Abstract
Simvastatin (SV), a drug of the statin class currently used orally as an anti-cholesterolemic via the inhibition of the 3-hydroxy-3-methyl-glutaryl-Coenzyme A (HMG-CoA) reductase, has been found not only to reduce cholesterol but also to have several other pharmacological actions that might be beneficial in airway inflammatory diseases. Currently, there is no inhalable formulation that could deliver SV to the lungs. In this study, a pressurised metered-dose inhaler (pMDI) solution formulation of SV was manufactured, with ethanol as a co-solvent, and its aerosol performance and physico-chemical properties investigated. A pMDI solution formulation containing SV and 6% w/w ethanol was prepared. This formulation was assessed visually and quantitatively for SV solubility. Furthermore, the aerosol performance (using Andersen Cascade impactor at 28.3 L/min) and active ingredient chemical stability up to 6 months at different storage temperatures, 4 and 25°C, were also evaluated. The physico-chemical properties of the SV solution pMDI were also characterised by differential scanning calorimetry (DSC), thermogravimetric analysis (TGA) and laser diffraction. The aerosol particles, determined using scanning electron microscopy (SEM), presented a smooth surface morphology and were spherical in shape. The aerosol produced had a fine particle fraction of 30.77 ± 2.44% and a particle size distribution suitable for inhalation drug delivery. Furthermore, the short-term chemical stability showed the formulation to be stable at 4°C for up to 6 months, whilst at 25°C, the formulation was stable up to 3 months. In this study, a respirable and stable SV solution pMDI formulation for inhalation has been presented that could potentially be used clinically as an anti-inflammatory therapy for the treatment of several lung diseases.
Key Words: lung inflammation, pMDI, pressurised metered dose inhaler, simvastatin
INTRODUCTION
Statins (HMG-CoA reductase inhibitors) are widely used as cholesterol-lowering drugs (1). These compounds inhibit the activity of 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase enzyme, which catalyses the rate-limiting step in mevalonate biosynthesis, a key intermediate in cholesterol metabolism. Studies have recently described other possible roles for statins as immuno-modulatory and anti-inflammatory compounds, suggesting a protective mechanism of action (2–5). Clinical studies have also shown that statins have been found to be effective in decreasing cardiac events in persons with average cholesterol levels and reduce inflammatory markers (6–8). Furthermore, in vitro, animal and human studies have found statin to have anti-inflammatory and mucolytic actions (9–14).
Observational studies, via both retrospective and prospective analysis, have shown that statins may be useful in reducing mortality rates in patients with chronic obstructive pulmonary disease (COPD) (15–18). Furthermore, it has been observed that, in a murine model of allergic asthma, statins had an immune-modulatory effect through several different anti-inflammatory pathways that still require further elucidation (19). In general, statins beneficial effects cannot entirely be attributed to reduction of lipid levels (20,21).
Statins have been investigated as an adjunct therapy to enhance current asthma therapies, i.e. the use of corticosteroids (22–24), although a meta-analysis of randomised controlled trials showed that statins did not improve lung functions in asthmatic patients (25). However, in these studies, the statin was taken via the oral systemic route, rather than topically. Indeed, this class of drugs has not been previously formulated as inhalation products, more specifically at low dose ranges, to be used as an anti-inflammatory, rather than their classical use as anti-cholesterolemic (26).
Among the drug delivery options available, i.e. nebulizers and dry powder inhalers, pressurised metered-dose inhalers (pMDIs) are the most common inhalation devices used for the treatment of respiratory diseases (27). Drug-dissolved formulations containing co-solvents (i.e. ethanol) are advantageous over suspension formulation, since the aerosol performance can be improved via modification of the ethanol concentration and actuator geometry (28,29). Therefore, the aim of this study was to formulate a statin, specifically simvastatin (SV), as a low-dose solution pMDI formulation. To evaluate its characteristics in terms of aerosol performance, and physico-chemical features, the Andersen Cascade Impactor (ACI), laser diffraction and thermo analysis techniques were used. In addition, the short-term stability of this SV pMDI solution formulation and its conversion into its active metabolite, simvastatin hydroxy acid (SVA), was also investigated.
MATERIALS AND METHODS
Materials
Simvastatin (SV) was used as supplied (Jayco Chemical Industries, Thane, India). The metabolite and degradation products, simvastatin hydroxy acid (SVA), were manufactured in house according the following procedure: 41.8 mg of SV was dissolved in 1 mL of absolute ethanol; 1.5 mL of NaOH 1 N was added to the SV ethanol solution. The solution was then incubated at 50°C. After 2 h, the pH of the solution was adjusted to 7.2 with HCL. Deionised water was then added to the solution up to 10 mL, and the solution was stored at −20°C (12) until further use.
The propellant 1,1,1,2-tetrafluoroethane (HFA-134a) was supplied from Solvay Chemicals (Germany). Ethanol and acetonitrile (100%) were purchased from Biolab (Clayton, Victoria, Australia) and Thermo Fisher Scientific Australia Pty Ltd (Scoresby, VIC, Australia), respectively. Water was purified by reverse osmosis (MilliQ, Millipore, France). All solvents were analytical grade and were supplied by Sigma (Sydney, NSW, Australia). Aluminium canisters, metering valves (50 μL) and actuators with orifice diameters of 0.33 mm were from Bespack Europe Limited (Norfolk, UK).
Determination of Solubility of Simvastatin Propellant/Ethanol Mixtures
Before preparing a suitable solution-based pMDI formulation, the solubility in propellant/ethanol mixtures was investigated visually. Solutions containing SV (0.5% w/w) and ethanol, at concentrations ranging from 2 to 12% w/w, were produced. An aliquot of these stock solutions (ranging from 300 to 1,500 μL) were directly weighed into pressure-resistant glass pMDI containers (Saint Gobain plc., London, UK), which were immediately crimped with a 50 μL metering valve (Bespack Europe Limited, Norfolk, UK) and pressure-filled with HFA-134a using a Pamasol Laboratory plant 02016 (Pamasol Willi Maäden AG, Paffikon, Schwyz, Switzerland) to a total mass of 10 g. After filling, each canister was sonicated for 10 min and stored for a minimum of 24 h at 25°C prior to further studies to assess SV solubility in HFA/ethanol visually.
Subsequently, based on the visual inspection of SV solubility in different concentrations of ethanol, the solubility of SV in HFA-134a/ethanol mixtures, containing 0 and 6% ethanol (w/w), was quantitatively determined using the solubility apparatus developed by Traini et al. (30). Briefly, excessive amounts of SV was first loaded into the secondary casing of the solubility apparatus and subsequently assembled with the primary casing using a stainless steel frit (0.1 μm) sandwiched between two slit O-rings. A 50-μL metering valve was then crimped onto the primary casing of the assembly, and the device was filled with approximately 7 g of HFA-134a/ethanol mixture. The filled apparatus was incubated at 25°C in a shaking water bath for 24 h prior to analysis. Finally, the formulation was fired to exhaustion into a dosage unit sampling apparatus (DUSA) as a collector device, using a 0.33-mm pMDI actuator (Bespack Europe Ltd). Each part of DUSA components, actuator, valve and primary casing was rinsed with acetonitrile:water (65:35 v/v) and chemically analysed using high performance liquid chromatography (HPLC). The tests were performed in triplicate, and results were interpreted in terms of SV to HFA-134a/ethanol weight fraction (w/w).
Simvastatin pMDI Formulation
According to results obtained from the solubility study of SV in HFA/ethanol mixture, only the formulation containing 6% (w/w) ethanol and 0.5% (w/w) SV (delivering 300 μg dose per actuation) was chosen for further studies. It is known that increasing ethanol concentration leads to an increase in droplet size and evaporation time, resulting in a decrease in aerosol performance (31). All test formulations were prepared in aluminium pMDI canisters (Valois, France). The SV and ethanol were directly weighed into the canister, which was immediately crimped with a 50-μL metering valve and pressure-filled with HFA-134a using a Pamasol Laboratory plant 02016. After filling, each canister was sonicated for 10 min to ensure complete miscibility among components. Once manufactured, the pMDI formulations were stored in a temperature-controlled cabinet (Napco, Model 5410) at 4 and 25°C.
Post-Throat Droplet Size Measurement of pMDI SV Formulation Using Laser Diffraction
The size distribution of droplets emitted from the SV solution pMDI formulation post-United States pharmacopoeia (USP) throat were determined by laser diffraction using the Malvern Spraytec (Malvern Instrument Ltd., Worcestershire, UK) with the USP induction port connected to the inhalation cell. The vacuum pump was connected to the assembly and an airflow, set at 28.3 L/min using a calibrated flow metre (TSI 3063, TSI instruments Ltd., Buckinghamshire, UK), was generated through the flow cell. The first five shots of each pMDI canister were fired to waste before three consecutive actuations were fired into the Spraytec. Samples were assessed in triplicate. Data were described as droplet size (Dv50) expressed by 50% of the cumulative volume undersize and the fraction of droplets with a volume diameter ≤5 μm.
Thermal Characterisation of Aerosols Generated from the SV pMDI
The thermal characteristics of droplets generated from the SV pMDI were characterised using differential scanning calorimetry (DSC) and thermogravimetric analyses (TGA). An Andersen Cascade impactor (ACI) was assembled to capture aerosolised particles after actuation. Two freshly prepared pMDIs (200 shots each) were actuated into the impactor at 28.3 L/min, within an approximately 20-min period. Powder samples were collected from stage 5 of the ACI and ∼20 mg of the collected powder samples were transferred to a 40-μl sealed aluminium pan, pierced with a 1-mm pinhole to ensure constant pressure before being transferred immediately to the DSC-1 (DSC-Mettler-Toledo Ltd, Switzerland). Samples were heated under a N2 atmosphere at a rate of 10°C/min between 25 and 190°C.
Thermogravimetric analyses were carried out using a TGA/DSC-1 thermal analysis system (Mettler-Toledo Ltd, Switzerland). Collection of sample followed the same procedure as for the DSC measurements. About 7 mg of collected sample was transferred to an open aluminium crucible pan immediately after collection. The samples were heated from 25 to 140°C, at a heating rate of 10°C/min under a N2 atmosphere. The volatile loss was defined as the amount of the residual solvent trapped within the solid particles post-actuation and calculated as the percentage of weight loss from the collected powder.
Scanning Electron Microscopy
The particle morphology of the formulation was studied using a field emission scanning electron microscope (SEM) (Zeiss Ultra Plus, Carl Zeiss NTS GmbH, Oberkochen, Germany). Particles collected on ACI stage 5 were selected for SEM imaging due to highest mass deposition during aerosolisation and impaction. Circular adhesive carbon tapes were placed at the centre of the impactor on stage five and used to collect particles during the impaction after one actuation of the pMDIs at 28.3 L/min. The corresponding carbon tapes were mounted on aluminium SEM stubs and then sputter coated with gold (Sputter coater S150B, Edwards High Vacuum, Sussex, UK) at 10 nm thicknesses prior to imaging. SEM images were taken at the magnification of 20,000×.
Quantitative High Performance Liquid Chromatography Analysis
Chemical quantification of SV and its metabolite SVA, collected from aerosol performance and stability studies, was performed using high performance liquid chromatography (HPLC). A Shimadzu HPLC system consisting of a LC20AT pump, the SIL20AHT autosampler and an SPD-20A UV-VIS detector (Shimadzu, Sydney, NSW, Australia) was used. Analysis of SV was achieved using a reverse phase C-18 column (Phenomenex ODS hypersclone) 250 × 4.6, 5-μm particle size. The mobile phase comprised a mixture of acetonitrile:water (65:35 v/v) and 0.025 M sodium dihydrogen phosphate with pH adjusted to pH 4.5 with phosphoric acid. The mobile phase was filtered under vacuum. The HPLC was set to the following conditions: flow rate of 1.5 mL/min and peaks analysed with a UV detector wavelength of 238 nm with 100 μL of injection volume. All collected samples were diluted in the mobile phase to be within the linearity region from 0.05 to 50 μg/mL for SV and SVA. Retention time for SV was 9.1 and for SVA was 5.5 min, respectively. Quantification was calculated based on peak area integration with fresh standard solutions prepared daily. The method was validated with regard to variability, recovery, linearity, detection limit and range, and proved to be suitable for this study. The regression coefficient (R2) was 0.997, and the calibration curve of HPLC assay for determining SV and SVA in the concentration range of 0.05–50 μg/mL.
Chemical Stability Study of Simvastatin
The chemical stability over time of the SV solution pMDI formulation was assessed using a dose uniformity apparatus (DUSA, Copley Scientific Limited, Nottingham, UK) up to 6 months of storage, at 4 and 25°C. Dose uniformity tests were performed at days 0, 7, 14, 22, 30, 60, 90 and 180. Ten doses from each canister stored at different temperature conditions were fired into a DUSA at the calibrated flow rate of 28.3 L/min. The canister was left to stand for 30 s between shots. Immediately before each experiment, the pMDI was shaken for 30 s. pMDI valves were primed by discharging five shots to waste. Each pMDI was weighed before and after each actuation to calculate total weight loss for each canister. After actuation into the DUSA, all components, actuator and mouthpiece adaptor were rinsed into suitable volumetric flasks with acetonitrile:water solution (65:35 v/v) for HPLC analysis. Each sample was centrifuged for 5 min at 2,000 rpm to remove filter matter and the supernatant collected for HPLC analysis. All experiments were performed in triplicate.
In Vitro Aerosolisation Studies by ACI
Evaluation of aerosol performance of the SV pMDI solution formulation was assessed using an ACI (Copley Scientific Limited, Nottingham, UK). The flow rate was set at 28.3 L/min using a calibrated flow metre (TSI 3063, TSI instruments Ltd., Buckinghamshire, UK). Prior to the aerosol characterisation, five shots of each canister were fired to waste. The pMDI was inserted into a rubber adaptor connected to the UPS throat. Before each pMDI was discharged into the ACI, the pump was switched on for 5 s to insure system equilibration. One shot was actuated into the impactor using a 0.33-mm actuator by pressing the canister and holding for 7 s. The actuator, rubber adaptor, USP throat, ACI stages and filter stage (Sartorius Stedim Pty Ltd, Australia) were each rinsed with 10 mL of acetonitrile:water solution (65:35 v/v) for SV and SVA chemical quantification. After each experiment, the ACI components and actuator were washed with distilled water followed by methanol, and dried in an oven at 40°C before cooling and reuse. Each experiment was performed in triplicate.
The concentration on each stage of the ACI and device was calculated, and stage data was plotted as log-probability plots of cumulative percentage versus the ACI cutoff diameters (9.0, 5.8, 4.7, 3.3, 2.1, 1.1, 0.7 and 0.4 μm, respectively). The mass median aerodynamic diameter (MMAD) was calculated as the 50th percentile mass distribution, whilst the geometric standard deviation (GSD) was calculated as the square root of the 84th/16th percentiles. The fine particle fraction (FPF) was defined as the percentage of SV mass deposited from stage 3 to filter (corresponding to the cutoff size ≤5 μm) as a function of the ex-valve dose (ED), and fine particle dose (FPD) is the mass of drug with aerodynamic diameter lower than 5 μm. The impaction study was carried out in triplicate for each formulation at specific time points during stability testing.
Statistical Analysis
All results are expressed as mean ± standard deviation for ≥3 separate determinants. Unpaired 2-tailed t tests were performed to determine significance (which was quoted at the level of P < 0.05) between treatment groups.
RESULTS AND DISCUSSION
Solubility of Simvastatin Propellant/Ethanol Mixture
SV is poorly soluble in HFA-134a propellant with a maximum solubility of 0.25 ± 0.12 mg/g. Hence, ethanol was introduced as a co-solvent to improve its solubility. The saturated ethanol solubility of SV was acquired from the literature (32). All the formulations prepared with SV in HFA/ethanol mixtures (2–12% w/w) were examined visually, and complete miscibility was observed among the components above 4% w/w. Previous studies have shown that increasing ethanol concentration leads to larger droplet sizes and evaporation times, and subsequently decreases the fine particle fraction and reduces aerosol performance (31). Hence, only the SV formulation containing 6% of EtOH was chosen for aerosol aerodynamic assessment and the stability. This concentration of ethanol allowed for the formulation to achieve a 300-μg SV dose when using a 50-μL valve. At this dose, the solubility of SV in the formulation is 7.14 mg/g, well below the saturated solubility of 16.34 ± 4.02 mg/g SV in the 6% EtOH formulation. This dose was selected based on a study by Xu L et al., where nebulisation of 1–20 mg/mL simvastatin presented potent anti-inflammatory effect in mice, with only 3 to 4% of simvastatin actually delivered to the lung tissue (33).
Post-USP Throat Droplet Size Measurement
Droplet/particle size distributions for SV pMDI solution formulation measured post-USP throat were determined by laser diffraction at 28.3 L/min (Fig. 1). Analysis of the percentile undersize values showed SV aerosol components to have values of D0.1 = 0.91 ± 0.04 μm, D0.5 = 2.13 ± 0.11 μm and D0.9 = 4.99 ± 0.29 μm, respectively, with a span of 1.92 ± 0.05, indicating that the aerosol possessed a suitable size for drug delivery to the lung.
Fig. 1.
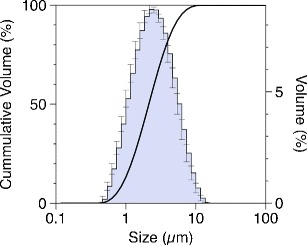
Particle size distribution of the SV pMDI solution formulation containing 6% w/w ethanol as co-solvent
Thermal Characterisation of Aerosols Generated from the SV pMDI
The thermal behaviour of the maturated particles, generated from the SV pMDI solution and collected on stage 5 of the ACI formulation, was characterised using DSC and TGA, respectively.
The DSC thermograph of both the aerosol particles and starting material is shown in Fig. 2. An endothermic peak that correlated to the melting point of the raw SV and SV pMDI was found to be 140°C for both materials and was in good correlation with previous study (34). For the pMDI aerosol particles, a broad endothermic event was observed between 60 and 113°C. To further investigate the individual events, first derivative of the DSC thermograph was performed. The glass transition step at around 30°C was followed by a crystallisation exothermic peak at 90°C (35). Furthermore, due to volatile loss of residual ethanol, melting point was at 113°C (36), suggesting the aerosol particles to be amorphous.
Fig. 2.
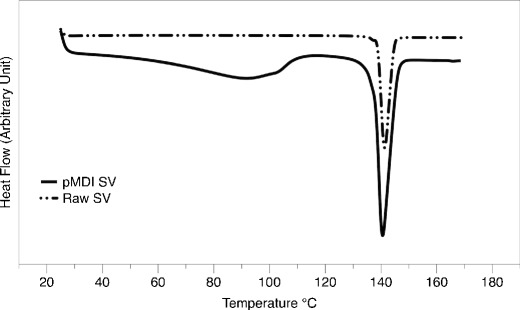
Differential scanning calorimetry of SV raw material and SV pMDI solution formulation containing 6% w/w ethanol
To further understand the physico-chemical characteristics of this formulation, the influence of weight lost was also investigated. The mass loss observed by TGA for SV pMDI solution from 25 to 140°C was around 3.25%, and was most likely attributed to the evaporation of ethanol, and moisture and coupled with glass transition step followed by crystallisation (figure not shown).
Morphological Evaluation of Aerosols
Representative scanning electron micrographs of particles captured on impactor stage 5 are shown in Fig. 3. Aerosols produced from the formulation had identical spherical shape with smooth morphology, suggesting the aerosol particles to be amorphous. The smooth surface morphology probably resulted from the co-evaporation process of ethanol and propellant components, confirming the observations made from the DSC thermogram of SV pMDI particles (37).
Fig. 3.
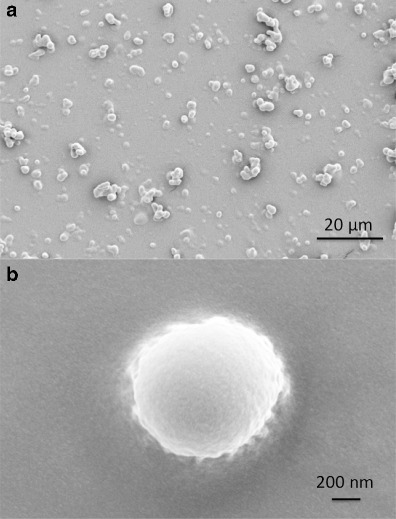
Representative SEM images of a a population of SV pMDI particles collected from stage 5 after actuation and deposition on stage 5 of the ACI and b a close-up image of a single particle of SV pMDI
Chemical Stability Study of SV
The uniformity of dose through use and consequently the chemical stability of the 6% EtOH SV solution pMDI formulation assessed by DUSA at 4 and 25°C, respectively, up to 180 days is presented in Fig. 4. The ED fell within the pharmacopoeia specification of ±25% except at 180 days for 25°C (38). In general, SV showed no chemical degradation to its active metabolite SVA, when compared to time point zero measurements, for the formulations stored at 4°C, over a 180-day period, and the formulations stored at 25°C up to 90 days.
Fig. 4.
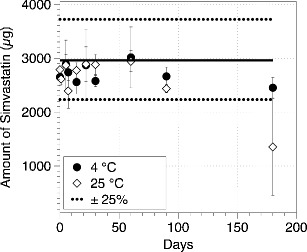
Drug dose recovery of SV pMDI formulation with 6% w/w ethanol collected using a DUSA at 28.3 L/min at 4 and 25°C for 180 days (n = 3, mean ± SD), each data point around mean target dose (300 mg)
Simvastatin, being a lactone, is susceptible by hydrolysis to be converted to SVA at room temperature, with anhydrous simvastatin as another possible degradation product that might appear during acid stress (i.e. under different conditions such as high pH, as well as under presence of different solvents i.e. ethanol) (39). However, from the chemical analysis, no noticeable degradation peaks were observed when compared to time zero during the stability study performed. Long-term stability of this formulation is warranted and is currently on going.
In Vitro Aerosol Performance
One actuation of the SV pMDI solution formulation was fired into the induction port of an ACI at 28.3 L/min, and samples collected from the induction port, stages and actuator housing were measured and the FPF, FPD, ED, MMAD and GSD calculated (Table I). Fig. 5 shows the percentage drug distribution of SV pMDI solution formulation on each stage of the ACI, at time 0, 60, 90 and 180 days of storage at 4 and 25°C, respectively.
Table I.
Fine Particle Fraction (FPF), Fine Particle Dose (FPD), Emitted Dose (ED), Mass Median Aerodynamic Diameter (MMAD) and Geometric Standard Deviations (GSD) of SV pMDI Formulations Stored at Different Temperatures up to 180 Days
| Days | FPF (%) | FPD (μg) | ED (μg) | MMAD (μm) | GSD |
|---|---|---|---|---|---|
| Day 0 | 31.88 ± 2.79 | 97.41 ± 8.62 | 103.45 ± 7.97 | 1.58 ± 0.08 | 2.12 ± 0.05 |
| Day 60, 4°C | 36.31 ± 3.12 | 97.12 ± 14.12 | 103.63 ± 13.11 | 1.87 ± 0.32 | 1.97 ± 0.11 |
| Day 90, 4°C | 39.24 ± 1.19 | 110.3 ± 9.6 | 112.51 ± 9.27 | 1.55 ± 0.06 | 1.98 ± 0.15 |
| Day 180, 4°C | 36.13 ± 0.69 | 112.62 ± 0. 32 | 121.15 ± 1.39 | 1.91 ± 0.37 | 2.017 ± 0.14 |
| Day 60, 25°C | 36.11 ± 2.56 | 97.45 ± 9.39 | 101.09 ± 9.31 | 1.56 ± 0.15 | 2.03 ± 0.25 |
| Day 90, 25°C | 34.77 ± 7.90 | 86.65 ± 26.96 | 91.35 ± 24.55 | 1.41 ± 0.13 | 2.08 ± 0.14 |
Fig. 5.
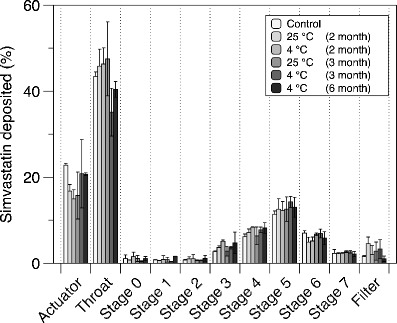
Aerosol mass deposition of the SV pMDI solution formulation containing 6% w/w ethanol at times: 0, 2, 3 and 6 months storage at 4 and 25°C temperature
At time zero, the total dose delivered per shot was 306 ± 8.2 μg (target dose = 300 μg). The FPF, MMAD and GSD were 31.88 ± 2.79%, 1.58 ± 0.08 μm and 2.12 ± 0.05, respectively. SV pMDI solution formulation presented a high pMDI actuator (22 ± 0.35%) and induction port (44 ± 0.86%) deposition. These results are not unexpected since it is known that solution-based pMDIs containing ethanol as co-solvent possess a plume with a high exit velocity and long drying times when compared to HFA alone. Indeed, previous studies using ethanol formulation of Qvar-100 reported ∼50% loss in the actuator and induction port (40).
The results of the FPF after 60, 90 and 180 days of storage at 4°C, whilst only 60 and 90 days of storage at 25°C are shown in Table I. The total dose delivered was around 253.1 ± 43.3 μg, and the FPF was 36.11 ± 2.56 and 34.77 ± 7.90%, after 60 and 90 days of storage at 25°C, respectively. Whilst the total dose delivered was around 265.07 ± 32.3 μg at 4°C, the FPF after 60 days was 36.31 ± 3.12%, after 90 days was 40.22 ± 1.19% and after 180 days was 36.13 ± 0.69%. There was no significant difference in SV aerosols performance (FPF) among the different days, whilst the SV formulations were still stable (P > 0.05). Therefore, the SV pMDI formulation in this study was found to be chemically stable and suitable for inhalation drug delivery.
CONCLUSIONS
In this study, the formulation of SV as pMDI solution as an anti-inflammatory therapeutic for the treatment of lung inflammations has been presented. This formulation was showed to be chemically stable up to 180 days at 4 °C and up to 90 days at 25 °C, in addition to its suitable in vitro aerosol performance for lung delivery. The thermal properties of the SV solution pMDI were also characterised. This investigation has the potential to open up the use of inhaled low-dose simvastatin as a new anti-inflammatory therapy for chronic lung diseases. Future studies will assess the effect of this formulation on cell viability, drug uptake, anti-inflammatory and mucolytic effects, as well as in vivo pharmacokinetic and toxicity using small animal models.
Acknowledgments
A/Professor Young is the recipient of an Australian Research Council Future Fellowship (project number FT110100996). A/Professor Traini is the recipient of an Australian Research Council Future Fellowship (project number FT12010063). The authors are grateful to Mr. Eric Zhu for his advice on the solubility study of the pMDI formulations. Alaa Tulbah is also appreciative to Umm Al-Qura University for the Scholarship and the Saudi Government for its financial support.
Abbreviations
- ACI
Andersen Cascade Impactor
- COPD
Chronic obstructive pulmonary disease
- °C/min
Temperature degree per minute
- DUSA
Dose uniformity apparatus
- DSC
Differential scanning calorimetry
- Dv0.1
10% of the volume distribution is below this value
- Dv0.5
The volume median diameter is the diameter where 50% of the distribution is above and 50% is below
- Dv0.9
90% of the volume distribution is below this value
- EtOH
Ethanol
- FPF
Fine particle fraction
- ED
Ex-valve dose
- GSD
Geometric standard deviation
- HCL
Hydrochloride
- HFA-134a
1,1,1,2-tetrafluoroethane
- HPLC
High performance liquid chromatography
- HMG-CoA
3-hydroxy-3-methyl-glutaryl-Coenzyme A
- h
Hour
- L
Litre
- L/min
Litres per minute
- mm
Millimetre
- mg
Milligramme
- mL
Millilitres
- mM
millimolar
- MMAD
Mass median aerodynamic diameter
- NaOH
Sodium hydroxide
- pMDI
Pressured metered-dose inhaler
- pH
Measure of the acidity or basicity of an aqueous solution
- rpm
Rounds per minute
- RH
Relative humidity
- R2
Regression coefficient
- SV
Simvastatin
- SVA
Simvastatin hydroxy acid
- SD
Standard deviation
- s
Second
- TGA
Thermogravimetric analyses
- μg/mL
Micrograms per millilitre
- μL
Microlitre
- μm
Micro metre
- μg
Microgram
- v/v
Volume per volume
- w/w
Weight per weight
- %
Percentage
References
- 1.Tobert JA. Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors. Nat Rev Drug Discov. 2003;2(7):517–526. doi: 10.1038/nrd1112. [DOI] [PubMed] [Google Scholar]
- 2.Endo A, Tsujita Y, Kuroda M, Tanzawa K. Inhibition of cholesterol synthesis in vitro and in vivo by ML-236A and ML-236B, competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Euro J Biochem/ FEBS. 1977;77(1):31–36. doi: 10.1111/j.1432-1033.1977.tb11637.x. [DOI] [PubMed] [Google Scholar]
- 3.Vaughan CJ, Murphy MB, Buckley BM. Statins do more than just lower cholesterol. Lancet. 1996;348(9034):1079–1082. doi: 10.1016/S0140-6736(96)05190-2. [DOI] [PubMed] [Google Scholar]
- 4.McAuley DF, O'Kane CM, Craig TR, Shyamsundar M, Herwald H, Dib K. Simvastatin decreases the level of heparin-binding protein in patients with acute lung injury. BMC Pulm Med. 2013;13:47. doi: 10.1186/1471-2466-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grommes J, Vijayan S, Drechsler M, Hartwig H, Morgelin M, Dembinski R, et al. Simvastatin reduces endotoxin-induced acute lung injury by decreasing neutrophil recruitment and radical formation. PLoS ONE. 2012;7(6):e38917. doi: 10.1371/journal.pone.0038917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plenge JK, Hernandez TL, Weil KM, Poirier P, Grunwald GK, Marcovina SM, et al. Simvastatin lowers C-reactive protein within 14 days: an effect independent of low-density lipoprotein cholesterol reduction. Circulation. 2002;106(12):1447–1452. doi: 10.1161/01.CIR.0000029743.68247.31. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) investigators. Circulation. 1998;98(9):839–844. doi: 10.1161/01.CIR.98.9.839. [DOI] [PubMed] [Google Scholar]
- 8.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. New Engl J Med. 1996;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 9.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6(12):1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 10.Sparrow CP, Burton CA, Hernandez M, Mundt S, Hassing H, Patel S, et al. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler Thromb Vasc Biol. 2001;21(1):115–121. doi: 10.1161/01.ATV.21.1.115. [DOI] [PubMed] [Google Scholar]
- 11.Weitz-Schmidt G. Statins as anti-inflammatory agents. Trends Pharmacol Sci. 2002;23(10):482–486. doi: 10.1016/S0165-6147(02)02077-1. [DOI] [PubMed] [Google Scholar]
- 12.Marin L, Colombo P, Bebawy M, Young PM, Traini D. Chronic obstructive pulmonary disease: patho-physiology, current methods of treatment and the potential for simvastatin in disease management. Expert Opin Drug Deliv. 2011;8(9):1205–1220. doi: 10.1517/17425247.2011.588697. [DOI] [PubMed] [Google Scholar]
- 13.Marin L, Traini D, Bebawy M, Colombo P, Buttini F, Haghi M, et al. Multiple dosing of simvastatin inhibits airway mucus production of epithelial cells: implications in the treatment of chronic obstructive airway pathologies. EurJ Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2013;84(3):566–572. doi: 10.1016/j.ejpb.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Shyamsundar M, McKeown ST, O'Kane CM, Craig TR, Brown V, Thickett DR, et al. Simvastatin decreases lipopolysaccharide-induced pulmonary inflammation in healthy volunteers. Am J Respir Crit Care Med. 2009;179(12):1107–1114. doi: 10.1164/rccm.200810-1584OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishida W, Kajiwara T, Ishii M, Fujiwara F, Taneichi H, Takebe N, et al. Decrease in mortality rate of chronic obstructive pulmonary disease (COPD) with statin use: a population-based analysis in Japan. Tohoku J Exp Med. 2007;212(3):265–273. doi: 10.1620/tjem.212.265. [DOI] [PubMed] [Google Scholar]
- 16.Soyseth V, Brekke PH, Smith P, Omland T. Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007;29(2):279–283. doi: 10.1183/09031936.00106406. [DOI] [PubMed] [Google Scholar]
- 17.Mancini GB, Etminan M, Zhang B, Levesque LE, FitzGerald JM, Brophy JM. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006;47(12):2554–2560. doi: 10.1016/j.jacc.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 18.Dobler CC, Wong KK, Marks GB. Associations between statins and COPD: a systematic review. BMC Pulm Med. 2009;9:32. doi: 10.1186/1471-2466-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKay A, Leung BP, McInnes IB, Thomson NC, Liew FY. A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J Immunol. 2004;172(5):2903–2908. doi: 10.4049/jimmunol.172.5.2903. [DOI] [PubMed] [Google Scholar]
- 20.Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Dis. 2007;7(5):358–368. doi: 10.1016/S1473-3099(07)70111-1. [DOI] [PubMed] [Google Scholar]
- 21.Kopterides P, Falagas ME. Statins for sepsis: a critical and updated review. Clin Microbiol Infect: Off Publ Eur Soc Clin Microbiol Infect Dis. 2009;15(4):325–334. doi: 10.1111/j.1469-0691.2009.02750.x. [DOI] [PubMed] [Google Scholar]
- 22.Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2,3-dioxygenase. J Allergy Clin Immunol. 2010;126(4):754–762 e1. doi: 10.1016/j.jaci.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Lokhandwala T, West-Strum D, Banahan BF, Bentley JP, Yang Y. Do statins improve outcomes in patients with asthma on inhaled corticosteroid therapy? A retrospective cohort analysis. BMJ open. 2012;2(3):e001279. doi:10.1136/bmjopen-2012-001279. [DOI] [PMC free article] [PubMed]
- 24.Walker DY, Edwards KL. Statins in the treatment of asthma. Am J Health-Syst Pharm : AJHP : Off J Am Soc Health-Syst Pharm. 2013;70(19):1661–1669. doi: 10.2146/ajhp120680. [DOI] [PubMed] [Google Scholar]
- 25.Si XB, Zhang S, Huo LY, Dai WL, Wang HL. Statin therapy does not improve lung function in asthma: a meta-analysis of randomized controlled trials. J Int Med Res. 2013;41(2):276–283. doi: 10.1177/0300060513477005. [DOI] [PubMed] [Google Scholar]
- 26.Yuan C, Zhou L, Cheng J, Zhang J, Teng Y, Huang M, et al. Statins as potential therapeutic drug for asthma? Respir Res. 2012;13:108. doi: 10.1186/1465-9921-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brocklebank D, Wright J, Cates C. Systematic review of clinical effectiveness of pressurised metered dose inhalers versus other hand held inhaler devices for delivering corticosteroids in asthma. BMJ. 2001;323(7318):896–900. doi: 10.1136/bmj.323.7318.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smyth H, Hickey AJ, Brace G, Barbour T, Gallion J, Grove J. Spray pattern analysis for metered dose inhalers I: orifice size, particle size, and droplet motion correlations. Drug Dev Ind Pharm. 2006;32(9):1033–1041. doi: 10.1080/03639040600637598. [DOI] [PubMed] [Google Scholar]
- 29.Gupta A, Stein SW, Myrdal PB. Balancing ethanol cosolvent concentration with product performance in 134a-based pressurized metered dose inhalers. J Aerosol Med. 2003;16(2):167–174. doi: 10.1089/089426803321919924. [DOI] [PubMed] [Google Scholar]
- 30.Traini D, Young PM, Price R, Rogueda P. A novel apparatus for the determination of solubility in pressurized metered dose inhalers. Drug Dev Ind Pharm. 2006;32(10):1159–1163. doi: 10.1080/03639040600920325. [DOI] [PubMed] [Google Scholar]
- 31.Stein SW, Myrdal PB. A theoretical and experimental analysis of formulation and device parameters affecting solution MDI size distributions. J Pharm Sci. 2004;93(8):2158–2175. doi: 10.1002/jps.20116. [DOI] [PubMed] [Google Scholar]
- 32.Brambilla G, Ganderton D, Garzia R, Lewis D, Meakin B, Ventura P. Modulation of aerosol clouds produced by pressurised inhalation aerosols. Int J Pharm. 1999;186(1):53–61. doi: 10.1016/S0378-5173(99)00137-4. [DOI] [PubMed] [Google Scholar]
- 33.Xu L, Dong XW, Shen LL, Li FF, Jiang JX, Cao R, et al. Simvastatin delivery via inhalation attenuates airway inflammation in a murine model of asthma. Int Immunopharmacol. 2012;12(4):556–564. doi: 10.1016/j.intimp.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Murtaza G. Solubility enhancement of simvastatin: a review. Acta Pol Pharm. 2012;69(4):581–590. [PubMed] [Google Scholar]
- 35.Graeser KA, Strachan CJ, Patterson JE, Gordon KC, Rades T. Physicochemical properties and stability of two differently prepared amorphous forms of simvastatin. Crystal Growth & Design. 2008;8:128–135. doi: 10.1021/cg700913m. [DOI] [Google Scholar]
- 36.Ma F, Hanna MA. Biodiesel production: a review. Bioresour Technol. 1999;70:1–15. doi: 10.1016/S0960-8524(99)00025-5. [DOI] [Google Scholar]
- 37.Zhu B, Traini D, Chan H, Young P. The effect of ethanol on the formation and physico-chemical properties of particles generated from budesonide solution-based pressurized metered dose inhalers. Drug Dev Ind Pharm. 2013;11:1625–1637. doi: 10.3109/03639045.2012.728230. [DOI] [PubMed] [Google Scholar]
- 38.British Pharmacopoeia Commission. Preparations for Inhalations, Volume III Formulated Preparations: General Monographs. The British Pharmacopoeia. 2013.
- 39.Malenovic A, Jancic-Stojanovic B, Ivanovic D, Medenica M. Forced degradation studies of simvastatin using microemulsion liquid chromatography. J Liq Chromatogr R T. 2010;33(4):536–547. doi: 10.1080/10826070903574576. [DOI] [Google Scholar]
- 40.Mitchell JP, Nagel MW, Wiersema KJ, Doyle CC. Aerodynamic particle size analysis of aerosols from pressurized metered-dose inhalers: comparison of Andersen 8-stage cascade impactor, next generation pharmaceutical impactor, and model 3321 Aerodynamic Particle Sizer aerosol spectrometer. AAPS PharmSciTech. 2003;4(4):E54. doi: 10.1208/pt040454. [DOI] [PMC free article] [PubMed] [Google Scholar]


