Abstract
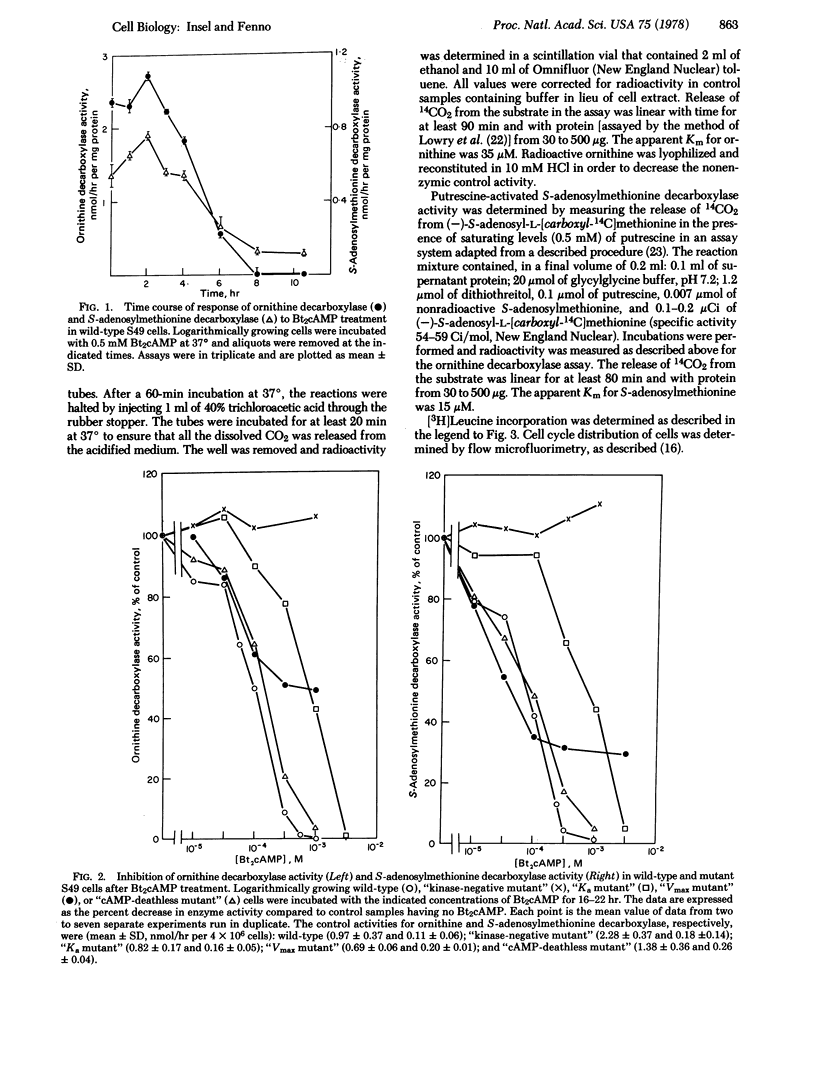
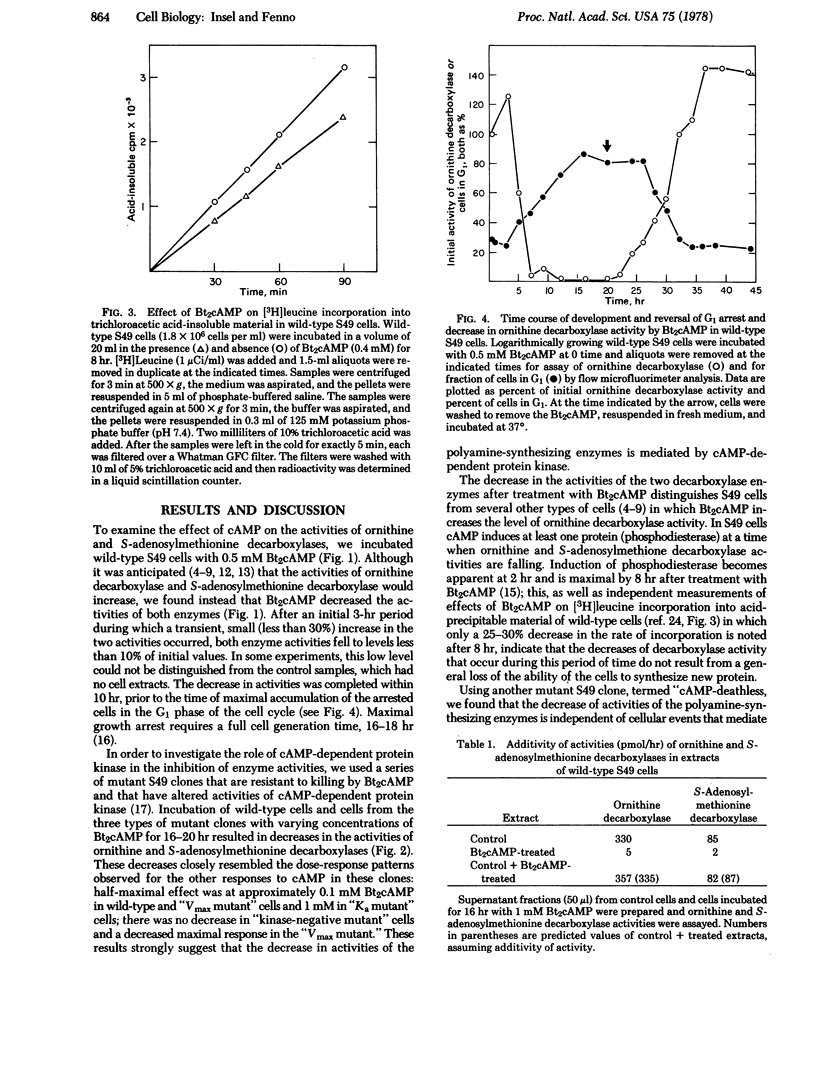
Incubation of S49 lymphoma cells with N6,O2'-dibutyryl cyclic AMP (Bt2cAMP) decreases the activities of ornithine decarboxylase (L-ornithine carboxy-lyase; EC 4.1.1.17) and S-adenosylmethionine decarboxylase (S-adenosyl-L-methionine carboxy-lyase; EC 4.1.1.50), the two principal enzymes in the pathway of polyamine synthesis. This decrease is dose-dependent, commences after a 3-hr delay, virtually abolishes the assayable activities of the two enzymes, and is not associated with a soluble inhibitor of the enzyme activities. Studies in mutant S49 clones that have altered protein kinase indicate that cAMP-dependent protein kinase mediates the decreases in enzyme activities. The dose-response pattern for the cAMP-stimulated decrease in enzyme activities parallels the pattern for the cAMP-stimulated, cell cycle-specific (G1) growth arrest of S49 cells. The activity of ornithine decarboxylase decreases faster than Bt2cAMP arrests wild-type S49 cells and, similarly, release of cells from the cAMP-stimulated arrest in G1 increases the activity of ornithine decarboxylase faster than cells exit from G1. These findings contrast with reports that cAMP induces ornithine decarboxylase in other cell types and further suggest that passage of cells through cell cycle is required for maintaining the activities of ornithine and S-adenosylmethionine decarboxylases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisbitt R. P., Barry J. M. Stimulation by insulin of ornithine decarboxylase activity in cultured mammary tissue. Biochim Biophys Acta. 1973 Oct 5;320(3):610–616. doi: 10.1016/0304-4165(73)90140-2. [DOI] [PubMed] [Google Scholar]
- Bachrach U. Cyclic AMP-mediated induction of ornithine decarboxylase of glioma and neuroblastoma cells. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3087–3091. doi: 10.1073/pnas.72.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrach U. Induction of S-adenosyl-L-methionine decarboxylase in glioma and neuroblastoma cells. FEBS Lett. 1977 Mar 15;75(1):201–204. doi: 10.1016/0014-5793(77)80086-0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Tomkins G. M., Dion S. Regulation of phosphodiesterase synthesis: requirement for cyclic adenosine monophosphate-dependent protein kinase. Science. 1973 Sep 7;181(4103):952–954. doi: 10.1126/science.181.4103.952. [DOI] [PubMed] [Google Scholar]
- Canellakis Z. N., Theoharides T. C. Stimulation of ornithine decarboxylase synthesis and its control by polyamines in regenerating rat liver and cultured rat hepatoma cells. J Biol Chem. 1976 Jul 25;251(14):4436–4441. [PubMed] [Google Scholar]
- Clark J. L., Fuller J. L. Protein inhibitor of ornithine decarboxylase does not account for effect of putrescine on 3T3 cells. Biochem Biophys Res Commun. 1976 Dec 6;73(3):785–790. doi: 10.1016/0006-291x(76)90878-0. [DOI] [PubMed] [Google Scholar]
- Clark J. L., Fuller J. L. Regulation of ornithine decarboxylase in 3T3 cells by putrescine and spermidine: indirect evidence for translational control. Biochemistry. 1975 Oct 7;14(20):4403–4409. doi: 10.1021/bi00691a010. [DOI] [PubMed] [Google Scholar]
- Coffino P., Bourne H. R., Friedrich U., Hochman J., Insel P. A., Lemaire I., Melmon K. L., Tomkins G. M. Molecular mechanisms of cyclic AMP action: a genetic approach. Recent Prog Horm Res. 1976;32:669–684. doi: 10.1016/b978-0-12-571132-6.50037-3. [DOI] [PubMed] [Google Scholar]
- Coffino P., Gray J. W., Tomkins G. M. Cyclic AMP, a nonessential regulator of the cell cycle. Proc Natl Acad Sci U S A. 1975 Mar;72(3):878–882. doi: 10.1073/pnas.72.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel V., Bourne H. R., Tomkins G. M. Altered metabolism and endogenous cyclic AMP in cultured cells deficient in cyclic AMP-binding proteins. Nat New Biol. 1973 Aug 8;244(136):167–169. doi: 10.1038/newbio244167a0. [DOI] [PubMed] [Google Scholar]
- Fong W. F., Heller J. S., Canellakis E. S. The appearance of an ornithine decarboxylase inhibitory protein upon the addition of putrescine to cell cultures. Biochim Biophys Acta. 1976 Apr 23;428(2):456–465. doi: 10.1016/0304-4165(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B., Shields R., Curtis D. Effect of cyclic nucleotides on the induction of ornithine decarboxylase in BHK cells by serum and insulin. Cell. 1974 Aug;2(4):229–233. doi: 10.1016/0092-8674(74)90015-4. [DOI] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Bourne H. R., Coffino P., Tomkins G. M. Cyclic AMP-dependent protein kinase: pivotal role in regulation of enzyme induction and growth. Science. 1975 Nov 28;190(4217):896–898. doi: 10.1126/science.171770. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemaire I., Coffino P. Cyclic AMP-induced cytolysis in S49 cells: selection of an unresponsive "deathless" mutant. Cell. 1977 May;11(1):149–155. doi: 10.1016/0092-8674(77)90325-7. [DOI] [PubMed] [Google Scholar]
- Mufson R. A., Astrup E. G., Simsiman R. C., Boutwell R. K. Dissociation of increases in levels of 3':5'-cyclic AMP and 3':5'-cyclic GMP from induction of ornithine decarboxylase by the tumor promoter 12-O-tetradecanoyl phorbol-13-acetate in mouse epidermis in vivo. Proc Natl Acad Sci U S A. 1977 Feb;74(2):657–661. doi: 10.1073/pnas.74.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Richman R., Park S., Akbar M., Yu S., Burke G. Regulation of thyroid ornithine ornithine decarboxylase (ODC) by thyrotropin. I. The rat. Endocrinology. 1975 Jun;96(6):1403–1412. doi: 10.1210/endo-96-6-1403. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Byus C. V., Manen C. A. Proposed model of major sequential biochemical events of a trophic response. Life Sci. 1976 Nov 1;19(9):1297–1305. [PubMed] [Google Scholar]
- Steinberg R. A., O'Farrell P. H., Friedrich U., Coffino P. Mutations causing charge alterations in regulatory subunits of the cAMP-dependent protein kinase of cultured S49 lymphoma cells. Cell. 1977 Mar;10(3):381–391. doi: 10.1016/0092-8674(77)90025-3. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Jänne J., Coppoc G. L., Geroch M. E., Schenone A. New aspects of polyamine biosynthesis in eukaryotic organisms. Adv Enzyme Regul. 1972;10:225–245. doi: 10.1016/0065-2571(72)90016-7. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y., Ichihara A. Induction of ornithine decarboxylase in cultured mouse L cells. I. Effects of cellular growth, hormones, and actinomycin D. J Biochem. 1976 Sep;80(3):557–562. doi: 10.1093/oxfordjournals.jbchem.a131311. [DOI] [PubMed] [Google Scholar]


