Abstract
Purpose
Prior phase II studies of intravesical gemcitabine have shown it to be active and well tolerated, but durable responses in patients with nonmuscle invasive bladder cancer who have experienced recurrence after bacillus Calmette-Guérin treatment are uncommon. We performed a multi-institutional phase II study within the SWOG (Southwest Oncology Group) cooperative group to evaluate the potential role of gemcitabine induction plus maintenance therapy in this setting.
Materials and Methods
Eligible patients had recurrent nonmuscle invasive bladder cancer, stage Tis (carcinoma in situ), T1, Ta high grade or multifocal Ta low grade after at least 2 prior courses of bacillus Calmette-Guérin. Patients were treated with 2 gm gemcitabine in 100 cc normal saline intravesically weekly × 6 and then monthly to 12 months. Cystoscopy and cytology were performed every 3 months, with biopsy at 3 months and then as clinically indicated. Initial complete response was defined as negative cystoscopy, cytology and biopsy at 3 months.
Results
A total of 58 patients were enrolled in the study and 47 were evaluable for response. Median patient age was 70 years (range 50 to 88). Of the evaluable patients 42 (89%) had high risk disease, including high grade Ta in 12 (26%), high grade T1 in 2 (4%) and carcinoma in situ in 28 (60%) with or without papillary lesions. At the initial 3-month evaluation 47% of patients were free of disease. At 1 year disease had not recurred in 28% of the 47 patients, all except 2 from the high risk group, and at 2 years disease had not recurred in 21%.
Conclusions
Intravesical gemcitabine has activity in high risk nonmuscle invasive bladder cancer and offers an option for patients with recurrence after bacillus Calmette-Guérin who are not suitable for cystectomy. However, less than 30% of patients had a durable response at 12 months even with maintenance therapy.
Keywords: urinary bladder neoplasms, administration, intravesical, gemcitabine, neoplasm invasiveness
INTRODUCTION
The treatment of nonmuscle invasive urothelial carcinoma continues to be a challenge for the practicing urologist. Patients with intermediate and high-risk cancers are usually treated with TUR followed by intravesical BCG instillations with or without maintenance therapy. 1-3 However, up to 20% of patients will not be able to tolerate the treatment and another 20% to 40% will fail to respond or will experience recurrence after the initial response.4 Intravesical treatments available for these patients include BCG plus interferon alpha-α, MMC, valrubicin, combination chemotherapy, and MMC with electromotive or hyperthermia techniques. Responses to these secondary treatments vary but generally range generally between 15% and 40% depending on the treatment group. For patients with the highest risk disease, cystectomy may ultimately be the most effective treatment. However, most patients would prefer to avoid cystectomy and many are too frail to be considered for this surgery. Urologists have long recognized the need for new alternative forms of therapy.
Gemcitabine was proposed as a reasonable choice for intravesical therapy based on its known activity against urothelial carcinoma when used systemically. Phase I trials of intravesical therapy showed a low incidence of local and systemic side effects with treatment, and have identified maximum tolerated doses generally based on volume and solubility rather than toxicity. 5-7 Several phase II studies using a 6-week induction course with weekly or twice weekly dosing have been reported to date. These tend to show a good initial response to treatment but a high failure rate by 1 to 2 years.8 Monthly maintenance has been shown to significantly improve durable responses with MMC.9 We hypothesized that intravesical gemcitabine would be effective for intermediate and high risk patients with recurrence after BCG therapy, and that the addition of monthly maintenance would improve durability of the response.
MATERIALS AND METHODS
Patients were informed of the investigational nature of the study and signed a written informed consent in accordance with institutional, federal and Declaration of Helsinki guidelines. The trial is registered with ClinicalTrials.gov Identifier as NCT00234039. Patients were enrolled at 16 sites from May 2007 through October 2009.
Patient Eligibility
Eligible patients had recurrent nonmuscle invasive urothelial carcinoma after at least 2 prior courses of intravesical BCG (6 + 6 weeks or 6 + 3 weeks) received up to 3 years before registration. The most recent biopsy, within 60 days of registration and at least 6 weeks after completion of BCG, must have shown high grade stage Ta or T1, multifocal Ta any grade or CIS with or without associated papillary lesions. Eligibility was confirmed by central pathology review. All patients underwent studies showing normal upper tracts within 2 years before registration. Patients were allowed to have received prior post-TUR chemotherapy instillations and no more than 1 induction course of other intravesical chemotherapy during the year before registration. Patients had a Zubrod performance status of 0 to 2, no prior malignancies except for nonmelanoma skin cancer, in situ cervical cancer, adequately treated stage I or II cancer in complete remission, or other treated cancer with remission for more than 5 years, no prior pelvic radiation, and adequate renal and hematologic function at baseline.
Treatment Plan and Patient Evaluation
Patients were treated with 2 gm intravesical gemcitabine in 100 cc saline for 1 hour once weekly for 6 weeks (induction). Patients who had no tumor after induction received maintenance treatments every 4 weeks for a total of 40 weeks (10 treatments). Urinalysis was performed before each treatment to rule out infection, and complete blood count was performed at baseline, weeks 3 and 6, and then monthly. Cystoscopy, cytology and biopsy were performed at 3 months, and then cystoscopy and cytology were performed every 3 months up to month 12, with biopsy performed as clinically indicated. Patients with disease recurrence (defined as the appearance of new lesions of any stage or grade) were removed from protocol treatment. Progression was defined as recurrence with biopsy proven stage T2 disease or greater, cystectomy, or death from any cause.
Study Design
The primary outcome was the absence of tumor at the 3-month evaluation (complete response was defined as negative cystoscopy, urinary cytology and bladder biopsy). It was determined that this regimen would be of further interest if the CR rate was 40% or greater, whereas further testing would not be pursued if the CR rate was 20% or less. The 2-stage study design of Green and Dahlberg was used.10 Planned accrual in the first stage was 25 eligible patients. If 5 or more CRs were observed, then accrual would continue to a total of 45 eligible patients, with 14 or more CRs considered sufficient activity to warrant further investigation of this regimen. This design had a significance level of 5.2% and a power of 91%.
Secondary end points included recurrence-free survival, progression-free survival and overall survival. Survival curves were plotted using the Kaplan-Meier product limit method. Overall survival was measured from the day of registration to death from any cause. RFS was defined as time from registration to first instance of disease recurrence or death from any cause and progression-free survival was defined similarly.
RESULTS
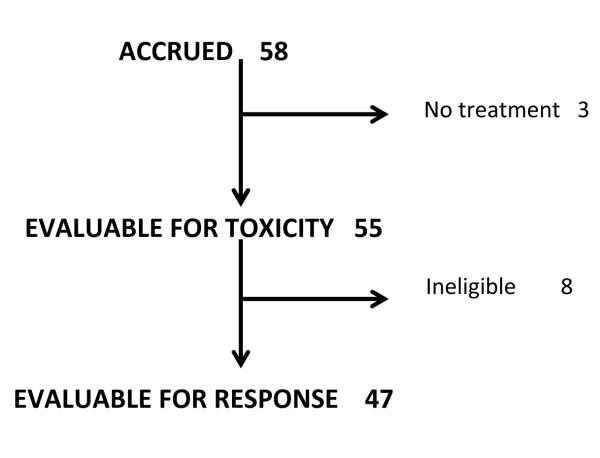
A total of 58 patients were accrued. Two eligible patients did not receive any protocol treatment, and 9 were ineligible, including 5 due to issues with biopsy timing or pathology specimen submission, 2 with no cancer on pathology review and 2 who had not received the minimum prior BCG treatments. The distribution of all enrolled patients is shown in Figure 1.
Figure 1.
All patients accrued
There were 55 patients who received at least some treatment and were evaluable for toxicity. Of these patients 18 had no toxicity, 34 (62%) had grade 1 to 2 toxicity, primarily dysuria and urinary frequency, and 3 had grade 3 toxicity (dysuria, frequency and neutropenia). No patient had grade 4 or 5 toxicity. Fifty-three patients (96%) completed 6 weeks of induction treatment, and 2 (4%) discontinued treatment after weeks 4 and 5 due to personal reasons and grade 2 dysuria, respectively. Twenty-eight patients received 1 or more maintenance treatments, including 4 who were ineligible and 2 who were not complete responders at first cystoscopy. Eleven patients completed all scheduled monthly maintenance treatments, whereas maintenance therapy was discontinued due to recurrence in 14, intercurrent illness in 2 and urethral stricture in 1 patient. No apparent increase in toxicity was observed with an increased number of treatments.
The 47 eligible patients who received any treatment were evaluable for response. Of these patients 42 (89%) had high risk disease, including high grade papillary disease alone (12 Ta and 2 T1), CIS alone (20) or with associated papillary disease (4 Ta and 4 T1). The remaining 5 patients had multifocal low grade disease. Median time from the most recent BCG treatment was 205 days (IQR 137, 382). Nine patients had BCG refractory disease, defined as persistent disease on first followup biopsy after the last BCG course before enrollment. The majority of patients (79%) had disease relapse within 12 months of the last BCG treatment. Twenty-six patients had received at least 2, 6-week courses of BCG, 15 received 6 + 3 weeks and 6 had received less treatment due to intolerance. Five patients had received interferon-α instillations along with some of the BCG treatments. Prior treatment with 1 or more induction courses of other chemotherapy agents (other than treatment after TUR) was reported in 17 (36%) patients using various regimens. The baseline characteristics of the evaluable patients are shown in the table.
TABLE.
| BASELINE PATIENT CHARACTERISTICS | |
|---|---|
| No. (%) | |
| Male | 33 (79) |
| Race: | |
| White | 43 (91) |
| Asian | 3 |
| Mixed | 1 |
| Zubrod Performance Status: | |
| 0 | 39 (83) |
| 1 | 7 (15) |
| 2 | 1 (2) |
| Multifocal low grade Ta | 6 (13) |
| High grade Ta | 11(23) |
| High grade T1 | 2 (4) |
| CIS: | 28 (60) |
| With papillary lesions | 8 (17) |
| Prior BCG treatments: | |
| 6 + 6 Wks or more | 26 (55) |
| 6 + 3 Wks | 15 (32) |
| Less than 6 + 3 Wks (intolerance) | 6 (13) |
| Mos since last BCG: | |
| 3 (refractory) | 9 (19) |
| 4–12 | 28 (60 |
| Greater than 12 | 10 (21) |
Twenty patients had negative cystoscopy, urinary cytology and biopsy after induction treatment for a CR rate at 3 months of 40% (95% CI 30–60). Two additional patients had negative cystoscopy and cytology at 3 months, did not undergo the required biopsy (a protocol violation) and were continuously free of disease to 12 months. If these patients were treated as presumed complete responders and were included in the CR group, the total CR rate at 3 months would be 47%. We did not observe a clear relationship between the number or timing of prior BCG treatments and response rates, but the study was not adequately powered to detect this association.
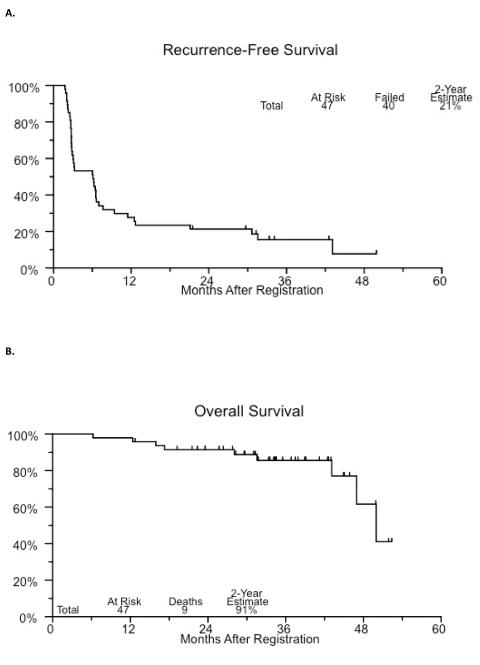
Median RFS was 6.1 months (95% CI 16–43). There were 13 evaluable patients who remained free of disease at 12 months, with 28% of the evaluable patients and 59% of those who had no disease at the 3-month evaluation. The 24-month estimated RFS was 21%. To date, recurrence was observed in 40 patients. Clinical disease progression (2) or intervention with cystectomy (15) occurred in 17 evaluable patients (36%). Of the patients who underwent cystectomy 12 had recurrent nonmuscle invasive disease on final pathology (less than pT2) and 3 had pT2 or greater disease. Eight patients (17%) died, although none died with documented metastatic disease. Cause of death was unknown in 1 patient with pT4 disease at cystectomy. Kaplan-Meier plots of recurrence-free and overall survival are shown in figure 2.
Figure 2.
Recurrence-free (A) and overall (B) survival
DISCUSSION
High risk nonmuscle invasive bladder cancer which has recurred after 2 cycles of intravesical BCG is an aggressive tumor with a high probability of progression and metastasis. Although many therapies have been suggested for these patients, all have a relatively low chance of long-term cancer control.
Phase I studies of intravesical gemcitabine have shown that it is well tolerated with little absorption and few systemic side effects.5-7 Activity of intravesical treatments was shown in marker studies, in which the marker lesion left in the bladder was eradicated in 40% to 50% of patients.11,12 Gunelli13 and Dalbagni8 et al used a twice weekly schedule in phase II trials based on preclinical studies suggesting better activity with that dosing.8 and 13 Gunelli et al treated 40 patients with papillary lesions TaG3 or T1 G1-3 that recurred after 2 cycles of BCG.13 They observed negative cystoscopy and cytology in 39 patients (95%) at 6 months and an estimated RFS of 66% at 24 months (CI 52–84). However, in a group of 30 patients Dalbagni et al reported that only 20% were still free of recurrence at 1 year without maintenance therapy.8 The latter study included 23 patients with CIS (75%)8 while these patients were apparently excluded from the study by Gunelli et al.13
Di Lorenzo et al performed a randomized phase II trial comparing intravesical gemcitabine to a second course of BCG in 80 high risk patients who had recurrence after an initial 6 weeks of BCG using a maintenance schedule in both arms.14 High risk was defined using the EORTC (European Organisation for Research and Treatment of Cancer) scoring system. However, in this population 30% had low grade disease and only 31% had CIS. The gemcitabine schedule included biweekly treatment for 6 weeks, and then weekly treatment for 3 weeks at 3, 6 and 12 months. BCG was given weekly × 6 with the same maintenance schedule. At a median followup of 15 months 52% of patients in the gemcitabine group had recurrence compared to 87.5% of those in the BCG arm, and progression occurred in 16 vs 27 patients, respectively. This study suggests that gemcitabine might be a better option for this group of patients than a second course of BCG. However, these results need to be confirmed in a larger study. This population is not directly comparable to patients in the trial by Dalbagni et al or our patients, who had predominantly CIS or high grade papillary disease and in whom 2 prior courses of BCG had failed.
The wide variation in reported results with intravesical gemcitabine is most likely due to differences in patient and tumor characteristics, but may also be explained in part by differences in dosing and schedules. A direct comparison of the results of these different doses is not possible except in a randomized clinical trial. In our study of predominantly high risk patients 47% were free of disease at 3 months, 28% remained continuously free of disease at 1 year and 21% remained continuously free of disease at 2 years. This appears to represent only a modest improvement in RFS with the addition of maintenance therapy. The patients who were disease-free at 3 months may have benefited from maintenance treatments since more than half continued to be free of recurrence at 1 year. Ultimately the determination of the ideal treatment schedule and the relative effect of maintenance treatments will require prospective, randomized studies.
The strengths of this study include the prospective, multi-institutional nature of the trial, which was conducted within the SWOG cooperative group, including academic and community centers. The weaknesses include the nonrandomized study design and the fact that, as is typical of these studies, the patients were not uniform in terms of tumor characteristics or prior therapy. The small number of patients does not allow for subgroup analysis, for example, of the impact of time since BCG treatment or the exact regimen of prior BCG or chemotherapy. Only a minority of patients completed the full maintenance schedule but this was mainly due to disease recurrence rather than toxicity. It is possible that the patients who were free of disease at 3 months did well with maintenance simply because of a selection effect rather than additional treatment. A large randomized trial would be required to be confident about the additional benefit of the maintenance treatments. Finally, several patients also did not have the required 3-month biopsy and were not included as responders unless they remained free of disease to 12 months. This likely resulted in an underestimation of the true response rate at 3 months.
CONCLUSIONS
Our study confirmed significant activity of this agent when used in high risk patients who had recurrence after 2 courses of BCG and were treated in a multicenter, cooperative group setting. The study met its predetermined end point of efficacy as 47% of patients had a complete response at 3 months after the initiation of induction therapy. While only 28% of the evaluable patients had a durable response at 1 year, more than half of the patients without positive cytology or biopsy at 3 months remained free of disease at 12 months with maintenance therapy, and most of those patients continued to be free of disease at 24 months. Cystectomy should still be considered the optimal therapy for these patients, but intravesical gemcitabine is an additional available treatment alternative for those who are not candidates for cystectomy. The challenge ahead is to identify and validate biomarkers as well as clinical and pathological factors associated with a response to intravesical gemcitabine, and to optimize the strategy to maintain that response.
Acknowledgments
Support: This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA27057, CA42777, CA63844, CA12644, CA58861, CA11083, CA35090, CA35119, CA58882, CA128567, CA46113, CA86780, CA14028, and in part by Eli Lilly and Company and Response Genetics, Inc.
Abbreviations and Acronyms
- BCG
bacillus Calmette-Guérin
- CIS
carcinoma in situ
- CR
complete response
- MMC
mitomycin C
- RFS
recurrence-free survival
- TUR
transurethral resection
REFERENCES
- 1.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Oosterlinck W, Sylvester R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59:997. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Malmstrom P-U, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomized studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non–muscle-invasive bladder cancer. Eur Urol. 2009;56:247. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 4.Witjes JA. Management of BCG failures in superficial bladder cancer: a review. Eur Urol. 2006;49:790. doi: 10.1016/j.eururo.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Laufer M, Ramalingam S, Schoenberg MP, et al. Intravesical gemcitabine therapy for superficial transitional cell carcinoma of the bladder: a phase I and pharmacokinetic study. J Clin Oncol. 2003;21:697. doi: 10.1200/JCO.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 6.DeBerardinis E, Antonini G, Peters GJ, et al. Intravesical administration of gemcitabine in superficial bladder cancer: a phase I study with pharmacodynamics evaluation. BJU Int. 2004;93:491. doi: 10.1111/j.1464-410x.2003.04656.x. [DOI] [PubMed] [Google Scholar]
- 7.Dalbagni G, Russo P, Sheinfeld J, et al. Phase I trial of intravesical gemcitabine in bacillius Calmette-Guerin-refractory transitional cell carcinoma of the bladder. J Clin Oncol. 2002;20:3193. doi: 10.1200/JCO.2002.02.066. [DOI] [PubMed] [Google Scholar]
- 8.Dalbagni G, Russo P, Bochner B, et al. Phase II trial of intravesical gemcitabine in bacilli Calmette-Guerin-refractory transitional cell carcinoma of the bladder. J Clin Oncol. 2006;24:2729. doi: 10.1200/JCO.2005.05.2720. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich MG, Pichlmeier U, Schwaibold H, et al. Long-term intravesical adjuvant chemotherapy further reduces recurrence rate compared with short-term intravesical chemotherapy and short-term therapy with bacillus Calmette-Guerin (BCG) in patients with non– muscle-invasive bladder carcinoma. Eur Urol. 2007;52:1123. doi: 10.1016/j.eururo.2007.02.063. [DOI] [PubMed] [Google Scholar]
- 10.Green SJ, Dahlberg S. Planned Versus Attained Design in Phase II Clinical Trials. Stat Med. 1992;11:853. doi: 10.1002/sim.4780110703. [DOI] [PubMed] [Google Scholar]
- 11.Gontero P, Casetta G, Maso G, et al. Phase II study to investigate the ablative efficacy of intravesical administration of gemcitabine in intermediate-risk superficial bladder cancer (SBC) Eur Urol. 2004;46:339. doi: 10.1016/j.eururo.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Gardmark T, Carringer M, Beckman E, et al. Intravesical gemcitabine study group. Randomized phase II marker lesion study evaluating effect of scheduling on response to intraveiscal gemcitabine in recurrent stage Ta urothelial cell carcinoma of the bladder. Urology. 2005;66:527. doi: 10.1016/j.urology.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 13.Gunelli R, Bercovich E, Nanni O, et al. Activity of endovesical gemcitabine in BCG-refractory bladder cancer patients: a translational study. BJ Cancer. 97:1499–1504. doi: 10.1038/sj.bjc.6604074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Lorenzo G, Perdona S, Damiano R, et al. Gemcitabine versus bacilli Calmette-Guerin after initial bacilli Calmette-Guerin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer. 2010;116:1893. doi: 10.1002/cncr.24914. [DOI] [PubMed] [Google Scholar]