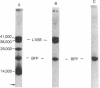
Abstract
A blue fluorescence protein has been isolated and purified from extracts of the luminous bacterium Photobacterium phosphoreum. It is a single polypeptide of molecular weight 22,000 with absorption maxima at 274 and 418 nm. It is efficiently fluorescent (ϕF 0.45), with a fully corrected spectral maximum (476 nm) and distribution identical to the in vivo bioluminescence from this same type of bacterium. At low concentration this fluorescence shifts towards the red and becomes identical to the in vitro bioluminescence emission. This spectral shift apparently results from a change in the protein pulled by dissociation of the chromophore (Kd [unk] 10-7 M). If the blue fluorescence protein is included in the in vitro bioluminescence reaction with reduced FMN, oxygen, aldehyde, and luciferase (P. phosphoreum), the bioluminescence spectrum is shifted towards the blue from its maximum at 490 nm to one at 476 nm, where it is again identical in all respects to the in vivo bioluminescence spectrum. This is accompanied by an increase in the initial light intensity by an order of magnitude at saturating levels of blue fluorescence protein, and the specific light yield of the luciferase is increased 4-fold. It is suggested that the blue fluorescence protein acts as a sensitizer of the bacterial bioluminescence reaction.
Keywords: emission spectra, blue fluorescence protein
Full text
PDF
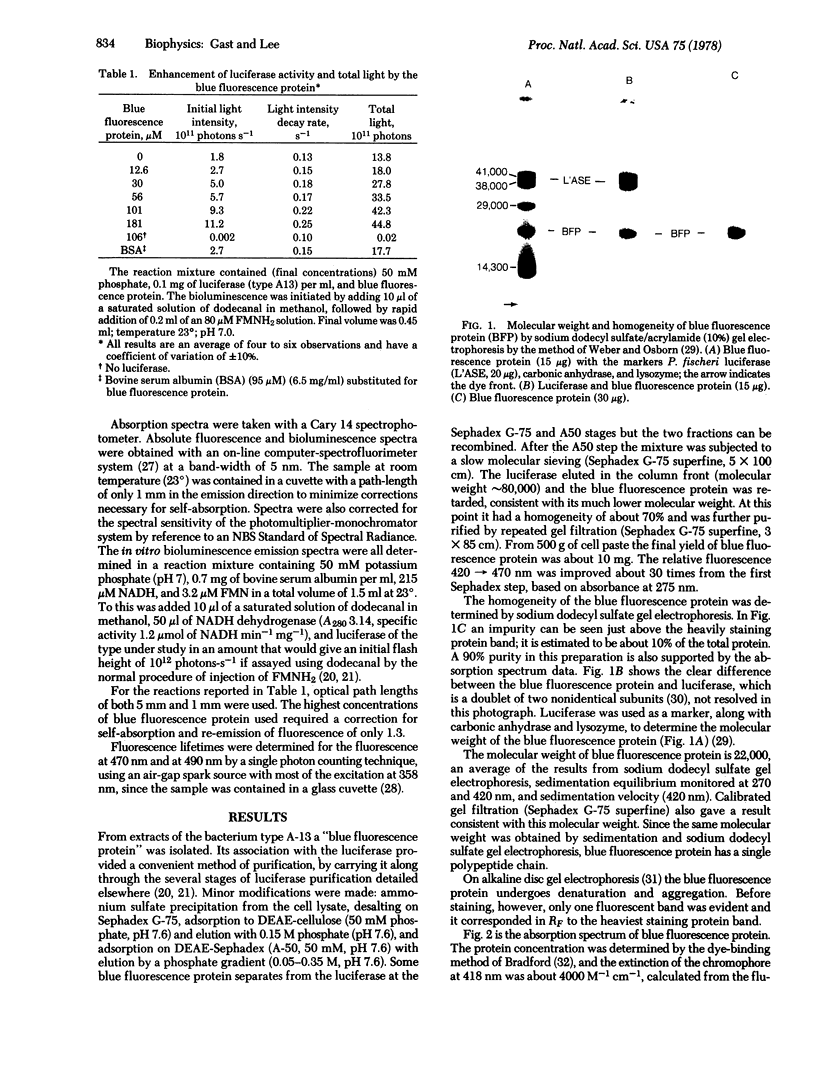
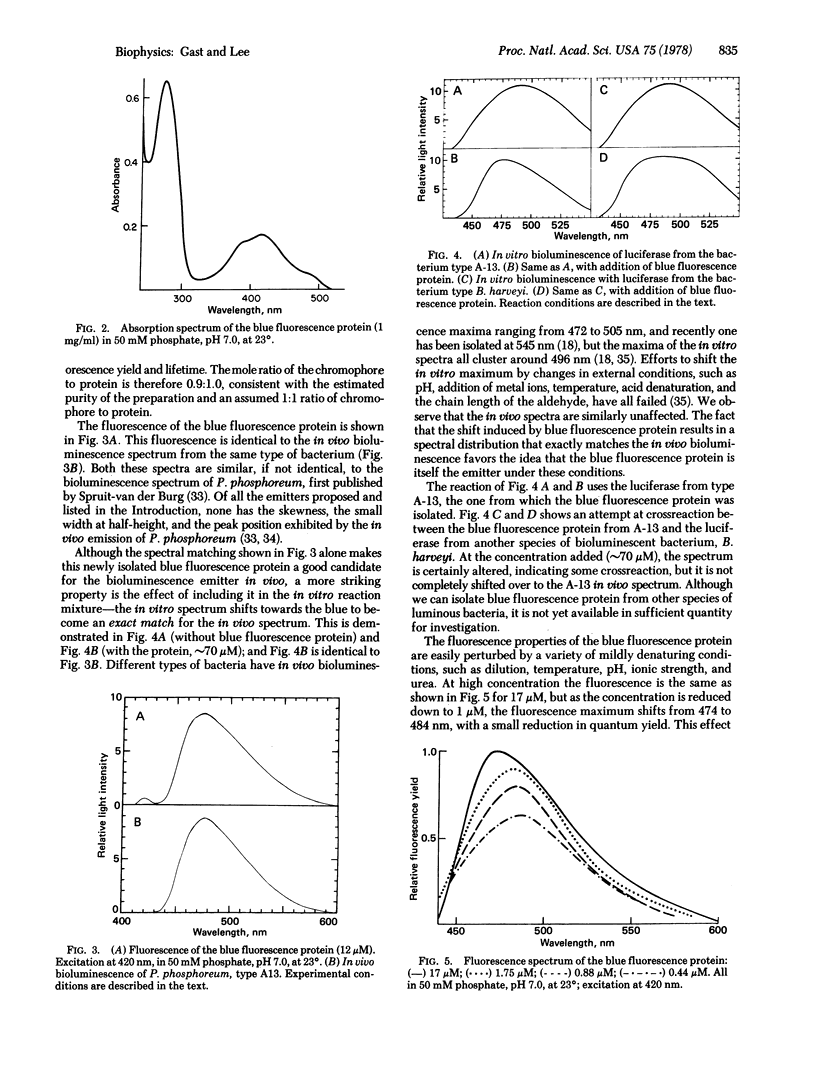


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balny C., Hastings J. W. Fluorescence and bioluminescence of bacterial luciferase intermediates. Biochemistry. 1975 Oct 21;14(21):4719–4723. doi: 10.1021/bi00692a024. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brewer J. M., Ashworth R. B. Disc electrophoresis. J Chem Educ. 1969 Jan;46(1):41–45. doi: 10.1021/ed046p41. [DOI] [PubMed] [Google Scholar]
- CORMIER M. J., ECKROADE C. B. Studies on the bioluminescence of Renilla reniformis. III. Some biochemical comparisons of the system to other Renilla species and determinations of the spectral energy distributions. Biochim Biophys Acta. 1962 Oct 22;64:340–344. doi: 10.1016/0006-3002(62)90742-4. [DOI] [PubMed] [Google Scholar]
- CORMIER M. J., TOTTER J. R. BIOLUMINESCENCE. Annu Rev Biochem. 1964;33:431–458. doi: 10.1146/annurev.bi.33.070164.002243. [DOI] [PubMed] [Google Scholar]
- Cormier M. J., Eley M., Abe S., Nakano Y. On the requirement and mode of action of long-chain aldehydes during bacterial bioluminescence. Photochem Photobiol. 1969 Apr;9(4):351–358. doi: 10.1111/j.1751-1097.1969.tb07299.x. [DOI] [PubMed] [Google Scholar]
- Cormier M. J., Lee J., Wampler J. E. Bioluminescence: recent advances. Annu Rev Biochem. 1975;44:255–272. doi: 10.1146/annurev.bi.44.070175.001351. [DOI] [PubMed] [Google Scholar]
- Eley M., Lee J., Lhoste J. M., Lee C. Y., Cormier M. J., Hemmerich P. Bacterial bioluminescence. Comparisons of bioluminescence emission spectra, the fluorescence of luciferase reaction mixtures, and the fluorescence of flavin cations. Biochemistry. 1970 Jul 7;9(14):2902–2908. doi: 10.1021/bi00816a023. [DOI] [PubMed] [Google Scholar]
- HASTINGS J. W., RILEY W. H., MASSA J. THE PURIFICATION PROPERTIES, AND CHEMILUMINESCENT QUANTUM YIELD OF BACTERIAL LUCIFERASE. J Biol Chem. 1965 Mar;240:1473–1481. [PubMed] [Google Scholar]
- Hastings J. W., Weber K., Friedland J., Eberhard A., Mitchell G. W., Gunsalus A. Structurally distinct bacterial luciferases. Biochemistry. 1969 Dec;8(12):4681–4689. doi: 10.1021/bi00840a004. [DOI] [PubMed] [Google Scholar]
- KUWABARA S., CORMIER M. J., DURE L. S., KREISS P., PFUDERER P. CRYSTALLINE BACTERIAL LUCIFERASE FROM PHOTOBERTERIUM FISCHERI. Proc Natl Acad Sci U S A. 1965 Apr;53:822–828. doi: 10.1073/pnas.53.4.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Bacterial bioluminescence. Quantum yields and stoichiometry of the reactants reduced flavin mononucleotide, dodecanal, and oxygen, and of a product hydrogen peroxide. Biochemistry. 1972 Aug 29;11(18):3350–3359. doi: 10.1021/bi00768a007. [DOI] [PubMed] [Google Scholar]
- Lee J., Murphy C. L. Bacterial bioluminescence: equilibrium association measurements, quantum yields, reaction kinetics, and overall reaction scheme. Biochemistry. 1975 May 20;14(10):2259–2268. doi: 10.1021/bi00681a034. [DOI] [PubMed] [Google Scholar]
- Lee J., Seliger H. H. Absolute spectral sensitivity of phototubes and the application to the measurement of the absolute quantum yields of chemiluminescence and bioluminescence. Photochem Photobiol. 1965 Dec;4(6):1015–1048. doi: 10.1111/j.1751-1097.1965.tb09293.x. [DOI] [PubMed] [Google Scholar]
- McCapra F., Hysert D. W. Bacterial bioluminescence-identification of fatty acid as product, its quantum yield and a suggested mechanism. Biochem Biophys Res Commun. 1973 May 1;52(1):298–304. doi: 10.1016/0006-291x(73)90987-x. [DOI] [PubMed] [Google Scholar]
- Mitchell G., Hastings J. W. The effect of flavin isomers and analogues upon the color of bacterial bioluminescence. J Biol Chem. 1969 May 25;244(10):2572–2576. [PubMed] [Google Scholar]
- Murphy C. L., Faini G. J., Lee J. Separation of the apoprotein and reconstitution of the holoprotein from the long-lived intermediate in bacterial bioluminescence. Biochem Biophys Res Commun. 1974 May 7;58(1):119–125. doi: 10.1016/0006-291x(74)90899-7. [DOI] [PubMed] [Google Scholar]
- Ohtsuka H., Rudie N. G., Wampler J. E. Structural identification and synthesis of luciferin from the bioluminescent earthworm, Diplocardia longa. Biochemistry. 1976 Mar 9;15(5):1001–1004. doi: 10.1021/bi00650a009. [DOI] [PubMed] [Google Scholar]
- Ruby E. G., Nealson K. H. A luminous bacterium that emits yellow light. Science. 1977 Apr 22;196(4288):432–434. doi: 10.1126/science.850787. [DOI] [PubMed] [Google Scholar]
- SPRUIT-van der BURG A. Emission spectra of luminous bacteria. Biochim Biophys Acta. 1950 Apr;5(2):175–178. doi: 10.1016/0006-3002(50)90161-2. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H., Kohama Y. Reactions involved in bioluminescence systems of limpet (Latia neritoides) and luminous bacteria. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2086–2089. doi: 10.1073/pnas.69.8.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERPSTRA W. Evidence for the presence of an unknown factor, active in the light reaction, in preparations of Photobacterium phosphoreum. Biochim Biophys Acta. 1962 Jul 16;60:580–590. doi: 10.1016/0006-3002(62)90876-4. [DOI] [PubMed] [Google Scholar]
- TERPSTRA W. INVESTIGATIONS ON THE IDENTITY OF THE LIGHT-EMITTING MOLECULE IN PHOTOBACTERIUM PHOSPHOREUM. Biochim Biophys Acta. 1963 Nov 29;75:355–364. doi: 10.1016/0006-3002(63)90623-1. [DOI] [PubMed] [Google Scholar]
- Tu S. C., Hastings J. W. Photoexcited bacterial luminescence. Spectral properties and mechanistic implication of a reduced flavine-like prosthetic group associated with photoexcitable luciferase. Biochemistry. 1975 May 6;14(9):1975–1980. doi: 10.1021/bi00680a027. [DOI] [PubMed] [Google Scholar]
- WEBER G. Rotational Brownian motion and polarization of the fluorescence of solutions. Adv Protein Chem. 1953;8:415–459. doi: 10.1016/s0065-3233(08)60096-0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]