Summary
Leishmaniasis covers a broad spectrum of diseases with distinct, and sometimes overlapping, characteristics. The common thread in all forms of leishmaniasis is the infection by the parasite Leishmania belonging to the genus Leishmania. Upon infection of humans there can be at least three outcomes, 1) control of Leishmania by the host immune response resulting in asymptomatic disease, 2) patent infection and development of a relatively mild form of leishmaniasis, and 3) patent infection and development of severe clinical forms. The factors that determine the outcome of an initial inoculation with Leishmania are many, with the species of Leishmania representing one of the strongest predictive factors for the development of a given clinical form of disease. This is seen with L. braziliensis and L. amazonensis, infection leading mostly to tegumentary forms of disease, and L. infantum with the potential to induce visceral disease. However, it is also clear that the host immune response is a key factor in disease progression, not only responsible for control of Leishmania, but also playing an important role in disease progression and pathology. This duality between protective and pathogenic immune responses in individuals infected with Leishmania in the Americas is the focus of this review.
Initial host-parasite interaction: disease development vs. asymptomatic exposure
Natural infection via a Leishmania contaminated sand-fly bite can lead to asymptomatic exposure or development of severe leishmaniasis in humans (1, 2). This first decision point holds for infection with species that eventually lead to either tegumentary or visceral disease (1). Upon natural infection, Leishmania metacyclic promastigotes gain access to the host where they encounter neutrophils, macrophages and monocytes, all of which can serve as targets for infection (2–5). It has become clear over the years that all three cell types can be infected by Leishmania and that the proportion of each cell type infected, the microenvironment present at the site of infection, the activation state of the cells, and the intensity of infection, all culminate in the development of a productive Leishmania infection, or possibly extinguish Leishmania before it progresses to a patent infection (Figure 1). Upon infection of host cells, Leishmania is likely shuttled to the nearest draining lymph nodes where again, depending on the microenvironment and cell types most involved, further replication and dissemination of the infection can occur, as well as the development of the adaptive immune response made up of B and T cells (CD4, CD8, double negative) (1, 2). Thus, these stages are the earliest events that determine whether an initial inoculation with Leishmania will go on to a form a patent infection, or be halted at this early stage.
Figure 1. Parasite species and host responses influence disease outcome.
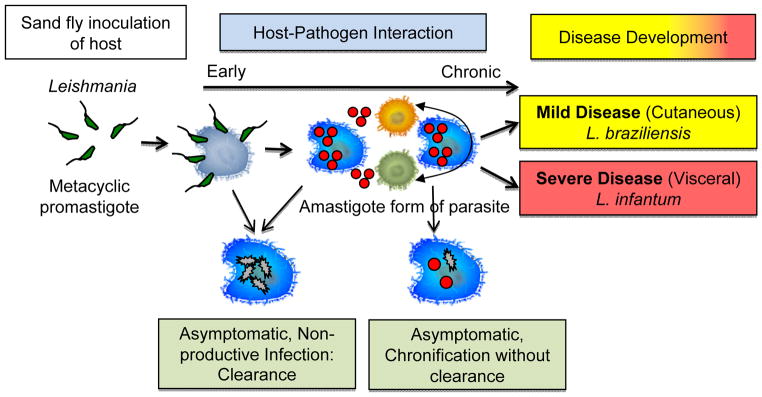
Leishmania infects macrophages early during infection: 1) infection via the introduction of infective metacyclic promastigotes via an insect bite, 2) initial infection of host macrophages, 3) conversion to intracellular replicating form of the parasite (amastigote), and 4) progression to a complex host-parasite interaction culminating in diseases with distinct clinical forms.
Interaction between Leishmania and a number of potential surface receptors expressed by host neutrophils, dendritic cells, macrophages and monocytes trigger phagocytic activities, allowing Leishmania to gain access into these cells (6–11). It was recently shown that infection of neutrophils can lead to Leishmania death, but also to the generation of apoptotic bodies that are capable of inhibiting effective responses of nearby macrophages (4). This initial contact can influence the replication of Leishmania in the critical early phases of infection. If macrophages are infected, there are also a variety of responses that can be triggered depending on the macrophage activation state and the species of Leishmania (2, 12–15). In addition to controlling parasite replication or not, the macrophage can also produce highly active immunoregulatory cytokines, such as the inflammatory cytokines TNF-alpha and IL-1 and the down modulatory cytokines, TGF-beta and IL-10 (2, 12, 16–20). Again, which cytokines and biological activities expressed by the infected macrophage depend on the activation state of the cell, the Leishmania species or strain, the sand-fly vector saliva activities present, and host genetics (1, 2, 21). Recent studies have shown that strain differences within the same species are associated with a given clinical form of disease (22, 23). Several studies have shown the importance of host genetic makeup in susceptibility to tegumentary and visceral forms of leishmaniasis (2, 24–30). These host genetic differences can play important roles in determining whether an individual will induce an effective leishmaniacidal response or not, as well as determine the further development of disease and pathology. Many gene polymorphisms identified as susceptibility factors for leishmaniasis are in genes that code for molecules associated with the immune response, especially cytokines and HLA alleles (25, 28, 31). One can postulate, for example, (32) that an individual with a high producer allele for TNF-alpha could be primed to respond more effectively with leishmaniacidal activity of infected macrophages early following infection. However, if the initial infection is not controlled, that same high production of TNF-alpha could later contribute to more severe immunopathology development as seen in mucosal leishmaniasis (30, 33–35).
Interestingly, different monocyte and macrophage profiles have been associated with distinct clinical forms of leishmaniasis. The majority of the studies have been performed when an active adaptive immune response is already induced in the infected individual and, thus, the host monocytes and macrophages are already in an environment dominated by inflammatory cytokines and active CD4+ T cells, DN T cells and CD8+ T cells, all producing bioactive cytokines and chemokines (2, 36–38). Nevertheless, clear differences are seen in monocytes and macrophages isolated from leishmaniasis patients as compared to non-infected controls and comparing between clinical forms of disease (39–41).
Thus, the initial Leishmania–host interaction culminates in either the control, with or without “sterilization,” of Leishmania likely leading to an asymptomatic non-productive infection, or it leads to a patent Leishmania infection expressed as either tegumentary diseases, such as cutaneous, mucosal or disseminated clinical forms (associated with L. amazonensis, L. braziliensis, L. guyanesis, L. mexicana and others in the Americas) of leishmaniasis, or visceral disease (L. infantum chagasi in the Americas) (Figure 1).
Pathogen control vs. pathology: immune balancing act in human leishmaniasis
Tegumentary disease
Once an adaptive cellular immune response develops following the presentation of classic peptide antigens by MHC class I for CD8+ T cells, MHC class II for CD4+ T cells, and CD1-presented lipid antigens to DN T cells and invariant NK T cells (iNK T cells), the balance between control of the pathogen and pathology development is amplified (1, 2, 33, 36, 42). The initial expansion of T cell subpopulations with distinct functional potentials begins in earnest within the draining lymph nodes following the initial infection (Figure 2). There, T cells differentiate towards effector T cells following activation through their interaction with antigen presenting cells (APCs). These differentiated T cells, capable of producing distinct cytokines can home to infection sites and drive efficient anti-Leishmania immune responses (Figure 2) both at the lesion site and in draining lymph nodes. These responses are greatly driven by Th1 CD4+ T cells producing monocyte and macrophage activating cytokines like IFN-gamma and TNF-alpha, together with regulatory cytokines like IL-10 (1, 2, 38). Importantly, the balance begins between controlling Leishmania replication within infected macrophages and monocytes, and the induction of immunopathology by excessive inflammatory responses (33, 35, 42–44). Activated Th1 cytokine producing CD4+ T cells have been correlated both with effective leishmaniacidal immune responses, as well as with greater pathology in cutaneous disease (35,45), and in mucosal disease, where an exacerbated Th1 type CD4+ T cell response is present (32–34, 46–49). Moreover, CD4+ T cells expressing the TCR V beta region, Vbeta5, have been associated with lesion size and differential lesion homing, indicating the possible role of these cells in pathology (44). In addition, the strong inflammatory CD4+ T cell response seen in cutaneous leishmaniasis (CL) is accompanied by increased IL-10 producing T cells (43), and the same is seen within the monocyte population (32). Thus, an ongoing regulation of the inflammatory response in CL disease is apparent, while that same co-regulation is faulty in mucosal leishmaniasis (ML) (32). Interestingly, while TNF-alpha and IFN-gamma are associated with the eventual resolution of disease in CL, lower levels of these cytokines are associated with disseminated leishmaniasis, indicating a weak cell mediated T cell response and thus, poor control of Leishmania, but also a lack of tissue destruction as seen in cutaneous and mucosal disease (50–52), both of which are associated with higher levels of TNF-alpha and IFN-gamma. Moreover, monitoring CD4+ T cell activation and cytokine production during active disease and following disease resolution has indicated their important role in finalization of disease and pathology (49, 53, 54). Recently, a correlation between IL-17 production and tegumentary and mucosal disease was found (46, 55). Thus, the nature of the CD4+ T cell response during active CL and through disease resolution is associated with robust production of inflammatory cytokines such as TNF-alpha and IFN-gamma in the presence of IL-10, a regulatory cytokine that acts to reduce production of TNF-alpha by host macrophages and monocytes (Figure 3) (43). Interestingly, treatment of leishmaniasis patients with pentoxifyline to reduce the activity of TNF-alpha leads to quicker resolution of lesions in both CL and ML (56–60). These groups of studies, and others not cited here, have produced a model of the role of CD4+ T cells in orchestrating the initial leishmaniacidal response, but also for contributing to lesion development in both cutaneous and mucosal disease (Figure 3).
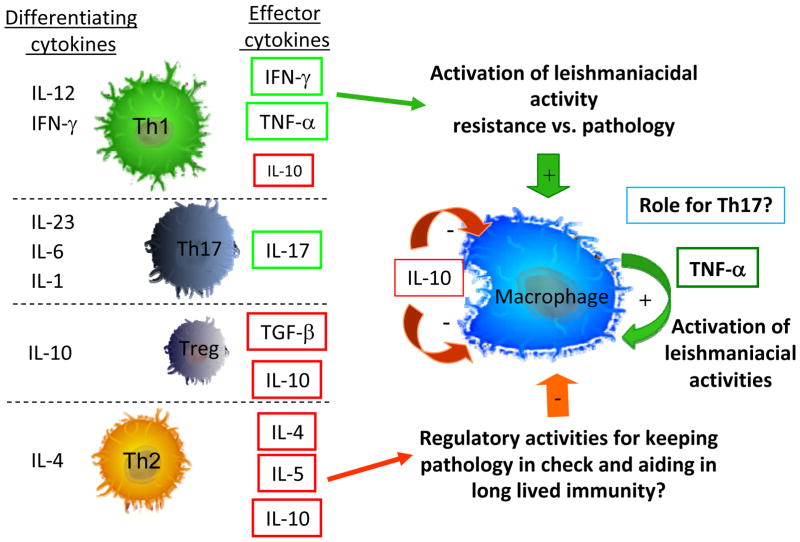
Figure 2. T cell subsets and macrophage interactions in cellular responses to Leishmania.
Effective cellular responses to combat Leishmania depend on the formation of CD4+ T cell subsets that are capable of activating leishmaniacidal responses by host macrophages and monocytes. The differentiation of CD4+ T cell subsets, Th1, Th2, Th3, Treg and Th17 all depend greatly on the cytokine microenvironment during the initial activation of naïve CD4+ T cells. Depending on the balance of these cytokines, co-stimulatory molecules, host genetics and antigenic stimuli, a given T cell will differentiate toward one of the Th subsets and produce the effector cytokines indicated in the figure. These cytokines in turn will act on host macrophages and monocytes to prime them for effective or ineffective control of Leishmania and subsequent control or not of immunopathology as well.
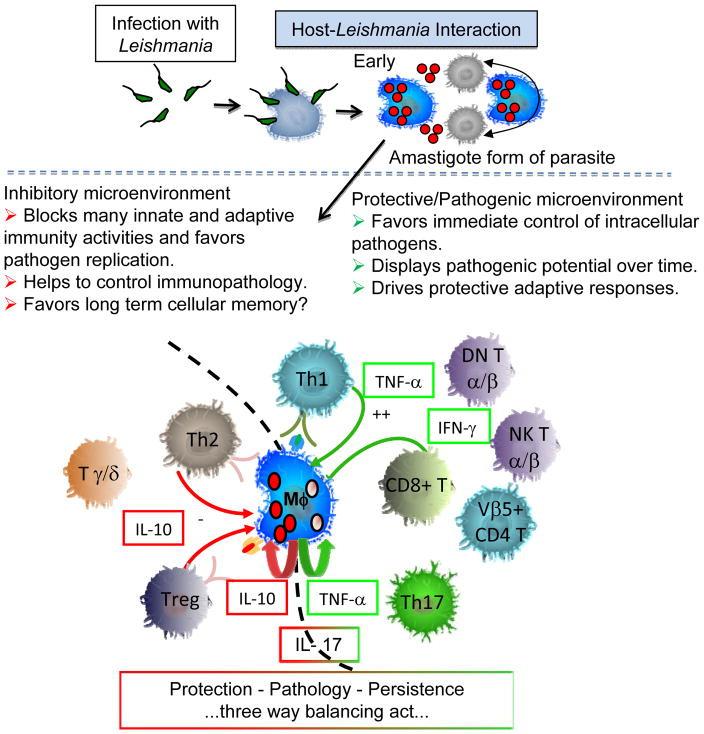
Figure 3. Protection - Pathology – Persistence, three way balancing act.
After the initial introduction of the pathogen within the host, the infective form of the parasite quickly parasitizes host macrophages and converts to the amastigote, intracellular replicating form of the parasite. This initial interaction between the host and parasite is paramount for establishing the infection and directing the subsequent adaptive immune response. Both parasite and host factors interact to culminate in a beneficial or detrimental host-parasite interaction, which is dependent on multiple factors. However, it is clear that the macrophage itself will be more effective at killing Leishmania under a cytokine environment rich in IFN-gamma and TNF-alpha, and that IL-10 can act to reduce macrophage production of TNF-alpha and parasite killing.
In addition to classic CD4+ T cells producing key regulatory cytokines for induction of leishmaniacidal responses, CD8+ T cells also play a role in both the establishment of the cytokine environment, as well as in cytolytic activity (2, 33). Several studies have shown that CD8+ T cells are associated with both protective immunity in CL, as well as greater pathology in CL and ML (61–65). In addition to cytokine production, which is lower in both frequency and intensity as compared to the CD4+ T cell subpopulation, CD8+ T cells also express cytolytic molecules such as granzyme and perforin. Progression of CL lesion has been associated with an increased frequency of CD8+ T cells expressing granzyme (63). Thus, the role of CD8+ T cells in both control of Leishmania and in lesion pathology is likely important.
Finally, a minority subpopulation of highly activated, cytokine producing T cells identified by their lack of both CD4 and CD8 co-receptors, termed double negative (DN) T cells, were shown to be the second most prevalent producers of inflammatory cytokines in active CL (38). This DN T cell population is a heterogenous population made up of T cells that recognize antigens presented by classic MHC molecules, class I and class II, as well as by non-classical presenting molecules, CD1. CD1 molecules have an antigen-binding cleft that is shallow and better suited for presentation of lipid and carbohydrate antigens than peptide antigens (66, 67). However, despite this heterogeneity, the DN T cell subpopulation is greatly committed toward the production of inflammatory cytokines (TNF-alpha and IFN-gamma) and, when subdivided into those T cells expressing the alpha/beta T cell receptor (TCR) vs. those expressing the gamma/delta TCR, one can see a clear division into T cell populations that produce a biased inflammatory environment (alpha/beta DN T cells) vs. those that produce a biased regulatory environment (gamma/delta DN T cells) (37). Thus, these T cell subpopulations may carry out distinct roles in parasite control vs. control of pathology in human CL.
Overall a picture of the duality of the T cell response on the broad view of Leishmania control vs. the development of pathology has been identified for the majority of T cell subpopulations studies in human CL and ML. This duality represents a classic immunological concept now clearly demonstrated in a complex human parasitic disease (Figure 3). The ability of immunotherapies to modulate these responses is key toward development of novel treatments that can accelerate healing and possibly reduce the toxicity of existing treatments by providing immune therapies that could serve as treatment adjuvants with existing therapies. Such treatment has been demonstrated effective by the use of pentoxifylline to reduce pathology and speed healing during CL and ML (56–60). In addition, the use of immune modulating compounds such as n-acetyl-l-cysteine may act in conjunction with glucantime to produce more effective leishmaniacidal responses while regulating some of the immunopathology induced during infection (68, 69). Further studies are needed to determine the suitability of this approach in human disease.
Visceral disease
Visceral leishmaniasis (VL) presents a case of a misbalanced immune response, but unlike in CL and ML where a strong inflammatory and leishmaniacidal response is poorly controlled leading to the formation of excessive immunopathology, VL is the result of an ineffective leishmaniacidal immune response. This defect leads to the dissemination of Leishmania in the host and a generalized immune disbalance dominated by increases in down modulatory cytokines like IL-10 and TGF-beta, both of which dampen the effectiveness of the cellular anti-Leishmania response (Figure 4). The reasons why VL can develop following infection with L. infantum in the Americas, while typically not following infection with L. amazonensis or L. braziliensis for example, are many fold and include the initial host-parasite interaction, temperature sensitivity of the parasite, the host immune response under the influence of host genetics and past immune experience, and even the sand fly – host interaction. Thus, the outcome of an estimated 5% of infections with L. infantum is VL, which culminates in the dissemination of Leishmania throughout the body concentrating within macrophages and monocytes in the bone marrow, liver, and spleen (1, 2, 70).
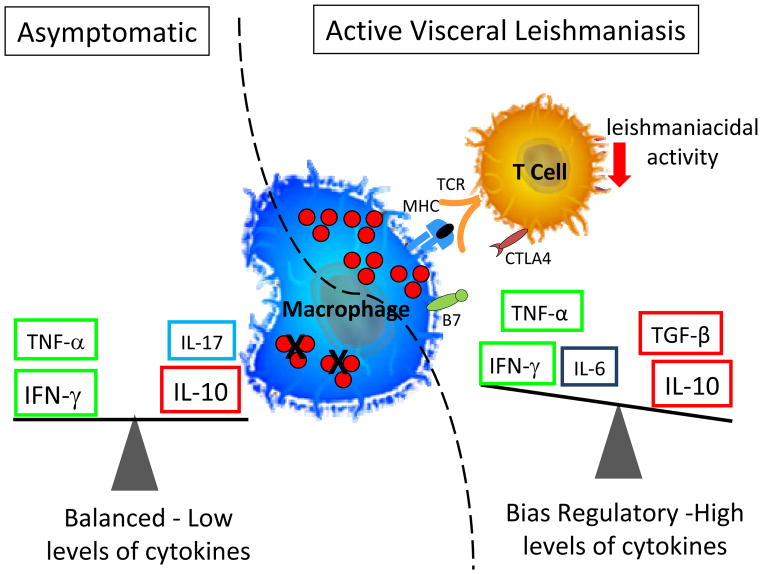
Figure 4. Representation of the two clinical outcomes following infection with L. infantum.
While an asymptomatic outcome is associated with lower levels cytokine production and a balance between the cytokines IFN-gamma and IL-10, patients with active VL are associated with higher production of both inflammatory (IFN-gamma and TNF-alpha) and the down regulatory cytokine IL-10. Thus, a suppression of an effective leishmaniacidal response in VL is correlated with disease development, as well as the presence of immunomodulatory molecules such as CTLA-4 leading to ineffective control of Leishmania.
The immune response in individuals following infection with L. infantum can be effective in controlling infection and generating a strong delayed type hypersensitivity response. In these cases, the infected individual is typically asymptomatic and the strong DTH is associated with high levels of IFN-gamma and TNF-alpha produced by CD4+ T cells (70, 71). IFN-gamma is an important cytokine involved with resistance of infection in VL due to its capacity to induce the production of ROS in phagocytic cells, which lead to destruction of the parasite (72).
Evaluation of cytokine levels in active VL caused by Leishmania donovani and Leishmania infantum have shown high levels of IFN-gamma in serum (73) and plasma (74–76). However, it is believed that despite the presence of high levels of IFN-gamma during infection, the host may fail to control Leishmania and mount an effective response to kill the parasite, partly due to an IFN-gamma receptor blockage in macrophage signaling (74). Other researchers have found a mixed profile of cytokines during active VL, associated with high levels of IFN-gamma, IL-10 and IL-6, demonstrating an exacerbation in immune response in these patients (74, 76). Following treatment, VL patients show a reduction in these cytokines and eventually return to levels similar to those observed in healthy individuals (74, 75). Another inflammatory cytokine associated with active VL is TNF-alpha (77). This cytokine seems to plays an important role in disease progression (77). IL-6, while at high levels during active VL before treatment (74, 75), also remains significantly elevated compared with control individuals even after treatment, indicating that it may have some beneficial role in disease resolution (74). Despite the high levels of inflammatory cytokines, increased levels of IL-10 in serum from active VL patients have been associated with severity of VL (75, 78–81) (Figure 4). Several articles have shown the importance of IL-10 in the pathogenesis of VL (82–84). Gautam et al. have shown that IL-10 is related to the chronicity of infection, suggesting a direct decrease in T cell activation and/or an inhibitory effect directly on APC. These studies demonstrated that blocking IL-10 in cultures of splenic asperates from VL patients led to a decrease in the parasite load (83). Thus, it is believed that IL-10 plays an important role in the suppression of the immune response, and thus is an important therapeutic target in VL (83, 84). TGF-beta also seems to be important in the development of VL, given its association with high levels of IL-10 in active VL (85).
Deciphering the mechanisms involved in the regulation of cytokine production during active VL infection as compared to asymptomatic individuals will contribute to a better understanding of the immunological phenomena that occur during disease progression. Immune responses amongst individuals with the subclinical form of VL have shown variable cytokine profiles, predominantly characterized by low levels of IL-10 (76, 77, 86), IL-12 and IFN-gamma (77), suggesting an important role for these cytokines in disease development (86). A follow-up study of patients with active and subclinical disease showed that the levels of IL-10 and TNF-alpha were higher in the acute form of the disease than in subclinical individuals (77). These studies suggest that production of both resistance and susceptibility cytokines may be important for contributing to distinct clinical manifestations (86). Although in recent years research has intensified concerning the role of the cytokine IL-17 in protozoa infection, very little is known about its activities in VL. Studies have indicated that IL-17 may be involved in the pathogenesis of CL (87, 88). Interestingly, the opposite is suggested in Chagas disease, given the association of IL-17 expression with the occurrence of the indeterminate (asymptomatic) clinical form (89). Studies seeking to understand the importance of Th1, Th2 and Th17 responses in Leishmania donovani induced VL have found that individuals who did not develop Kalazar (KA), or who were protected against KA during a severe outbreak, produced higher levels of IL-17 and Th1 cytokines than those with active KA, suggesting their role in protection from KA (90).
Effective T cell immunity requires activation and differentiation of T cell subpopulations into active effector cells. This activation and differentiation depends on efficient antigen presentation, as well as co-stimulatory activation via molecules like CD28, which is balanced by inhibitory networks such as those provided by CTLA-4 (91), PD1, and PD1-L (92). Through the analysis of markers associated with anergy/exhaustion in CD8+ T cells, it was demonstrated that VL subjects before treatment had higher expression of CTLA-4 and PD-1, than after treatment, or as compared to control individuals (93). These authors went on to suggest that this inhibitory network limits the ability of the individuals to produce IFN-gamma and effectively combat Leishmania (93). In experimental models of VL, blockade of CTLA-4 can result in enhanced host resistance to intracellular pathogens. The administration of monoclonal antibodies anti-CTLA-4 in BALB/c mice infected with Leishmania donovani enhanced the frequency of IFN-gamma and IL-4 producing cells in both spleen and liver and, thus, indicated a potent immunomodulatory activity of CTLA-4 in vivo (94). CTLA-4 suppression is important because of its capacity to mediate TGF-beta production. However, higher production is critically involved in intracellular parasite replication (95). Finally, given CTLA-4’s role in maintenance of T cell homeostasis, it seems clear that more research on this molecule’s role in human VL is warranted (95).
The Programmed Death 1 (PD-1) receptor: PD Ligand (PD-L) pathway is another major receptor–ligand network that functions primarily to provide an inhibitory signal. The inhibitory receptor PD-1 and its ligand PD-L (B7H1) have been shown to play an important role in T cell regulation (96). Joshi et al. demonstrated the importance of CD8+ T cells in the control of Leishmania infection in animal models where CD8+ T cells produce cytokines like IFN-gamma that may aid in parasite killing. However, the PD-1/PD-L interaction seems to induce apoptosis and inhibit proliferation of CD8+ T cells, and thereby reduce Leishmania control as seen by recovering T cell activity upon blocking the PD-1/PD-L interaction (96). Figure 4 summarizes the results found above in patients during active VL as compared to the asymptomatic infections.
Several studies have shown the importance of immunoregulatory cytokines described above in experimental mouse models of visceral leishmaniasis and some are discussed breifly in Box 1.
Box 1. Experimental murine models of visceral leishmaniasis.
To better understand the role of immnoregulation and immunopathology in VL induced following infection with Leishmania donovani and Leishmania chagasi/infantum, experimental mouse models have been extensively studied. A study evaluating the immune response in BALB/c mice with an inoculum of different concentrations of L. chagasi showed that those mice infected with low doses seemed to respond with the production of IFN-gamma. However, when higher doses where administrated the type of immune response changed and IL-10 prevailed, together with progression of severe disease indicating the importance of immunoregulatory cytokines in disease development in this model (97). The importance of IFN-gamma in macrophage activation and Leishmania killing has been studied in BALB/c IFN-gamma knockout mice and highlighted a fundamental role for this cytokine in disease resistance (98). Finally, mice deficient in TNF-alpha have increased susceptibility to hepatic L. donovani infection (99, 100).
Studies using mice lacking the gene for IL-10 showed that this cytokine plays a central role in susceptibility to L. donovani infection. In the absence of IL-10, mice rapidly developed an enhanced Th1 type response. The consequence of this response is a highly effective control of visceral parasites (101, 102). Experiments blocking IL-10-receptor or using anti-IL-10 monoclonal antibodies showed that the immune response and the production of higher levels of IFN-gamma could be restored, which led to activation of macrophages, and consequently parasite death (102–105). Interestingly, IL-6 knockout mice infected with L. donovani, had better control of Leishmania in the liver and higher IFN-gamma production with effective granuloma formation, thus suggesting that IL-6 played a role in pathology in this model (106). Experiments using anti-TGF-beta mAb did not demonstrate significant effects on IFN-gamma levels, showing that the ability to modulate IFN-gamma levels is clearly a hallmark of IL-10 activity (107, 108).
Pathology resolution and protection: role of memory and pathogen persistence
Once active leishmaniasis is diagnosed treatment typically begins within a week or so after the initial diagnosis, or sooner depending on the clinical form and patient complications (co-infections, other diseases, etc.). Most studies of disease resolution have been performed before and after treatment, with a few during treatment. Many factors determine an individual’s response to treatment and disease resolution. As stated above, the adaptive immune response plays important roles in both parasite control and development of pathology. Studies in animal models have suggested that sterilizing immune responses early after infection with Leishmania can lead to poor maintenance of memory responses (109). In addition, the development and maintenance of effector memory vs. central memory T cell populations can be influenced by the continuing presence of Leishmania, even after disease resolution, and in the absence of parasite (110, 111). In human disease, we know that Leishmania often persists in chronic infection and is held at bay due to the active immune response controlling further replication and expansion of Leishmania in vivo. This is most clearly seen in cases of immunosuppression via chemical means or naturally due to HIV infection. In active CL disease, there is often long-lived immunity following disease resolution, and this immunity is most likely related to maintenance of Leishmania within the host in the form of a cryptic infection (109). Thus, for any effective vaccine against Leishmania, the ability to induce a long-lived protective response in the absence of live persistent parasites is a key hurdle to be solved.
Concluding remarks
Overall it has been clear during the study of human and animal leishmaniasis that the immune response not only plays a key role in control of the parasite, but also in the development of pathology. While this review has focused on ATL and only briefly referred to studies on leishmaniasis in the Old World, there are many parallels between CL due to L. major infection. Moreover, visceral leishmaniasis in the Americas also has similar immunoregulatory aspects with KA. Thus, through continued studies designed to understand the immunological aspects of leishmaniasis, these findings will continue to aid in the development and discovery of novel therapies, vaccines, and diagnostics.
Acknowledgments
Our work related to this review was supported by NIH/Tropical Medicine Research Center Program, NIH-NIAID (R03AI066253-03), INCT-DT/CNPq and FAPEMIG. We would like to thank all authors whose contributions have allowed for a greater understanding of the pathogenesis of human leishmaniasis and apologize to others whose work was not cited here due to size and format limitations.
Footnotes
Disclosures: NONE
References
- 1.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366(9496):1561–77. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 2.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9(8):604–15. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 3.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321(5891):970–4. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afonso L, Borges VM, Cruz H, Ribeiro-Gomes FL, DosReis GA, Dutra AN, et al. Interactions with apoptotic but not with necrotic neutrophils increase parasite burden in human macrophages infected with Leishmania amazonensis. J Leukoc Biol. 2008;84(2):389–96. doi: 10.1189/jlb.0108018. [DOI] [PubMed] [Google Scholar]
- 5.Novais FO, Santiago RC, Bafica A, Khouri R, Afonso L, Borges VM, et al. Neutrophils and macrophages cooperate in host resistance against Leishmania braziliensis infection. J Immunol. 2009;183(12):8088–98. doi: 10.4049/jimmunol.0803720. [DOI] [PubMed] [Google Scholar]
- 6.Brandonisio O, Spinelli R, Pepe M. Dendritic cells in Leishmania infection. Microbes Infect. 2004;6(15):1402–9. doi: 10.1016/j.micinf.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–44. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 8.Ueno N, Bratt CL, Rodriguez NE, Wilson ME. Differences in human macrophage receptor usage, lysosomal fusion kinetics and survival between logarithmic and metacyclic Leishmania infantum chagasi promastigotes. Cell Microbiol. 2009;11(12):1827–41. doi: 10.1111/j.1462-5822.2009.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty P, Ghosh D, Basu MK. Modulation of macrophage mannose receptor affects the uptake of virulent and avirulent Leishmania donovani promastigotes. J Parasitol. 2001;87(5):1023–7. doi: 10.1645/0022-3395(2001)087[1023:MOMMRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Saraiva EM, Andrade AF, de Souza W. Involvement of the macrophage mannose-6-phosphate receptor in the recognition of Leishmania mexicana amazonensis. Parasitol Res. 1987;73(5):411–6. doi: 10.1007/BF00538197. [DOI] [PubMed] [Google Scholar]
- 11.Mosser DM, Vlassara H, Edelson PJ, Cerami A. Leishmania promastigotes are recognized by the macrophage receptor for advanced glycosylation endproducts. J Exp Med. 1987;165(1):140–5. doi: 10.1084/jem.165.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raes G, Beschin A, Ghassabeh GH, De Baetselier P. Alternatively activated macrophages in protozoan infections. Curr Opin Immunol. 2007;19(4):454–9. doi: 10.1016/j.coi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsiao CH, Ueno N, Shao JQ, Schroeder KR, Moore KC, Donelson JE, et al. The effects of macrophage source on the mechanism of phagocytosis and intracellular survival of Leishmania. Microbes Infect. 2011;13(12–13):1033–44. doi: 10.1016/j.micinf.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Almeida MC, Cardoso SA, Barral-Netto M. Leishmania (Leishmania) chagasi infection alters the expression of cell adhesion and costimulatory molecules on human monocyte and macrophage. Int J Parasitol. 2003;33(2):153–62. doi: 10.1016/s0020-7519(02)00266-7. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez NE, Chang HK, Wilson ME. Novel program of macrophage gene expression induced by phagocytosis of Leishmania chagasi. Infect Immun. 2004;72(4):2111–22. doi: 10.1128/IAI.72.4.2111-2122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribeiro-Gomes FL, Otero AC, Gomes NA, Moniz-De-Souza MC, Cysne-Finkelstein L, Arnholdt AC, et al. Macrophage interactions with neutrophils regulate Leishmania major infection. J Immunol. 2004;172(7):4454–62. doi: 10.4049/jimmunol.172.7.4454. [DOI] [PubMed] [Google Scholar]
- 18.Fromm PD, Kling J, Mack M, Sedgwick JD, Korner H. Loss of TNF signaling facilitates the development of a novel Ly-6C(low) macrophage population permissive for Leishmania major infection. J Immunol. 2012;188(12):6258–66. doi: 10.4049/jimmunol.1100977. [DOI] [PubMed] [Google Scholar]
- 19.Chandra D, Naik S. Leishmania donovani infection down-regulates TLR2-stimulated IL-12p40 and activates IL-10 in cells of macrophage/monocytic lineage by modulating MAPK pathways through a contact-dependent mechanism. Clin Exp Immunol. 2008;154(2):224–34. doi: 10.1111/j.1365-2249.2008.03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gantt KR, Schultz-Cherry S, Rodriguez N, Jeronimo SM, Nascimento ET, Goldman TL, et al. Activation of TGF-beta by Leishmania chagasi: importance for parasite survival in macrophages. J Immunol. 2003;170(5):2613–20. doi: 10.4049/jimmunol.170.5.2613. [DOI] [PubMed] [Google Scholar]
- 21.Costa DJ, Favali C, Clarencio J, Afonso L, Conceicao V, Miranda JC, et al. Lutzomyia longipalpis salivary gland homogenate impairs cytokine production and costimulatory molecule expression on human monocytes and dendritic cells. Infect Immun. 2004;72(3):1298–305. doi: 10.1128/IAI.72.3.1298-1305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Queiroz A, Sousa R, Heine C, Cardoso M, Guimaraes LH, Machado PR, et al. Association between an emerging disseminated form of leishmaniasis and Leishmania (Viannia) braziliensis strain polymorphisms. J Clin Microbiol. 2012;50(12):4028–34. doi: 10.1128/JCM.02064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schriefer A, Guimaraes LH, Machado PR, Lessa M, Lessa HA, Lago E, et al. Geographic clustering of leishmaniasis in northeastern Brazil. Emerg Infect Dis. 2009;15(6):871–6. doi: 10.3201/eid1506.080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ettinger NA, Duggal P, Braz RF, Nascimento ET, Beaty TH, Jeronimo SM, et al. Genetic admixture in Brazilians exposed to infection with Leishmania chagasi. Annals of human genetics. 2009;73(Pt 3):304–13. doi: 10.1111/j.1469-1809.2009.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blackwell JM, Fakiola M, Ibrahim ME, Jamieson SE, Jeronimo SB, Miller EN, et al. Genetics and visceral leishmaniasis: of mice and man. Parasite Immunol. 2009;31(5):254–66. doi: 10.1111/j.1365-3024.2009.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salhi A, Rodrigues V, Jr, Santoro F, Dessein H, Romano A, Castellano LR, et al. Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis. J Immunol. 2008;180(9):6139–48. doi: 10.4049/jimmunol.180.9.6139. [DOI] [PubMed] [Google Scholar]
- 27.Peacock CS, Collins A, Shaw MA, Silveira F, Costa J, Coste CH, et al. Genetic epidemiology of visceral leishmaniasis in northeastern Brazil. Genet Epidemiol. 2001;20(3):383–96. doi: 10.1002/gepi.8. [DOI] [PubMed] [Google Scholar]
- 28.Fakiola M, Strange A, Cordell HJ, Miller EN, Pirinen M, Su Z, et al. Common variants in the HLA-DRB1-HLA-DQA1 HLA class II region are associated with susceptibility to visceral leishmaniasis. Nat Genet. 2013;45(2):208–13. doi: 10.1038/ng.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramasawmy R, Menezes E, Magalhaes A, Oliveira J, Castellucci L, Almeida R, et al. The −2518bp promoter polymorphism at CCL2/MCP1 influences susceptibility to mucosal but not localized cutaneous leishmaniasis in Brazil. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2010;10(5):607–13. doi: 10.1016/j.meegid.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castellucci L, Menezes E, Oliveira J, Magalhaes A, Guimaraes LH, Lessa M, et al. IL6-174 G/C promoter polymorphism influences susceptibility to mucosal but not localized cutaneous leishmaniasis in Brazil. J Infect Dis. 2006;194(4):519–27. doi: 10.1086/505504. [DOI] [PubMed] [Google Scholar]
- 31.Sakthianandeswaren A, Foote SJ, Handman E. The role of host genetics in leishmaniasis. Trends Parasitol. 2009;25(8):383–91. doi: 10.1016/j.pt.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Gaze ST, Dutra WO, Lessa M, Lessa H, Guimaraes LH, Jesus AR, et al. Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population. Scand J Immunol. 2006;63(1):70–8. doi: 10.1111/j.1365-3083.2005.01707.x. [DOI] [PubMed] [Google Scholar]
- 33.Dutra WO, de Faria DR, Lima Machado PR, Guimaraes LH, Schriefer A, Carvalho E, et al. Immunoregulatory and Effector Activities in Human Cutaneous and Mucosal Leishmaniasis: Understanding Mechanisms of Pathology. Drug Dev Res. 2011;72(6):430–6. doi: 10.1002/ddr.20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, et al. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70(12):6734–40. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliveira F, Bafica A, Rosato AB, Favali CB, Costa JM, Cafe V, et al. Lesion size correlates with Leishmania antigen-stimulated TNF-levels in human cutaneous leishmaniasis. Am J Trop Med Hyg. 2011;85(1):70–3. doi: 10.4269/ajtmh.2011.10-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gollob KJ, Antonelli LR, Faria DR, Keesen TS, Dutra WO. Immunoregulatory mechanisms and CD4−CD8− (double negative) T cell subpopulations in human cutaneous leishmaniasis: a balancing act between protection and pathology. Int Immunopharmacol. 2008;8(10):1338–43. doi: 10.1016/j.intimp.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antonelli LR, Dutra WO, Oliveira RR, Torres KC, Guimaraes LH, Bacellar O, et al. Disparate immunoregulatory potentials for double-negative (CD4− CD8−) alpha beta and gamma delta T cells from human patients with cutaneous leishmaniasis. Infect Immun. 2006;74(11):6317–23. doi: 10.1128/IAI.00890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bottrel RL, Dutra WO, Martins FA, Gontijo B, Carvalho E, Barral-Netto M, et al. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect Immun. 2001;69(5):3232–9. doi: 10.1128/IAI.69.5.3232-3239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soares G, Barral A, Costa JM, Barral-Netto M, Van Weyenbergh J. CD16+ monocytes in human cutaneous leishmaniasis: increased ex vivo levels and correlation with clinical data. J Leukoc Biol. 2006;79(1):36–9. doi: 10.1189/jlb.0105040. [DOI] [PubMed] [Google Scholar]
- 40.Giudice A, Vendrame C, Bezerra C, Carvalho LP, Delavechia T, Carvalho EM, et al. Macrophages participate in host protection and the disease pathology associated with Leishmania braziliensis infection. BMC Infect Dis. 2012;12:75. doi: 10.1186/1471-2334-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vieira EL, Keesen TS, Machado PR, Guimaraes LH, Carvalho EM, Dutra WO, et al. Immunoregulatory profile of monocytes from cutaneous leishmaniasis patients and association with lesion size. Parasite Immunol. 2013;35(2):65–72. doi: 10.1111/pim.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carvalho LP, Passos S, Schriefer A, Carvalho EM. Protective and pathologic immune responses in human tegumentary leishmaniasis. Frontiers in immunology. 2012;3:301. doi: 10.3389/fimmu.2012.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Gollob KJ. Antigen specific correlations of cellular immune responses in human leishmaniasis suggests mechanisms for immunoregulation. Clin Exp Immunol. 2004;136(2):341–8. doi: 10.1111/j.1365-2249.2004.02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keesen TS, Antonelli LR, Faria DR, Guimaraes LH, Bacellar O, Carvalho EM, et al. CD4(+) T cells defined by their Vbeta T cell receptor expression are associated with immunoregulatory profiles and lesion size in human leishmaniasis. Clin Exp Immunol. 2011;165(3):338–51. doi: 10.1111/j.1365-2249.2011.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett. 2005;101(2):226–30. doi: 10.1016/j.imlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Boaventura VS, Santos CS, Cardoso CR, de Andrade J, Dos Santos WL, Clarencio J, et al. Human mucosal leishmaniasis: neutrophils infiltrate areas of tissue damage that express high levels of Th17-related cytokines. Eur J Immunol. 2010;40(10):2830–6. doi: 10.1002/eji.200940115. [DOI] [PubMed] [Google Scholar]
- 47.Carvalho LP, Passos S, Bacellar O, Lessa M, Almeida RP, Magalhaes A, et al. Differential immune regulation of activated T cells between cutaneous and mucosal leishmaniasis as a model for pathogenesis. Parasite Immunol. 2007;29(5):251–8. doi: 10.1111/j.1365-3024.2007.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faria DR, Gollob KJ, Barbosa J, Jr, Schriefer A, Machado PR, Lessa H, et al. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun. 2005;73(12):7853–9. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Da-Cruz AM, Bittar R, Mattos M, Oliveira-Neto MP, Nogueira R, Pinho-Ribeiro V, et al. T-cell-mediated immune responses in patients with cutaneous or mucosal leishmaniasis: long-term evaluation after therapy. Clin Diagn Lab Immunol. 2002;9(2):251–6. doi: 10.1128/CDLI.9.2.251-256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guimaraes LH, Machado PR, Lago EL, Morgan DJ, Schriefer A, Bacellar O, et al. Atypical manifestations of tegumentary leishmaniasis in a transmission area of Leishmania braziliensis in the state of Bahia, Brazil. Trans R Soc Trop Med Hyg. 2009;103(7):712–5. doi: 10.1016/j.trstmh.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Machado PR, Rosa ME, Costa D, Mignac M, Silva JS, Schriefer A, et al. Reappraisal of the immunopathogenesis of disseminated leishmaniasis: in situ and systemic immune response. Trans R Soc Trop Med Hyg. 2011;105(8):438–44. doi: 10.1016/j.trstmh.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silveira FT, Lainson R, Corbett CE. Further observations on clinical, histopathological, and immunological features of borderline disseminated cutaneous leishmaniasis caused by Leishmania (Leishmania) amazonensis. Mem Inst Oswaldo Cruz. 2005;100(5):525–34. doi: 10.1590/s0074-02762005000500013. [DOI] [PubMed] [Google Scholar]
- 53.Vieira-Goncalves R, Pirmez C, Jorge ME, Souza WJ, Oliveira MP, Rutowitsch MS, et al. Clinical features of cutaneous and disseminated cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis in Paraty, Rio de Janeiro. International journal of dermatology. 2008;47(9):926–32. doi: 10.1111/j.1365-4632.2008.03701.x. [DOI] [PubMed] [Google Scholar]
- 54.Coutinho SG, Pirmez C, Da-Cruz AM. Parasitological and immunological follow-up of American tegumentary leishmaniasis patients. Trans R Soc Trop Med Hyg. 2002;96 (Suppl 1):S173–8. doi: 10.1016/s0035-9203(02)90072-6. [DOI] [PubMed] [Google Scholar]
- 55.Bacellar O, Faria D, Nascimento M, Cardoso TM, Gollob KJ, Dutra WO, et al. Interleukin 17 production among patients with American cutaneous leishmaniasis. J Infect Dis. 2009;200(1):75–8. doi: 10.1086/599380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ojha RP, Cervantes D, Fischbach LA. Oral pentoxifylline and pentavalent antimony for treatment of leishmaniasis: promising but inconclusive evidence of superiority, compared with antimony monotherapy. Clin Infect Dis. 2007;45(8):1104. doi: 10.1086/521938. author reply 005–6. [DOI] [PubMed] [Google Scholar]
- 57.Machado PR, Lessa H, Lessa M, Guimaraes LH, Bang H, Ho JL, et al. Oral pentoxifylline combined with pentavalent antimony: a randomized trial for mucosal leishmaniasis. Clin Infect Dis. 2007;44(6):788–93. doi: 10.1086/511643. [DOI] [PubMed] [Google Scholar]
- 58.Sadeghian G, Nilforoushzadeh MA. Effect of combination therapy with systemic glucantime and pentoxifylline in the treatment of cutaneous leishmaniasis. International journal of dermatology. 2006;45(7):819–21. doi: 10.1111/j.1365-4632.2006.02867.x. [DOI] [PubMed] [Google Scholar]
- 59.Bafica A, Oliveira F, Freitas LA, Nascimento EG, Barral A. American cutaneous leishmaniasis unresponsive to antimonial drugs: successful treatment using combination of N-methilglucamine antimoniate plus pentoxifylline. International journal of dermatology. 2003;42(3):203–7. doi: 10.1046/j.1365-4362.2003.01868.x. [DOI] [PubMed] [Google Scholar]
- 60.Lessa HA, Machado P, Lima F, Cruz AA, Bacellar O, Guerreiro J, et al. Successful treatment of refractory mucosal leishmaniasis with pentoxifylline plus antimony. Am J Trop Med Hyg. 2001;65(2):87–9. doi: 10.4269/ajtmh.2001.65.87. [DOI] [PubMed] [Google Scholar]
- 61.da Santos CS, Boaventura V, Ribeiro Cardoso C, Tavares N, Lordelo MJ, Noronha A, et al. CD8(+) granzyme B(+)-mediated tissue injury vs. CD4(+)IFNgamma(+)-mediated parasite killing in human cutaneous leishmaniasis. J Invest Dermatol. 2013;133(6):1533–40. doi: 10.1038/jid.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nateghi Rostami M, Keshavarz H, Edalat R, Sarrafnejad A, Shahrestani T, Mahboudi F, et al. CD8+ T cells as a source of IFN-gamma production in human cutaneous leishmaniasis. PLoS Negl Trop Dis. 2010;4(10):e845. doi: 10.1371/journal.pntd.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Faria DR, Souza PE, Duraes FV, Carvalho EM, Gollob KJ, Machado PR, et al. Recruitment of CD8(+) T cells expressing granzyme A is associated with lesion progression in human cutaneous leishmaniasis. Parasite Immunol. 2009;31(8):432–9. doi: 10.1111/j.1365-3024.2009.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruiz JH, Becker I. CD8 cytotoxic T cells in cutaneous leishmaniasis. Parasite Immunol. 2007;29(12):671–8. doi: 10.1111/j.1365-3024.2007.00991.x. [DOI] [PubMed] [Google Scholar]
- 65.Da-Cruz AM, Conceicao-Silva F, Bertho AL, Coutinho SG. Leishmania-reactive CD4+ and CD8+ T cells associated with cure of human cutaneous leishmaniasis. Infect Immun. 1994;62(6):2614–8. doi: 10.1128/iai.62.6.2614-2618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Voelkl S, Gary R, Mackensen A. Characterization of the immunoregulatory function of human TCR-alphabeta+ CD4− CD8− double-negative T cells. Eur J Immunol. 2011;41(3):739–48. doi: 10.1002/eji.201040982. [DOI] [PubMed] [Google Scholar]
- 67.D’Acquisto F, Crompton T. CD3+CD4−CD8− (double negative) T cells: saviours or villains of the immune response? Biochem Pharmacol. 2011;82(4):333–40. doi: 10.1016/j.bcp.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 68.Monteiro MC, Marques FC, Blazius RD, Santos da Silva O, de Queiroz Cunha F, Bento DB, et al. N-acetyl-L: -cysteine reduces the parasitism of BALB/c mice infected with Leishmania amazonensis. Parasitol Res. 2008;102(4):801–3. doi: 10.1007/s00436-007-0827-x. [DOI] [PubMed] [Google Scholar]
- 69.Rocha-Vieira E, Ferreira E, Vianna P, De Faria DR, Gaze ST, Dutra WO, et al. Histopathological outcome of Leishmania major-infected BALB/c mice is improved by oral treatment with N-acetyl-l-cysteine. Immunology. 2003;108(3):401–8. doi: 10.1046/j.1365-2567.2003.01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goto H, Prianti MG. Immunoactivation and immunopathogeny during active visceral leishmaniasis. Rev Inst Med Trop Sao Paulo. 2009;51(5):241–6. doi: 10.1590/s0036-46652009000500002. [DOI] [PubMed] [Google Scholar]
- 71.Kaye PM, Aebischer T. Visceral leishmaniasis: immunology and prospects for a vaccine. Clin Microbiol Infect. 2011;17(10):1462–70. doi: 10.1111/j.1469-0691.2011.03610.x. [DOI] [PubMed] [Google Scholar]
- 72.Kaye P, Scott P. Leishmaniasis: complexity at the host–pathogen interface. Nature reviews. 2011;9:604–15. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 73.Hailu A, Baarle DV, Knol GJ, Berhe N, Miedema F, Kager PA. T cell subset and cytokine profiles in human visceral leishmaniasis during active and asymptomatic or sub-clinical infection with Leishmania donovani. Clinical Immunology. 2005;117(2):182–91. doi: 10.1016/j.clim.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 74.Ansari NA, Saluja S, Salotra P. Elevated levels of interferon-γ, interleukin-10, and interleukin-6 during active disease in Indian kala azar. Clinical Immunology. 2006;119(3):339–45. doi: 10.1016/j.clim.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 75.Caldas A, Favali C, Aquino D, Vinhas V, Weyenbergh Jv, Brodskyn C, et al. Balance of IL-10 and Interferon-γ plasma levels in human visceral leishmaniasis: Implications in the pathogenesis. BMC Infectious Diseases. 2005;113:1–9. doi: 10.1186/1471-2334-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costa ASA, Costa GC, Aquino DMCd, Mendonça VRRd, Barral A, Barral-Netto M, et al. Cytokines and visceral leishmaniasis: a comparison of plasma cytokine profiles between the clinical forms of visceral leishmaniasis. Memórias do Instituto Oswaldo Cruz. 2012;107:735–9. doi: 10.1590/s0074-02762012000600005. [DOI] [PubMed] [Google Scholar]
- 77.Gama MEA, Costa JML, Pereira JCR, Gomes CMC, Corbett CEP. Serum cytokine profile in the subclinical form of visceral leishmaniasis. Brazilian Journal of Medical and Biological Research. 2004;37:129–36. doi: 10.1590/s0100-879x2004000100018. [DOI] [PubMed] [Google Scholar]
- 78.Holaday B, Pompeu M, SJ, Texeira M, Sousa AA, Vasconcelos A, et al. Potential role for interleukin-10 in the immunosuppression associated with kala azar. The Journal of Clinical Investigation. 1993;92(6):2626–32. doi: 10.1172/JCI116878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karp CL, El-Safi SH, Wynn TA, Satti MMH, Kordofani AM, Hashim FA, et al. In Vivo Cytokine Profiles in Patients with Kala-azar Marked Elevation of Both Interleukin-10 and Interferon-gamma. The Journal of Clinical Investigation. 1993;91:1644–8. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghalib HW, Piuvezam MR, Skeiky YAW, Siddig M, Hashim FA, El-Hassan AM, et al. Interleukin 10 Production Correlates with Pathology in Human Leishmania donovani Infections. Journal clinical investigation. 1993;92:324–9. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kurkjian KM, Mahmutovic AJ, Kellar KL, Haque R, Bern C, Secor WE. Multiplex Analysis of Circulating Cytokines in the Sera of Patients with Different Clinical Forms of Visceral Leishmaniasis. Cytometry Part A. 2006;69:353–8. doi: 10.1002/cyto.a.20256. [DOI] [PubMed] [Google Scholar]
- 82.Nylen S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. TRENDS in Immunology. 2007;28(9):378–84. doi: 10.1016/j.it.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Gautam S, Kumar R, Maurya R, Nyle S, Ansari N, Rai M, et al. IL-10 Neutralization Promotes Parasite Clearance in Splenic Aspirate Cells From Patients With Visceral Leishmaniasis. The Journal of Infectious Diseases. 2011;204:1134–7. doi: 10.1093/infdis/jir461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rai AK, PC, Singh A, Seth T, Srivastava SK, Singh P, et al. Regulatory T Cells Suppress T Cell Activation at the Pathologic Site of Human Visceral Leishmaniasis. Plos One. 2012;7(2):1–11. doi: 10.1371/journal.pone.0031551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saha S, Mondal S, Ravindran R, Bhowmick S, Modak D, Mallick S, et al. IL-10- and TGF-b-Mediated Susceptibility in Kala-azar and Post-kala-azar Dermal Leishmaniasis: The Significance of Amphotericin B in the Control of Leishmania donovani Infection in India. The Journal of Immunology. 2007;179:5592–603. doi: 10.4049/jimmunol.179.8.5592. [DOI] [PubMed] [Google Scholar]
- 86.Khoshdel A, Alborzi A, Rosouli M, Taheri E, Kiany S, Javadian M. Increased levels of IL-10, IL-12, and IFN- in patients with visceral leishmaniasis. Brazilian Journal of Infectious Diseases. 2009;13(1):44–6. doi: 10.1590/s1413-86702009000100010. [DOI] [PubMed] [Google Scholar]
- 87.Novoa R, Bacellar O, Nascimento M, Cardoso TM, Ramasawmy R, Oliveira WN, et al. IL-17 and Regulatory Cytokines (IL-10 and IL-27) in L. braziliensis Infection. Parasite immunology. 2011;33:132–6. doi: 10.1111/j.1365-3024.2010.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bacellar O, Faria D, Nascimento M, Cardoso TM, Gollob KJ, Dutra WO, et al. IL-17 Production in Patients with American Cutaneous Leishmaniasis. Journal infectious disease. 2009;200:75–8. doi: 10.1086/599380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Magalhães LMD, Villani FNA, Nunes MdCP, Gollob KJ, Rocha MOC, Dutra WO. High Interleukin 17 Expression Is Correlated With Better Cardiac Function in Human Chagas Disease. The Journal of infectious diseases. 2013;208:661–5. doi: 10.1093/infdis/jis724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pitta MGR, Romano A, Cabantous S, Henri S, Hammad A, Kouriba B, et al. IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. Research article The Journal of Clinical Investigation. 2009;119:2379–89. doi: 10.1172/JCI38813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zubairi S, Sanos SL, Hill2 S, Kaye PM. Immunotherapy with OX40L-Fc or anti-CTLA-4 enhances local tissue responses and killing of Leishmania donovani. European Journal of Immunology. 2004;34:1433–40. doi: 10.1002/eji.200324021. [DOI] [PubMed] [Google Scholar]
- 92.Liang SC, Greenwald RJ, Latchman YE, Rosas L, Satoskar A, Freeman GJ, et al. PD-L1 and PD-L2 have distinct roles in regulating host immunity to cutaneous leishmaniasis. European Journal of Immunology. 2006;36:58–64. doi: 10.1002/eji.200535458. [DOI] [PubMed] [Google Scholar]
- 93.Gautam S, Kumar R, Singh N, Singh AK, Rai M, Sacks D, et al. CD8 T Cell Exhaustion in Human Visceral Leishmaniasis. The Journal of Infectious Diseases. 2013:1–10. doi: 10.1093/infdis/jit401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murph ML, Cotterell SEJ, Gorak PMA, Engwerda CR, Kaye M. Blockade of CTLA-4 Enhances Host Resistance to the Intracellular Pathogen, Leishmania donovani. The Journal of Immunology. 1998;161:4153–60. [PubMed] [Google Scholar]
- 95.Gomes NA, Gattass CR, Barreto-de-Souza V, Wilson ME, DosReis GA. TGF-b Mediates CTLA-4 Suppression of Celular Immunity in Murine Kalaazar. The Journal of Immunology. 2000;164:2001–8. doi: 10.4049/jimmunol.164.4.2001. [DOI] [PubMed] [Google Scholar]
- 96.Joshi T, Rodriguez S, Perovic V, Cockburn IA, Stager1 S. B7-H1 Blockade Increases Survival of Dysfunctional CD8+T Cells and Confers Protection against Leishmania donovani Infections. Plos Pathogens. 2009;5:1–14. doi: 10.1371/journal.ppat.1000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oliveira DM, Costa MAF, Chavez-Fumagalli MA, Valadares DG, Duarte MC, Costa LE, et al. Evaluation of parasitological and immunological parameters of Leishmania chagasi infection in BALB/c mice using different doses and routes of inoculation of parasites. Parasitology research. 2012;110:1277–85. doi: 10.1007/s00436-011-2628-5. [DOI] [PubMed] [Google Scholar]
- 98.Wadhone P, Maiti M, Agarwal R, Kamat V, Martin S, Saha B. Miltefosine Promotes IFN-g-Dominated Anti-Leishmanial Immune Response. The journal of immunology. 2009;182:7146–54. doi: 10.4049/jimmunol.0803859. [DOI] [PubMed] [Google Scholar]
- 99.Engwerda CR, Ato M, Cotterell SEJ, Mynott TL, Tschannerl A, Gorak-Stolinska PMA, et al. A Role for Tumor Necrosis Factor-alpha in Remodeling the Splenic Marginal Zone during Leishmania donovani Infection. American Journal of Pathology. 2002;161:429–37. doi: 10.1016/s0002-9440(10)64199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Engwerda CR, Ato M, Stager S, Alexander CE, Stanley AC, Kaye PM. Distinct Roles for Lymphotoxin- and Tumor Necrosis Factor in the Control of Leishmania donovani Infection. American Journal of Pathology. 2004;165:2123–33. doi: 10.1016/s0002-9440(10)63262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Murphy ML, Wille U, Villegas EN, Hunter CA, Farrell JP. IL-10 mediates susceptibility to Leishmania donovani infection. European Journal of Immunology. 2001;31:2848–56. doi: 10.1002/1521-4141(2001010)31:10<2848::aid-immu2848>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 102.Bhattacharjee S, Gupta G, Bhattacharya P, Adhikari A, Majumdar SB, Majumdar S. Anti-IL-10 mAb protection against experimental visceral leishmaniasis via induction of Th1 cytokines and nitric oxide. Indian Journal of Experimental Biology. 2009;47:489–97. [PubMed] [Google Scholar]
- 103.Murray HW, Lu CM, Mauze S, Freeman S, Moreira AL, Kaplanan G, et al. Interleukin-10 (IL-10) in Experimental Visceral Leishmaniasis and IL-10 Receptor Blockade as Immunotherapy. Infection and immunity. 2002;70:6284–93. doi: 10.1128/IAI.70.11.6284-6293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murray HW, Moreira AL, Lu CM, DeVecchio JL, Matsuhashi M, Ma X, et al. Determinants of Response to Interleukin-10 Receptor Blockade Immunotherapy in Experimental Visceral Leishmaniasis. Journal of infectious disease. 2003;188:458–64. doi: 10.1086/376510. [DOI] [PubMed] [Google Scholar]
- 105.Murray HW, Flanders KC, Donaldson DD, Sypek JP, Gotwals PJ, Liu J, et al. Antagonizing Deactivating Cytokines To Enhance Host Defense and Chemotherapy in Experimental Visceral Leishmaniasis. Infection and immunity. 2005;2005:3903–11. doi: 10.1128/IAI.73.7.3903-3911.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murray HW. Accelerated Control of Visceral Leishmania donovani Infection in Interleukin-6-Deficient Mice. Infection and immunity. 2008;76:4088–91. doi: 10.1128/IAI.00490-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murray HW. Interleukin 10 receptor blockade—pentavalent antimony treatment in experimental visceral leishmaniasis. Acta tropica. 2005;93:295–301. doi: 10.1016/j.actatropica.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 108.Rodrigues Virmons, Jr , Silva JSD, Campos-Neto A. Transforming Growth Factor-b and Immunosuppression in Experimental Visceral Leishmaniasis. Infection and immunity. 1998;66:1233–6. doi: 10.1128/iai.66.3.1233-1236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Okwor I, Uzonna J. Persistent parasites and immunologic memory in cutaneous leishmaniasis: implications for vaccine designs and vaccination strategies. Immunol Res. 2008;41(2):123–36. doi: 10.1007/s12026-008-8016-2. [DOI] [PubMed] [Google Scholar]
- 110.Gollob KJ, Antonelli LR, Dutra WO. Insights into CD4+ memory T cells following Leishmania infection. Trends Parasitol. 2005;21(8):347–50. doi: 10.1016/j.pt.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 111.Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat Med. 2004;10(10):1104–10. doi: 10.1038/nm1108. [DOI] [PubMed] [Google Scholar]