Abstract
Background
Tight junction proteins in the cell organize paracellular permeability and they play a critical role in apical cell-to-cell adhesion and epithelial polarity. Claudins are major integral membrane proteins of tight junctions, especially Claudin 1, 4, and 7, which are known as the impermeability Claudins. In this study, we investigated the importance of loss of Claudin 1, 4, and 7 expression, and their relation to tumor progression in colorectal cancer patients.
Material/Methods
Loss of Claudin 1, 4, and 7 expression was examined by immunohistochemical method in 70 patients diagnosed with colorectal cancer. Cases with loss of Claudin expression in <1/3 of tumor cells were classified as mild loss, whereas cases with loss of Claudin expression ≥1/3 of tumor cells were classified as moderate-to-marked loss in order to evaluate the relation between loss of Claudin 1, 4, and 7 expression and clinicopathologic data.
Results
The severe suppression of Claudin 1, 4, and 7 expression was found to be significantly related to the depth of tumor invasion, positive regional lymph nodes, histological grade, lymphovascular invasion, perineural invasion, and lymphocytic response. Additionally, severity of loss in Claudin 4 expression was found to have a relation with distant metastasis.
Conclusions
Claudin 1, 4, and 7 are important building blocks of paracellular adhesion molecules. Their decreased expression in colorectal cancer seems to have critical effects on cell proliferation, motility, invasion, and immune response against the tumor.
MeSH Keywords: Claudin 1, Claudin 4, Claudin 7, Colorectal Neoplasms, Prognosis, Tight Junctions
Background
Due to its high frequency, colorectal carcinoma (CRC) is the third most common cause of death in cancer-related deaths [1]. Studies to estimate the prognosis in CRC are still in progress. In the 7th edition of the AJCC (American Joint Committee on Cancer), the following have been added to the previously known high level of preoperative CEA: satellite tumor deposits without residue lymph node (LN) characteristics and durability with the infiltrative limit of the carcinoma, tumor regression against neoadjuvant chemotherapy, circumferential surgical margin, microsatellite instability, perineural invasion (PNI), lymphovascular invasion (LVI), and KRAS mutation status [2].
Paracellular tight junctions (TJs) organize paracellular permeability, and they have a critical role in apical cell-to-cell adhesion and epithelium cell polarity. As a result of the intense studies on the molecular architecture of TJs, it has been demonstrated that the Claudin family is an important component of this structure. There are 24 known members of the Claudin family, each with distinct a dispersion pattern [3,4]. Claudin 1, 4, 5, 7, 8, 11, 14, and 19 are impermeability Claudins and the increment in their expressions strengthens the density between epithelial cells [5–8]. Claudin 2, 10, and 16 are pore-forming Claudins and their increased expression reduces the density of epithelial cells [9]. Other Claudins have the ability to form paracellular anion/cation pores and water channels [3,7].
The invasive carcinoma development process from normal colon epithelium lasts 7–12 years. During this time, many genetic and epigenetic factors are active [10,11]. Loss of cell-to-cell adhesion is important in cellular transformation and acquisition of metastatic potential [12]. It has been recently shown that the Claudin protein family has significant roles in a series of pathophysiological events such as carcinoma development [13].
Claudin 1, 4, and 7 are important members of the Claudin protein family. It has been demonstrated that their expressions are altered in many malignancies. In the present study, we investigated the significance of the loss of Claudin 1, 4, and 7 expression and its relation with tumor progression.
Material and Methods
Patient selection
Our study included 110 cases histopathologically diagnosed with CRC from tissue samples acquired by surgical resection in Antalya Education and Research Hospital between 2008 and 2012. We excluded 20 patients whose follow-ups and treatments were not carried out in our hospital. Samples of the remaining 90 patients were histopathologically re-staged according to the 7th edition of the American Joint Committee on Cancer (AJCC). Claudin 1, 4, and 7 expressions were examined by immunohistochemical method. For technical reasons, we excluded 20 cases in which the immunohistochemical expression was not eligible for evaluation. As a result, samples of 70 patients were examined. Consent was obtained from the local ethics committee. Demographic data such as age, sex, stage of disease, and treatments were obtained by retrospectively searching patient files.
Tissue preparation and immunohistochemical staining
Resection materials obtained after colorectal surgery were placed in 10% formaldehyde immediately after the process and fixed for 24 h. After fixation, pathologically sampled tumor samples were embedded in paraffin after routine tissue follow-up. Immunohistochemical staining was applied on cross-sections containing nominal tumor samples that were evaluated with hematoxylin and eosin staining. Cross-sections of 4-μm thickness prepared for immunohistochemical staining were deparaffinized in an oven at 60°C for 2 h. Subsequently, they were kept in xylene for 30 min and 100% alcohol for 30 min, and then washed with water. Laminas were kept in a solution buffered with 10% citrate in the microwave at maximum power (800 watts) for 15 min. Afterwards, the power was decreased by half for an additional 20 min in the microwave. Laminas brought out of the microwave were kept at room temperature for 20 min. Endogenous peroxidase activity was removed by being kept in 3% hydrogen peroxide for 10 min. After being kept in primary antibodies Claudin 1 (rabbit polyclonal, clone ab15098, dilution 1:200, Abcam, Cambridge, MA, USA), Claudin 4 (rabbit polyclonal, clone ab15104, dilution 1:200, Abcam, Cambridge, MA, USA), and Claudin 7 (rabbit polyclonal, clone ab27487, dilution 1:200, Abcam, Cambridge, MA, USA) for 60 min, they were washed in PBS for 5 min. Afterwards, they were treated with biotinylated secondary antibody (Vector Laboratories, Burlingham, CA) for 20 min and washed with PBS for 5 min. They were kept with peroxidase conjugated antibody for 20 min. Then they were washed in PBS for 5 min. They were kept in chromogenic DAB for 5 min. Laminas were washed under tap water and then counterstained with hematoxylin. They were dehydrated, dried, and covered with Entellan. In each case, normal colorectal mucosa adjacent to the carcinoma was used as an internal positive control for each Claudin. For negative controls, the primary antibodies were omitted.
Evaluation of immunohistochemically stained sections
Expression rates for the tumor cells in the samples were evaluated by 2 pathologists (DS, ASA) blinded to patient clinical features. In each case, normal colorectal mucosa adjacent to the carcinoma showed complete membranous staining with each Claudin. This membranous staining observed in the non-neoplastic surface and gland epithelium was used as the positive internal control for each Claudin in the evaluation of cases. Vascular endothelial and smooth-muscle cells, fibroblasts, inflammatory cells, smooth-muscle cells of muscular layers, neural structures, and adipocytes within the cross-section showed no staining. Absence of expression in these structures was used as the negative internal control for each Claudin in immunohistochemical evaluation. We were careful to ensure that the staining pattern for tumor cells was membranous with each Claudin. Immunohistochemical evaluation was performed by the method that Matsuoka et al. used [14]. Tumors with all tumor cells showing complete membranous staining were classified as score 0. Cases with loss of Claudin expression were divided into 3 grades: cases with loss of expression in <1/3 of tumor cells were classified as score 1, cases with 1/3–2/3 losses were classified as score 2, and cases with ≥2/3 loss were classified as score 3. To evaluate the relation between loss of Claudin expressions and clinicopathologic data, score 0 and 1 patients were further grouped as mild loss, and score 2 and 3 patients were categorized as moderate-to-marked loss. Figure 1 shows various staining patterns for each Claudin in non-neoplastic mucosa and tumor.
Figure 1.
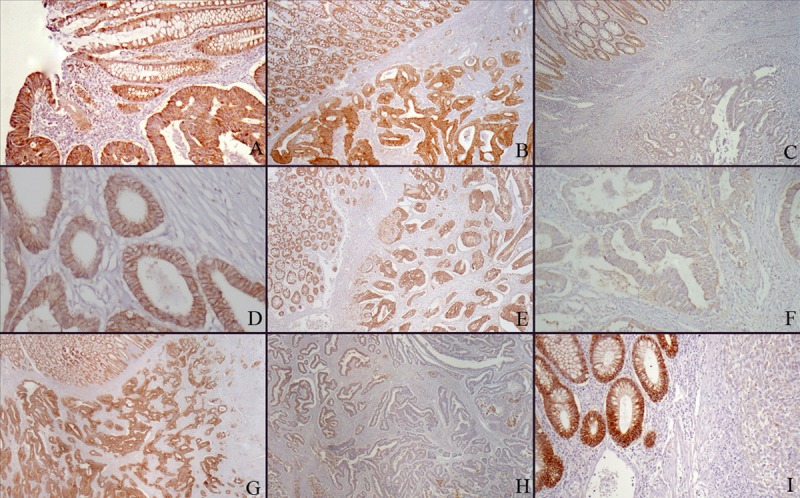
(A) Claudin 1 positivity in non-neoplastic surface epithelium, in situ carcinoma, and adenocarcinoma (Claudin 1 ×100). (B) Claudin 1 score 0: Same expression in non-neoplastic mucosa and adenocarcinoma (Claudin 1 ×40). (C) Claudin 1 score 3: Positive staining in non-neoplastic mucosa and complete loss of expression in adenocarcinoma (Claudin 1 ×40). (D) Claudin 1 score 0: Complete membranous Claudin 4 positivity in adenocarcinoma (Claudin 4 ×200). (E) Claudin 4 score 0: Same membranous expression in adenocarcinoma and non-neoplastic mucosa (Claudin 4 ×40). (F) Claudin 4 score 3: Complete loss of expression in adenocarcinoma (Claudin 4 ×200). (G) Claudin 7 score 0: Same membranous expression in adenocarcinoma and non-neoplastic mucosa (Claudin 7 ×40). (H) Claudin 7 score 2: Loss of expression of more than 2/3 in adenocarcinoma (Claudin 7 ×40). (I) Claudin 7 score 3: Membranous staining in non-neoplastic mucosal glands and complete expression loss in poorly differentiated adenocarcinoma (Claudin 7 ×100).
Statistical analysis
Statistical analyses were carried out by SPSS software for Windows 15.0. Suitability of variables to normal dispersion was observed by using visual (histograms and probability graphics) and analytical (Kolmogorov-Smirnov, and Shapiro-Wilk tests) methods. In Kolmogorov-Smirnov testing, p values above 0.05 are considered as normal dispersion. Differences between groups were observed by using chi-square and Mann-Whitney U test. Kaplan-Meier survival analysis was performed for the relation of each immunohistochemical positive and negative result with survival. Statistical differences were confirmed by log-ranking test. P values under 0.05 were considered to be significant.
Results
A total of 70 CRC patients – 30 (42.9%) females and 40 (57.1%) males – were included in the study. Mean age of patients was 62.1±13.8 years (range 32–83 years). Conventional adenocarcinoma was determined in 57 (81.4%) patients, mucinous adenocarcinoma was determined in 10 (14.3%), and signet ring cell carcinoma was determined in 3 (4.3%) patients. T2 disease was detected in 2 patients (2.9%), and T3 and T4 disease were determined in 49 (70%) and 19 (27.1%) patients, respectively. Regional lymph node (LN) metastasis was positive in 42 (60%) patients, and negative in 28 (40%) patients. Distant metastasis was found in 16 (22.9%) patients, and there were no distant metastasis in 54 (77.1%) patients. The most frequent metastasis site was the liver, with a determination rate of 14.3% (10 patients).
When patients were evaluated according to their disease stage, we found that 1 patient (1.4%) was in stage 1, 25 patients (35.7%) were in stage 2, 29 patients (41.4%) were in stage 3, and 15 patients (21.5%) were in stage 4. When patient samples were evaluated in terms of histological grades, 46 patients (65.7%) had grade 1 tumor and 24 patients (34.3%) had grade 2 tumor. Lymphocytic response (LR) was determined in 23 (32.9%), and PNI and LVI was determined in 54 (77.1%) and 55 (78.6%) patients, respectively.
Loss of Claudin 1 expression was evaluated as score 1 in 17 (24.3%) patients, score 2 in 26 (37.1%) patients, and score 3 in 27 (38.6%) patients. There was no relation between the severity of loss of Claudin 1 expression and sex, metastasis status, or histological type. There was a significant relation between loss of Claudin 1 expression with tumor invasion depth, LN status, stage of disease, tumor grade, PNI, LVI, and LR. In patients with moderate-to-marked loss of Claudin 1, T stage was advanced and there were relatively more patients with LN metastasis. Also, there was more LVI and PNI in these patients, because the tumor grade was higher and LR against tumor was lower (Table 1).
Table 1.
Relation between demographic and tumor characteristics of patients based on Claudin 1 expression.
| Claudin 1 mild loss N (%) | Claudin 1 modarate to marked loss N (%) | P value | |
|---|---|---|---|
| Sex | 0.334 | ||
| Female | 9 (52.9) | 21 (39.6) | |
| Male | 8 (47.1) | 32 (60.4) | |
|
| |||
| Age | 62.5±13.5 | 61.9±14 | 0.8 |
|
| |||
| T stage | 0.004 | ||
| T2 | 1 (5.9) | 1 (1.9) | |
| T3 | 16 (94.1) | 33 (62.3) | |
| T4 | 0 | 19 (27.1) | |
|
| |||
| N status | |||
| Node negative | 15 (88.2) | 13 (25.4) | <0.001 |
| Node positive | 2 (11.8) | 40 (75.5) | |
|
| |||
| M status | 0.214 | ||
| Metastasis negative | 15 (88.2) | 39 (73.6) | |
| Metastasis positive | 2 (11.8) | 14 (26.4) | |
|
| |||
| Stage | |||
| Stage 1 | 1 (5.9) | 0 | |
| Stage 2 | 14 (82.4) | 11 (20.8) | <0.001 |
| Stage 3 | 0 | 29 (54.7) | |
| Stage 4 | 2 (11.8) | 13 (24.5) | |
|
| |||
| Histological sub-type | 0.552 | ||
| Conventional adenocarcinoma | 15 (88.2) | 42 (79.2) | |
| Mucinous adenocarcinoma | 2 (11.8) | 8 (15.1) | |
| Signet ring cell adenocarcinoma | 0 | 3 (5.7) | |
|
| |||
| Grade | 0.026 | ||
| Grade 1 | 15 (88.2) | 31 (58.5) | |
| Grade 2 | 2 (11.8) | 22 (41.5) | |
|
| |||
| Perineural invasion | 0.04 | ||
| Negative | 7 (41.1) | 9 (17) | |
| Positive | 10 (58.8) | 44 (83) | |
|
| |||
| Lymphovascular invasion | 0.024 | ||
| Negative | 7 (41.1) | 8 (15.1) | |
| Positive | 10 (58.8) | 45 (84.9) | |
|
| |||
| Lymphocytic response | 0.009 | ||
| Negative | 7 (41.1) | 40 (%75.5) | |
| Positive | 10 (58.8) | 13 (24.5) | |
Loss of Claudin 4 expression was evaluated as score 1 in 28 (40%) patients, score 2 in 33 (47.1%) patients, and score 3 in 9 (12.9%) patients. There were no significant relations between the severity of loss of Claudin 4 expression and sex, metastasis status, and histological type. There was a significant relation between loss of Claudin 4 expression, and tumor invasion depth, LN status, stage of the disease, tumor grade, PNI, LVI, and LR. Patients with moderate-to-marked loss of Claudin 4 expression had characteristics similar to those in the moderate-to-marked loss of Claudin 1 expression group, but there was a high ratio of distant metastasis in this group (Table 2).
Table 2.
Relation between demographic and tumor characteristics of patients based on Claudin 4 expression.
| Claudin 4 mild loss N (%) | Claudin 4 modarate to marked loss N (%) | P value | |
|---|---|---|---|
| Sex | 0.334 | ||
| Female | 13 (46.4) | 17 (40.5) | |
| Male | 15 (53.6) | 25 (59.5) | |
|
| |||
| Age | 65.4±13.5 | 60.4±13.8 | 0.8 |
|
| |||
| T stage | 0.004 | ||
| T2 | 2 (7.2) | 0 | |
| T3 | 21 (75) | 28 (66.7) | |
| T4 | 5 (17.9) | 14 (33.3) | |
|
| |||
| N status | |||
| Node negative | 19 (67.9) | 9 (21.4) | <0.001 |
| Node positive | 9 (32.1) | 33 (78.6) | |
|
| |||
| M status | 0.214 | ||
| Metastasis negative | 25 (89.3) | 29 (69) | |
| Metastasis positive | 3 (10.7) | 13 (31) | |
|
| |||
| Stage | |||
| Stage 1 | 1 (3.6) | 0 | |
| Stage 2 | 18 (64.3) | 7 (16.7) | <0.001 |
| Stage 3 | 6 (21.4) | 23 (54.8) | |
| Stage 4 | 3 (10.7) | 12 (28.6) | |
|
| |||
| Histological sub-type | 0.552 | ||
| Conventional adenocarcinoma | 23 (82.1) | 34 (81) | |
| Mucinous adenocarcinoma | 5 (17.9) | 5 (11.9) | |
| Signet ring cell adenocarcinoma | 0 | 3 (7.1) | |
|
| |||
| Grade | 0.026 | ||
| Grade 1 | 25 (89.3) | 21 (50) | |
| Grade 2 | 3 (10.7) | 21 (50) | |
|
| |||
| Perineural invasion | 0.04 | ||
| Negative | 11 (39.3) | 5 (11.9) | |
| Positive | 17 (60.7) | 37 (88.1) | |
|
| |||
| Lymphovascular invasion | 0.024 | ||
| Negative | 7 (41.1) | 8 (15.1) | |
| Positive | 10 (58.8) | 45 (84.9) | |
|
| |||
| Lymphocytic response | 0.009 | ||
| Negative | 14 (50) | 33 (78.6) | |
| Positive | 14 (50) | 9 (21.9) | |
Loss of Claudin 7 expression was evaluated as score 0 in 16 (22.9%) patients, score 1 in 30 (42.9%) patients, and score 3 in 24 (34.2%) patients. There was no significant relation between the severity of loss of Claudin 7 expression and sex, metastasis status, and histological type. There was a significant relation between loss of Claudin 7 expression and tumor invasion depth, LN status, stage of the disease, tumor grade, PNI, LVI, and LR. Patients with moderate-to-marked loss of Claudin 7 expression had characteristics similar to those of the patients in the moderate-to-marked loss of Claudin 1 expression group (Table 3).
Table 3.
Relation between demographic and tumor characteristics of patients based on Claudin 7 expression.
| Claudin 7 mild loss N (%) | Claudin 7 modarate to marked loss N (%) | P value | |
|---|---|---|---|
| Sex | 0.511 | ||
| Female | 8 (50) | 22 (40.7) | |
| Male | 8 (50) | 32 (59.3) | |
|
| |||
| Age | 65.4±13.5 | 60.4±13.8 | 0.177 |
|
| |||
| T stage | 0.005 | ||
| T2 | 1 (6.3) | 1 (1.9) | |
| T3 | 15 (93.8) | 34 (63) | |
| T4 | 0 | 19 (35.2) | |
|
| |||
| N status | |||
| Node negative | 14 (87.5) | 14 (25.9) | <0.001 |
| Node positive | 2 (12.5) | 40 (74.1) | |
|
| |||
| M status | 0.265 | ||
| Metastasis negative | 14 (87.5) | 40 (74.1) | |
| Metastasis positive | 2 (12.5) | 14 (25.9) | |
|
| |||
| Stage | |||
| Stage 1 | 1 (6.3) | 0 | |
| Stage 2 | 13 (81.3) | 12 (22.2) | <0.001 |
| Stage 3 | 0 | 29 (53.7) | |
| Stage 4 | 2 (12.5) | 13 (24.1) | |
|
| |||
| Histological sub-type | 0.444 | ||
| Conventional adenocarcinoma | 14 (87.5) | 43 (79.6) | |
| Mucinous adenocarcinoma | 2 (12.5) | 8 (14.8) | |
| Signet ring cell adenocarcinoma | 0 | 3 (5.6) | |
|
| |||
| Grade | 0.038 | ||
| Grade 1 | 14 (87.5) | 32 (59.3) | |
| Grade 2 | 2 (12.5) | 22 (34.3) | |
|
| |||
| Perineural invasion | 0.024 | ||
| Negative | 7 (43.8) | 9 (16.7) | |
| Positive | 9 (56.3) | 45 (83.3) | |
|
| |||
| Lymphovascular invasion | 0.014 | ||
| Negative | 7 (43.8) | 8 (14.8) | |
| Positive | 9 (56.3) | 46 (85.2) | |
|
| |||
| Lymphocytic response | 0.004 | ||
| Negative | 6 (37.5) | 41 (75.9) | |
| Positive | 10 (62.5) | 13 (24.1) | |
The average follow-up time was 38.1 months, and median follow-up time was 37.6 months. Due to inadequate follow-up time, we were unable to gain information about the median survival period in survival analysis. Average survival of the patients was 66.3±4.5 months (95% confidence interval 57.3–75.2 months). No statistically significant relation was detected between Claudin 1, 4, and 7 and survival (p=0.589, =: 0.749, and p=0.755, respectively).
Discussion
We have demonstrated that Claudin 1, 4, and 7, which are important TJs proteins, are related with tumor invasion depth, LN status, stage of the disease, tumor grade, PNI, LVI, and LR. The losses of expressions of these proteins caused more aggressive cancer behavior.
TJs are the most important intracellular joints in the epithelium and endothelial cells. There are 2 important functions determined for TJs: the paracellular permeability regulation and the maintenance of cell polarization with window function. The functions of TJs closely related to cancer cell biology are epithelial paracellular permeability and loss of cell polarization [15,16].
Tan et al. demonstrated that Claudin 1 release and dispersion were related to the dissociation of pancreatic cancer cells [17]. Although it is not clear in which way the increased Claudin expression contributes to carcinogenesis, it is suggested that cancer aggression is increased by the matrix metalloproteinase activation due to over-expression [18]. Studies have demonstrated that loss of expression plays a role in carcinogenesis beyond Claudin over-expression. Dysregulation of Claudin 7 expression was determined in breast-invasive ductal carcinoma, head-neck carcinoma, and metastatic breast carcinoma [6,18–20].
Claudin over-expression or loss of expression varies in different cancer types. In hepatocellular carcinoma and renal cell carcinoma, Claudin 4 and 5 expressions are lost; whereas Claudin 3 and 4 over-expressions are detected in various cancer types, including pancreatic ductal adenocarcinoma, and prostate, uterus, ovary, and breast cancers [21,22]. The weak release of Claudin 2 is observed in breast and prostate carcinomas, and it has been demonstrated that Claudin 1 and 7 expressions, which cannot be detected in normal cervical squamous epithelium, increases in cervical neoplasia [23,24]. Research has suggested that loss of Claudin expression leads to repression of TJs and that this repression culminates in cell proliferation, motility and invasion; consequently, loss of Claudin expression has a role in carcinogenesis [13].
Claudin 1 expression in CRC has been researched: Shibutani et al. demonstrated that loss of Claudin 1 expression was related to LVI, histological grade, decreased disease-free survival (DFS), and overall survival (OAS) in stage 2 and 3 CRC, and reported that it was an independent predictor for recurrence in multivariate analysis [25].
In a study by Abdelzaher et al., 62% less Claudin 1 was found in CRC cases, and this decrement was found to be related to grade, depth of tumor invasion, LN involvement and stage, suggesting that it has a predictive value in demonstrating stage and LN involvement [26]. Yoshida et al. determined that loss of Claudin 1 in stage 2 and 3 was related with recurrence and decreased survival [27].
Nakagawa et al. showed that high Claudin 1 expression was related with a better prognosis, and decreased expression was related with poor OAS and DFS. In univariate analyses, loss of Claudin 1 expression was found to be significantly related to increased histological grade, morphologically aggressive sub-type, increased tumor size, tumor invasion depth, LVI, LN, and distant metastasis [28]. Moreover, Resnick et al. found that weak Claudin 1 expression was related to grade and poor survival, and it was an independent predictor of recurrence [29]. Ersöz et al. reported that Claudin 1 found expression was significantly decreased in LN-positive cases [30].
In our study, similar to the results of previous studies, loss of Claudin 1 expression was found to be related to depth of tumor invasion, LN involvement, stage of the disease, histological grade, LVI, and PNI. In addition, we determined that LR against tumor was increased in parallel with the loss of Claudin 1 expression. This finding suggests that loss of Claudin 1 expression might play a role in the distortion of immune response against the tumor.
In contrast with our findings, there are some studies demonstrating that Claudin 1 expression unfavorably affected prognosis in CRC. In their series on Claudin 1 mRNA and protein analyses, Huo et al. reported that increased Claudin 1 levels, compared to normal tissue, were related with tumor depth [31]. Tang et al. demonstrated that Claudin 1 was increased in CRC cases [32]. Some studies report that Claudin 1 mRNA levels are upregulated in CRC [31,33–35]. An in vitro study reported that Claudin 1 over-expression increased the cancer aggressiveness by matrix metalloproteinase activation [36]. In these studies, Claudin 1 upregulation was demonstrated by increased mRNA levels. In our study, loss of Claudin 1 expression was demonstrated with immunohistochemistry. The results of our study were similar with the those of Matsuoka et al., possibly because our immunohistochemical evaluation methods were the same [14].
In studies in which Claudin 4 expression in CRC was researched, findings similar to data showing loss of Claudin 1 expression were obtained. In agreement with our results, Matsuoka et al. found the decreased Claudin 4 expression was related with advanced stage, poor tumor differentiation, LVI, distant metastasis, and poor prognosis [14]. Ersöz et al. demonstrated that Claudin 4 was significantly lower in LN-positive cases [14]. Additionally, Ueda et al. reported 57% less Claudin 4 in CRC cases, and suggested that Claudin could be a biomarker for use in determining distant metastasis [15]. We have also demonstrated that Claudin 4 expression was related with distant metastasis.
We found loss of Claudin 7 expression resulted in similar tumor behaviors as in Claudin 1 and 4. Bornholdt et al. determined a 2.7-fold reduction in Claudin 7 levels in CRC cases compared to healthy individuals. Also, they found significantly less in colorectal dysplasia cases and suggested that in dysplasia cases the decrement in Claudin 7 is an early phase of colorectal carcinogenesis [9]. Similarly, Tang et al. found that a Claudin 7 decrement was related to LN metastasis [32].
Nakayama et al. determined a significant relation between LVI and clinical stage with degree of loss of Claudin 7 [38]. Oshima et al. reported that loss of Claudin 7 expression was related to venous invasion and liver metastasis in CRC cases [39]. In our study, we did not find a relation between Claudin 7 expression and distant metastasis.
Conclusions
Carcinogenesis and its final phase – metastasis – create a complex process requiring certain steps such as decreased adhesion, increased motility and invasion, proteolysis, and resistance to apoptosis. Claudin 1, 4, and 7 are important building blocks of cell-to-cell adhesion molecules. Therefore, the suppression of expression in cancer cells seems to have effects on cell proliferation, motility, invasion, and immune response to tumor.
In light of all these findings, we suggest that the determination of a significant relation between loss of Claudin 1, 4, and 7 expressions, and depth of tumor invasion, positive LN metastasis, and histological grade, LVI, PNI, and LR supports our hypothesis. Introduction of these specific members of the Claudin family in CRC cases into molecular reagent profiles may significantly affect future clinical approaches to this issue.
Footnotes
Conflict of interest
We have no financial interest or conflict of interest in association with this work.
Source of support: Departmental sources
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC (American Joint Committee on Cancer) Cancer Staging Manual. 7th edition. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 3.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nature Reviews Molecular Cell Biology. 2001;2:285–93. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 4.Rahner C, Mitic L, Anderson JM. Heterogenity in expression and subcellular localization of Claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–22. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 5.Krause G, Winkler L, Mueller SL, et al. Structure and function of Claudins. Biochim Biophys Acta. 2008;1778:631–45. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Ouban A, Ahmed AA. Claudins in human cancer: a review. Histol Histopathol. 2010;25:83–90. doi: 10.14670/HH-25.83. [DOI] [PubMed] [Google Scholar]
- 7.Will C, Fromm M, Muller D. Claudin tight junction proteins: novel aspects in paracellular transport. Perit Dial Int. 2008;28:577–84. [PubMed] [Google Scholar]
- 8.Oliveira SS, Morgado-Diaz JA. Claudins: multifunctional players in epithelial tight junctions and their role in cancer. Cell Mol Life Sci. 2007;64:17–28. doi: 10.1007/s00018-006-6314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornholdt J, Friis S, Godiksen S, et al. The level of Claudin-7 is reduced as an early event in colorectal carcinogenesis. BMC Cancer. 2011;11:65. doi: 10.1186/1471-2407-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han DP, Zhu QL, Cui JT, et al. Polo-like kinase 1 is overexpressed in colorectal cancer and participates in the migration and invasion of colorectal cancer cells. Med Sci Monit. 2012;18(6):BR237–46. doi: 10.12659/MSM.882900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao L, Bai L, Nan QZ. Activation of Rho GTPase Cdc42 promotes adhesion and invasion in colorectal cancer cells. Med Sci Monit. 2013;19:201–7. doi: 10.12659/MSMBR.883983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritzsche FR, Oelrich B, Johannsen M, et al. Claudin-1 protein expression is a prognostic marker of patient survival in renal cell carcinomas. Clin Cancer Res. 2008;14:7035–42. doi: 10.1158/1078-0432.CCR-08-0855. [DOI] [PubMed] [Google Scholar]
- 13.Singh AB, Sharma A, Dhawan P. Claudin family of proteins and cancer: an overview. J Oncol. 2010;2010:541957. doi: 10.1155/2010/541957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuoka T, Mitomi H, Fukui N, et al. Cluster analysis of Claudin-1 and -4, E-cadherin, and β-catenin expression in colorectal cancers. J Surg Oncol. 2011;103:674–86. doi: 10.1002/jso.21854. [DOI] [PubMed] [Google Scholar]
- 15.Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochimica et Biophysica Acta. 2009;4:872–91. doi: 10.1016/j.bbamem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Miyoshi J, Takai Y. Molecular perspective on tight junction assembly and epithelial polarity. Advanced Drug Delivery Reviews. 2005;57:815–25. doi: 10.1016/j.addr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Tan C, Cruet-Hennequart S, Troussard A, et al. Regulation of tumor angiogenesis by integrin-linked kinase (ILK) Cancer Cell. 2004;5:79–90. doi: 10.1016/s1535-6108(03)00281-2. [DOI] [PubMed] [Google Scholar]
- 18.Kominsky SL, Argani P, Korz D, et al. Loss of the tight junction protein Claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22:2021–33. doi: 10.1038/sj.onc.1206199. [DOI] [PubMed] [Google Scholar]
- 19.Usami Y, Chiba H, Nakayama F, et al. Reduced expression of Claudin-7 correlates with invasion and metastasis in squamous cell carcinoma of the esophagus. Human Pathology. 2006;37:569–77. doi: 10.1016/j.humpath.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Sauer T, Pedersen, Ebeltoft K, Næss O. Reduced expression of Claudin-7 in fine needle aspirates from breast carcinomas correlate with grading and metastatic disease. Cytopathology. 2005;16:193–98. doi: 10.1111/j.1365-2303.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 21.Michl P, Barth C, Buchholz M, et al. Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res. 2003;63:6265–71. [PubMed] [Google Scholar]
- 22.Rangel LBA, Agarwal R, D’Souza T, et al. Tight junction proteins Claudin-3 and Claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin Cancer Res. 2003;9:2567–75. [PubMed] [Google Scholar]
- 23.Soini Y. Expression of Claudins 1, 2, 3, 4, 5 and 7 in various types of tumours. Histopathology. 2005;46:551–60. doi: 10.1111/j.1365-2559.2005.02127.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee JW, Lee SJ, Seo J, et al. Increased expressions of Claudin-1 and Claudin-7 during the progression of cervical neoplasia. Gynecol Oncol. 2005;97:53–59. doi: 10.1016/j.ygyno.2004.11.058. [DOI] [PubMed] [Google Scholar]
- 25.Shibutani M, Noda E, Maeda K, et al. Low expression of Claudin-1 and presence of poorly-differentiated tumor clusters correlate with poor prognosis in colorectal cancer. Anticancer Res. 2013;33:3301–6. [PubMed] [Google Scholar]
- 26.Abdelzaher E, Rizk AM, Bessa SS, Omer KM. Predictive value of immunohistochemical expression of Claudin-1 in colonic carcinoma. J Egypt Natl Canc Inst. 2011;23:123–31. doi: 10.1016/j.jnci.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida T, Kinugasa T, Akagi Y, et al. Decreased expression of Claudin-1 in rectal cancer: a factor for recurrence and poor prognosis. Anticancer Res. 2011;31:2517–25. [PubMed] [Google Scholar]
- 28.Nakagawa S, Miyoshi N, Ishii H, et al. Expression of CLDN1 in colorectal cancer: a novel marker for prognosis. Int J Oncol. 2011;39:791–96. doi: 10.3892/ijo.2011.1102. [DOI] [PubMed] [Google Scholar]
- 29.Resnick MB, Konkin T, Routhier J, et al. Claudin-1 is a strong prognostic indicator in stage II colonic cancer: a tissue microarray study. Mod Pathol. 2005;18:511–18. doi: 10.1038/modpathol.3800301. [DOI] [PubMed] [Google Scholar]
- 30.Ersoz S, Mungan S, Cobanoglu U, et al. Prognostic importance of Claudin-1 and Claudin-4 expression in colon carcinomas. Pathol Res Pract. 2011;15:285–89. doi: 10.1016/j.prp.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Huo Q, Kinugasa T, Wang L, et al. Claudin-1 protein is a major factor involved in the tumorigenesis of colorectal cancer. Anticancer Res. 2009;29:851–57. [PubMed] [Google Scholar]
- 32.Tang W, Dou T, Zhong M, Wu Z. Dysregulation of Claudin family genes in colorectal cancer in a Chinese population. Biofactors. 2011;37:65–73. doi: 10.1002/biof.138. [DOI] [PubMed] [Google Scholar]
- 33.Dhawan P, Singh AB, Deane NG, et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765–76. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gröne J, Weber B, Staub E, et al. Differential expression of genes encoding tight junction proteins in colorectal cancer: frequent dysregulation of Claudin-1, -8 and -12. Int J Colorectal Dis. 2007;22:651–59. doi: 10.1007/s00384-006-0197-3. [DOI] [PubMed] [Google Scholar]
- 35.Miwa N, Furuse M, Tsukita S, et al. Involvement of Claudin-1 in the b-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol Res. 2001;12:469–76. doi: 10.3727/096504001108747477. [DOI] [PubMed] [Google Scholar]
- 36.Takehara M, Nishimura T, Mima S, et al. Effect of Claudin expression on paracellular permeability, migration and invasion of colonic cancer cells. Biol Pharm Bull. 2009;32:825–31. doi: 10.1248/bpb.32.825. [DOI] [PubMed] [Google Scholar]
- 37.Ueda J, Semba S, Chiba H. Heterogeneous expression of Claudin-4 in human colorectal cancer: decreased Claudin-4 expression at the invasive front correlates cancer invasion and metastasis. Pathobiology. 2007;74:32–41. doi: 10.1159/000101049. [DOI] [PubMed] [Google Scholar]
- 38.Nakayama F, Semba S, Usami Y, et al. Hypermethylation-modulated downregulation of Claudin-7 expression promotes the progression of colorectal carcinoma. Pathobiology. 2008;75:177–85. doi: 10.1159/000124978. [DOI] [PubMed] [Google Scholar]
- 39.Oshima T, Kunisaki C, Yoshihara K. Reduced expression of the Claudin-7 gene correlates with venous invasion and liver metastasis in colorectal cancer. Oncol Rep. 2008;19:953–59. [PubMed] [Google Scholar]


