] [Google Scholar]
- 35.Kawamura DH, Johnson PJ, Moore AM, Magill CK, Hunter DA, Ray WZ, Tung TH, Mackinnon SE. Matching of motor-sensory modality in the rodent femoral nerve model shows no enhanced effect on peripheral nerve regeneration. Exp Neurol. 2010;223:496–504. doi: 10.1016/j.expneurol.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braun S, Croizat B, Lagrange MC, Warter JM, Poindron P. Neurotrophins increase motoneurons’ ability to innervate skeletal muscle fibers in rat spinal cord--human muscle cocultures. J Neurol Sci. 1996;136:17–23. doi: 10.1016/0022-510x(95)00315-s. [DOI] [PubMed] [Google Scholar]


