Abstract
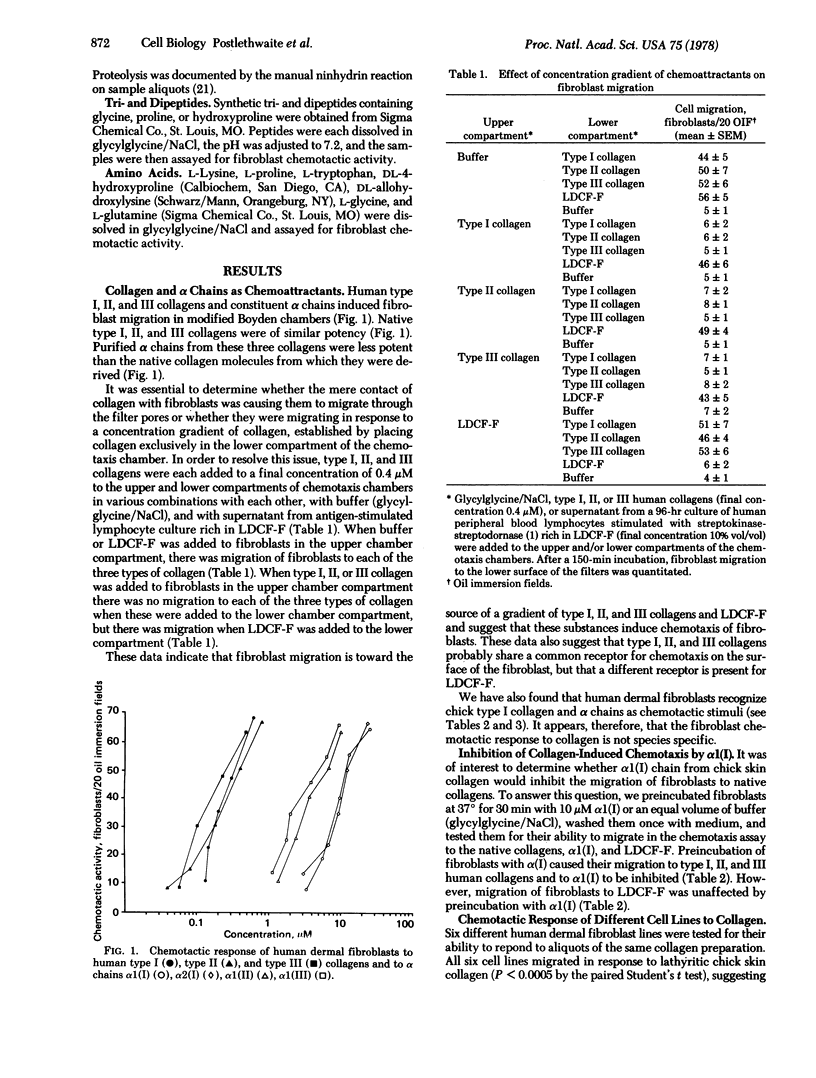
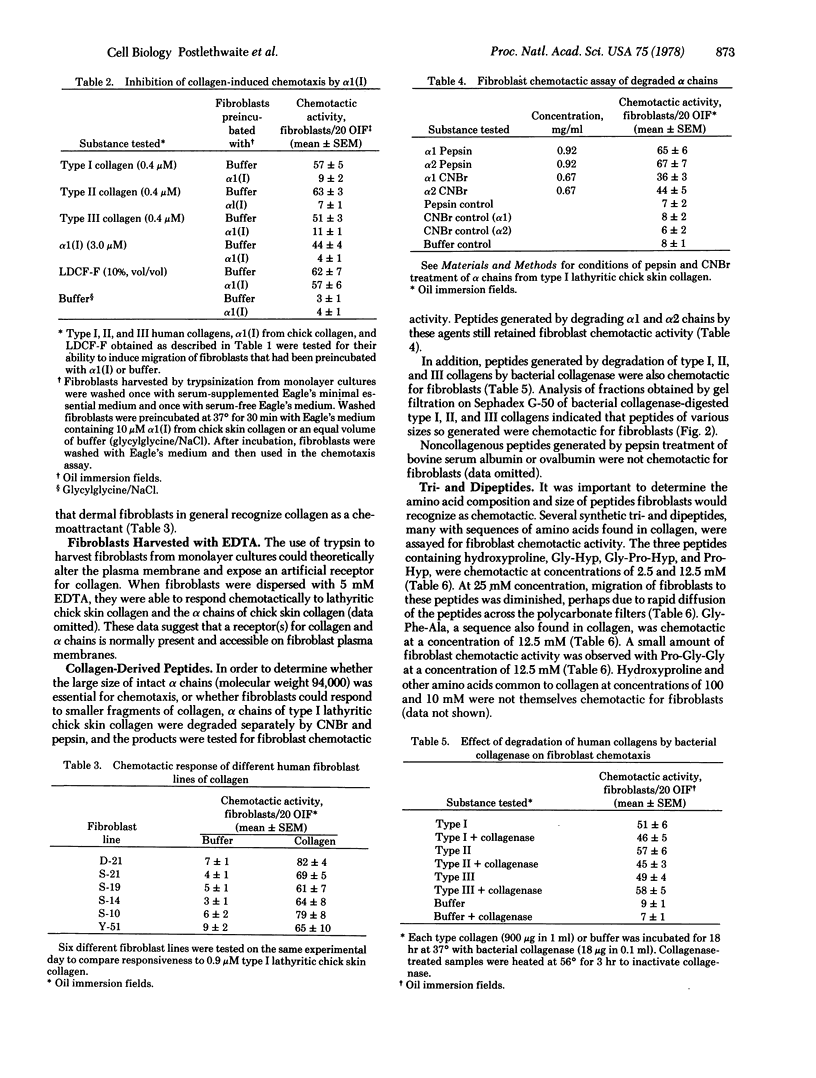
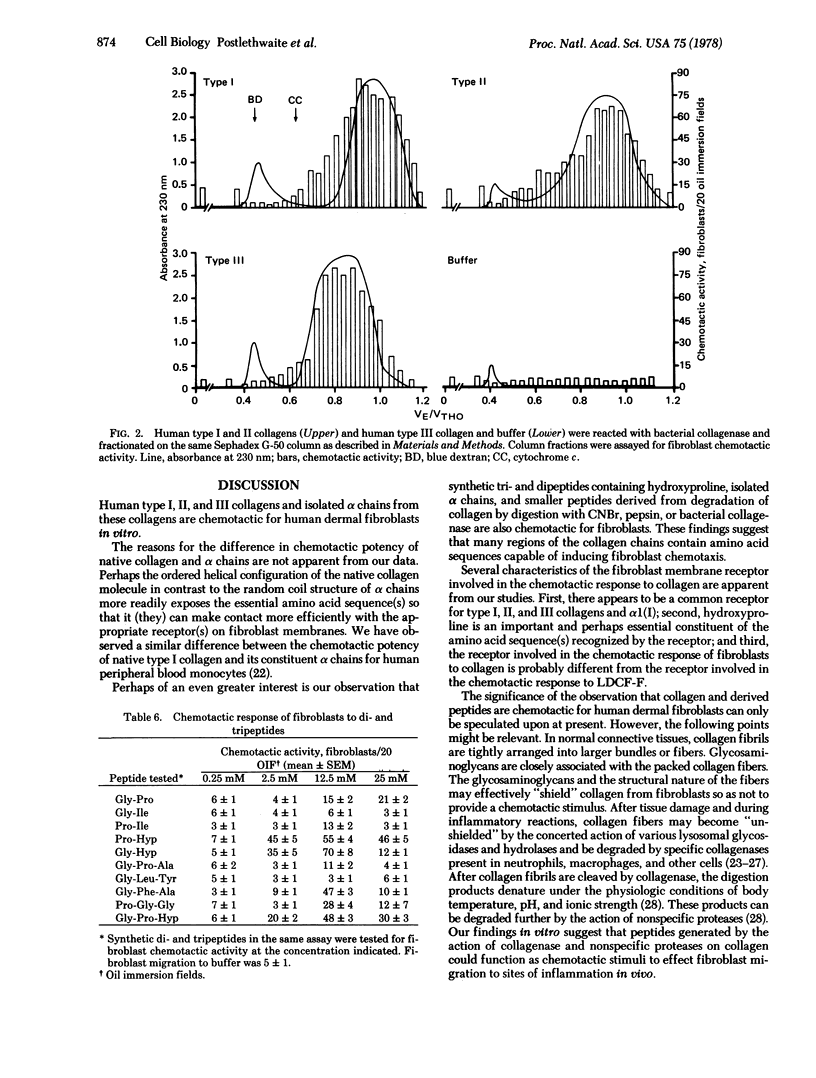
The chemotactice response of human dermal fibroblasts of type I, II, and III human collagens and collagen-derived peptides was quantitated by an in vitro assay. All three native human collagens and constituent alpha chains can serve as chemoattractants for fibroblasts in vitro. When type I, II, and III collagens were digested by bacterial collagenase, the resulting peptides were also chemotactic. In addition, synthetic di- and tripeptides containing hydroxyproline were also chemotactic for fibroblasts. Since collagen is degraded and remodeled at sites of tissue injury and inflammation, these findings suggest that collagen and collagen-degradation peptides might function as chemotactic stimuli for fibroblasts in vivo and attract these cells to effect repair of damaged tissue.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler W. T., Birkedal-Hansen H., Beegle W. F., Taylor R. E., Chung E. Proteins of the periodontium. Identification of collagens with the [alpha1(I)]2alpha2 and [alpha1(III)]3 structures in bovine periodontal ligament. J Biol Chem. 1975 Dec 10;250(23):8907–8912. [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Chung E., Rhodes K., Miller E. J. Isolation of three collagenous components of probable basement membrane origin from several tissues. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1167–1174. doi: 10.1016/0006-291x(76)90776-2. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr, Munderloh N. H. Isolation and characterization of CNBr peptides of human (alpha 1 (III) )3 collagen and tissue distribution of (alpha 1 (I) )2 alpha 2 and (alpha 1 (III) )3 collagens. J Biol Chem. 1975 Dec 25;250(24):9304–9312. [PubMed] [Google Scholar]
- Gallop P. M., Blumenfeld O. O., Seifter S. Structure and metabolism of connective 801 tissue proteins. Annu Rev Biochem. 1972;41:617–672. doi: 10.1146/annurev.bi.41.070172.003153. [DOI] [PubMed] [Google Scholar]
- Gay S., Fietzek P. P., Remberger K., Eder M., Kühn K. Liver cirrhosis: immunofluorescence and biochemical studies demonstrate two types of collagen. Klin Wochenschr. 1975 Mar 1;53(5):205–208. doi: 10.1007/BF01468808. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, DiBona D. R., Krane S. M. Collagenases in human synovial fluid. J Clin Invest. 1969 Nov;48(11):2104–2113. doi: 10.1172/JCI106177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (first of three parts). N Engl J Med. 1974 Sep 12;291(11):557–563. doi: 10.1056/NEJM197409122911105. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Igarashi S., Gross J. Characterization of the cyanogen bromide peptides from the alpha-2 chain of chick skin collagen. Biochemistry. 1969 Aug;8(8):3200–3204. doi: 10.1021/bi00836a011. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Piez K. A., Gross J. Characterization of the alpha-chains of chick skin collagen and the nature of the NH2-terminal cross-link region. Biochemistry. 1969 Sep;8(9):3648–3655. doi: 10.1021/bi00837a023. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Piez K. A., Gross J. Characterization of the cyanogen bromide peptides from the alpha 1 chain of chick skin collagen. Biochemistry. 1969 Apr;8(4):1506–1514. doi: 10.1021/bi00832a029. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Isolation and characterization of cyanogen bromide peptides from basement membrane collagen. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1151–1158. doi: 10.1016/0006-291x(72)90955-2. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Isolation of a collagen from basement membranes containing three identical - chains. Biochem Biophys Res Commun. 1971 Oct 1;45(1):226–234. doi: 10.1016/0006-291x(71)90073-8. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Daniels J. R., Brown R. S., Bladen H. A., Fullmer H. M. Degradation of collagen by a human granulocyte collagenolytic system. J Clin Invest. 1968 Dec;47(12):2622–2629. doi: 10.1172/JCI105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of the cyanogen bromide peptides from the l(II) chain of chick cartilage collagen. Biochemistry. 1971 Aug 3;10(16):3030–3035. doi: 10.1021/bi00792a007. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Kang A. H. Collagen-and collagen peptide-induced chemotaxis of human blood monocytes. J Exp Med. 1976 Jun 1;143(6):1299–1307. doi: 10.1084/jem.143.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med. 1976 Nov 2;144(5):1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remberger K., Gay S., Fietzek P. P. Immunohistochemische Untersuchungen zur Kollagencharakterisierung in Lebercirrhosen. Virchows Arch A Pathol Anat Histol. 1975 Aug 12;367(3):231–240. doi: 10.1007/BF00430710. [DOI] [PubMed] [Google Scholar]
- Sakai T., Gross J. Some properties of the products of reaction of tadpole collagenase with collagen. Biochemistry. 1967 Feb;6(2):518–528. doi: 10.1021/bi00854a021. [DOI] [PubMed] [Google Scholar]
- Seyer J. M., Hutcheson E. T., Kang A. H. Collagen polymorphism in idiopathic chronic pulmonary fibrosis. J Clin Invest. 1976 Jun;57(6):1498–1507. doi: 10.1172/JCI108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyer J. M., Hutcheson E. T., Kang A. H. Collagen polymorphism in normal and cirrhotic human liver. J Clin Invest. 1977 Feb;59(2):241–248. doi: 10.1172/JCI108634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L. Human aorta collagens: evidence for three distinct species. Biochem Biophys Res Commun. 1974 Apr 8;57(3):717–725. doi: 10.1016/0006-291x(74)90605-6. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Kang A. H., Toole B. P., Gross J. Collagen heterogeneity. High resolution separation of native ( 1(I) 2 2 and ( 1(II) 3 and their component chains. J Biol Chem. 1972 Oct 25;247(20):6469–6473. [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by lymphokine-activated macrophages. Science. 1975 Jan 24;187(4173):261–263. doi: 10.1126/science.163038. [DOI] [PubMed] [Google Scholar]


