Abstract
Background
Increasing evidence indicates that GABAergic neurons in the nucleus of the solitary tract (NTS) play a significant role in the arterial baroreceptor reflex and in the control of cardiovascular homeostasis. However, the role of these neurons in the development of hypertension is not yet fully clear.
Methods and Results
In the present study, we first confirmed that GABA B receptor (GBR) expression is enhanced in the NTS of SHR as compared with WKY rats using real time RT-PCR and Western Blots. To study the functional consequence of upregulated GBR expression, GBR was overexpressed in the NTS by bilateral microinjection of the AAV2-GBR1 viral vector into the NTS of WKY rats. Immunofluorescence staining and Western blots demonstrated that microinjection of AAV2-GBR1 into the NTS of WKY rats resulted in a significant increase in GBR1 expression in the NTS neurons. Overexpression of GBR in the NTS induced a chronic elevation in blood pressure and heart rate in the normotensive WKY rats. In an acute study, the pressor response to baclofen microinjected into the NTS was enhanced in SHR as compared with WKY rats.
Conclusion
GBR1 expression is enhanced in the NTS of SHR versus WKY rats and overexpression of this gene in the NTS results in chronic elevation of blood pressure and heart rate in normotensive rats.
Keywords: GABAB receptor, blood pressure, hypertension
INTRODUCTION
The nucleus tractus solitarius (NTS) is a termination site for primary afferent fibers from baroreceptors, and other peripheral cardiovascular receptors, that contain blood pressure-sensitive neurons. As such, it mediates the inhibitory actions of baroreceptors on sympathetic discharge (1). The intermediate portion of NTS is richly innervated by fibers from different brain nuclei that are known to have an important role in cardiovascular control, including the area postrema and hypothalamic nuclei (2). Thus, it is believed that NTS plays an important role in blood pressure regulation. In addition, NTS also contributes to the development and maintenance of hypertension. Several studies demonstrate that the commissural NTS may be altered in spontaneous hypertensive rat (SHR) (3). These cardiovascular regulatory actions are carried out by several neuronal transmitters and modulators, such as γ-aminobutyric acid (GABA) (4).
GABA is well known as a neurotransmitter that exerts inhibitory actions in the brain. These actions of GABA are mediated through its receptors, which include ionotropic GABA A receptor (GAR) and metabotropic GABAB receptor (GBR) that are defined on the basis of pharmacological and physiological studies (5). The ionotropic GAR has an intrinsic Cl− channel, which is responsible for inducing fast inhibitory postsynaptic potentials. The GBR is a G-protein-coupled receptor, and regulates neuronal activity via activation of K+ channels. The activation of K+ channels induces hyperpolarization of the neuronal membrane, which produces chronic inhibition of neuronal activity. A high density of both GAR and GBR and a high density of GABA-containing nerve terminals have been found within the NTS (6). The GBR is a heterodimer comprised of GBR1 and GBR2 proteins. GBR1 is critical for ligand activation of the heterodimer, while GBR2 merely serves as a shuttle protein to transfer GBR1 onto the surface of the cell membrane (7). A large number of studies have demonstrated that both GAR and GBR play an important role in the integration of baroreceptor afferent inputs and baroreflex function. Microinjection of the GAR agonist, muscimol, or the GBR agonist, baclofen, into the NTS produces a marked pressor response via inhibition of baroreflexes, elevation of sympathetic tone, and vasopression release (8). Conversely, microinjection of GBR antagonists removes tonic GABAergic inhibition of NTS neurons and results in a fall in arterial pressure (9). More interestingly, the pressor response to microinjection of baclofen into the NTS is enhanced in several hypertensive animal models, such as DOCA-salt hypertensive rats (10), rats with Ang II-infusion induced hypertension (11), and 1-kidney renal wrap hypertensive rats (9). These studies demonstrate that enhanced GBR function could be associated with the high blood pressure observed in those animals. However, whether GBR expression is enhanced in the NTS during hypertension, and whether the enhanced expression of GBR in the NTS would lead to elevated BP, remains unanswered.
In the present study, we examined the GBR and GAR expression in the NTS of SHR and WKY rats. Our results demonstrate that GBR expression is enhanced in the NTS of SHR as compared with WKY rats. Next, we investigated the functional consequences of enhanced GBR expression in the NTS during hypertension by microinjection of a viral vector containing the GBR gene into the NTS. Our data demonstrate that overexpression of GBR in the NTS of normotensive rats induced a significant elevation in arterial pressure and heart rate.
METHODS
Animals
Adult male SHR and WKY rats (Charles River Laboratories, Wilmington, MA) weighing 300–350g were used in this study. All animals were housed under controlled conditions with a 12:12-h light-dark cycle in a climate-controlled room. Rat chow (Harlan Tekland, Madison, WI) and water were provided ad libitum. All protocols were approved by the North Dakota State University Institutional Animal Care and Use Committee.
Recording of arterial pressure and sympathetic nerve activity
Chronic BP recording was carried out with a radiotelemetry system as described in our previous publications (11,12). Continuous recordings were started 5 days after transducer implantation, in order to allow for recovery from the surgical procedure. Acute BP, HR recording was carried out using PE-10 catheters fused to PE-50 catheters and performed under urethane (1 g/kg, ip) anesthesia. The catheter was perfused with heparinized saline (100 IU/ml), placed in the right femoral artery, and connected to a BP transducer and a bridge amplifier (AD Instrument, Colorado Springs, CO). The BP and HR data were collected and analyzed with Powerlab software (AD Instrument, Colorado Springs, CO). Renal sympathetic nerve activity (RSNA) was recorded in SHR and WKY rats, before and after NTS injection, as described in our previous publication (12).
NTS microinjection and GBR1 gene transfer into the NTS
Agonists or antagonists of GBR or GAR were bilaterally microinjected into the NTS of SHR and WKY rats, according to procedures described in our previous publication (11).
For GBR1 gene transfer into the NTS, WKY rats were anesthetized with a mixture of O2 and isoflurane. AAV2-GBR1 or AAV2-GFP (all in 1×109 gc in 50 nl) was microinjected bilaterally into the NTS as described in our previous publication (11). The adeno-associated virus type2 (AAV2) containing the rat GBR1a gene (AAV2-GBR1) or green fluorescent protein (AAV2-GFP), as control, driven by a chicken β-actin promoter with a human cytomegalovirus enhancer, were used to induce endogenous GBR1 expression in the NTS. AAV2-GBR1 and AAV2-GFP plasmids were constructed and prepared as described previously (12).
Real-time RT-PCR
Real-time PCR was used to detect changes in the expression of GAR and GBR in the NTS of SHR rats and WKY rats. Isolation of total RNA from NTS tissue, reverse transcription, and the specific probes for GBR1 and GBR2 were described in our previous study (13). In each experiment, samples were analyzed in triplicate.
Western blot analysis
GBR1 and GARγ2 protein levels in rat brain sections NTS were assessed by Western blot analysis as described in our previous publication (13).
Immunohistochemistry
Immunofluorescence staining of brain NTS sections was performed as we described previously (13). In brief, the NTS sections, identified with a rat brain atlas, were incubated with PBS plus 0.5% Tween 20 (PBS-T) containing 5 % goat serum for 60 min. Slices were incubated with primary antibodies (anti-NeuN monoclonal antibody, 1:500; rabbit anti-GFP, 1:500) overnight at 4°C. After being washed with PBS-T, the sections were incubated with secondary antibodies (Alexa Fluor 488 goat anti-rabbit IgG, 1:1,000; Alexa Fluor 594 goat anti-mouse IgG, 1:1,000) for 2 h. The sections were then washed with PBS-T, and detected with a confocal fluorescence microscope (Olympus, Fluoview FV300). The fluorescent images were collected and analyzed with Flow-view software.
Statistical Analyses
All data are presented as means ± SE. Statistical significance was evaluated by 1-or 2-way ANOVA, as appropriate, followed by either a Newman-Keuls or Bonferroni post hoc analysis, where appropriate. Differences were considered significant at P<0.05, and individual probability values are noted in the figure legends.
RESULTS
GAR and GBR expression in the NTS of SHR versus WKY rats
The aim of our first experiment was to confirm whether GBR expression is increased in the NTS of SHR. GBR1 and GBR2 mRNA levels in the NTS and the paraventricular nucleus (PVN) of SHR and WKY rats were detected with real-time RT-PCR. The results shown in Figure 1A demonstrate that both GBR1 and GBR2 mRNA levels in the NTS of SHR were significantly higher than those of WKY rats; however, GBR1 and GBR2 mRNA levels in the PVN were comparable between SHR and WKY rats. For comparison, we also determined GAR expression in the NTS of SHR and WKY rats. The data shown in Figure 1B indicates that both GARα1 and GARγ2 are expressed in the NTS of SHR and WKY rats and that the mRNA levels of GARα1 and GARγ2 in the NTS were comparable between the two strains of rats.
Figure 1.
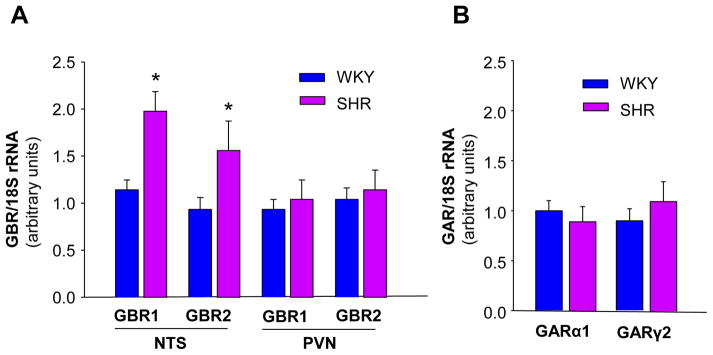
GAR and GBR mRNA levels in the NTS of SHR and WKY rats. A: GBR mRNA levels in the NTS and the PVN of SHR and WKY rats. GBR mRNA levels were detected with real-time RT-PCR as described in Methods. Data were normalized with 18S rRNA and represent means ± SE of the fold increase over WKY GBR1 (n=3 rats in each group). *P<0.05 vs. WKY rats. B: GAR mRNA levels in the NTS of SHR and WKY rats. Data were normalized with 18S rRNA and represent means ± SE of the fold increase over WKY GARα1 (n=3 rats in each group).
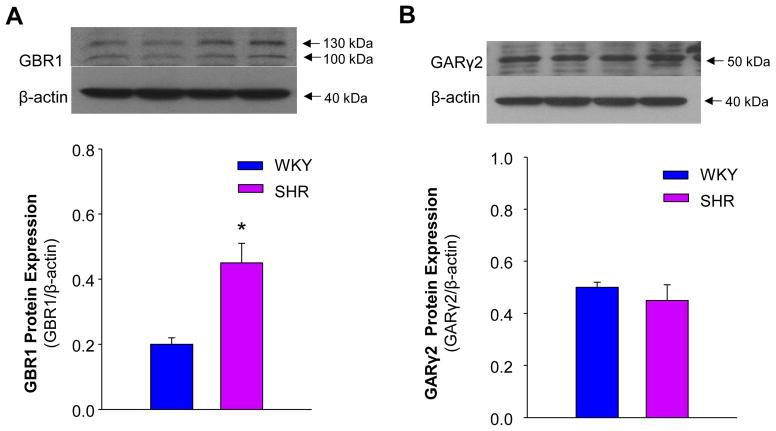
GBR1 and GAR γ2 protein levels in the NTS were determined by Western blot analysis in SHR and WKY rats. The results are shown in Figure 2A, indicating that GBR1 protein levels were enhanced more than 2-fold in SHR, as compared with WKY rats. In contrast, GAR γ2 expression was comparable between SHR and WKY rats (Figure 2B). These results demonstrate that GBR1 expression in NTS is enhanced in the NTS of SHR and that GAR γ2 expression is not significantly altered between SHR and WKY rats.
Figure 2.
GBR1 and GARγ2 protein levels in the NTS of SHR and WKY rats. Western blot was used to measure GBR1 and GARγ2 protein levels in the NTS from brain sections of SHR and WKY rats. A: upper panel, representative blot showing GBR1 protein levels in the NTS of SHR and WKY rats; lower panel, bar graphs summarizing GBR1 protein levels in the NTS of SHR and WKY rats. Data were normalized to β-actin and present means ± SE (n=3 experiments from 6 rats in each group). *p<0.05 vs. WKY rats. B: Upper panel, the autoradiogram showing GARγ2 protein levels in the NTS of SHR and WKY rats; lower panel, bar graphs summarizing GARγ2 protein levels in the NTS of SHR and WKY rats. Data were normalized to β-actin and presented as means ± SE (n=3 experiments from 6 rats in each group).
Overexpression of GBR1 in the NTS by gene transfer
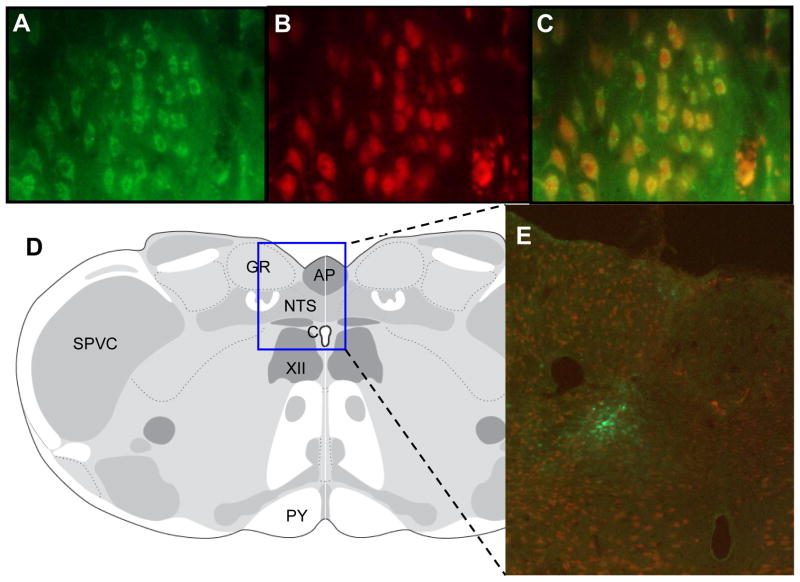
To study the functional consequence of enhanced GBR1 expression in the NTS of SHR, and whether enhanced GBR1 expression in the NTS could results in an elevation of BP, the GBR1 gene was overexpressed using AAV2 viral vector-mediated gene transfer (AAV2-GBR1) into the NTS of normotensive WKY rats. The results presented in Figure 3 show immunofluorescence of GFP expression in the NTS on the seventh day after microinjection of AAV2-GFP. Figure 3A through 3C shows a high magnification view indicating that GFP expression is localized to neurons of the NTS. Figure 3D and 3E demonstrate a strong immunoreactive signal in the NTS after transfection. These results indicate that the microinjection of AAV2-GFP significantly increases the GFP gene expression in the neurons within the NTS.
Figure 3.
Gene transfer into the NTS of WKY rats. A–C: Immunofluorescence images showing overexpression of GFP in the NTS of rats after 7 days of microinjection of AAV2-GFP into the NTS. A, Fluorescence micrograph (×40 magnification) demonstrating localization of GFP in the NTS (green). B. Same field of cells as in A, immunostained with a neuron-specific anti-NeuN antibody (red). C. Overlap of A and B, indicating that GFP is located on neurons. D: location of the stained NTS brain sections shown in A, B, C, and E, based on the rat brain atlas of Swanson (17). E: fluorescence micrograph (×10magnification) demonstrating location of GFP within the NTS, indicated by the square in D.
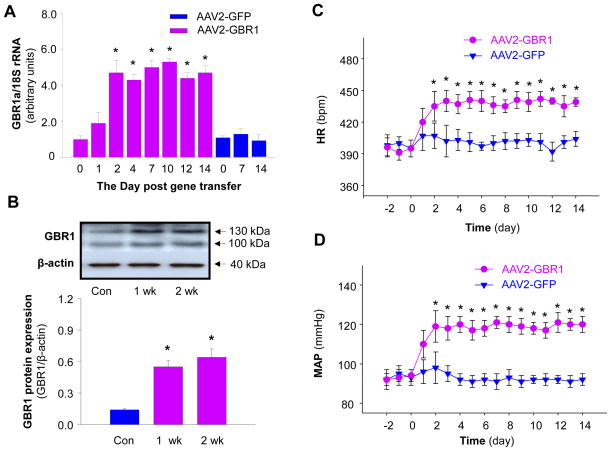
We, next, examined GBR1 mRNA levels in the NTS at the time points indicated in Figure 4A after microinjection of AAV2-GBR1 or AAV2-GFP into the NTS. The results are shown in Figure 4A, demonstrating that GBR1 mRNA levels in the NTS were significantly increased by AAV2-GBR1 gene transfer. The elevation of GBR1 gene expression started on day 1, reached a peak in 2 days, and lasted at least for 14 days after NTS injection of this viral vector. In contrast, microinjection of AAV2-GFP did not alter GBR1 expression in the NTS. GBR1a protein levels were examined in the NTS after microinjection of AAV2-GBR1 and AAV2-GFP. The results are presented in Figure 4B, demonstrating that GBR1a protein levels were significantly enhanced in the NTS after gene transfer, as compared with control rats, which received NTS microinjection of AAV-GFP. The results indicate that AAV2-mediated GBR1 gene transfer induced a significant increase in GBR1 protein expression. However, microinjection of AAV2-GFP control vector intro the NTS did not alter GBR1 protein levels (data not shown).
Figure 4.
GBR1 mRNA and protein levels in the NTS after GBR1 gene transfer and effect of overexpression of GBR1 in the NTS on BP and HR. A, Bar graphs showing the GBR1 mRNA levels after AAV2-GBR1 and AAV2-GFP transfer. The GBR1 mRNA levels were detected using real-time RT-PCR. Data are means ± SE (n=3 rats in each group). *p<0.05 compared with respective control. B, Upper panel showing a representative autoradiogram of GBR1 protein levels in the NTS. Lower panel, bar graphs summarizing the quantitation of GBR1 protein levels in the NTS. Data are normalized using β-actin. Values are means ± SE (n=3 experiments from 6 rats in each group).*p<0.05 vs. control. C and D, AAV2-GBR1 or AAV2-GFP was microinjected bilaterally into the NTS of WKY rats, and MAP (C) and HR (D) were recorded in a conscious state using radiotelemetry (11, 12, 28). BP and HR were elevated after GBR1 gene transfer into the NTS of conscious rats. Data are means ± SE from 6 rats (AAV2-GBR1 group) and 6 rats (AAV2-GFP group). *p<0.05 vs corresponding time point in the AAV2-GFP group.
Effect of GBR1 gene transfer on arterial pressure, heart rate, and norepinephrine plasma levels
Based on the above expression data, we determined whether increased expression of GBR1 in the NTS of normotensive rats would alter basal BP in normotensive rats. One week after implantation of the BP-transducers, either AAV2-GBR1 (1×109 gc in 50 nl) or AAV2-GFP (1×109 gc in 50 nl) was microinjected bilaterally into the NTS of WKY rats. Mean arterial pressure (MAP) and HR were recorded via telemetry before and after NTS microinjection. The data are summarized in figure 4C and 4D. Before NTS microinjections, there was no difference in basal MAP and HR between the AAV2-GBR1 and AAVE2-GFP groups of rats. After NTS microinjection of AAV2-GBR1, both MAP and HR increased steadily, reaching a peak level of 119±6 mmHg and 441±12 bpm at 2–3days after microinjection and remained elevated throughout the 14-day recording period. In contrast, microinjection of AAV2-GFP into the NTS of WKY rats did not alter basal MAP and HR. These results indicate that overexpression of GBR1 in the NTS induces a chronic elevation in BP and HR in normotensive rats, mimicking the high blood pressure observed in SHR rats. In addition, plasma norepinephrine (NE) levels were analyzed using high sensitivity ELISA kits (Rocky Mountain Diagnostics, Colorado Springs, Colo) in WKY rats receiving NTS injection of AAV2-GBR1 or AAV2-GFP. Plasma NE levels were significantly elevated in rats that received NTS injection of AAV2-GBR1 (426±31 pg/ml and 438±27 pg/ml at 7 and 14 days after NTS injection as compared with control rats (271±19 pg/ml) (n=4 rats in each group, P<0.01). NTS injection of AAV2-GFP did not alter plasma NE levels (288±25 pg/ml in control rats; 301±32 pg/ml and 292± 31 pg/ml in rats at 7 and 14 days after NTS injection of AAV2-GFP, n=4 rats in each group, p>0.05). These results indicate that overexpression of GBR1 in the NTS elevates plasma levels of NE, which is a marker of chronic sympathetic nerve activation.
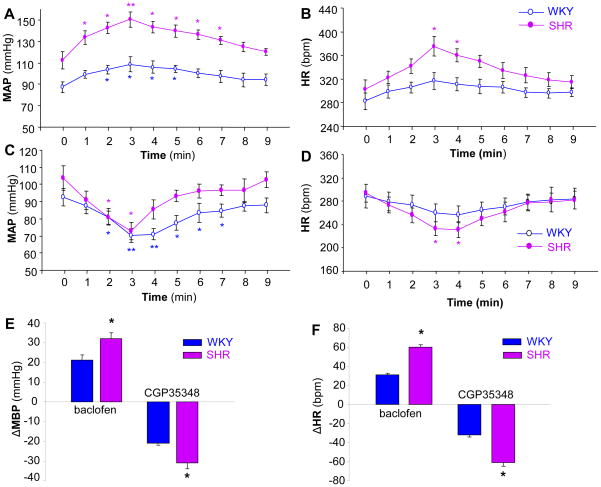
Effect of agonists and antagonists of GBR and GAR on BP, HR, and RSNA in SHR and WKY rats
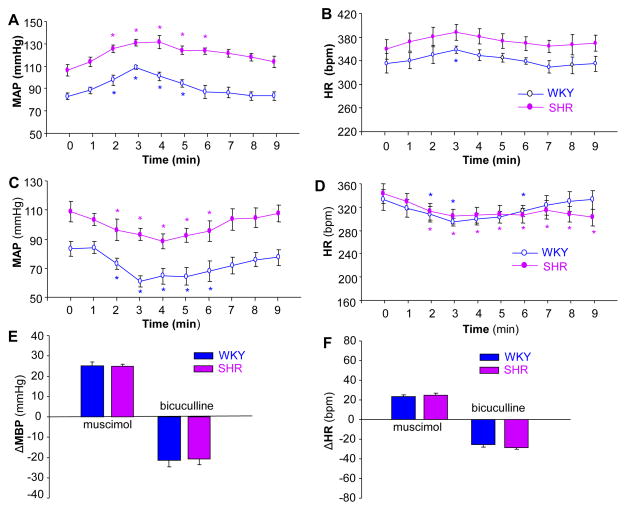
The experiments described above indicate that GBR1 expression is significantly enhanced in the NTS of SHR rats. To examine whether these enhanced GBR1 are functional in the regulation of BP, we further investigated the effects of agonists and antagonists of GBR and GAR on blood pressure and heart rate in SHR and WKY rats. The BP and HR were recorded before and after NTS microinjection of muscimol (a GAR agonist), bicuculline (GAR antagonist), baclofen (a GBR agonist), and CGP35348 (a GBR antagonist) as described in the Methods. The effects of these agents on BP and HR are shown in Figures 5 and 6. NTS microinjection of muscimol (100 pmol, 50 nl) significantly increased BP from 106±5 to 131±6 mmHg (n=6, P<0.05) in SHR and from 83±3 to 108±2 mmHg (n=6, P<0.05) in WKY rats (Figure 5A). The pressor responses evoked by NTS microinjection of muscimol were comparable between SHR and WKY rats (Figure 5E). The HR in SHR was significantly increased from 359±17 to 388±16 bpm (n=6, P<0.05), and the HR in WKY rats was also significantly increased from 335±18 to 358±17 bpm (n=6, P<0.05) by muscimol NTS injection (Figure 5B). However, the HR increases evoked by microinjection of muscimol into the NTS were comparable between SHR and WKY rats (Figure 5F). In addition, microinjection of an equal volume of saline at the same site in the NTS did not alter MAP and HR in both strains of rats (data not shown). These data indicate that the effect of muscimol microinjected into the NTS on BP and HR is comparable between WKY and SHR.
Figure 5.
Effect of the GAR antagonist, bicuculline, and the agonist, muscimol on MAP and HR in SHR or WKY rats. A and B: Time course data showing the MAP (A) and HR (B) changes evoked by muscimol (100 pmol in 50 nl) microinjected into the NTS of SHR (filled dots) and WKY rats (open dots). Values are means±SE (n=6 rats in each group). *P<0.05 as compared with basal condition. C and D: Time course data showing the MAP (C) and HR (D) changes evoked by bicuculline (10 pmol in 50 nl) microinjected into the NTS of SHR (filled dots) and WKY rats (open dots). Values are means±SE (n=5 or 6 rats in each group). *P<0.05 as compared with respective basal condition. E and F: Bar graphs showing MAP (E) and HR (F) changes evoked by microinjection of muscimol or bicuculline into the NTS in SHR and WKY rats. Values are means±SE (n=6 or 5 rats in each group). *p< 0.05 vs. WKY in each group of rats.
Figure 6.
Effect of the GBR antagonist, CGP35348, and the agonist, baclofen, on MAP and HR in SHR or WKY rats. A and B: Time course data showing the MAP (A) and HR (B) changes evoked by baclofen (50 pmol in 50 nl) microinjected into the NTS of SHR (filled dots) and WKY rats (open dots). Values are means±SE (n=6 rats in each group). *P<0.05 or **P<0.01 as compared with respective basal condition. C and D: time course data showing the MAP (C) and HR (D) changes evoked by CGP35348 (100 pmol in 50 nl) microinjected into the NTS of SHR (filled dots) and WKY rats (open dots). Values are means±SE (n=5 or 6 rats in each group). *P<0.05 or **P<0.01 as compared with respective basal condition. E and F: Bar graphs showing MAP (E) and HR (F) changes evoked by microinjection of baclofen or CGP35348 into the NTS of SHR and WKY rats. Values are means± SE (n=6 or 5 rats in each group). *p< 0.05 vs. WKY in each group of rats.
The effect of a GAR antagonist, bicuculline, microinjected into the NTS on BP and HR was also examined in SHR and WKY rats. Microinjection of bicuculline (10 pmol in 50 nl) into the NTS resulted in a significant decrease in MAP from 110±7 to 89±5 mmHg (n=6, P<0.05) in SHR and from 84±5 to 62±4 mmHg (n=5, P<0.05) in WKY rats (Figure 5C). However, the depressor responses to bicuculline microinjected into the NTS were not different between SHR and WKY rats (Figure 5E). The HR in both SHR and WKY rats was significantly reduced from 338±17 to 308±13 bpm (n=4, P<0.05) and from 332±17 to 295±6 bpm (n=5, P<0.05) respectively before and after NTS administration of bicuculline (Figure 5D). The HR response evoked by bicuculline was comparable between SHR and WKY rats (Figure 5F). These results demonstrate that the depressor response to the GAR antagonist, bicuculline, microinjected into the NTS is comparable between SHR and WKY rats.
In another group of rats, we examined the effect of microinjection of a selective GBR agonist, baclofen, into the NTS of SHR and WKY rats. Microinjection of baclofen (50 pmol, 50 nl) into the NTS caused BP increases in both SHR (from 112±8 to 150±7 mmHg, n=6, P<0.01) and WKY rats (from 87±5 to 108±7 mmHg, n=6, P<0.05) (Figure 6A). The pressor response to baclofen microinjected into the NTS was significantly increased in SHR as compared to WKY rats (Figure 6E). In addition, NTS administration of baclofen also increased the HR in SHR (from 302±17 to 375±17 bpm, n=6, P<0.05) and WKY rats (from 282±14 to 317±14 bpm, n=6, P<0.05) (Figure 6B). The tachycardic response to baclofen was significantly increased in SHR as compared to WKY rats (n=6, P<0.05) (Figure 6F). In addition, RSNA was recorded in SHR and WKY rats before and after NTS microinjection of baclofen (50 pmol, 50 nl). Microinjection of baclofen into the NTS increased RSNA by 21±3% and 13±2% in SHR and WKY rats, respectively. The sympathoexcitatory effect of baclofen was significantly enhanced in SHR (n=4 rats in each group, P<0.05) as compared with WKY rats. In summary, these data indicate that both pressor and sympathoexcitatory effects of baclofen in the NTS are enhanced in SHR as compared with WKY rats.
The effect of a GBR antagonist, CGP-35348, microinjected into the NTS on BP and HR was examined in SHR and WKY rats. Bilateral microinjection of CGP-35348 (100 pmol, 50 nl) into the NTS significantly reduced the MAP in SHR (from 103±7 to 72±5 mmHg, n=6, P<0.01) and WKY rats (from 92±5 to 70±4 mmHg, n=5, P<0.05) (Figure 6C). The depressor response to CGP-35348 was significantly enhanced in SHR rats as compared with WKY rat (Figure 6E). The HR was also reduced by microinjection of CGP-35348 into the NTS in both SHR (from 294±15 to 233±12 bpm, n=6, P<0.01) and WKY rats (from 289±20 to 257±15 bpm, n=6, P<0.05) (Figure 6D). The bradycardic response to CGP-35348 was significantly enhanced in SHR as compared with WKY rats (Figure 6F). The data demonstrate that the depressor response to the GBR antagonist is enhanced in SHR as compared with WKY rats.
DISCUSSION
The current investigation demonstrates that the BP response to both a GBR agonist and antagonist is elevated in the NTS of SHR and that GBR expression in the NTS is increased in SHR as compared with WKY rats. This study also provides the first evidence that enhanced endogenous GBR expression in the NTS leads to elevated blood pressure in normotensive rats, indicating that enhanced GBR expression or activity in the NTS may contribute to the development of hypertension in SHR. This conclusion is supported by the following observations: 1) GBR expression is enhanced in the NTS of SHR compared with normotensive WKY rats; 2) GBR1 gene transfer into the NTS significantly increases GBR1 expression and results in chronic increases in BP in WKY rats; and 3) Acute microinjection of either exogenous GBR agonist or antagonist into the NTS induces pressor or depressor responses, respectively. These responses were enhanced in SHR, as compared with WKY rats. In contrast, the expression of GAR in the NTS and the responses to the agonist/antagonist microinjected into the NTS were comparable between SHR and WKY rats.
The pressor response to baclofen microinjected into the NTS is elevated in deoxycorticosterone acetate salt-induced hypertensive rats, renal wrap hypertensive rats, and angiotensin II-infusion induced hypertensive rats (9,10,11). Consistent with these observations, the present study indicates that acute microinjection of the exogenous GBR agonist (baclofen) into the NTS significantly induces an elevated pressor response in SHR as compared with normotensive WKY rats. The elevated response to GBR agonist could be explained by the current observation that GBR expression is enhanced in the NTS of SHR as compared with WKY rats. Enhanced expression of GBR in the NTS has also been reported in other hypertensive rat models, such as Ang II-induced hypertensive rats, and renal wrap hypertensive rats (9,10,11). Taken together, these studies demonstrate that enhanced GBR expression in the NTS is associated with hypertension; however, the cellular mechanism(s) underlying enhanced GBR expression in the NTS of SHR is not clear. One possibility is that enhanced GBR expression in the NTS is caused by high blood pressure, since elevated peripheral arterial pressure may increase the central input signal, leading to altered gene expression in brain cardiovascular regulatory areas. This seems unlikely, however, since we observed previously that GBR expression is not altered in the NTS of rats made hypertensive by peripheral infusion of L-NAME (11). A second possibility is that enhanced GBR expression in SHR is caused by angiotensin II, since angiotensin II activity in the NTS is increased in SHR and Ang II increases GBR expression (11, 14, 15). A third possibility is that enhanced GBR expression in the NTS of SHR may be caused by other neural modulators, such as nitric oxide (NO) or reactive oxygen species (ROS). It has been reported that NO production in the NTS of SHR is reduced as compared with WKY rats (16), and enhanced NO production in this brain area by overexpression of eNOS lowers blood pressure in SHR (17). On the other hand, ROS generation in the NTS is increased in SHR. Reducing ROS levels either by blockade of ROS generating enzymes or overexpression of superoxide dismutase (SOD) decreases blood pressure in those rats (18,19). However, whether impaired NO production and/or increased oxidative stress in the NTS contributes to enhanced GBR expression in SHR is still not clear. Identifying the exact signaling pathways controlling gene regulation that are altered and lead to elevated GBR expression in the NTS of SHR requires further investigation.
Data from our laboratory, as well as other research groups, demonstrates that the pressor response to baclofen injected into the NTS is enhanced in the NTS of several hypertensive animal models (9, 10, 11). This phenomenon could be explained by several observations showing that GBR expression is elevated in the NTS of these hypertensive rats (9, 11). However, it is still not clear whether enhanced GBR expression contributes to the development of hypertension in these animals. Here, we provide the first evidence showing that viral vector-mediated overexpression of GBR in the NTS results in chronic elevation in blood pressure and heart rate in normotensive rats, indicating that enhanced GBR expression or activity could contribute to the development of high blood pressure in SHR. However, the exact cellular mechanisms responsible for the elevated BP observed in these rats with overexpressed GBR in the NTS are still not clear. Several possible neuronal pathways could be involved. Anatomic studies showed projections from the NTS to neurons in the PVN of hypothalamus, a brain area that controls cardiovascular system via the sympathetic nervous system and hormone release from the pituitary gland. The notion that this pathway is involved is supported by the current observations that microinjection of baclofen into the NTS increases renal sympathetic nerve activity in both SHR and WKY rats and that the sympathoexcitatory response to baclofen is enhanced in SHR. This hypothesis is also supported by studies from other research groups that show the pressor response to baclofen microinjected into the NTS is attenuated by a vasopressin antagonist (20). In addition, the NTS is well known as a termination site for primary afferent fibers from baroreceptors and other peripheral cardiovascular receptors that contain blood pressure-sensitive neurons. As such, the NTS mediates the inhibitory actions of baroreceptors on sympathetic discharge though neurons in the caudal and rostral ventrolateral medulla (CVLM and RVLM). Therefore, enhanced GABAergic neuronal transmission in the NTS could inhibit baroreceptor afferent input signals triggered by increased blood pressure, leading to dampening of the baroreflex (21). The inhibited baroreflex may contribute to the long-term elevation of blood pressure. This notion is supported by several experimental studies and clinical observations, including: 1) chronically decreasing baroreceptor afferent input to the brain induces sustained hypertension in dogs (22); 2) chronic electrical stimulation of the carotid sinus in dogs produces a sustained decrease in arterial pressure (23); 3) baroreflex input signal reduction induced by sinoaortic denervation causes salt-dependent hypertension in normotensive rats (24); and 4) patients with baroreflex failure suffer from volatile hypertension with increased sympathetic nervous system activity (25,26). The preponderance of evidence indicates that enhanced GBR-mediated inhibition of the baroreflex may cause central desensitization to elevations in peripheral blood pressure and, thus, reset the blood pressure regulatory set point to a higher level. This concept is further supported by the observation in the present study that enhanced expression of GBR leads to elevated BP and may contribute to the development of hypertension.
In conclusion, the results of the current study demonstrate that GBR expression is enhanced in the NTS of SHR, as compared with normotensive WKY rats. Moreover, overexpression of GBR in the NTS of normotensive rats results in chronic elevations in BP and HR, suggesting that elevated GBR in the NTS is able to elevate blood pressure. Taken together, these findings suggest that the GABAergic system in the NTS contributes not only to the regulation of blood pressure, but also to central resetting of blood pressure and the development of hypertension.
Acknowledgments
GRANTS
This work was supported by the Amercian Heart Association (SDG 0635050 and 10GRNT3170012) and NIH (NS55008 and P30 GM103332-01).
Footnotes
DISCLOSURES
None
References
- 1.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–46. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 2.Colombari E, Sato MA, Cravo SL, Bergamaschi CT, Campos RR, Jr, Lopes OU. Role of the medulla oblongata in hypertension. Hypertension. 2001;38:549–54. doi: 10.1161/01.hyp.38.3.549. [DOI] [PubMed] [Google Scholar]
- 3.Moreira TS, Takakura AC, Colombari E. Important GABAergic mechanism within the NTS and the control of sympathetic baroreflex in SHR. Auton Neurosci. 2011;159:62–70. doi: 10.1016/j.autneu.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Zubcevic J, Potts JT. Role of GABAergic neurones in the nucleus tractus solitarii in modulation of cardiovascular activity. Exp Physiol. 2010;95:909–18. doi: 10.1113/expphysiol.2010.054007. [DOI] [PubMed] [Google Scholar]
- 5.Kuriyama K, Hirouchi M, Kimura H. Neurochemical and molecular pharmacological aspects of the GABA(B) receptor. Neurochem Res. 2000;25:1233–9. doi: 10.1023/a:1007640027977. [DOI] [PubMed] [Google Scholar]
- 6.Maqbool T, Batten FC, Mc William PN. Ultrastructural relationship between GABAergic terminals and cardiac vagal preganglionic motoneurons and vagal afferents in the cat; a combined HRP tracing and immunogold labeling study. Eur J Neurosci. 1991;3:51–53. doi: 10.1111/j.1460-9568.1991.tb00837.x. [DOI] [PubMed] [Google Scholar]
- 7.Möhler H, Benke D, Fritschy JM. GABA(B)-receptor isoforms molecular architecture and distribution. Life Sci. 2001;68:2297–300. doi: 10.1016/s0024-3205(01)01018-9. [DOI] [PubMed] [Google Scholar]
- 8.Callera JC, Bonagamba LG, Nosjean A, Laguzzi R, Machado BH. Activation of GABA receptors in the NTS of awake rats reduces the gain of baroreflex bradycardia. Auton Neurosci. 2000;84:58–67. doi: 10.1016/S1566-0702(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 9.Durgam VR, Vitela M, Mifflin SW. Enhanced gamma-aminobutyric acid-B receptor agonist responses and mRNA within the nucleus of the solitary tract in hypertension. Hypertension. 1999;33:530–536. doi: 10.1161/01.hyp.33.1.530. [DOI] [PubMed] [Google Scholar]
- 10.Tsukamoto K, Sved AF. Enhanced gamma-aminobutyric acid-mediated responses in nucleus tractus solitaries of hypertensive rats. Hypertension. 1993;22:819–825. doi: 10.1161/01.hyp.22.6.819. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Yao F, Rourke ST, Qian SY, Sun C. Angiotensin II enhances GABAB receptor-mediated responses and expression in nucleus tractus solitarii of rats. Am J Physiol Heart Circ Physiol. 2009;297:H1837–H1844. doi: 10.1152/ajpheart.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Yao F, Raizada MK, O’Rourke ST, Sun C. Apelin gene transfer into the rostral ventrolateral medulla induces chronic blood pressure elevation in normotensive rats. Circ Res. 2009;104:1421–8. doi: 10.1161/CIRCRESAHA.108.192302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao F, Sumners C, O’Rourke ST, Sun C. Angiotensin II increases GABAB receptor expression in nucleus tractus solitarii of rats. Am J Physiol Heart Circ Physiol. 2008;294:H2712–H2720. doi: 10.1152/ajpheart.00729.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumura K, Averill DB, Ferrario CM. Angiotensin II acts at AT1 receptors in nucleus of the solitary tract to attenuate the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1611–R1619. doi: 10.1152/ajpregu.1998.275.5.R1611. [DOI] [PubMed] [Google Scholar]
- 15.Katsunuma N, Tsukamoto K, Ito S, Kanmatsuse K. Enhanced angiotensin-mediated responses in the nucleus tractus solitarii of spontaneously hypertensive rats. Brain Res Bull. 2003;60:209–14. doi: 10.1016/s0361-9230(03)00013-3. [DOI] [PubMed] [Google Scholar]
- 16.Murphy MN, Mizuno M, Downey RM, Squiers JJ, Squiers KE, Smith SA. Neuroanl nitric oxide synthase expression is lower in areas of the nucleus tractus solitarius excited by skeletal muscle relexes in hypertensive rats. [Accessed May 15, 2013];Am J Physiol Heart Circ Physiol. 2013 Apr 5; doi: 10.1152/ajpheart.00235.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirooka Y, Sakai K, Kishi T, Ito K, Shimokawa H, Takeshita A. Enhanced depressor response to endothelial nitric oxide synthase gene transfer into the nucleus tractus solitarii of spontaneously hypertensive rats. Hypertens Res. 2003;26:325–31. doi: 10.1291/hypres.26.325. [DOI] [PubMed] [Google Scholar]
- 18.Nozoe M, Hirooka Y, Koga Y, Sagara Y, Kishi T, Engelhardt JF, Sunagawa K. Inhibition of Rac1-derived reactive oxygen species in nucleus tractus solitarius decreases blood pressure and heart rate in stroke-prone spontaneously hypertensive rats. Hypertension. 2007;50:62–8. doi: 10.1161/HYPERTENSIONAHA.107.087981. [DOI] [PubMed] [Google Scholar]
- 19.Shinohara K, Hirooka Y, Kishi T, Sunagawa K. Reduction of nitric oxide-mediated γ-amino butyric acid release in rostral ventrolateral medulla is involved in superoxide-induced sympathoexcitation of hypertensive rats. Circ J. 2012;76:2814–21. doi: 10.1253/circj.cj-12-0399. [DOI] [PubMed] [Google Scholar]
- 20.Landulpho CD, Dias AC, Colombari E. Cardiovascular mechanisms activated by microinjection of baclofen into NTS of conscious rats. Am J Physiol Heart Circ Physiol. 2003;284:H987–93. doi: 10.1152/ajpheart.00447.2002. [DOI] [PubMed] [Google Scholar]
- 21.Callera Joao Carlos, Leni G, Bonagamba H. Activation of GABA receptors in the NTS of awake rats reduces the gain of baroreflex bradycardia. Autonomi Neuroscience: Basic and Clinical. 2000;84:58–67. doi: 10.1016/S1566-0702(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 22.Thrasher TN. Unloading arterial baroreceptors causes neurogenic hypertension. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1044–53. doi: 10.1152/ajpregu.00431.2001. [DOI] [PubMed] [Google Scholar]
- 23.Lohmeier TE, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43:306–11. doi: 10.1161/01.HYP.0000111837.73693.9b. [DOI] [PubMed] [Google Scholar]
- 24.Howe PR, Rogers PF, Minson JB. Dietary sodium loading elevates blood pressure in baroreceptor denervated rats. J Auton Nerv Syst. 1990;29:151–6. doi: 10.1016/0165-1838(90)90180-q. [DOI] [PubMed] [Google Scholar]
- 25.Heusser K, Tank J, Luft FC, Jordan J. Baroreflex failure. Hypertension. 2005;45:834–9. doi: 10.1161/01.HYP.0000160355.93303.72. [DOI] [PubMed] [Google Scholar]
- 26.Jordan J, Toka HR, Heusser K, Toka O, Shannon JR, Tank J, Diedrich A, Stabroth C, Stoffels M, Naraghi R, Oelkers W, Schuster H, Schobel HP, Haller H, Luft FC. Severely impaired baroreflex-buffering in patients with monogenic hypertension and neurovascular contact. Circulation. 2000;102:2611–8. doi: 10.1161/01.cir.102.21.2611. [DOI] [PubMed] [Google Scholar]
- 27.Swanson LW. Brain Maps: Structure of the Rat Brain. 3. Vol. 153. San Diego. Calif: Elsevier; 2004. [Google Scholar]
- 28.Kishi T, Sunagawa K. Combination therapy of atorvastatin and amlodipine inhibits sympathetic nervous system activation and improves cognitive function in hypertensive rats. Circ J. 2012;76:1934–41. doi: 10.1253/circj.cj-12-0276. [DOI] [PubMed] [Google Scholar]