Abstract
Titanate nanotubes can be used as drug delivery systems, but limited information is available on their interactions with intestinal cells. In this study, we investigated the cytotoxicity and cellular uptake of titanate nanotubes on Caco-2 monolayers and found that up to 5 mg/ml concentration, these nanotubes are not cytotoxic and not able to permeate through the intestinal cell layer. Transmission electron microscopic experiments showed that titanate nanotubes are not taken up by cells, only caused a high-density granulation on the surface of the endoplasmic reticulum. According to these results, titanate nanotubes are suitable systems for intestinal drug delivery.
KEY WORDS: Caco-2 cells, cytotoxicity, titanate nanotubes
INTRODUCTION
Carbon and titanate nanotubes (TiNT) are specific types of nanoparticles (1,2) with the advantage that small particles of active pharmaceutical ingredients (APIs) can be incorporated into the nanotube cavity (3). With nanotube technology, it is possible to prepare stable drug delivery systems, but their safety is a key issue that remains to be resolved. Several publications have reported on the application of carbon nanotubes (4,5), but toxicity studies have not been conclusive (6). Accordingly, the absorption, toxicity and cellular effects of nanotubes should be investigated for a full characterization of their effects. Only limited information is available concerning the cellular effects of TiNT in the gastrointestinal tract, and we have therefore studied their toxicity and absorption through the use of the Caco-2-cell line.
MATERIALS AND METHODS
Preparation of Titanate Nanotubes
TiNTs were synthesized by a simple alkali hydrothermal method involving the alkaline recrystallization of anatase TiO2, as described previously (7,8). The material obtained was characterized by transmission electron microscopy (TEM; Philips CM10, 100 kV), scanning electron microscopy (SEM; Hitachi S4700; Hitachi Scientific Instruments Ltd., Japan) and X-ray diffractometry (XRD; Rigaku miniflex 2000, CuKα). Its specific surface area was determined from nitrogen adsorption measurements performed at 77 K in a Quantachrome Nova 3000e instrument and analysed by the BET method.
Cell Culture and MTT Cell Viability Test
Caco-2 cells were used for permeability and cytotoxicity experiments. Cells were seeded on Transwell® (Corning Costar, USA) filters as reported previously (9). The cellular uptake of TiNT was examined by TEM as described in Fig. 3. To test TiNT cytotoxicity by the MTT method (10), Caco-2 cells were seeded in 96-well plates, and cells were exposed to increasing TiNT concentrations in Hank’s balanced salt solution (HBSS) at 37°C for 120 min. Dye absorbance was measured at 570 nm with a FLUOstar OPTIMA microplate reader (BMG LABTECH, Offenburg, Germany), and the values were corrected for the background absorbance, measured at 690 nm. Cell viability was expressed as a percentage of the untreated control. All reagents were purchased from Sigma-Aldrich (Budapest, Hungary).
Fig. 3.
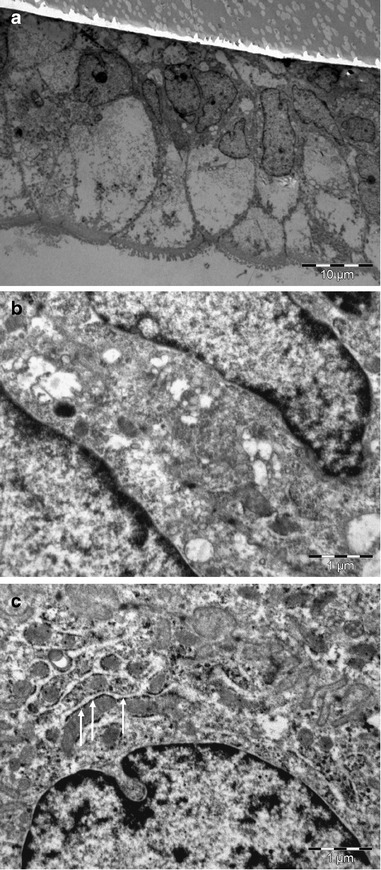
TEM images of Caco-2 cells. Untreated (a and b) or titanate nanotube-treated (c) Caco-2 monolayers were fixed with 4% glutaraldehyde and examined by TEM. a A 45° cross section of an untreated Caco-2 monolayer. High-density granules (arrows) can be observed on the surface of the endoplasmic reticulum in titanate nanotube-treated cells (c) after the 30-min treatment. TEM: For the detection of cellular titanate nanotubes uptake, confluent monolayers were treated with 0.5 mg/ml titanate nanotubes for 30–120 min, washed three times with HBSS and fixed with 4% glutaraldehyde. For TEM, the samples were embedded in Embed812 (EMS, USA), and 70-nm thin sections were cut with an Ultracut S ultra-microtome (Leica, Austria). After staining with uranyl acetate and lead citrate, the sections were observed with a Phillips CM10 electron microscope (Eindhoven, the Netherlands) equipped with a Mega-view G2 digital camera and iTEM imaging analysis software (Olympus, Münster, Germany)
Caco-2 Permeability Experiments
In permeability experiments, Caco-2 monolayers were incubated apically with TiNT at 2 mg/ml for 120 min. Then, the permeated amount of Ti was measured with an energy dispersive X-ray fluorescence analyser (Philips MiniPal PW 4025, Philips Analytical, the Netherlands).
Morphology Studies
TEM
The morphology of the synthesized titanate nanotubes was characterized with the aid of a TECNAI G2 20 X-Twin high-resolution transmission electron microscope operating at an accelerating voltage of 200 kV. Samples for TEM measurements were drop casted onto carbon-coated copper grids from an acetone suspension.
SEM
The surface of the nanotubes was tested with a scanning electron microscope (Hitachi S4700; Hitachi Scientific Instruments Ltd., Japan). A SEM sputter coating unit (Polaron E5100; VG Microtech, UK) was used to charge the surfaces for the SEM measurements.
RESULTS
The formation of the TiNT was examined by TEM and SEM. Figure 1 shows that the length of the nanotubes was 50–150 nm and their diameter was 6–10 nm. The tubular structure can also be identified. A typical TiNT has four walls and an interlayer of spacing approximately 0.7 nm. The specific surface area of the TiNT is ∼185 m2g−1 due to the specific morphology. The as-synthesized sample (Na-form) exhibited broad peaks of low intensity, which are quite difficult to index, but the profile could be referred to as reflections (around 10°, 25°, 28° and 49°) of sodium trititanate, Na2Ti3O7 (JCPDS no. 31-1329).
Fig. 1.
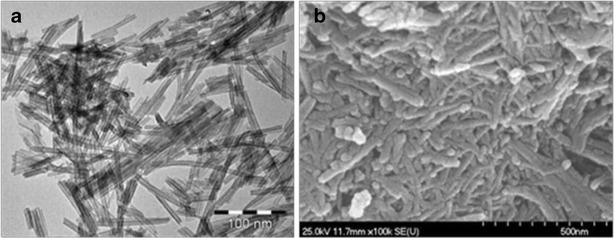
TEM (a) and SEM (b) images of TiNT
Cytotoxic effects could not be detected with the MTT method up to a nanotube concentration of 5 mg/ml (Fig. 2). This is in accordance with the findings that titanium dioxide (TiO2) nanoparticles at 1 mg/ml did not cause the death of Caco-2 cells (11) and that titania nanotubes up to 1.1 mg/ml are non-cytotoxic on A549 lung epithelial cells (12).
Fig. 2.
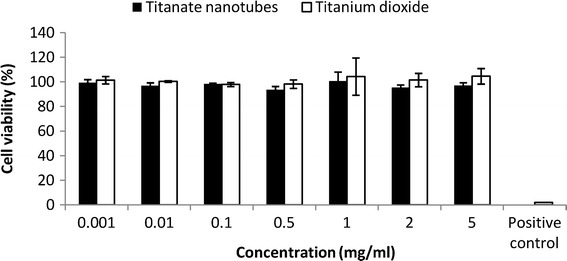
Cytotoxicity of TiNT and TiO2. Caco-2 cells were treated with nanotubes and titanium dioxide in different concentrations for 120 min, and their viability was determined by MTT tests. Untreated control was considered as 100%, and data are expressed as the percentage of untreated control. Positive control, 2% Triton X-100 solution. There were no significant differences among TiNT- and TiO2-treated samples (p > 0.05), while Triton X-100 exerted complete cell death (p < 0.05). Data are means of three independent experiments ± SD
In permeability experiments, no detectable amount of Ti was found in the basolateral side of the monolayers, indicating that intestinal cells are impermeable for TiNT. Monolayers were also treated with 0.5 mg/ml TiNT, fixed with 4% glutaraldehyde and processed for TEM investigations. Nanotubes could not be identified in the Caco-2 cells demonstrating (Fig. 3) that these nanotubes were not taken up by the cells. Nevertheless, high-density granules which had no nanotubular morphology (Fig. 3c, arrows) could be observed on the surface of the endoplasmic reticulum in the treated cells. These granules may be titanium dioxide particles formed from the nanotubes during the incubation or TiO2 impurities. This is in accordance with a previous report that at ≥10 μg/ml, TiO2 nanoparticles are able to enter Caco-2 cells and cross a Caco-2 monolayer by transcytosis (11).
DISCUSSION
TiO2 is a safe, widely used excipient in pharmaceutical technology, whereas TiNT is not yet applied. Our study has furnished evidence that TiNT does not cause cellular toxicity in short-term treatment and does not penetrate Caco-2 cells, but does lead to a high-density granulation on the surface of the endoplasmic reticulum. In vitro cytotoxicity assays (i.e. MTT, LDH) can predict irritancy and delayed toxicity of harmful agents (13). Nevertheless, cytotoxicity data alone are not necessarily predictive of in vivo issues (14), but complemented with results of morphology studies, the in vivo toxicity data may be estimated (15). Even if titanate nanotubes are unpermeable on intestinal cell layer, they deliver drug particles to intestinal cell surface and can also increase the solubility of active substances. The aqueous solutions are stable for months. That may be the reason that TiNTs provide new possibilities for the formulation of oral drug delivery systems (16).
CONCLUSION
It may be concluded that TiNT is a safe system for intestinal formulations, as they are practically not absorbed from the intestine.
Acknowledgments
This research was supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP-4.2.2.A-11/1/KONV-2012-0047 and TÁMOP 4.2.4. A/2-11-1-2012-0001 “National Excellence Program—Elaborating and operating an inland student and researcher personal support system”.
References
- 1.Chen P, Lin J, Tan KL. Carbon nanotubes: a future material of life. IUBMB Life. 2000;49(2):105–8. doi: 10.1080/15216540050022403. [DOI] [PubMed] [Google Scholar]
- 2.Kasuga T, Hiramatsu M, Hoson A, Sekino T, Niihara K. Formation of titanium oxide nanotube. Langmuir. 1998;14:3160–3. doi: 10.1021/la9713816. [DOI] [Google Scholar]
- 3.Li Y, Wang T, Wang J, Jiang T, Cheng G, Wang S. Functional and unmodified MWNTs for delivery of the water-insoluble drug carvedilol—a drug-loading mechanism. Appl Surf Sci. 2011;257(13):5663–70. doi: 10.1016/j.apsusc.2011.01.071. [DOI] [Google Scholar]
- 4.Im JS, Bai BC, Lee YS. The effect of carbon nanotubes on drug delivery in an electrosensitive transdermal drug delivery system. Biomater. 2010;31(6):1414–9. doi: 10.1016/j.biomaterials.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Naficy S, Razal JM, Spinks GM, Wallace GG. Modulated release of dexamethasone from chitosan-carbon nanotube films. Sens Actuators A. 2009;155(1):120–4. doi: 10.1016/j.sna.2009.07.021. [DOI] [Google Scholar]
- 6.Sayes CM, Liang F, Hudson JL, Mendez J, Guo W, Beach JM, et al. Functionalization density dependence of single walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett. 2006;161(2):135–42. doi: 10.1016/j.toxlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Horváth E, Kukovecz Á, Kónya Z, Kiricsi I. Hydrothermal conversion of self-assembled TiNT into nanowires in a revolving autoclave. Chem Mater. 2007;19:927–31. doi: 10.1021/cm062413q. [DOI] [Google Scholar]
- 8.Kukovecz Á, Hodos M, Horváth E, Radnóczi G, Kónya Z, Kiricsi I. Oriented crystal growth model explains the formation of titania nanotubes. J Phys Chem B. 2005;109:17781–3. doi: 10.1021/jp054320m. [DOI] [PubMed] [Google Scholar]
- 9.Fenyvesi F, Kiss T, Fenyvesi É, Szente L, Veszelka S, Deli MA, et al. Randomly methylated β-cyclodextrin derivatives enhance taxol permeability through human intestinal epithelial Caco-2 cell monolayer. J Pharm Sci. 2011;100(11):4734–44. doi: 10.1002/jps.22666. [DOI] [PubMed] [Google Scholar]
- 10.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 11.Koeneman BA, Zhang Y, Westerhoff P, Chen Y, Crittenden JC, David G, et al. Toxicity and cellular responses of intestinal cells exposed to titanium dioxide. Cell Biol Toxicol. 2010;26:225–38. doi: 10.1007/s10565-009-9132-z. [DOI] [PubMed] [Google Scholar]
- 12.Wadhawa S, Rea C, O’Hare P, Mathur A, Roy SS, Dunlop PSM, et al. Comparative in vitro cytotoxicity study of carbon nanotubes and titania nanostructures on human lung epithelial cells. J Hazard Mater. 2011;191:56–61. doi: 10.1016/j.jhazmat.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 13.Fotakis G, Timbrell JA. In vitro cytotoxicity assays. Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Tox. Lett. 2006;160:171–7. doi: 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Konsoula R, Barile FA. Correlation of in vitro cytotoxicity with paracellular permeability in Caco-2 cells. Toxicol in Vitro. 2005;19:675–84. doi: 10.1016/j.tiv.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Sun Y, Wang D, Liu H, Boughton IR. In situ fabrication of silver nanoparticle-filled hydrogen titanate nanotube layer on metallic titanium surface for bacteriostatic and biocompatible implantation. Int. J. Nanomed. 2013;8:2903–16. doi: 10.2147/IJN.S45742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zennaro L, Magro M, Vianello F, Rigo A, Mariotto G, Giarola M, et al. Stable aqueous solutions of naked nanotubes. ChemPhysChem. 2013;14:2786–92. doi: 10.1002/cphc.201300292. [DOI] [PubMed] [Google Scholar]


