Abstract
Transcription factors play an important role in the pathophysiology of many neurological disorders, including stroke. In the past three decades, an increasing number of transcription factors and their related gene signaling networks have been identified, and have become a research focus in the stroke field. Krüppel-like factors (KLFs) are members of the zinc finger family of transcription factors with diverse regulatory functions in cell growth, differentiation, proliferation, migration, apoptosis, metabolism, and inflammation. KLFs are also abundantly expressed in the brain where they serve as critical regulators of neuronal development and regeneration to maintain normal brain function. Dysregulation of KLFs has been linked to various neurological disorders. Recently, there is emerging evidence that suggests KLFs have an important role in the pathogenesis of stroke and provide endogenous vaso- or neuro- protection in the brain’s response to ischemic stimuli. In this review, we summarize the basic knowledge and advancement of these transcriptional mediators in the central nervous system, highlighting the novel roles of KLFs in stroke.
Keywords: Krüpple-like factors, transcription factors, gene regulation, central nervous system, neurological disorders, cerebrovascular diseases, ischemic stroke
1. Introduction
Currently, thrombolytic therapy within a narrow time window is the only acute therapeutic intervention for ischemic stroke and development of effective therapies is urgently required (Stapf and Mohr, 2002; Schellinger et al., 2004; Schellinger and Warach, 2004). Extensive research has demonstrated that stroke triggers abnormal cerebral gene expression to activate complex cellular biochemical events that eventually lead to apoptotic and necrotic neuronal death in the ischemic regions (Love, 2003; Yuan, 2009). However, the delicate mechanisms of stroke-induced neuronal death and neurological dysfunction are not completely understood.
Transcription factors are proteins that control which genes are turned on or off in the genome, which are essential for the regulation of gene function in a transcriptional manner. During acute stroke, the brain responds to ischemic insults very quickly by activating many transcription factors in various neural cells to mediate diverse cell signaling cascades and pathogenic processes including but not limited to inflammation, oxidative stress, neuronal survival, neuroprotection, neurogenesis, and angiogenesis (Chang and Huang, 2006; Scholzke and Schwaninger, 2007; Yi et al., 2007). It is becoming increasingly evident that suppression or activation of the stroke-responsive cerebral transcription factors may ameliorate stroke damage and improve clinical outcomes (Yi et al., 2007). For example, inhibition of NF-κB effectively prevents ischemic stroke-induced brain damage by regulating inflammation and cell survival (Schneider et al., 1999; Nurmi et al., 2004), whereas activation of peroxisome proliferator-activated receptor γ (PPARγ) (Sundararajan et al., 2005; Zhao et al., 2005; Luo et al., 2006; Tureyen et al., 2007; Zhao et al., 2009) was shown to reduce post-ischemic inflammation and neuronal damage in rodent experimental stroke models. Obviously, a better understanding of the functional importance of novel transcription factors in the regulation of post-ischemic pathophysiological events as well as their activation or inactivation through small molecules or drugs may eventually lead us to discover novel pharmaceutical targets for the treatment of ischemic stroke.
Krüppel-like factors (KLFs) have been discovered as a novel family of transcription factors that trans-activate or trans-suppress gene expression in various organisms. KLFs are highly distributed in various neural cells in the brain and have been implicated in a variety of human neurological diseases (Moore et al., 2009; Moore et al., 2011). Within this review, we will focus on recent advances describing the role and mechanisms of KLFs in neurological disorders, with a focus on ischemic stroke. We will also discuss the potential clinical applications of these transcriptional molecules as novel therapeutic targets for ischemic stroke.
2. Biology and function of Krüpple-like factors
Krüppel-like factors (KLFs) are members of the zinc finger family of transcription factors that have been shown as important regulators in cellular growth and differentiation (Black et al., 2001; Kaczynski et al., 2003; Pearson et al., 2008; McConnell and Yang, 2010). They are named based on their sequence homology with the DNA-binding domain of the Drosophila melanogaster Krüppel protein, an embryonic pattern regulator. Drosophila embryos deficient in Kruppel die as a result of abnormal thoracic and abdominal segmentation and thus appear “crippled” (Nusslein-Volhard and Wieschaus, 1980; Jackle et al., 1985; Preiss et al., 1985). The first KLF gene, erythroid Krüppel-like factor (KLF1/EKLF), was initially discovered from the red blood cell lineage in 1993 (Miller and Bieker, 1993). To date, a total 17 members of the mammalian KLF family have been identified in a chronological order of discovery, and referred to as KLF1 to KLF17 (Black et al., 2001; Kaczynski et al., 2003; Pearson et al., 2008; McConnell and Yang, 2010).
Similar to other classical transcription factors, KLF proteins feature a DNA-binding domain at the carboxyl termini, which consist of three highly conserved Cys2/His2 zinc-finger motifs. These fingers allow KLFs to bind to CACCC elements and GC-rich regions of DNA in the promoters of their downstream target genes. Although conservation within their DNA binding domains means that KLFs can interact with the same DNA sequences, it should be noted that different family members have preferences for different sequences (Black et al., 2001; Kaczynski et al., 2003; Pearson et al., 2008; McConnell and Yang, 2010). It is worth noting that the functional domains of the KLFs are at the amino-terminal regions, which are not conserved and much more variable among the family members. These regions contain transcriptional activation and/or repression domains to permit KLF interaction with cofactor complexes (i.e. co-activators or co-repressors), thus allowing KLFs to activate and/or repress downstream gene expression, and regulate epigenetic modification and diverse functional phenotypes. According to the diversity of the amino termini in structural and functional features, KLF transcription factors have been classified into several subgroups and differ in their transcriptional activities (Black et al., 2001; Kaczynski et al., 2003; Pearson et al., 2008; McConnell and Yang, 2010). In addition, KLF family members have nuclear localization signal sequences, which are located within or adjacent to the zinc finger motifs (Shields and Yang, 1997; Song et al., 2002).
The biological functions of KLFs are mainly identified by gene knockout studies. Through manipulating the expression of a large number of downstream target genes, KLF transcription factors regulate cellular processes in many distinct cell types in virtually all cellular functions, including cell proliferation, differentiation, migration, metabolism, apoptosis, cell growth/death, and reprogramming somatic cells into induced pluripotent stem (iPS) cells (Black et al., 2001; Kaczynski et al., 2003; Pearson et al., 2008; McConnell and Yang, 2010). Of note, KLFs regulate gene expression profiles and cellular function in a cell/tissue- and/or promoter-specific manner (Kaczynski et al., 2003). Generally, individual members of the KLF family can function as transcriptional activators or repressors depending on which promoter they bind to and the recruited coregulators which they interact with (Kaczynski et al., 2003).
KLFs are involved in many physiological and pathological processes such as hematopoiesis, embryogenesis/organ development, cardiac remodeling, blood vessel and lung development, neurite outgrowth, angiogenesis, neurogenesis, metabolic regulation, bone metabolism, gluconeogenesis, adipogenesis, cardiac fibrosis, cardiac hypertrophy, neoplasia, thrombosis, restenosis, atherosclerosis, carcinogenesis, tumor suppression, tumor progression, shear stress, and inflammation (Black et al., 2001; Kaczynski et al., 2003; Atkins and Jain, 2007; Haldar et al., 2007; Pearson et al., 2008; McConnell and Yang, 2010; Moore et al., 2011; Nayak et al., 2011; Wu and Wang, 2013). Thus, KLFs are critical regulators of physiological systems that include the hematological, skeletal, digestive, central nervous, cardiovascular, respiratory, and immune systems. Numerous studies have also been published that demonstrate their contributions to various human diseases including cancers, diabetes, obesity, cerebrovascular diseases, neurodegenerative diseases, cardiovascular diseases, and inflammatory conditions (reviewed previously (McConnell and Yang, 2010)). As research on KLFs is in progress, additional KLF functions and associations with human diseases are likely to be discovered soon. Here, we are only reviewing and discussing studies that implicate those KLFs as important players in neurobiology and neurological disorders.
3. Role of KLFs in nervous system development and regeneration
Generally, KLFs are expressed ubiquitously throughout other body tissues, and single cells normally contain multiple KLFs that may act as a functional network (Turner and Crossley, 1999; Black et al., 2001; Eaton et al., 2008). In the nervous system, there are few systematic examinations of KLF expression but a number of KLFs have been found in various neural cell types including neurons, astrocytes, microglia, oligodendrocytes, and cerebral vascular cells (Table 1, and Figure 1). Of note, retinal ganglion cells (RGCs) express almost all 17 KLFs (Moore et al., 2009), and the expression patterns and abundance of these KLFs change during neural developmental to correlate with their different effects on neurite growth (Moore and Goldberg, 2011). In addition to RGCs, it is unclear if other brain cells express all 17 KLF transcription factors as well. Also, cell-type specific functions of KLFs have been demonstrated in other systems, as evidenced by the findings that KLF4 inhibits proliferation and promotes differentiation in the skin cells of the epidermis (Segre et al., 1999) but has opposite effects in the induction of pluripotency in somatic cells (Takahashi and Yamanaka, 2006; Zhao and Daley, 2008). However, the cell-type specific expression patterns and related functional differences of KLFs have not been determined in the nervous system (Kaczynski et al., 2003; Moore et al., 2011).
Table 1.
Biological function of KLFs in the nervous system
| KLFs | Function in the nervous system | GeneTargets | Cell Types | References |
|---|---|---|---|---|
| KLF4 |
|
JAK-SAT pathway Cytokines/iNOS/Cox-2 TRH Unknown Unknown Unknown |
Neurons, neural stem cells Microglia Hypothalamic neurons Astrocytes Cortical and retinal ganglion cells Postnatal cortical neurons |
Qin S 2012; Qin S 2011 Kaushik DK 2012, 2010 Pérex-Monter C 2011 Qin S 2011 Moore DL 2009 Kim J 2011 |
| KLF6 | Promotes neurite outgrowth/axon regeneration | Unknown | Cortical and retinal ganglion cells | Moore DL 2009 |
| KLF7 |
|
TH, Dat Unknown Map2, NGF, TrkA |
Dopaminergic neurons Neuroectodermal/mesodermal cells Neurons, retinal ganglion cells, PC12 cells |
Caiazzo M 2011; Laub F 2006 Caiazzo M 2010 Moore DL 2009; Caiazzo M 2010; Blackmore MG 2012 |
| KLF9 |
|
Unknown Unknown Unknown Unknown |
Purkinje, cortical and retinal ganglion cells Hippocampal neurons Oligodendrocyte precursor cells HT-22 cell line, neurons, tadpole brain |
Moore DL 2009; Avci HX 2012 Scobie KN 2009 Dugan JC 2012 Bagamasbad P 2012; Scobie KN 2009; Bonett RM 2009 |
| KLF11 | Transactivates neurotransmitter gene to regulate neuronal differentiation | Dopamine D2 receptor | PC12 cells, PANC1 cells, DRG neurons | Seo S 2012 |
| KLF15 | Promotes neuronal differentiation | Unknown | Neural stem cells | Ohtsuka T 2011 |
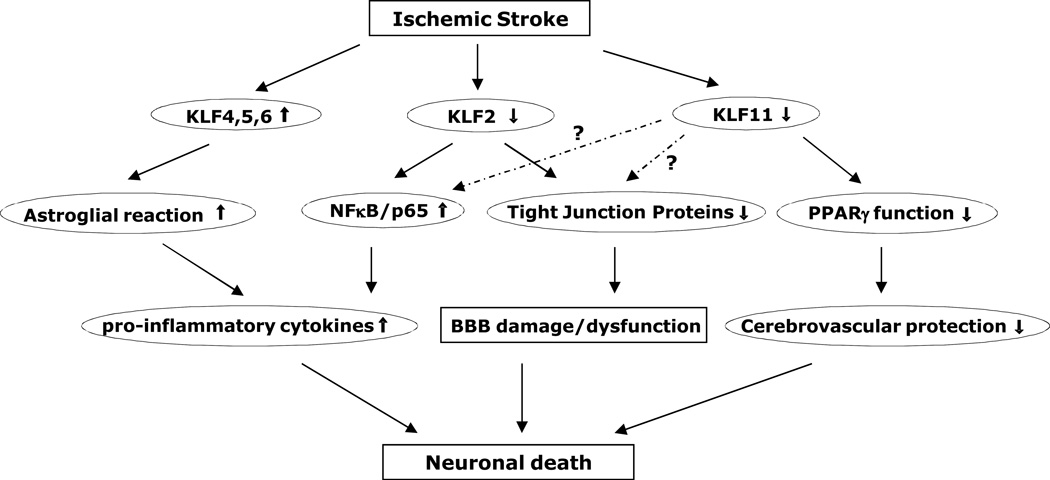
Figure 1.
Schematic representation of stroke-associated KLFs and signaling cascades. KLF4 is induced in reactive astrocytes in rat hippocampus after global cerebral ischemia, and may transcriptionally regulate astroglial reaction and cerebral inflammation following ischemic injury (Park et al., 2013). KLF2 level is decreased in blood outgrowth endothelial cells (BOECs) derived from stroke children with sickle cell anemia (SCA) than in non-SCA BOECs, thus changes the ratio between proinflammatory NF-κB/p65 to anti-inflammatory factor KLF2 and leads to a proinflammatory phenotype within the cerebral vasculature (Enenstein et al., 2010). In mouse brain vasculature, ischemic stimuli induce KLF2 (Shi et al., 2013) or KLF11 dysfunction (Yin et al., 2013), featuring attenuated KLF2 transactivation of tight junction protein occludin or reduced KLF11-mediated PPARγ vascular function, thereby resulting in a series of cerebral vascular pathologies, such as BBB damage/dysfunction, disturbed cerebral vascular protection, and final neuronal cell death.
It has been shown that KLFs can regulate and contribute to a variety of normal neurobiological activities, including neurite outgrowth and axon regeneration (Moore et al., 2009; Caiazzo et al., 2010; Avci et al., 2012; Blackmore et al., 2012), neuronal differentiation and migration (Caiazzo et al., 2010; Ohtsuka et al., 2011; Qin et al., 2011; Qin and Zhang, 2012; Seo et al., 2012), neuronal development and plasticity (Laub et al., 2006; Bonett et al., 2009; Scobie et al., 2009; Caiazzo et al., 2011; Bagamasbad et al., 2012), astroglial activation (Qin et al., 2011), microglial activation (Kaushik et al., 2010; Kaushik et al., 2012), oligodendrocyte differentiation and myelin regeneration (Duncan et al., 2012), neurogenesis (Scobie et al., 2009; Perez-Monter et al., 2011), and cell reprogramming (Kim et al., 2011) (Table 1). Interestingly, more attention has been paid to the physiological functions of KLF 4, 6, 7, and 9 in the nervous system.
KLF7 has strictly-restricted high levels of expression in many embryonic brain regions such as the olfactory bulb (OB), cerebral cortex, hippocampus, mesencephalon, and subventricular zone and reflects an essential role in neural development. It has been reported that KLF7 is required for OB dopaminergic neuron development. Deletion of Klf7 in mice results in a significant reduction of tyrosine hydroxylase and dopamine receptors in OB neurons (Laub et al., 2006; Caiazzo et al., 2011). Genetic deficiency of Klf7 also leads to deficits in neurite outgrowth and axonal regeneration which eventually cause neonatal lethality in mice (Moore et al., 2009; Caiazzo et al., 2010; Blackmore et al., 2012). KLF7 is also needed for the differentiation of neuroectodermal and mesodermal cells by regulating several neuronal markers, including the microtubule-associated protein 2, nerve growth factor tyrosine kinase receptor A, and brain lipid-binding protein/fatty acid-binding protein 7 in the PC12 cell line or neural stem cells (Caiazzo et al., 2010).
KLF4 is another KLF transcription factor that exhibits a broad range of regulatory functions in the nervous system. Outside the nervous system, Klf4 is one of four key pluripotency genes responsible for reprogramming fibroblasts or other somatic cells into induced pluripotent stem (iPS) cells (Takahashi et al., 2007). Recently, Moore and others (Moore et al., 2009) examined the role of 17 KLF members in the regulation of neuronal regenerative capacity, and revealed that KLF4 is the most effective suppressor of neurite outgrowth and intrinsic axon regeneration in primary retinal ganglion cells and cortical neuron cultures. In contrast, KLF6 and -7 enhance neurite growth and axon regeneration. Interestingly, these effects are not related to cell survival status since all tested KLF family members had no significant effect on neuronal death by in vitro gain- or loss-of-KLF function (Moore et al., 2009). KLF4 expression was also shown to be upregulated by NMDA or AMPA treatment in cortical neuron cultures (Zhu et al., 2009), which led to increased caspase-3 levels but not effecting neuronal survival. Moreover, earlier findings also demonstrated that KLF4 is involved in microglial activation and promotes release of proinflammatory cytokines, tumor necrosis factor-alpha, macrophage chemoattractant protein-1, and interleukin-6 as well as proinflammatory enzymes, inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide-treated microglial cells (Kaushik et al., 2010; Kaushik et al., 2012). In addition, dysregulation of KLF4 during mouse brain development results in elevated GFAP expression and hypertrophy of astrocytes, leading to hydrocephalus (Qin et al., 2011). Most recently, Qin et al. also reported that KLF4 regulates neurogenesis and radial migration of neurons in the developing cerebral cortex by regulating the JAK-STAT signaling pathway (Qin and Zhang, 2012).
KLF9 has also appeared as an important transcription factor regulating the neural regenerative potential in the CNS. Similar to KLF4, KLF9 overexpression significantly decreases neurite growth and axon regeneration in primary retinal ganglion cell and cortical neuron cultures (Moore et al., 2009). During hippocampal development and neurogenesis in adults, KLF9 is required for late-phase maturation of dentate granule neurons. A lack of KLF9 in mice results in delayed maturation of hippocampal neurons, impaired neuronal differentiation, reduced neurogenesis, and synaptic plasticity, as well as abnormal learning and memory ability (Bonett et al., 2009; Scobie et al., 2009; Bagamasbad et al., 2012). KLF9 was also recently found to promote oligodendrocyte differentiation in cultured oligodendrocyte precursor cells (OPCs). Loss-of-KLF9 function in mice does not affect normal myelin development but significantly impairs the ability of the mature CNS to regenerate lost myelin in both the cortex and corpus callosum after cuprizone-induced demyelinated lesions (Dugas et al., 2012).
4. Role of KLFs in neurologic disorders: focus on stroke
Although the biological functions and molecular mechanisms of most KLF family proteins are largely unknown, a growing body of evidence links several KLFs to multiple human disease statuses in the nervous system (Table 2), such as carcinoma in the CNS (Jeng and Hsu, 2003; Kimmelman et al., 2004; Camacho-Vanegas et al., 2007; Nakahara et al., 2010; Ying et al., 2011; Schnell et al., 2012), Alzheimer’s disease (Wu et al., 2013), depression and alcoholism (Duncan et al., 2012), chronic stress (Grunewald et al., 2012), hydrocephalus (Qin et al., 2011), epilepsy (Jeong et al., 2011), NMDA neurotoxicity (Zhu et al., 2009), cerebral malaria (Srivastava et al., 2010), neuroinflammation (Kaushik et al., 2010; Kaushik et al., 2012; Kaushik et al., 2013), and schizophrenia (Yanagi et al., 2008). This list (Table 2) is expected to expand quickly as more studies are performed. Of importance, the functional mechanisms and potentially clinically-relevant intervention through targeting KLFs in these neurological disorders need to be uncovered.
Table 2.
KLFs involved in neurological disorders
| KLFs | Neulogical Diseases | Function | Targets | References |
|---|---|---|---|---|
| KLF2 | Alzheimer's Disease | Decreased expression in the AD mice brains. Overexpression of KLF2 rescues cerebrovascular dysfunction in HBMECs. | Occludin | Wu C 2013 |
| KLF4 | Neuroinflammation Medulloblastoma Hydrocephalus Cerebral malaria NMDA neurotocxicity |
|
Cytokines, iNOS, Cox-2 Unknown Astrocyte hypertrophy Unknown Caspase-3, cyclin D1 |
Kaushik DK 2013, 2012, 2010 Nakahara Y 2010 Qin S 2011 Srivastava K 2010 Zhu S 2009 |
| KLF5 | Schizophrenia | Decreased expression in the brains of schizophrenia patients | Glutamate transmission | Yanagi M 2008 |
| KLF6 | Epilepsy Glioblastoma |
|
HSP47 Unknown |
Jeong KH 2011 Jeng YM 2003;Kimmelman AC 2004;Camacho-Vanegas O 2007 |
| KLF8 | Gliomas | Promotes tumor cell proliferation | Unknown | Schnell O 2012 |
| KLF9 | Gliobalstoma | Suppresses gliblastoma-intiating stem cells | Notch1 | Ying M 2011 |
| KLF11 | Depression, alcoholism, stress | Transactivates MAO-A and -B gene in ethanol dependence, chronic stress and major depressive disorders | Monoamine oxidase (MAO) |
Duncan J 2012; Grunewald M 2012 |
As described above, dysregulated cerebral KLFs have been linked to a variety of neurological diseases. However only a few of them have been studied in the ischemic brain (Figure 1) (Enenstein et al., 2010; Park et al., 2013; Shi et al., 2013; Yin et al., 2013). Our laboratory, together with another research team, is among the first to determine the role of KLFs in cerebrovascular function and the pathogenesis of ischemic stroke (Shi et al., 2013; Yin et al., 2013). During cerebral ischemia, cerebral vascular endothelial cells are a major target for the generation of cerebrovascular damage. There is increasing evidence showing that ischemia-induced cerebral endothelial injury or death increases vascular permeability and BBB disruption, leading to primary and secondary ischemic brain injury (del Zoppo and Hallenbeck, 2000; Wang and Lo, 2003; Ishikawa et al., 2004; Huang et al., 2006; Sandoval and Witt, 2008; Yin et al., 2010). Therefore, protection of the cerebral endothelium becomes an important therapeutic target for stroke (Fagan et al., 2004; Rodriguez-Yanez et al., 2006; Fisher, 2008). Recent studies have highly documented that partial KLF family members (KLF2, KLF4, KLF5, KLF6, and KLF11) are implicated in developmental and pathological vascular processes (Suzuki et al., 2005; Atkins and Jain, 2007; Fan et al., 2012; Yin et al., 2013). However, the function of the KLF family in the cerebrovascular pathologies following ischemic stroke is largely unexplored. In a recent publication (Yin et al., 2013) to investigate the role of PPARγ and its coregulators in cerebrovascular endothelial dysfunction after stroke, we screened for PPARγ coregulators using a genome-wide and high-throughput coactivation system and revealed one of the KLF transcription factors, KLF11, as a novel PPARγ coregulator, which was further confirmed to physically interact with PPARγ and enhance its transcriptional function in mouse BMEC cultures. Moreover, we also found that loss-of-KLF11 function via genetic deficiency effectively abolished PPARγ agonist pioglitazone-mediated cytoprotection in mouse BMECs after exposure to oxygen glucose deprivation (OGD). Similarly, cerebrovascular protection derived from PPARγ activation by pioglitazone was also significantly reduced in KLF11 knockout mice after middle cerebral artery occlusion (MCAO) in comparison with its wild-type controls. Mechanistically, we demonstrated that KLF11 enhanced PPARγ transcriptional suppression of the microRNA-15a (miR-15a) gene, resulting in endothelial protection in BMEC cultures and cerebral microvasculature after ischemic stimuli. Thus, our data clearly indicate that recruitment of KLF11 as a novel PPARγ coregulator is required for PPARγ-mediated cerebrovascular protection in ischemic stroke. It is anticipated that elucidating the coordinated actions of KLF11 and PPARγ will provide new insights into understanding the molecular mechanisms underlying PPARγ function in the cerebral vasculature and help to develop a novel therapeutic strategy for the treatment of stroke.
It is worth noting that KLF11 is a member of the KLF family with high expression in vascular endothelium (Fan et al., 2012), and emerging data from population genetics studies suggest that KLF11 gene single nucleotide polymorphisms (SNPs) are significantly associated with type 2 diabetes (Neve et al., 2005; Fernandez-Zapico et al., 2009). Indeed, MODY7, an early-onset type 2 diabetes mellitus, is caused by mutations in the KLF11 gene (Fernandez-Zapico et al., 2009). These findings suggest KLF11 as a diabetes-associated transcription factor. Since human type 2 diabetes mellitus is a major risk factor for stroke (Ringelstein and Nabavi, 2000; Allen and Bayraktutan, 2008), and PPARγ agonists (thiazolidinediones, rosiglitazone and pioglitazone) show efficacy in treating type 2 diabetes clinically (Yki-Jarvinen, 2004) and appear to reduce brain injury after stroke in rodent models (Sundararajan et al., 2005; Luo et al., 2006; Victor et al., 2006; Tureyen et al., 2007; Wilcox et al., 2007; Zhao et al., 2009), our identification of KLF11 as a key co-activator in the regulation of PPARγ cerebrovascular protection after ischemic stroke has established an essential link between ischemic stroke and its major risk factors, such as diabetes. In addition, it is worth noting that in our recent publication, the functional significance and mechanisms of KLF11 itself as a novel diabetes mellitus-associated transcription factor in regulating cerebrovascular pathogenesis in ischemic stroke have not been revealed, especially in comorbidities of interest such as diabetes. Also, to better understand the functional importance of KLF11 in stroke-induced pathophysiological paradigms in the brain, neural cell-specific genetically-manipulated mice must be generated and employed in future studies.
Concurrently, another group led by Drs. Atkins and Jain investigated the role of KLF2 in cerebrovascular dysfunction and the pathogenesis of ischemic stroke in mice (Shi et al., 2013). KLF2 is one of the most highly investigated KLF members in endothelial biology, and has been reported to regulate endothelial inflammation (SenBanerjee et al., 2004), thrombotic function (Lin et al., 2005), atherosclerotic progression (Atkins et al., 2008), endothelial proliferation, migration, angiogenesis (Bielenberg et al., 2004; Bhattacharya et al., 2005; Dekker et al., 2006), and endothelial barrier integrity (Lin et al., 2010) as well as vasoreactivity and vascular tone (Dekker et al., 2005; Dekker et al., 2006; Parmar et al., 2006). In this study, ischemia-induced brain infarction and BBB leakage were significantly increased in tamoxifen inducible global KLF2 knockout mice, whereas markedly decreased in the inducible KLF2 transgenic mice following 1h MCAO and 48h reperfusion. Consistent with these in vivo findings, adenoviral-mediated in vitro gain- or loss-of-KLF2 function in primary human brain microvascular EC cultures was able to reduce or increase OGD-induced endothelial barrier injury, respectively.
Mechanistically, KLF2 was shown to trans-activate a key BBB tight junction factor, occludin both in in vitro and in vivo studies. These data are the first to identify KLF2 as a novel stroke-protective factor in the cerebrovasculature through its regulation of BBB structure and function (Shi et al., 2013). As the authors discussed in the article, the use of endothelial cell-selective transgenic and conditional knockout mice will be important for fully investigating the specific role of endothelial KLF2 in the regulation of BBB and subsequent neuroprotection after ischemic stroke. Also, KLF2 is expressed in several blood immune cells (e.g., monocytes and macrophages (8, 23) and cerebral microglia. Additional contributions from other cells may also be important to the overall KLF2-mediated vaso- and neuro-protection in ischemic stroke.
In humans, KLF2 was found at lower levels in blood outgrowth endothelial cells (BOECs) derived from stroke children with sickle cell anemia (SCA) than in non-SCA BOECs, changing the dynamic balance between proinflammatory NF-κB/p65 and anti-inflammatory KLF2. This imbalance of KLF2 and NF-κB/p65 may lead to a proinflammatory phenotype within the cerebral vasculature and potentially increase the risk of stroke in children with sickle cell anemia (Enenstein et al., 2010).
Reactive astrogliosisis, a characteristic response of astrocytes to brain injury and neurological disorders, contributes to the pathogenesis of ischemic stroke (Jorgensen et al., 1993; Chen and Swanson, 2003; Pekny and Nilsson, 2005; Sofroniew, 2009). Accumulating evidence has shown that KLF transcription factors are actively involved in this pathological process. A recent study using genomic analysis has demonstrated that KLF5 and KLF6 are induced in fluorescence activated cell sorting (FACS)-isolated reactive astrocytes from mouse brains following MCAO (Zamanian et al., 2012), suggesting a potential role of KLF5 and KLF6 in the regulation of the astroglial reaction in the injured brain. Also in another recent study, the involvement of KLF4 in the astroglial reaction after global ischemic injury was investigated. The observation from this group was that KLF4 immunoreactivity was not detected in resting astrocytes in the hippocampus. However, three days after ischemia, KLF4 immunoreactivity appeared to be increased in reactive astrocytes preferentially located in the CA1 regions, and this increase persisted until at least 4 weeks after ischemia. Interestingly, no significant KLF4 immunoreactivity was observed in microglial cells. Consistent with the in vivo findings, the induction of both KLF4 mRNA and protein levels was also observed in cultured rat primary cortical astrocytes after exposure to OGD. These data are the first to demonstrate that KLF4 expression was specifically induced in astroglia by ischemic injury both in vivo and in vitro (Park et al., 2013). Although the exact role and underlying mechanisms of KLF4 in global ischemia are not addressed in this article, their findings of upregulated KLF4 expression in astrocytes may imply KLF4 as a transcriptional regulator following ischemic brain injury. Further investigation in-depth needs to be performed in the future.
Taken together, KLFs are highly expressed in various neurovascular cells in the brain. Recent studies have highlighted the emerging role of KLF11 and KLF2 in the regulation of cerebrovascular endothelial dysfunction and subsequent brain injury in ischemic stroke. New experimental data have also shown a potential transcriptional regulation of KLF4, KLF5, and KLF6 on reactive astrogliosis after focal or global ischemia. These data reveal KLF transcription factors as novel mediators in the pathophysiology of stroke. However, KLFs have not been fully investigated for their roles in the central nervous system, and even less attention has been paid to the functional importance of stroke-associated or responsible KLFs. More experimental evidence regarding KLF function in neurons, microglia, oligodendrocytes, and other neural cells in the in vitro and in vivo cerebral ischemic stimuli remains totally undetermined, and needs to be uncovered in-depth.
5. Future Prospects for investigating KLFs in stroke pathologies
Although increasing evidence has shown that dysregulated KLF profiles relate to a variety of neurological diseases including stroke, it is worth noting that we are still at the very early stages in understanding the functional importance of KLF transcription factors in the stroke research field. Future studies will focus on elucidating the functions of different KLF members in cell cultures and experimental animals after cerebral ischemia. In particular, utilization of general or neural cell-specific KLF transgenic and knockout animals will be necessary to further clarify the role and underlying mechanisms of individual KLFs in the pathogenesis of stroke (Shi et al., 2013; Yin et al., 2013). In addition to exploring the roles of KLFs in ischemic stroke by using animal and cell culture models, data from human stroke patients or from population-based stroke epidemiology studies will better address the functional importance of KLFs in stroke and other neurological disorders (Enenstein et al., 2010). Also, the neuron-specific functions of KLFs remain unclear in ischemic brains (Shi et al., 2013; Yin et al., 2013) and need to be uncovered in future studies.
Moreover, the molecular regulatory mechanisms of particular KLFs in ischemic brain damage may also need to be elucidated to fully understand the function of these essential transcription factors in stroke. Specific attention will be paid to the careful identification of KLF downstream target genes, associated signaling pathways/networks, and also neural cell-type specificity of KLFs.
Furthermore, understanding the upstream factors that are able to modulate KLF expression and function will become necessary in the pathophysiology of stroke, which will provide new insights and potential for the development of pharmaceutical agents that affect KLF activity in the central nervous system and intervene against neurological disorders (Yin et al., 2013). Based on the molecular structures of KLF proteins that contain transcriptional activation and/or repression domains at their amino termini, coregulators including co-activators and co-repressors are essentially required for the regulation of function of KLF transcription factors (Kaczynski et al., 2003). Up to now, only a few interacting coregulators with KLFs have been discovered, such as p300/CBP, SWI/SNF, mSin3A, and CtBP2 (Kaczynski et al., 2003), making it another important research focus in stroke.
On the other hand, due to similarity in KLFs’ DNA recognition sequences and their potential co-localization in neural cells (Black et al., 2001; Kaczynski et al., 2003; Pearson et al., 2008; McConnell and Yang, 2010), further investigation is also needed to explore the interaction among different KLFs (synergistic, competitive, or antagonistic) as a transcriptional regulatory network in ischemic brain injury. It is also worth noting that the precise mechanisms of these interacting networks in regulating neural cell survival as well as post-ischemic remodeling are still elusive.
6. Conclusion
In the present review, we have demonstrated KLF transcription factors as important players in neuronal development, regeneration, and plasticity in the brain. Especially, we have summarized the essential role of KLFs in the regulation of PPARγ-mediated cerebral vascular protection, blood-brain barrier dysfunction, and reactive astrogliosis in response to focal or global cerebral ischemia.
However, the functional importance and molecular control of the KLF family in the etiology of ischemic stroke is still in its infancy. The significant role of the KLF family of transcription factors in stroke studies is largely unknown, and many individual targets of KLFs within ischemic neural cells have not yet been identified. The elucidation of KLF-related mechanisms involved in ischemic brain damage may be important for our understanding in-depth the pathogenesis of cerebral ischemia. It is our notion that more insights into the molecular mechanisms of brain function mediated by KLF transcription factors are essential for us to identify novel therapeutic targets for further development of KLF-based therapies in ischemic stroke.
Acknowledgments
This work was partially funded by the National Institutes of Health (NS066652, and HL089544). K.J.Y. was supported by the American Heart Association National Scientist Development Grant 0630209N.
References
- Allen CL, Bayraktutan U. Risk factors for ischaemic stroke. Int J Stroke. 2008;3:105–116. doi: 10.1111/j.1747-4949.2008.00187.x. [DOI] [PubMed] [Google Scholar]
- Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- Atkins GB, Wang Y, Mahabeleshwar GH, Shi H, Gao H, Kawanami D, Natesan V, Lin Z, Simon DI, Jain MK. Hemizygous deficiency of Kruppel-like factor 2 augments experimental atherosclerosis. Circ Res. 2008;103:690–693. doi: 10.1161/CIRCRESAHA.108.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avci HX, Lebrun C, Wehrle R, Doulazmi M, Chatonnet F, Morel MP, Ema M, Vodjdani G, Sotelo C, Flamant F, Dusart I. Thyroid hormone triggers the developmental loss of axonal regenerative capacity via thyroid hormone receptor alpha1 and kruppel-like factor 9 in Purkinje cells. Proc Natl Acad Sci U S A. 2012;109:14206–14211. doi: 10.1073/pnas.1119853109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagamasbad P, Ziera T, Borden SA, Bonett RM, Rozeboom AM, Seasholtz A, Denver RJ. Molecular basis for glucocorticoid induction of the Kruppel-like factor 9 gene in hippocampal neurons. Endocrinology. 2012;153:5334–5345. doi: 10.1210/en.2012-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya R, Senbanerjee S, Lin Z, Mir S, Hamik A, Wang P, Mukherjee P, Mukhopadhyay D, Jain MK. Inhibition of vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis by the Kruppel-like factor KLF2. J Biol Chem. 2005;280:28848–28851. doi: 10.1074/jbc.C500200200. [DOI] [PubMed] [Google Scholar]
- Bielenberg DR, Hida Y, Shimizu A, Kaipainen A, Kreuter M, Kim CC, Klagsbrun M. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest. 2004;114:1260–1271. doi: 10.1172/JCI21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–160. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- Blackmore MG, Wang Z, Lerch JK, Motti D, Zhang YP, Shields CB, Lee JK, Goldberg JL, Lemmon VP, Bixby JL. Kruppel-like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proc Natl Acad Sci U S A. 2012;109:7517–7522. doi: 10.1073/pnas.1120684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonett RM, Hu F, Bagamasbad P, Denver RJ. Stressor and glucocorticoid-dependent induction of the immediate early gene kruppel-like factor 9: implications for neural development and plasticity. Endocrinology. 2009;150:1757–1765. doi: 10.1210/en.2008-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M, Colucci-D'Amato L, Esposito MT, Parisi S, Stifani S, Ramirez F, di Porzio U. Transcription factor KLF7 regulates differentiation of neuroectodermal and mesodermal cell lineages. Exp Cell Res. 2010;316:2365–2376. doi: 10.1016/j.yexcr.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Caiazzo M, Colucci-D'Amato L, Volpicelli F, Speranza L, Petrone C, Pastore L, Stifani S, Ramirez F, Bellenchi GC, di Porzio U. Kruppel-like factor 7 is required for olfactory bulb dopaminergic neuron development. Exp Cell Res. 2011;317:464–473. doi: 10.1016/j.yexcr.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Camacho-Vanegas O, Narla G, Teixeira MS, DiFeo A, Misra A, Singh G, Chan AM, Friedman SL, Feuerstein BG, Martignetti JA. Functional inactivation of the KLF6 tumor suppressor gene by loss of heterozygosity and increased alternative splicing in glioblastoma. Int J Cancer. 2007;121:1390–1395. doi: 10.1002/ijc.22809. [DOI] [PubMed] [Google Scholar]
- Chang YC, Huang CC. Perinatal brain injury and regulation of transcription. Curr Opin Neurol. 2006;19:141–147. doi: 10.1097/01.wco.0000218229.73678.a8. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Dekker RJ, Boon RA, Rondaij MG, Kragt A, Volger OL, Elderkamp YW, Meijers JC, Voorberg J, Pannekoek H, Horrevoets AJ. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, de Vries CJ, Biessen EA, van Berkel TJ, Pannekoek H, Horrevoets AJ. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol. 2005;167:609–618. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thrombosis research. 2000;98:73–81. doi: 10.1016/s0049-3848(00)00218-8. [DOI] [PubMed] [Google Scholar]
- Dugas JC, Ibrahim A, Barres BA. The T3-induced gene KLF9 regulates oligodendrocyte differentiation and myelin regeneration. Mol Cell Neurosci. 2012;50:45–57. doi: 10.1016/j.mcn.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Johnson S, Ou XM. Monoamine oxidases in major depressive disorder and alcoholism. Drug Discov Ther. 2012;6:112–122. [PubMed] [Google Scholar]
- Eaton SA, Funnell AP, Sue N, Nicholas H, Pearson RC, Crossley M. A network of Kruppel-like Factors (Klfs). Klf8 is repressed by Klf3 and activated by Klf1 in vivo. J Biol Chem. 2008;283:26937–26947. doi: 10.1074/jbc.M804831200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enenstein J, Milbauer L, Domingo E, Wells A, Roney M, Kiley J, Wei P, Hebbel RP. Proinflammatory phenotype with imbalance of KLF2 and RelA: risk of childhood stroke with sickle cell anemia. Am J Hematol. 2010;85:18–23. doi: 10.1002/ajh.21558. [DOI] [PubMed] [Google Scholar]
- Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35:2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- Fan Y, Guo Y, Zhang J, Subramaniam M, Song CZ, Urrutia R, Chen YE. Kruppel-like factor-11, a transcription factor involved in diabetes mellitus, suppresses endothelial cell activation via the nuclear factor-kappaB signaling pathway. Arterioscler Thromb Vasc Biol. 2012;32:2981–2988. doi: 10.1161/ATVBAHA.112.300349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Zapico ME, van Velkinburgh JC, Gutierrez-Aguilar R, Neve B, Froguel P, Urrutia R, Stein R. MODY7 gene, KLF11, is a novel p300-dependent regulator of Pdx-1 (MODY4) transcription in pancreatic islet beta cells. J Biol Chem. 2009;284:36482–36490. doi: 10.1074/jbc.M109.028852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. Injuries to the vascular endothelium: vascular wall and endothelial dysfunction. Rev Neurol Dis. 2008;5(Suppl 1):S4–S11. [PubMed] [Google Scholar]
- Grunewald M, Johnson S, Lu D, Wang Z, Lomberk G, Albert PR, Stockmeier CA, Meyer JH, Urrutia R, Miczek KA, Austin MC, Wang J, Paul IA, Woolverton WL, Seo S, Sittman DB, Ou XM. Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J Biol Chem. 2012;287:24195–24206. doi: 10.1074/jbc.M112.373936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar SM, Ibrahim OA, Jain MK. Kruppel-like Factors (KLFs) in muscle biology. Journal of molecular and cellular cardiology. 2007;43:1–10. doi: 10.1016/j.yjmcc.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Zhang JH, Nanda A, Granger DN. Inflammatory responses to ischemia and reperfusion in the cerebral microcirculation. Front Biosci. 2004;9:1339–1347. doi: 10.2741/1330. [DOI] [PubMed] [Google Scholar]
- Jackle H, Rosenberg UB, Preiss A, Seifert E, Knipple DC, Kienlin A, Lehmann R. Molecular analysis of Kruppel, a segmentation gene of Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1985;50:465–473. doi: 10.1101/sqb.1985.050.01.058. [DOI] [PubMed] [Google Scholar]
- Jeng YM, Hsu HC. KLF6, a putative tumor suppressor gene, is mutated in astrocytic gliomas. Int J Cancer. 2003;105:625–629. doi: 10.1002/ijc.11123. [DOI] [PubMed] [Google Scholar]
- Jeong KH, Lee KE, Kim SY, Cho KO. Upregulation of Kruppel-like factor 6 in the mouse hippocampus after pilocarpine-induced status epilepticus. Neuroscience. 2011;186:170–178. doi: 10.1016/j.neuroscience.2011.02.046. [DOI] [PubMed] [Google Scholar]
- Jorgensen MB, Finsen BR, Jensen MB, Castellano B, Diemer NH, Zimmer J. Microglial and astroglial reactions to ischemic and kainic acid-induced lesions of the adult rat hippocampus. Exp Neurol. 1993;120:70–88. doi: 10.1006/exnr.1993.1041. [DOI] [PubMed] [Google Scholar]
- Kaczynski J, Cook T, Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4:206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik DK, Gupta M, Das S, Basu A. Kruppel-like factor 4, a novel transcription factor regulates microglial activation and subsequent neuroinflammation. J Neuroinflammation. 2010;7:68. doi: 10.1186/1742-2094-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik DK, Mukhopadhyay R, Kumawat KL, Gupta M, Basu A. Therapeutic targeting of Kruppel-like factor 4 abrogates microglial activation. J Neuroinflammation. 2012;9:57. doi: 10.1186/1742-2094-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik DK, Thounaojam MC, Kumawat KL, Gupta M, Basu A. Interleukin-1beta orchestrates underlying inflammatory responses in microglia via Kruppel-like factor 4. J Neurochem. 2013 doi: 10.1111/jnc.12382. [DOI] [PubMed] [Google Scholar]
- Kim J, Lengner CJ, Kirak O, Hanna J, Cassady JP, Lodato MA, Wu S, Faddah DA, Steine EJ, Gao Q, Fu D, Dawlaty M, Jaenisch R. Reprogramming of postnatal neurons into induced pluripotent stem cells by defined factors. Stem Cells. 2011;29:992–1000. doi: 10.1002/stem.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmelman AC, Qiao RF, Narla G, Banno A, Lau N, Bos PD, Nunez Rodriguez N, Liang BC, Guha A, Martignetti JA, Friedman SL, Chan AM. Suppression of glioblastoma tumorigenicity by the Kruppel-like transcription factor KLF6. Oncogene. 2004;23:5077–5083. doi: 10.1038/sj.onc.1207662. [DOI] [PubMed] [Google Scholar]
- Laub F, Dragomir C, Ramirez F. Mice without transcription factor KLF7 provide new insight into olfactory bulb development. Brain Res. 2006;1103:108–113. doi: 10.1016/j.brainres.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, Gimbrone MA, Jr, Balasubramanian V, Garcia-Cardena G, Jain MK. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res. 2005;96:e48–e57. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- Lin Z, Natesan V, Shi H, Dong F, Kawanami D, Mahabeleshwar GH, Atkins GB, Nayak L, Cui Y, Finigan JH, Jain MK. Kruppel-like factor 2 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2010;30:1952–1959. doi: 10.1161/ATVBAHA.110.211474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S. Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:267–282. doi: 10.1016/S0278-5846(03)00022-8. [DOI] [PubMed] [Google Scholar]
- Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J Neurochem. 2006;97:435–448. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiological reviews. 2010;90:1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller IJ, Bieker JJ. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Kruppel family of nuclear proteins. Mol Cell Biol. 1993;13:2776–2786. doi: 10.1128/mcb.13.5.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DL, Goldberg JL. Multiple transcription factor families regulate axon growth and regeneration. Dev Neurobiol. 2011;71:1186–1211. doi: 10.1002/dneu.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DL, Apara A, Goldberg JL. Kruppel-like transcription factors in the nervous system: novel players in neurite outgrowth and axon regeneration. Mol Cell Neurosci. 2011;47:233–243. doi: 10.1016/j.mcn.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science (New York, NY. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara Y, Northcott PA, Li M, Kongkham PN, Smith C, Yan H, Croul S, Ra YS, Eberhart C, Huang A, Bigner D, Grajkowska W, Van Meter T, Rutka JT, Taylor MD. Genetic and epigenetic inactivation of Kruppel-like factor 4 in medulloblastoma. Neoplasia. 2010;12:20–27. doi: 10.1593/neo.91122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak L, Lin Z, Jain MK. "Go with the flow": how Kruppel-like factor 2 regulates the vasoprotective effects of shear stress. Antioxidants & redox signaling. 2011;15:1449–1461. doi: 10.1089/ars.2010.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve B, et al. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc Natl Acad Sci U S A. 2005;102:4807–4812. doi: 10.1073/pnas.0409177102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N, Schwaninger M, Koistinaho J. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke. 2004;35:987–991. doi: 10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Shimojo H, Matsunaga M, Watanabe N, Kometani K, Minato N, Kageyama R. Gene expression profiling of neural stem cells and identification of regulators of neural differentiation during cortical development. Stem Cells. 2011;29:1817–1828. doi: 10.1002/stem.731. [DOI] [PubMed] [Google Scholar]
- Park JH, Riew TR, Shin YJ, Park JM, Cho JM, Lee MY. Induction of Kruppel-like factor 4 expression in reactive astrocytes following ischemic injury in vitro and in vivo. Histochem Cell Biol. 2013 doi: 10.1007/s00418-013-1134-5. [DOI] [PubMed] [Google Scholar]
- Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr, Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S. Kruppel-like transcription factors: a functional family. Int J Biochem Cell Biol. 2008;40:1996–2001. doi: 10.1016/j.biocel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Perez-Monter C, Martinez-Armenta M, Miquelajauregui A, Furlan-Magaril M, Varela-Echavarria A, Recillas-Targa F, May V, Charli JL, Perez-Martinez L. The Kruppel-like factor 4 controls biosynthesis of thyrotropin-releasing hormone during hypothalamus development. Mol Cell Endocrinol. 2011;333:127–133. doi: 10.1016/j.mce.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Preiss A, Rosenberg UB, Kienlin A, Seifert E, Jackle H. Molecular genetics of Kruppel, a gene required for segmentation of the Drosophila embryo. Nature. 1985;313:27–32. doi: 10.1038/313027a0. [DOI] [PubMed] [Google Scholar]
- Qin S, Zhang CL. Role of Kruppel-like factor 4 in neurogenesis and radial neuronal migration in the developing cerebral cortex. Mol Cell Biol. 2012;32:4297–4305. doi: 10.1128/MCB.00838-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Liu M, Niu W, Zhang CL. Dysregulation of Kruppel-like factor 4 during brain development leads to hydrocephalus in mice. Proc Natl Acad Sci U S A. 2011;108:21117–21121. doi: 10.1073/pnas.1112351109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringelstein EB, Nabavi D. Long-term prevention of ischaemic stroke and stroke recurrence. Thrombosis research. 2000;98:83–96. doi: 10.1016/s0049-3848(00)00230-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Yanez M, Castellanos M, Blanco M, Mosquera E, Castillo J. Vascular protection in brain ischemia. Cerebrovasc Dis. 2006;212(Suppl):21–29. doi: 10.1159/000091700. [DOI] [PubMed] [Google Scholar]
- Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Schellinger PD, Warach S. Therapeutic time window of thrombolytic therapy following stroke. Current atherosclerosis reports. 2004;6:288–294. doi: 10.1007/s11883-004-0060-3. [DOI] [PubMed] [Google Scholar]
- Schellinger PD, Kaste M, Hacke W. An update on thrombolytic therapy for acute stroke. Curr Opin Neurol. 2004;17:69–77. doi: 10.1097/00019052-200402000-00012. [DOI] [PubMed] [Google Scholar]
- Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- Schnell O, Romagna A, Jaehnert I, Albrecht V, Eigenbrod S, Juerchott K, Kretzschmar H, Tonn JC, Schichor C. Kruppel-like factor 8 (KLF8) is expressed in gliomas of different WHO grades and is essential for tumor cell proliferation. PLoS One. 2012;7:e30429. doi: 10.1371/journal.pone.0030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzke MN, Schwaninger M. Transcriptional regulation of neurogenesis: potential mechanisms in cerebral ischemia. J Mol Med (Berl) 2007;85:577–588. doi: 10.1007/s00109-007-0196-z. [DOI] [PubMed] [Google Scholar]
- Scobie KN, Hall BJ, Wilke SA, Klemenhagen KC, Fujii-Kuriyama Y, Ghosh A, Hen R, Sahay A. Kruppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. J Neurosci. 2009;29:9875–9887. doi: 10.1523/JNEUROSCI.2260-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA, Jr, Garcia-Cardena G, Jain MK. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Lomberk G, Mathison A, Buttar N, Podratz J, Calvo E, Iovanna J, Brimijoin S, Windebank A, Urrutia R. Kruppel-like factor 11 differentially couples to histone acetyltransferase and histone methyltransferase chromatin remodeling pathways to transcriptionally regulate dopamine D2 receptor in neuronal cells. J Biol Chem. 2012;287:12723–12735. doi: 10.1074/jbc.M112.351395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Sheng B, Zhang F, Wu C, Zhang R, Zhu J, Xu K, Kuang Y, Jameson SC, Lin Z, Wang Y, Chen J, Jain MK, Atkins GB. Kruppel-like factor 2 protects against ischemic stroke by regulating endothelial blood brain barrier function. Am J Physiol Heart Circ Physiol. 2013;304:H796–H805. doi: 10.1152/ajpheart.00712.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields JM, Yang VW. Two potent nuclear localization signals in the gut-enriched Kruppel-like factor define a subfamily of closely related Kruppel proteins. J Biol Chem. 1997;272:18504–18507. doi: 10.1074/jbc.272.29.18504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends in neurosciences. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A, Patel A, Thamatrakoln K, Liu C, Feng D, Clayberger C, Krensky AM. Functional domains and DNA-binding sequences of RFLAT-1/KLF13, a Kruppel-like transcription factor of activated T lymphocytes. J Biol Chem. 2002;277:30055–30065. doi: 10.1074/jbc.M204278200. [DOI] [PubMed] [Google Scholar]
- Srivastava K, Field DJ, Aggrey A, Yamakuchi M, Morrell CN. Platelet factor 4 regulation of monocyte KLF4 in experimental cerebral malaria. PLoS One. 2010;5:e10413. doi: 10.1371/journal.pone.0010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapf C, Mohr JP. Ischemic stroke therapy. Annual review of medicine. 2002;53:453–475. doi: 10.1146/annurev.med.53.082901.104106. [DOI] [PubMed] [Google Scholar]
- Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Aizawa K, Matsumura T, Nagai R. Vascular implications of the Kruppel-like family of transcription factors. Arterioscler Thromb Vasc Biol. 2005;25:1135–1141. doi: 10.1161/01.ATV.0000165656.65359.23. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, Vemuganti R. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- Turner J, Crossley M. Basic Kruppel-like factor functions within a network of interacting haematopoietic transcription factors. Int J Biochem Cell Biol. 1999;31:1169–1174. doi: 10.1016/s1357-2725(99)00067-9. [DOI] [PubMed] [Google Scholar]
- Victor NA, Wanderi EW, Gamboa J, Zhao X, Aronowski J, Deininger K, Lust WD, Landreth GE, Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur J Neurosci. 2006;24:1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol. 2003;28:229–244. doi: 10.1385/MN:28:3:229. [DOI] [PubMed] [Google Scholar]
- Wilcox R, Bousser MG, Betteridge DJ, Schernthaner G, Pirags V, Kupfer S, Dormandy J. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04) Stroke. 2007;38:865–873. doi: 10.1161/01.STR.0000257974.06317.49. [DOI] [PubMed] [Google Scholar]
- Wu C, Li F, Han G, Liu Z. Abeta(1–42) disrupts the expression and function of KLF2 in Alzheimer's disease mediated by p53. Biochem Biophys Res Commun. 2013;431:141–145. doi: 10.1016/j.bbrc.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Wu Z, Wang S. Role of kruppel-like transcription factors in adipogenesis. Dev Biol. 2013;373:235–243. doi: 10.1016/j.ydbio.2012.10.031. [DOI] [PubMed] [Google Scholar]
- Yanagi M, Hashimoto T, Kitamura N, Fukutake M, Komure O, Nishiguchi N, Kawamata T, Maeda K, Shirakawa O. Expression of Kruppel-like factor 5 gene in human brain and association of the gene with the susceptibility to schizophrenia. Schizophr Res. 2008;100:291–301. doi: 10.1016/j.schres.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Yi JH, Park SW, Kapadia R, Vemuganti R. Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage. Neurochemistry international. 2007;50:1014–1027. doi: 10.1016/j.neuint.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin KJ, Deng Z, Hamblin M, Xiang Y, Huang H, Zhang J, Jiang X, Wang Y, Chen YE. Peroxisome proliferator-activated receptor delta regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J Neurosci. 2010;30:6398–6408. doi: 10.1523/JNEUROSCI.0780-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin KJ, Fan Y, Hamblin M, Zhang J, Zhu T, Li S, Hawse JR, Subramaniam M, Song CZ, Urrutia R, Lin JD, Chen YE. KLF11 mediates PPARgamma cerebrovascular protection in ischaemic stroke. Brain. 2013;136:1274–1287. doi: 10.1093/brain/awt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying M, Sang Y, Li Y, Guerrero-Cazares H, Quinones-Hinojosa A, Vescovi AL, Eberhart CG, Xia S, Laterra J. Kruppel-like family of transcription factor 9, a differentiation-associated transcription factor, suppresses Notch1 signaling and inhibits glioblastoma-initiating stem cells. Stem Cells. 2011;29:20–31. doi: 10.1002/stem.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Jarvinen H. Thiazolidinediones. The New England journal of medicine. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- Yuan J. Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis. 2009;14:469–477. doi: 10.1007/s10495-008-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Daley GQ. From fibroblasts to iPS cells: induced pluripotency by defined factors. J Cell Biochem. 2008;105:949–955. doi: 10.1002/jcb.21871. [DOI] [PubMed] [Google Scholar]
- Zhao X, Strong R, Zhang J, Sun G, Tsien JZ, Cui Z, Grotta JC, Aronowski J. Neuronal PPARgamma deficiency increases susceptibility to brain damage after cerebral ischemia. J Neurosci. 2009;29:6186–6195. doi: 10.1523/JNEUROSCI.5857-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J. The intracerebral application of the PPARgamma-ligand pioglitazone confers neuroprotection against focal ischaemia in the rat brain. Eur J Neurosci. 2005;22:278–282. doi: 10.1111/j.1460-9568.2005.04200.x. [DOI] [PubMed] [Google Scholar]
- Zhu S, Tai C, MacVicar BA, Jia W, Cynader MS. Glutamatergic stimulation triggers rapid Krupple-like factor 4 expression in neurons and the overexpression of KLF4 sensitizes neurons to NMDA-induced caspase-3 activity. Brain Res. 2009;1250:49–62. doi: 10.1016/j.brainres.2008.11.013. [DOI] [PubMed] [Google Scholar]