Abstract
The present study was aimed at synthesizing an imidazole-based ionic liquid 1-butyl-3-methylimidazolium bromide (BMIMBr) and subsequent development of a novel ionic liquid-in-oil (IL/o) microemulsion (ME) system for dermal delivery of a poorly permeating drug 5-fluorouracil (5-FU). A significant enhancement in the solubility of 5-FU was observed in BMIMBr. IL/o MEs of 5-FU were prepared using isopropyl myristate, Tween 80/Span 20, and BMIMBr. Results of ex vivo skin permeation studies through mice skin indicated that the selected IL/o ME exhibited 4-fold enhancement in percent drug permeation as compared to aqueous solution, 2.3-fold as compared to hydrophilic ointment, and 1.6-fold greater permeation than water in oil (w/o) ME. The results of in vivo studies against dimethylbenz(a)anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mice skin carcinogenesis demonstrated that the IL/o ME could effectively treat skin cancer in 4 weeks. In addition, the side effects such as erythema and irritation associated with the conventional formulations were not observed. Histopathological studies showed that the use of IL/o ME caused no anatomic and pathological changes in the skin structure of mice. These studies suggest that the use of IL-based ME system can efficiently enhance the solubility and permeability of 5-FU and hence its therapeutic efficacy.
Key words: 5-fluorouracil, dermal delivery, ionic liquids, ionic liquid in oil (IL/o) microemulsion, skin cancer
INTRODUCTION
Ionic liquids (ILs) refer to organic salts synthesized by combining asymmetric cations with wide variety of anions whose melting point is below 100°C. During the last two decades, ILs have attracted escalating interest in miscellaneous research genera including synthetic chemistry, separation techniques, engineering fluids, analytical chemistry, and food sciences. Due to their outstanding properties including nonvolatility, high ionic conductivity, and recyclability, these are regarded as greener solvents in comparison to conventional organic solvents (1–4). Moreover, recently, the possibilities of using ILs in pharmaceutical sciences have been investigated due to their modifiable physicochemical properties like viscosity and polarity, reaction media and catalytic role in active pharmaceutical ingredient (PI) synthesis, and, most importantly, the excellent solubilization ability for hydrophobic and hydrophilic drugs (5–9).
One of the most fascinating and promising pharmaceutical application of ILs is to use them as a component of microemulsion (ME) carrier system. MEs are thermodynamically stable isotropic mixtures of polar, nonpolar, and amphiphilic phase, which provide unique advantages like nanosized aggregates, pharmacodynamic stability, enhanced drug solubilization, and ease of preparation (10,11). Depending on the polarity, ILs can be employed to prepare ionic liquid in oil (IL/o) (12,13) or ionic liquid in water (14,15) type of MEs.
Recently, it has been found that hydrophilic ILs are very effective in enhancing the solubility of an antiviral drug, acyclovir (ACV), which is practically insoluble in water (16). Another study reports the influence of the type of ILs on the formation and drug encapsulation efficiency of IL/o MEs (17). It was observed that such ionic liquid-based MEs are capable of solubilizing higher amount of poorly soluble drugs like acyclovir, methotrexate, and 1-[(5-(p-nitro-phenyl)furfurylidene)amino]hydantoin sodium than their individual components and water.
The above-mentioned studies suggest that the IL-based MEs couple the individual advantages of ILs and MEs by overcoming the inability of conventional MEs to dissolve a number of chemicals including some hydrophilic and hydrophobic substances. ILs acts as solvents for poorly water-soluble drugs and also as drug reservoirs in controlled release systems. In addition, IL-based MEs facilitate the solubilization of large amounts, a feature particularly favorable to develop MEs for drugs required in high doses to produce pharmacological effects. Furthermore, IL/o MEs can be attractive alternative for formulation and delivery of water-labile drugs (18).
Previous reports illustrate attempts on dermal delivery of poorly soluble drugs using IL-based carrier system (7,17). In contrast to this, we propose to develop a novel IL-based carrier system for a water-soluble BCS class III drug 5-fluorouracil (5-FU), which is soluble in water, but the permeation through skin is the rate-limiting factor of this drug (19).
5-FU is a halogenated pyrimidine in which fluorine at position 5 allows the molecule to mimic uracil biochemically, which enables it to act as antimetabolite. It is the thymidylate synthase enzyme inhibitor, which gets converted into 5-fluorodeoxyuridylates, which disrupts RNA synthesis in addition to inhibiting the enzyme.
The available commercial topical preparation is the cream containing 5% 5-FU, established as a cheap, convenient, and selective dosage form for the treatment of skin cancer (20). For the treatment of precancerous skin lesions (actinic keratosis), topical application of 5-FU twice daily for approximately 3–4 weeks is recommended with the desired end point being a matter of clinical judgment. However, the disadvantage of using the cream is poor permeation of the drug and patient noncompliance because of associated pain and redness in the affected areas (21).
Thus, in order to circumvent these limitations, IL/o ME-based delivery system is proposed as it may result in enhanced drug permeation through the skin, thus leading to reduction in dose and side effects and, consequently, better patient compliance. The entrapment of drug in the internal core of the ME system is expected to overcome the undesirable skin reactions associated with the commercial topical 5-FU cream formulation.
In current study, an imidazolium cation-based IL (BMIMBr) was used for constituting the hydrophilic core of the IL/o microemulsion systems. The selected microemulsion systems were characterized using various analytical techniques and evaluated by ex vivo permeation through excised mice skin using Franz diffusion cell assembly. Finally, the anticancer effect was evaluated against dimethylbenz(a)anthracene/12-O-tetradecanoylphorbol-13-acetate (DMBA/TPA)-induced skin tumors in mice, and the results were compared with conventional formulations and commercial cream (Flonida®).
MATERIALS AND METHODS
Chemicals
DMBA/TPA were obtained from Sigma Aldrich Co., St. Louis, MO, USA. 1-Bromobutane was procured from Sisco Research Laboratories Pvt. Ltd., Mumbai, India. Isopropyl myristate (IPM), oleic acid, castor oil, Span 20, Tween 20, Tween 40, Tween 60, and Tween 80 were purchased from S.D. Fine Chemicals Ltd., Mumbai, India. All other reagents used were of analytical grade. 5-FU (Biochem Pharmaceutical Industries, Mumbai, India), Captex 200, and Captex 300 (Abitec, Janesville, WI, USA) were received as gift samples. Topical 5-FU cream (5%, w/w), Flonida® (Shalak Pharmaceuticals Ltd., New Delhi, India), was procured from a local pharmacy store.
Animals
Female Swiss albino Laca mice 4–6 weeks old, weighing 20–25 g, were obtained from Central Animal House, Panjab University Chandigarh, India. These were housed in polypropylene cages and were kept at ambient temperature with a 12–h night/day cycle, and supplied with a standard pellet diet and water ad libitum. Ethical approval to perform the animal studies was obtained from Institutional Animal Ethics Committee (IAEC), Panjab University, Chandigarh, India and their guidelines were followed throughout the studies.
Synthesis of 1-Butyl-3-Methylimidazolium Bromide
The synthesis of BMIMBr was carried out by single-step quaternization of 1-methylimidazole following a previously reported method (18). Briefly, 1-methylimidazole (1.219 mmol) was dissolved in acetonitrile (10 mL) in an appropriately sized round-bottom flask, and, to this solution, 1-bromobutane (1.436 mmol) was added drop-wise. The reaction mixture was allowed to stir under reflux conditions. The reaction progress was monitored at intermittent time intervals using silica gel G254 plates developed in lid-closed chamber containing appropriate volume of 5% methanol in chloroform (v/v) as a mobile phase (Merck KGaA, Darmstadt, Germany). The developed plates were then dried in oven and consequently visualized by exposing them to iodine vapors. Upon completion of the reaction, acetonitrile and unreacted 1-bromobutane were evaporated from the reaction mixture using flash rotary evaporator (RV10 Basic V, IKA Inc. Staufen, Germany) to afford purified ionic liquid, which appeared as a viscous light brown liquid (BMIMBr). The characterization of BMIMBr was carried out using proton nuclear magnetic resonance (1H NMR), Fourier transform infrared, and mass spectroscopic techniques.
Solubility Studies
Solubility of 5-FU was determined in water, BMIMBr, and various oils and surfactants using the shake flask method (22). Excess drug was added to 5 mL of each solvent in screw-capped glass vials and kept for 24 h in a thermostat water shaker at 37°C. The saturated suspensions were then filtered through a membrane filter (0.45 μm Nylon, Millipore Millex-GN) and analyzed using previously validated spectrophotometric method at λmax 267 nm after suitable dilution (23). Studies were carried out in triplicate.
Screening of Formulation Ingredients
Screening of Oils
The oil phase for developing MEs of 5-FU was selected on the basis of drug solubility and surfactant efficiency (Smin). The solubility of 5-FU in various oils (IPM, oleic acid, Captex 200, Captex 300, and castor oil) was determined as mentioned under solubility studies. Smin % (w/w) was determined as the minimum amount of surfactant required for completely homogenizing equal masses of IL and oil mixtures to form a single phase (24).
Screening of Surfactants
Four different surfactants namely, Tween 20, Tween 40, Tween 60, and Tween 80 were screened in terms of their emulsification capabilities. A 1:1 mixture of surfactant and IPM was homogenized, and 50 mg of this isotropic mixture was accurately weighed and diluted with BMIMBr to 50 ml to yield fine emulsion. The emulsions were allowed to stand for 2 h and their transmittance was assessed at 638.2 nm by UV spectrophotometer using BMIMBr as blank (25).
Screening of Cosurfactants
Different cosurfactants such as ethanol, isopropyl alcohol, n-butanol, propylene glycol, and Span 20 were investigated. The values of cosurfactant efficiency (SCoSmin) were determined using titration method. The mixtures of the selected surfactant and cosurfactants in predetermined weight ratios (Km) were made. Each surfactant/cosurfactant mixture was added to the mixture of IL and IPM (1:1), in a drop-wise manner, stirred using a magnetic stirrer. SCoSmin % (w/w) was determined as the minimum amount of surfactant/cosurfactant mixture required for completely homogenizing IL and IPM mixture (1:1) to form a single phase. The transparent samples were maintained at 25 ± 1°C for a minimum of 72 h to attain the equilibrium.
Construction of Phase Diagrams
Pseudoternary phase diagrams were constructed by titrating varying amounts of IPM and surfactant mixture (Smix) consisting of Tween 80 and Span 20 with the IL phase at 25°C (26,27). The Smix of Tween 80/Span 20 used was in weight ratios of 1:1, 2:1, and 1:2, respectively. At specific surfactant/cosurfactant weight ratio, the ratios of IPM to the Smix were varied as 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1. BMIMBr was added drop-wise to these mixtures under moderate magnetic stirring and assessed visually for being a ME, a crude emulsion or a gel. The transparent fluid systems were characterized as MEs, whereas, the opaque systems were considered as coarse emulsions. Likewise, surfactant/cosurfactant mixture and BMIMBr, taken in various ratios, were titrated with oil and assessed for the parameters as mentioned above. Also, pseudoternary phase diagram using IPM, Smix, and water was constructed using the above described method in order to find out the ME region for water-in-oil (w/o) ME.
Preparation of IL/o ME Formulations
5-FU-loaded IL/o ME formulations with varied composition were prepared after identification of IL/o ME region from the phase diagram (Table I). Tween 80/premix of Tween 80 and Span 20 was added and stirred to the solution of 5-FU in BMIMBr in a beaker. Then, IPM was added drop-wise under constant stirring into the above solution; clear IL/o ME was obtained spontaneously. Blank MEs without drug were also prepared in a similar way. For comparative study, w/o ME was prepared similarly using water in place of BMIMBr.
Table I.
Composition of Various Microemulsion Formulations of 5-FU
| Code | Components (% w/w) | Mass ratio Tween-80/Span-20 |
Surfactant content (% w/w) | |||
|---|---|---|---|---|---|---|
| 5-FU | IL | Water | IPM | |||
| ME-1 | 0.2 | 5 | – | 65.0 | 1:0 | 30 |
| ME-2 | 0.2 | 5 | – | 69.8 | 2:1 | 25 |
| ME-3 | 0.2 | 5 | – | 69.8 | 1:2 | 25 |
| ME-4 | 0.4 | 10 | – | 49.6 | 2:1 | 40 |
| ME-5 | 0.4 | 10 | – | 49.6 | 1:2 | 40 |
| C3 | 0.2 | – | 20 | 34.8 | 2:1 | 45 |
Characterization of 5-FU-Loaded IL/oME Formulations
Morphology and Structure
Transmission electron microscopy (TEM) (H-7500, Hitachi, Japan) was performed after depositing the selected IL/o MEs on a film-coated 200-mesh gold specimen grid. Photomicrographs at suitable magnifications under an electron microscope were obtained using negative staining with 1% phosphotungstic acid (28).
Micromeritics
Globule size, polydispersity index, and zeta potential of selected IL/o MEs were analyzed using Malvern’s ZetasizerTM (Malvern Instruments Ltd., UK). Sample (5 mL) was placed in the cuvette and the instrument recorded the intensity of fluctuation of laser beam, correlated with the particle size of emulsion droplet. Zeta potential was measured at 25°C and the electric field strength was around 23.2 V/cm.
Drug Content
The percent drug content of selected 5-FU-loaded MEs was determined by diluting 100 times with ethanol and analyzing spectrophotometrically at λmax 267 nm.
Rheological Behavior and Viscosity Measurements
The viscosity of the selected IL/o MEs was measured at different angular velocities at 32.0 ± 0.1°C with a rotating-spindle Brookfield DV-II+ pro viscometer (PaarPhysica MC1, Brookfield DV-II, UK) using spindle number 21. Shear stress was measured within the shear rate ranging from 0 to 100 s−1 for both up and down curves, and rheograms were plotted (29).
Thermodynamic Stability Studies
Thermodynamic stability of MEs was assessed by the three-step procedure as reported by Shafiq et al. with slight modification (30).
Heating Cooling Cycle
Six cycles between refrigerator temperature (4°C) and 45°C with storage at each temperature for 48 h was studied.
Centrifugation
The formulations passed in previous step were centrifuged at 3,500 rpm for 30 min.
Freeze-Thaw Cycle
Three freeze-thaw cycles between −20°C and +25°C with storage at each temperature for 48 h was done for the formulations.
Microemulsion Stability Studies
Selected IL/o MEs were subjected to different temperature conditions, i.e., 4°C, 25°C, and 40°C, for 3 months and periodically examined for any physical change (like clarity, phase separation, precipitation of drug, and color change) and drug content.
Ex Vivo Studies
Drug Permeation Study
The ex vivo permeation studies were carried out employing excised abdominal skin (full thickness, 0.5–0.7 mm) of mice. The animals were sacrificed by cervical dislocation method and shaved; the shaven part of the skin was separated. After removal of excess fat and connective tissue, the excised skin was washed with normal saline and used. Permeation studies were conducted using Franz diffusion cell PermeGear, Inc., USA (31). The skin was mounted on the diffusion cell assembly with an effective diffusion area of 3.14 cm2 keeping the stratum corneum towards the donor compartment (32). The receptor compartment consisted of 30-ml phosphate buffer saline pH 6.4 kept at 32°C. The amount of different formulations equivalent to1.0 mg of drug was applied on to the skin in the donor compartment. An aliquot of 2-ml sample was withdrawn at suitable time intervals and replaced immediately with an equal volume of fresh receptor medium (33). The samples were analyzed spectrophotometrically at 267 nm after suitable dilution. At the end of the permeation experiment (24 h), the skin surface in the donor compartment was rinsed with ethanol to remove excess drug from the surface. The receptor medium was then replaced with ethanol and stirred for the next 24 h, followed by spectrophotometric determination. Ethanol is reported to extract drug deposited in the skin, thus giving a measure of skin retention. Similar permeation and skin retention studies were performed using blank formulations (without drug), and the absorbance values were subtracted from the test formulations to account for the effect of excipients. The cumulative amount permeated per unit area (μg/cm2), flux (μg/h/cm2), and skin retention (μg/cm2) were calculated. Each experiment was conducted in triplicate.
Statistical Analysis
One-way analysis of variance (ANOVA) with Tukey’s test was used for statistical comparison using GraphPadInStatTM software (GraphPad Software Inc., California, USA). Results were considered as significant where P < 0.05.
In Vivo Studies
Skin tumors were induced in female Swiss Albino Laca mice using DMBA and TPA. The hairs were removed from the back of the mice with the help of an animal hair clipper. The animals were divided into seven groups of four mice each and administered respective treatments (Table II). The control group (group 1) was treated with acetone (100 μL), which was topically applied twice a week on the depilated dorsal area of the mice. To the other groups (group 2–7), DMBA (100 nM/100 μL of acetone) was applied topically on the depilated dorsal region of each mice twice a week for 2 weeks, followed by application by TPA (1.7 nM/100 μL of acetone) per week for the next 18 weeks. Onset of tumor induction was observed after the 10th week of the above-mentioned cancer-inducing treatment. Thereafter, group 2 was not treated with any formulation, and groups 3–7 were treated with test formulations (amount sufficient to cover the affected area) twice daily for a period of 4 weeks. Groups 3, 4, 5, 6, and 7 were treated with ME1, ME2, ME4, C3, and commercial cream, respectively (Table II).
Table II.
Details of Treatments Employed for Evaluation of Anticancer Activity
| Treatment group | Treatment details |
|---|---|
| Group 1 | Treatment with acetone (control) |
| Group 2 | Treatment with DMBA and TPA |
| Group 3 | Treatment with DMBA and TPA + 0.2% w/w IL/O Microemulsion (ME-1) |
| Group 4 | Treatment with DMBA and TPA + 0.2% w/w IL/O Microemulsion (ME-2) |
| Group 5 | Treatment with DMBA and TPA + 0.4% w/w IL/O Microemulsion (ME-4) |
| Group 6 | Treatment with DMBA and TPA + 0.2% w/w W/O Microemulsion (C3) |
| Group 7 | Treatment with DMBA and TPA + 5% commercial cream (Flonida®) (C4) |
The formulations were applied for a period of 4 weeks, and vital observations, like changes in tumor size, animal body weight, and mortalities, were periodically recorded. After completion of the aforementioned study, the mice were sacrificed by cervical dislocation. Skin papillomas and skin tissues were removed immediately and were fixed in formalin, processed, and embedded in paraffin. Sections were stained with hematoxylin and eosin and observed under a high-power light microscope.
Skin Sensitivity and Histopathological Studies
The hairs on the dorsal side of the mice were removed with electric clipper in the direction of tail to head without damaging the skin. Test formulations (0.5 g) were applied uniformly on the dorsal region by uniform spreading within the area of 4 cm2. Observations were made for any visible change such as erythema 4 h after formulation application and compared to the control group untreated (34). The mean erythemal scores were recorded (ranging from 0 to 4) depending on the degree of erythema. The animals were then sacrificed by spinal dislocation method, and the exposed dorsal surface was cut and histopathological samples (skin sections) were prepared and studied as described earlier.
RESULTS AND DISCUSSION
Synthesis and Characterization of BMIMBr
The brown viscous ionic liquid, BMIMBr, was synthesized in good yield and characterized using various analytical techniques (18).
The 1H NMR exhibited the characteristic peaks of 3-butyl-1-methylimidazolium cation. The various peaks at respective chemical shift values can be summarized as: δ 0.935–0.972 (t, 3H, CH3, J = 7.36 Hz), 1.338–1.412 (m, 2H, CH2, J = 7.32), 1.878–1.953 (m, 2H, CH2, J = 7.60 Hz), 4.131 (s, 3H, N-CH3), 4.353–4.390 (t, 2H, N-CH2, J = 7.60), 7.677–7.685 (d, 1H, Ar-H, J = 3.20 Hz), and 7.774–7.782 (d, 1H, Ar-H, J = 3.20 Hz), 9.970 (s, 1H Ar-H).
FTIR spectrum showed characteristic peaks including, C-H stretching bands at 3,080 and 2,960 cm−1, C=C aromatic stretch at 1,629 and 1,570 cm−1, and C–N stretch band at 1,169 cm−1. Also, the mass spectrum was comprised of a molecular ion peak at 139.1, which was in agreement with the molecular weight of 1-butyl-3-methylimidazolium cation.
Solubility Studies
Aqueous solubility of 5-FU was found to be 12.2 ± 0.84 mg/mL, whereas the solubility of 5-FU in BMIMBr was determined to be 31.19 ± 1.43 mg/mL; in other words, in 2.6 times, the aqueous solubility was achieved using IL (Table III). Enhancement in solubility of 5-FU observed in BMIMBr can be attributed to solute-solvent interactions such as hydrogen bonds, van der Waal’s forces, and π-π interactions between aromatic rings of imidazolium cation and 5-FU (8).
Table III.
Drug Solubility in Various Media
| Media | Drug solubility (mg/mL; n = 3) |
|---|---|
| Water | 12.21 ± 0.84 |
| BMIMBr | 31.19 ± 1.43 |
| IPM | 0.36 ± 0.02 |
| Oleic acid | 0.21 ± 0.03 |
| Captex 300 | 0.08 ± 0.01 |
| Captex 200 | 0.06 ± 0.01 |
| Castor oil | 0.06 ± 0.02 |
| Tween 20 | 20.23 ± 1.01 |
| Tween 40 | 22.42 ± 1.08 |
| Tween 60 | 18.49 ± 0.98 |
| Tween 80 | 27.21 ± 1.26 |
Screening of Formulation Ingredients
Screening of Oils
The solubility of 5-FU in different oils was determined and was found to be highest in IPM (Table III). The physicochemical properties of oils influence the area of existence of ME; therefore, oils were also screened with respect to Smin for developing the ME formulations. It was observed that the vegetable oil (castor oil) resulted in formation of ME at extremely high-surfactant concentration (78%, w/w) which could be ascribed to its large molecular weight (35). However, medium-chain triglycerides (Captex 300 & Captex 200) were solubilized with lower surfactant concentration than vegetable oils (54%, w/w). These results could be explained by the fact that in medium-chain triglycerides, about 95% of fatty acids are made of 8–10 carbon atoms and therefore their molecular weights are less than vegetable oils (36). Further, oleic acid was solubilized completely with still lesser concentration of Tween 80 (42.5%, w/w), and IPM was solubilized using the least amount of surfactant (28.1%, w/w). In general, it was concluded that the lower the molecular weight of oil, the greater the surfactant efficiency. Moreover, IPM also acts as penetration enhancer that is thought to provide benefit by fluidizing stratum corneum lipids and partially dissolving them. IPM as a permeation enhancer had a strong permeation-enhancing effect and could increase the diffusion coefficient in skin which could result in the increase of permeation coefficient (37). Thus, on the basis of solubility and Smin, IPM was selected as oil phase.
Screening of Surfactant
Choice of the surfactant is critical for the formulation of MEs as it helps in the reduction of the interfacial tension by forming a film at the oil–water interface resulting in the spontaneous formation of MEs (37). According to the Bancroft rule, a predominantly hydrophilic emulsifier stabilizes an oil-in-water emulsion whereas a predominantly hydrophobic emulsifier stabilizes a water-in-oil emulsion. Literature also reports that surfactant(s) having HLB range 10–14 is desired for preparing IL/O ME formulation (38). There are literature reports regarding the selection of surfactant on the basis of drug solubility; however, the solubilization of oil with the surfactant is also an important factor. It is not necessary that the surfactant, having good solubilizing property for drug, would also have equally good affinity for the selected oil phase (34). The emulsification ability of the investigated surfactants in terms of percent transmittance was observed to be Tween 80 (96.4) > Tween 60 (95.4) > Tween 40 (94.7) > Tween 20 (94.3).
The differences between the Tween variants in terms of emulsification capacity can be explained on the basis of structure of the alkyl chain group. Ideally, the lipophilic chains of an amphiphile should be short or at least containing a fluidizing group such as double bonds in order to allow oil uptake. The surfactants containing longer saturated alkyl chain (Tween 20, Tween 40, and Tween 60) may not exhibit the required fluidity for ME formation as compared to Tween 80 which contains a double bond in its lipophilic chain. The choice of Tween 80 as surfactant was also based on the fact that Tween 80 has hydrophilic polyoxyethylene (PEO) groups, which have a strong affinity with the imidazolium cation appended in ILs through the electrostatic interaction (39). Therefore, on the basis of drug solubility (Table III) and emulsification capability, Tween 80 was selected as the surfactant for formulating MEs.
Screening of Cosurfactant
The presence of cosurfactants decrease the interface bending stress and allows the interfacial film sufficient flexibility to take up different curvatures required to form ME over a wide range of composition (40). The effect of different cosurfactants (Table IV) on the efficiency of Tween 80 in microemulsion development was investigated. The minimum concentration of Tween 80/cosurfactant mixture required to produce balanced microemulsions (SCoSmin) was determined varying Tween 80/cosurfactant mass ratio (Km) (Table IV). Emulsification studies clearly demonstrated the enhanced microemulsification ability of Tween 80 in the presence of various cosurfactants with Span 20 (in the ration 2:1) exhibited most pronounced effect (Table IV). Therefore, Span 20 was selected as the cosurfactant.
Table IV.
Cosurfactant Efficiency (SCoSmin) of Different Cosurfactants
| Cosurfactant | Tween 80: co-surfactant (Km) | SCoSmin (%, w/w) |
|---|---|---|
| n-Butanol | 1:1 | 29.50 |
| Ethanol | 1:1 | 30.00 |
| Isopropyl alcohol | 1:1 | 30.22 |
| Propylene glycol | 1:1 | 32.87 |
| Span 20 | 1:1 | 23.30 |
| Span 20 | 2:1 | 18.53 |
| Span 20 | 1:2 | 25.35 |
Pseudo-ternary Phase Diagram and Preparation of MEs
Figure 1a–d depicts the pseudoternary phase diagrams with various weight ratios of Tween 80 to Span 20 constructed to find out the microemulsion region. The ME single-phase region area observed in the plot constructed using water in place of BMIMBr was found to be significantly less. By comparing the BMIMBr/Tween 80-Span 20/IPM ternary plots with that of water/Tween 80-Span 20/IPM, it can be said that much lower weight fractions of surfactants are required to solubilize a large amount of polar phase in MEs using the mixture of Tween 80 and Span 20. These interesting findings can be explained in terms of the favorable interfacial properties provided by the mixtures of two different nonionic surfactants. The area of the single-phase zone slightly varied with the Tween 80/Span 20 ratio in the order of 1:1 > 2:1 > 1:2 which indicated the importance of the surfactant cosurfactant mass ratio and also highlighted that formation of MEs with IL as a polar core facilitated by gradual replacement of Span 20 by an equivalent amount of Tween 80. MEs employing minimum amount of surfactant and cosurfactant were selected from phase diagram for the preparation of ME formulations.
Fig. 1.
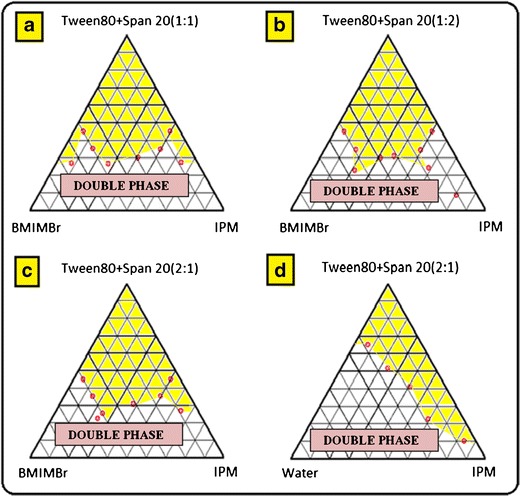
Pseudo ternary phase diagrams constructed with IPM, BMIMBr and mixture of Tween 80 with Span 20 in 1:1 (a), 1:2 (b), and 2:1 (c) ratio (w/w); pseudoternary phase diagram with IPM, water and mixture of Tween 80 with Span 20 in 2:1 w/w ratio (d). The area shaded in yellow depicts microemulsion region
Characterization of Prepared Formulations
Formulations ME3 and ME5, containing Tween 80:Span 20 in ratio of 1:2 were not physically stable; hence, these were not characterized and evaluated further. The IL/o ME formulations, ME2 and ME4, were characterized for various parameters (Table I).
Morphology and Structure
TEM images are depicted in Fig. 2. Positive images were obtained in which spherical dark globules were observed against bright background.
Fig. 2.
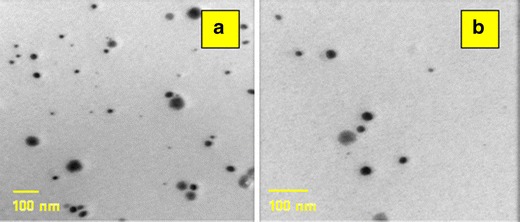
Transmission electron micrographs of ME2 (a) and ME4 (b)
Micromeritics and Zeta Potential
The mean globule sizes and polydispersity indices of ME2 and ME4 were found to be 10.2 and 11.5 nm, respectively, and 0.101 and 0.141 nm, respectively (Fig. 3). In MEs, the surfactant mixture lowers the interfacial tension, and hence reduce the curvature of nanodroplets, therefore providing them a very low globule size (11). Tween 80 as a surfactant also has significant influence on the droplet size due to effective interfacial activity. Polydispersity index values of ME2 and ME4 were less than 0.5 which described the homogeneity of globule size. Zeta potential of the optimized formulations (ME2 and ME4) was found to be 0.0854 and 0.0395 mV.
Fig. 3.

Globule size distribution of microemulsion ME2 (a) and ME4 (b)
Drug Content
The drug content was found to be in the range of 99.79 ± 0.35 to 99.87 ± 0.36%. This indicated that the drug was uniformly distributed throughout the formulations and drug loss was minimum while preparation of MEs.
Rheological Behavior and Viscosity Measurement
MEs exhibited proportionality between shear stress and shear rate and their viscosity values were calculated from the slopes of the rheograms. Viscosity of ME2 and ME4 was found to be 31.9 and 34.2 cP, respectively. The linear regression (r2) values were found to be more than 0.99, exhibiting their Newtonian flow behavior. This may be accounted due to the uniformity of the nanostructures, with respect to size and shape, which does not allow change in the flow behavior on changing the shear rate and maintains constant viscosity of the system (41).
Thermodynamic Stability Studies
The selected ME2 and ME4 formulations when centrifuged at 3,500 rpm for 30 min and subjected to heating cooling and freeze-thaw cycles showed no phase separation or drug precipitation, indicating that the ME formulations were physically stable.
Microemulsion Stability Studies
The selected ME2 and ME4 remained clear even after a period of 3 months at 4°C, 25°C and 40°C temperature and were found to be consistent with respect to their drug content, viscosity, and transparency during the stability study.
Ex Vivo Permeation Studies
Figure 4 shows a comparison of permeation profiles of 5-FU through mice skin in 24 h from IL/o MEs (ME1, ME2 and ME4) with different control formulations. C1, C2, C3, and C4 refer to 0.2% 5-FU aqueous solution, 0.2% 5-FU hydrophilic ointment, 0.2% 5-FU w/o ME, and commercial 5-FU cream (Flonida®), respectively. It was observed that 5-FU loaded w/o ME C3 (51.84 ± 0.24%) showed 2.5-fold enhancement in percent drug permeation in 24 h compared to the aqueous solution C1 (20.70 ± 0.23%) and a 1.48-fold increase as compared to the drug-loaded hydrophilic ointment C2 (36.31 ± 0.23%). Although the physicochemical properties of 5-FU, a BCS class III drug, highly water soluble, possessing a log P value of −0.78 are not suitable for dermal partitioning, the results indicate that a more effective skin penetration of 5-FU can be achieved using ME carrier. Different mechanisms can be stated to explain the enhanced transdermal drug delivery which include the penetration-enhancing effect of the ME components (42). Firstly, in the present study, IPM which was used as the oil phase for the ME is a penetration enhancer and it penetrates within lipid bilayers of the stratum corneum by disrupting their order and arrangement and results in drug permeation through it (43). Secondly, the improved drug permeation through skin may be due to the ME components entering into the skin as globules, which resulted in an increased drug concentration in the skin (44). Consequently, this increases the partitioning of the drug in the skin, leading to increased drug concentration in the upper layers of the skin. This concentration gradient acts as a driving force for transdermal drug delivery. Thirdly, there may be direct drug release from the ME droplets to the stratum corneum (45). The drug molecules are distributed within the nanosized globules of microemulsion systems. This small droplet size with increased surface area results in enhanced drug release. Further, addition of cosurfactant increases the flexibility of the surfactant film and reduces the droplet size and contact angle, resulting in greater increase in surface area and consequently higher enhancement of drug permeation. Fourthly, the supersaturation process increases the thermodynamic activity and the driving force for the transdermal drug transfer (45). This may be due to the fact that ME systems undergo phase transition upon dilution with aqueous phase or evaporation of any volatile constituents which can influence the drug loading with the possibility of formation of supersaturated systems. This process is more likely to occur after open application on a relatively large surface area.
Fig. 4.

Comparison of skin permeation profiles of various formulations in 24 h
The percent permeation of 5-FU from the IL-based ME1 (63.6 ± 0.23%) was significantly greater than w/o ME (C3) and it was drastically enhanced using the formulations ME2 (91.43 ± 1.26%) and ME4 (93.34 ± 1.48%). It was observed that using a combination of two surfactants (Tween 80 and Span 20) as in ME2 and ME4, provided better permeation results than the ME1 prepared using a single surfactant (Tween 80). However, using higher amount of 5-FU in ME4 (0.4%, w/w) produced no significant permeation enhancement compared to formulation ME2, containing 0.2%, w/w drug. Permeation flux through ME2 (49.99 ± 0.293 μg/h/cm2) and ME4 (50.19 ± 0.210 μg/h/cm2) was found to be much higher than the controls C1 (8.31 ± 0.152 μg/h/cm2), C2 (11.04 ± 0.42 μg/h/cm2), C3 (15.71 ± 0.216 μg/h/cm2), and C4 (11.78 ± 0.234 μg/h/cm2). Imidazole moiety has been reported to have skin permeation-enhancing effect (46,47). The penetration-enhancing effect of imidazole and imidazole derivatives have been reported by Helman et al. in as early as 1993. The group reported the skin penetration enhancement of various drugs like ibuprofen, naphazoline, hydrochlorthiazide, and aspirin using imidazole (48). As imidazole is the basic ring structure in BMIMBr ionic liquid, it is expected to be the responsible for the skin penetration enhancement. In addition, many reports discuss the use of compounds containing quaternized nitrogen atom like quaternary ammonium compounds as efficient skin penetration enhancers (49,50). This indicates a possible role of charged nitrogen atom for enhanced dermal delivery. As BMIMBr contains a quaternized nitrogen atom, this may be another contributing factor for the enhanced skin permeation of IL/o MEs.
Figure 5 depicts the comparative skin retention profile of the various formulations. It was observed that the skin retention values of 5-FU were found to be below 5.00 μg/cm2 for C1, C2, and C4. However, owing to its better skin penetration characteristics, w/o ME system (C3) showed drug a skin retention value of 8.24 μg/cm2. The IL/o-based systems ME1, ME2, and ME4 showed the mean skin retention values of 6.82, 7.55, and 7.25 μg/cm2, respectively. Therefore, microemulsion-based systems provided greater skin deposition of drug as compared to the conventional ointment systems and aqueous solution of drug. The w/o ME, however, showed slightly better drug skin retention value in comparison to that of IL/o MEs.
Fig. 5.

Comparison of drug skin retention values of various formulations
The above results clearly indicate the remarkable enhancement in permeation profile of 5-FU when formulated into the IL/o ME system. The IL/o ME system thus enhances the percutaneous permeation of hydrophilic drugs like 5-FU because it can increase the compatibility between the drug and the stratum corneum, change the structure of the lipid bilayer, and easily cross the pores of the skin.
Skin Sensitivity and Histopathological Studies
ME2 and ME4 formulations showed zero erythema score index. The microscopic appearance of the control mice skin sections (without treatment) and the sections of skin treated with formulation ME2 and ME4 are shown in Fig. 6. No observed anatomical and pathological changes established the safety of tested formulations on mice skin.
Fig. 6.
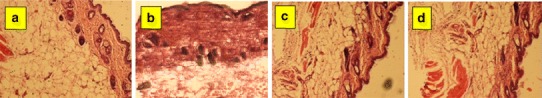
Histopathology of normal skin (a), skin treated with commercial cream C4 (b), ME2 (c), and skin treated with ME4 (d)
In Vivo Studies
Periodic weekly observations of skin tumor on treatment with test formulations of 5-FU were done in terms of reduction in number, size, tumor color, and erythema produced during the period of 4 weeks. Reduction in the size of tumor was observed in treatment with all microemulsion formulations (ME1, ME2, ME4, and C3). The cancerous lesions were observed to be completely cured in groups 4 and 5 (ME2 and ME4 treated), and the skin regained its normal physiology. Skin sections of groups 4 and 5 (treated with ME2 and ME4) showed normal physiology confirming the effectiveness of IL/o MEs as compared to the controls (Fig. 7).
Fig. 7.

Histopathology skin sections of mice treated with TPA/DMBA, i.e., cancer induced (a) and skin treated with commercial cream C4 (b), ME2 (c), and ME4 (d)
The macroscopic changes observed in the skin tumor during treatment with different formulations have been depicted in Fig. 8. Results of in vivo study clearly indicate that all the IL/o MEs (ME1, ME2, and ME4) were highly effective in reducing the size of the tumor within a period of 4 weeks. This was however not observed with the commercial cream (C4) indicating the ineffectiveness of the conventional formulation. Also the irritancy potential/erythema due to 5-FU was observed with the C3 as well as C4. However, the developed IL/o formulations (ME1, ME2, and ME4) did not produce any erythema indicating superior performance of IL-based formulation systems which may result in better patient compliance. Also, it was observed that among all the formulations evaluated, ME2 and ME4 were the only systems to have completely removed the tumor off the mice’s skin leaving behind no observable signs of cancer, and the skin resumed its normal physiology. Both the formulations treated skin cancer within a span of 4 weeks. Higher concentration of 5-FU (0.4% w/w) in formulation ME4 produced the same results as 0.2% 5-FU in ME2. Very importantly, the IL/o-based ME2 was effective even at a concentration of only 0.2% 5-FU while the commercial cream C4 containing 5% 5-FU was inefficient in treating the skin cancer in 4 weeks.
Fig. 8.
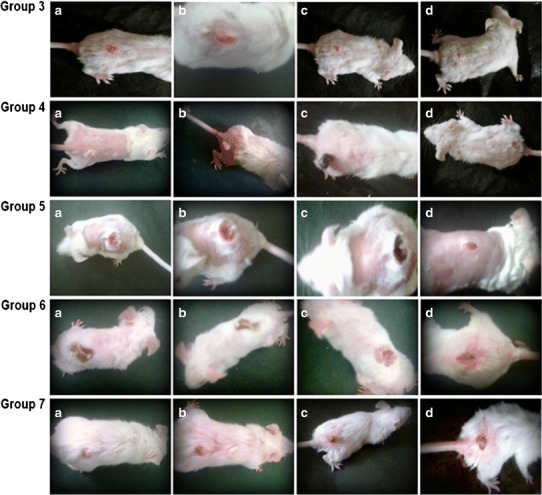
Macroscopic changes in skin of DMBA-TPA induced cancer mice after appropriate treatments. Group 3 Tumor at end of 10th week (before treatment) (a); mice treated with ME1 at the end of 2 weeks (tumor shape deformation) (b), 3 weeks (slight reduction in tumor size) (c), and 4 weeks (further reduction in tumor size) (d). Group 4 Tumor at the end of 10th week (before treatment) (a); mice treated with ME2 at the end of 2 weeks (decrease in the number of cancerous lesions) (b), 3 weeks (blackening of tumor) (c), and 4 weeks (point of tumor detachment) (d). Group 5 Tumor at the end of 10th week (before treatment) (a); mice treated with ME4 at the end of 2 weeks (no appreciable size reduction) (b), 3 weeks (blackening of tumor) (c), and 4 weeks (point of tumor detachment) (d). Group 6 Tumor at the end of 10th week (before treatment) (a); mice treated C3 at the end of 2 weeks (tumor shape deformation and size reduction) (b), 3 weeks (tumor size reduction and appearance of erythema) (c), and 4 weeks (appreciable size reduction and persistence of erythema) (d). Group 7 Tumor at the end of 10th week (before treatment) (a); mice treated with 5% commercial cream (C4) at the end of 2 weeks (appearance of erythema, tumor turns slight pink but no shape and size change) (b); 3 weeks (slight blackening of tumor, slight size reduction and persistence of erythema) (c); and 4 weeks (no appreciable change in tumor size observed) (d)
The body weight of mice was recorded during the entire 20-week duration of the study following various treatments. Significant reduction in the body weight of mice was observed in DMBA/TPA-treated group (group 2). Reduction in body weight was also observed in groups 3–7 during the first 10 weeks (during the treatment with carcinogens) but as the treatment protocol reached its final, the mice treated with ME2 and ME4 regained their normal body weight. Survival rate of the mice decreased significantly in DMBA/TPA-treated mice as compared to the control group treated with acetone alone and the developed formulations (Fig. 9). Among all the formulations evaluated, the percent survival of mice was maximum (100%) for IL/o microemulsions (ME2 and ME4), i.e., no mortality was observed in groups 4 and 5. However, the percent survival of mice was observed to be 50% for ME1-, C3-, and C4-treated groups. These results clearly suggest the superior performance of the IL/o-based ME2 and ME4 formulations in treatment of skin cancer. Also, ME2 despite containing half the amount of drug as in ME4 was equally effective in treating the skin tumors.
Fig. 9.
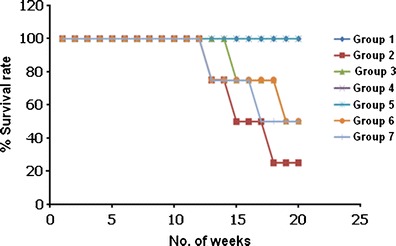
Survival rate of mice of various treatment groups
CONCLUSION
In this study, an attempt was made to develop and characterize an ionic liquid based novel nanocarrier system for topical delivery of 5-FU with a view to improve its solubility, permeability, and hence bioavailability for skin cancer treatment. The solubility of 5-FU was remarkably enhanced in BMIMBr as compared to water and, hence, it was selected as the internal phase for the ME. Ex vivo permeation studies suggest significant enhancement in flux as well as cumulative amount of drug permeated in 24 h using IL/o ME formulations. The evaluation of various formulations using the DMBA/TPA induced cancer in mice showed that the IL/o MEs were successful in treating skin tumors in a period of 4 weeks, whereas, the aqueous solution (C1), conventional ointment (C2), and the commercial cream (C4) were ineffective in regaining the normal physiology of skin after a period of 4 weeks of treatment. Also, the side effect (erythema) associated with the conventional formulations was not observed in case of IL/o-based ME formulations. This was due to the fact that IL/o formulations incorporated 5-FU inside the internal core of the ME systems. The preliminary investigations suggest that developed novel IL/o ME may be used topically for the treatment of actinic keratosis and basal cell carcinoma. However, the complete usefulness can only be realized after elucidating the comprehensive mechanisms of action and further preclinical and clinical investigations.
Acknowledgments
The authors acknowledge the kind help provided by Biochem Pharmaceutical Industries, Mumbai, in the form of free gift sample of 5-flurouracil.
Declaration of Interest
The authors report no declaration of interest.
REFERENCES
- 1.Sun P, Daniel W, Armstrong W. Ionic liquids in analytical chemistry. Anal Chim Acta. 2010;661(1):1–16. doi: 10.1016/j.aca.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Zhou F, Liang Y, Liu W. Ionic liquid lubricants: designed chemistry for engineering applications. Chem Soc Rev. 2009;38:2590–2599. doi: 10.1039/b817899m. [DOI] [PubMed] [Google Scholar]
- 3.Berthod A, Ruiz-Angel MJ, Carda-Broch S. Ionic liquids in separation techniques. J Chromatogr A. 2008;1184:6–18. doi: 10.1016/j.chroma.2007.11.109. [DOI] [PubMed] [Google Scholar]
- 4.Roy SR, Chakraborti AK. Supramolecular assemblies in ionic liquid catalysis for aza-Michael reaction. Org Lett. 2010;12:3866–3869. doi: 10.1021/ol101557t. [DOI] [PubMed] [Google Scholar]
- 5.Stoimenovski J, MacFarlane DR, Bica K, Rogers RD. Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: a position paper. Pharm Res. 2010;27:521–526. doi: 10.1007/s11095-009-0030-0. [DOI] [PubMed] [Google Scholar]
- 6.Pernak J, Sobaszkiewicz K, Mirska I. Anti-microbial activities of ionic liquids. Green Chem. 2003;5:52–56. doi: 10.1039/b207543c. [DOI] [Google Scholar]
- 7.Jaitely V, Karatas A, Florence AT. Water-immiscible room temperature ionic liquids (RTILs) as drug reservoirs for controlled release. Int J Pharm. 2008;354:168–173. doi: 10.1016/j.ijpharm.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Mizuuchi H, Jaitely V, Murdan S, Florence A. Room temperature ionic liquids and their mixtures: potential pharmaceutical solvents. Eur J Pharm Sci. 2008;33:326–331. doi: 10.1016/j.ejps.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Viau L, Tourné-Péteilh C, Devoisselle J-M, Vioux A. Ionogels as drug delivery system: one-step sol–gel synthesis using imidazolium ibuprofenate ionic liquid. Chem Commun. 2010;46:228–230. doi: 10.1039/b913879j. [DOI] [PubMed] [Google Scholar]
- 10.Shah DO, Bagwe RP, Kanicky JR, Palla BJ, Patanjali PK. Improved drug delivery using microemulsions: rationale, recent progress, and new horizons. Crit Rev Ther Drug Carrier Syst. 2001;18:77–140. [PubMed] [Google Scholar]
- 11.Tenjarla S. Microemulsions: an overview and pharmaceutical applications. Crit Rev Ther Drug Carrier Syst. 1999;16:461–521. doi: 10.1615/CritRevTherDrugCarrierSyst.v16.i5.20. [DOI] [PubMed] [Google Scholar]
- 12.Mandal S, Ghosh S, Banerjee C, Kuchlyan J, Banik D, Sarkar N. A novel ionic liquid-in-oil microemulsion composed of biologically acceptable components: an excitation wavelength dependent fluorescence resonance energy transfer study. J Phys Chem B. 2013;117(11):3221–3231. doi: 10.1021/jp4009515. [DOI] [PubMed] [Google Scholar]
- 13.Eastoe J, Gold S, Rogers SE, Paul A, Welton T, Heenan RK, et al. Ionic liquid-in-oil microemulsions. J Am Chem Soc. 2005;127(20):7302–7303. doi: 10.1021/ja051155f. [DOI] [PubMed] [Google Scholar]
- 14.Cao J, Qu H, Cheng Y. The use of novel ionic liquid-in-water microemulsion without the addition of organic solvents in a capillary electrophoretic system. Electrophoresis. 2010;31(20):3492–3498. doi: 10.1002/elps.201000168. [DOI] [PubMed] [Google Scholar]
- 15.Pavlidis IV, Tzafestas K, Stamatis H. Water-in-ionic liquid microemulsion-based organogels as novel matrices for enzyme immobilization. Biotechnol J. 2010;5(8):805–812. doi: 10.1002/biot.201000052. [DOI] [PubMed] [Google Scholar]
- 16.Moniruzzaman M, Noriho K, Masahiro G. Ionic liquid based microemulsion with pharmaceutically accepted components: formulation and potential applications. J Colloid Interface Sci. 2010;352:136–142. doi: 10.1016/j.jcis.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Moniruzzaman M, Tahara Y, Tamura M, Kamiya N, Goto M. Ionic liquid assisted transdermal delivery of sparingly soluble drugs. Chem Commun. 2010;47:1452–1454. doi: 10.1039/b907462g. [DOI] [PubMed] [Google Scholar]
- 18.Kumar N, Goindi S, Kumar S, Jana AK. The effect of N-alkyl substituents on the usability of imidazolium cation-based ionic liquids in microemulsion systems: a technical note. AAPS PharmSciTech. 2013. In press. DOI:10.1208/s12249-013-9939-z. [DOI] [PMC free article] [PubMed]
- 19.Heng CL, Yu LX, Lee HL, Yang CY, Lue CS, Chou CH. Biowaiver extension potential to BCS class III high solubility-low permeability drugs: bridging evidence for metformin immediate-release tablet. Eur J Pharm Sci. 2004;22(4):297–304. doi: 10.1016/j.ejps.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Kurwa HA, Yong-Gee SA, Seed PT, Markey AC, Barlow RJ. A randomized paired comparison of photodynamic therapy and topical 5-fluorouracil in the treatment of actinic keratoses. J Am Acad Dermatol. 1999;41:414–418. doi: 10.1016/S0190-9622(99)70114-3. [DOI] [PubMed] [Google Scholar]
- 21.Epstein E. Does intermittent "pulse" topical 5-fluorouracil therapy allow destruction of actinic keratoses without significant inflammation? J Am Acad Dermatol. 1998;38:77–80. doi: 10.1016/S0190-9622(98)70542-0. [DOI] [PubMed] [Google Scholar]
- 22.Avdeef A. Solubility of sparingly-soluble ionizable drugs. Adv Drug Deliv Rev. 2007;59:568–90.23. doi: 10.1016/j.addr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Shishu K, Maheshwari M. Development and evaluation of novel microemulsion based oral formulations of 5-fluorouracil using non-everted rat intestine sac model. Drug Dev Ind Pharm. 2012;38(3):294–300. doi: 10.3109/03639045.2011.602407. [DOI] [PubMed] [Google Scholar]
- 24.Tchakalova V, Testard F, Wong K, Parker A, Benczedi D, Zemb T. Solubilization and interfacial curvature in microemulsions: II. Surfactant efficiency and PIT. Colloids Surf A Physicochem Eng Asp. 2008;331:40–47. doi: 10.1016/j.colsurfa.2008.07.060. [DOI] [Google Scholar]
- 25.Wankhade V, Pande S, Tapar K, Bobade N. Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for Gliclazide. Der. Pharmacia Lett. 2010;2(4):132–143. [Google Scholar]
- 26.Alani R, Tucker I, Davies N, Rades T. Characterizing colloidal structures of pseudoternary phase diagrams formed by oil/water/amphiphile systems. Drug Dev Ind Pharm. 2001;27:31–38. doi: 10.1081/DDC-100000125. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Chang X, Weng T, Zhao X, Gao Z, Yang Y, et al. A study of microemulsion systems for transdermal delivery of triptolide. J Controlled Release. 2004;98:427–436. doi: 10.1016/j.jconrel.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Oyewumi M, Yokel R, Jay M, Coakley T, Mumper R. Comparison of cell uptake, biodistribution and tumor retention of folate-coated and PEG-coated gadolinium nanoparticles in tumor-bearing mice. J Controlled Release. 2004;95:613–626. doi: 10.1016/j.jconrel.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Welin-Berger K, Neelissen B, Bergenstahl B. The effect of rheological behaviour of a topical anaesthetic formulation on the release and permeation rates of the active compound. Eur J Pharm Sci. 2001;13:309–318. doi: 10.1016/S0928-0987(01)00118-X. [DOI] [PubMed] [Google Scholar]
- 30.Shafiq S, Shakeel F, Talegaonkar S, Ahmad F, Khar R, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007;66:227–243. doi: 10.1016/j.ejpb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Bolzinger MA, Briancon S, Pelletier J, Fessi H, Chevalier Y. Percutaneous release of caffeine from microemulsion, emulsion and gel dosage forms. Eur J Pharm Biopharm. 2008;68:446–451. doi: 10.1016/j.ejpb.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Venter J, Muller D, du Plessis J, Goosen C. A comparative study of an in situ adapted diffusion cell and an in vitro Franz diffusion cell method for transdermal absorption of doxylamine. Eur J Pharm Sci. 2001;13:169–177. doi: 10.1016/S0928-0987(01)00110-5. [DOI] [PubMed] [Google Scholar]
- 33.Braun E, Wagner A, Furnschlief E, Katinger H, Vorauer-Uhl K. Experimental design for in vitro skin penetration study of liposomal superoxide dismutase. J Pharm Biomed Anal. 2006;40:1187–1197. doi: 10.1016/j.jpba.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal N, Goindi S, Mehta SD. Preparation and evaluation of dermal delivery system of griseofulvin containing vitamin E-TPGS as penetration enhancer. AAPS PharmSciTech. 2012;13:67–74. doi: 10.1208/s12249-011-9722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basti′c M, Bastl′c L, Jovanovi′c JJ, Spiteller G. Hydrocarbons and other weakly polar unsaponifiables in some vegetable oils. J Am Oil Chem Soc. 2007;55(12):886–891. doi: 10.1007/BF02671413. [DOI] [Google Scholar]
- 36.Warisnoicharoen W, Lansley A, Lawrence M. Nonionic oil-in-water microemulsions: the effect of oil type on phase behaviour. Int J Pharm. 2000;198(1):7–27. doi: 10.1016/S0378-5173(99)00406-8. [DOI] [PubMed] [Google Scholar]
- 37.Shah D, Khandavilli S, Panchagnula R. Alteration of skin hydration and its barrier function by vehicle and permeation enhancers: a study using TGA, FTIR, TEWL and drug permeation as markers. Methods Find Exp Clin Pharmacol. 2008;30(7):499–512. doi: 10.1358/mf.2008.30.7.1159653. [DOI] [PubMed] [Google Scholar]
- 38.Moniruzzaman M, Tumara M, Tahara Y, Kamiya N, Goto M. Ionic liquid-in-oil microemulsion as apotential carrier of sparingly soluble drug: characterization and cytotoxicity evaluation. Int J Pharm. 2010;400(1–2):243–250. doi: 10.1016/j.ijpharm.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 39.Moniruzzaman M, Tahara Y, Tamura M, Kamiya N, Goto M. Ionic liquid assisted transdermal delivery of sparingly soluble drugs. Chem Commun. 2010;47(9):1452–1454. doi: 10.1039/b907462g. [DOI] [PubMed] [Google Scholar]
- 40.Baboota S, Alam M, Sharma S, Sahni JK, Kumar A, Ali J. Nanocarrier-based hydrogel of betamethasone dipropionate and salicylic acid for treatment of psoriasis. Int J Pharm Investig. 2011;1(13):139–147. doi: 10.4103/2230-973X.85963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CH, Moturi V, Lee Y. Thixotropic property in pharmaceutical formulations. J Controlled Release. 2009;136:88–98. doi: 10.1016/j.jconrel.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Dreher F, Walde P, Walther P, Wehrli E. Interaction of a lecithin microemulsion gel with human stratum corneum and its effect on transdermal transport. J Controlled Release. 1997;45:131–140. doi: 10.1016/S0168-3659(96)01559-3. [DOI] [Google Scholar]
- 43.Suh H, Jun HW. Effectiveness and mode of action of isopropyl myristate as a permeation enhancer for naproxen through shed snake skin. J Pharm Pharmacol. 1996;48:812–816. doi: 10.1111/j.2042-7158.1996.tb03979.x. [DOI] [PubMed] [Google Scholar]
- 44.Kreilgaard M. Influence of microemulsions on cutaneous drug delivery. Adv Drug Deliv Rev. 2002;54:77–98. doi: 10.1016/S0169-409X(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 45.Peltola S, Saarinen-Savolainen P, Kiesvaara J, Suhonen TM, Urtti A. Microemulsions for topical delivery of estradiol. Int J Pharm. 2003;254:99–107. doi: 10.1016/S0378-5173(02)00632-4. [DOI] [PubMed] [Google Scholar]
- 46.Buyuktimkin N, Buyuktimkin S, Rytting JH. Chemical means of transdermal drug permeation enhancement. In: Ghosh T, Yum S, Pfister W, editors. Transdermal drug permeation enhancement. Buffalo Grove, IL, USA: Interpharm Press; 1997. pp. 357–475. [Google Scholar]
- 47.Asbill CS, Michniak BB. Percutaneous penetration enhancers: local versus transdermal activity. Pharm Sci Technol Today. 2000;3(1):36–41. doi: 10.1016/S1461-5347(99)00225-4. [DOI] [PubMed] [Google Scholar]
- 48.Helman MD, Lukacsko AB, Thomas A, Zusi FC, inventors; Bristol-Myers Squibb Co., assignee. Method for enhancing transdermal penetration and compositions useful therein. USA patent US 5,164,406. 1992.
- 49.Sinha VR, Kaur MP. Permeation enhancers for transdermal drug delivery. Drug Dev Ind Pharm. 2000;26:1131–1140. doi: 10.1081/DDC-100100984. [DOI] [PubMed] [Google Scholar]
- 50.Ahad A, Aqil M, Kohli K, Chaudhary H, Sultana Y, Mujeeb M, et al. Chemical penetration enhancers: a patent review. Expert Opin Ther Pat. 2009;19:969–988. doi: 10.1517/13543770902989983. [DOI] [PubMed] [Google Scholar]


